Abstract
The response of lymphocytes from 17 patients with primary, secondary, and tertiary syphilis to phytohaemagglutinin (PHA) and to allogeneic lymphocytes was normal in heterologous serum; however, the responsiveness of cells from some patients with primary and secondary disease was significantly reduced in the presence of autologous serum. As cells from healthy controls invariably responded better to these stimuli in autologous serum, the sera from 81 patients with syphilis were screened for immunosuppressive properties. Sera from 25 primary, 32 secondary, two tertiary, six congenital, and 16 latent cases of syphilis were examined for their ability to reduce the responsiveness of normal cells to PHA. These experiments were performed with test sera as the sole source of serum for the cultures or with test sera added to cultures containing optimally supportive amounts of pooled human plasma.
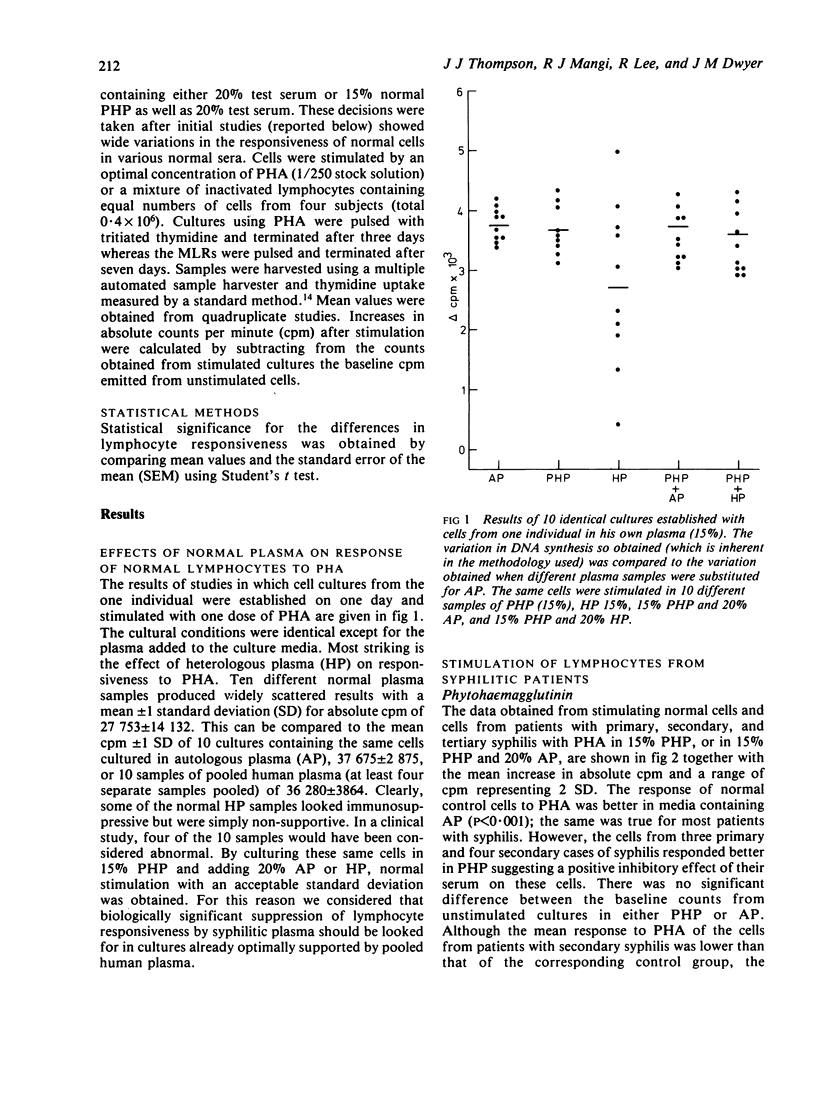
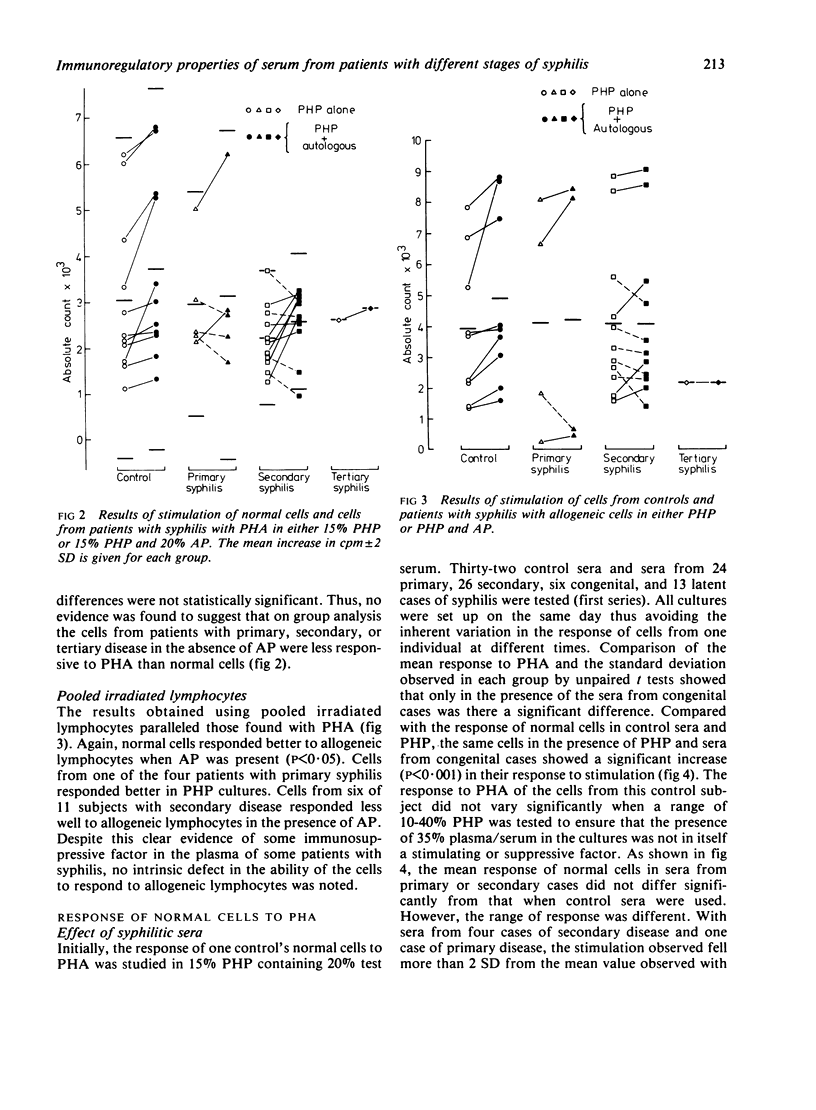
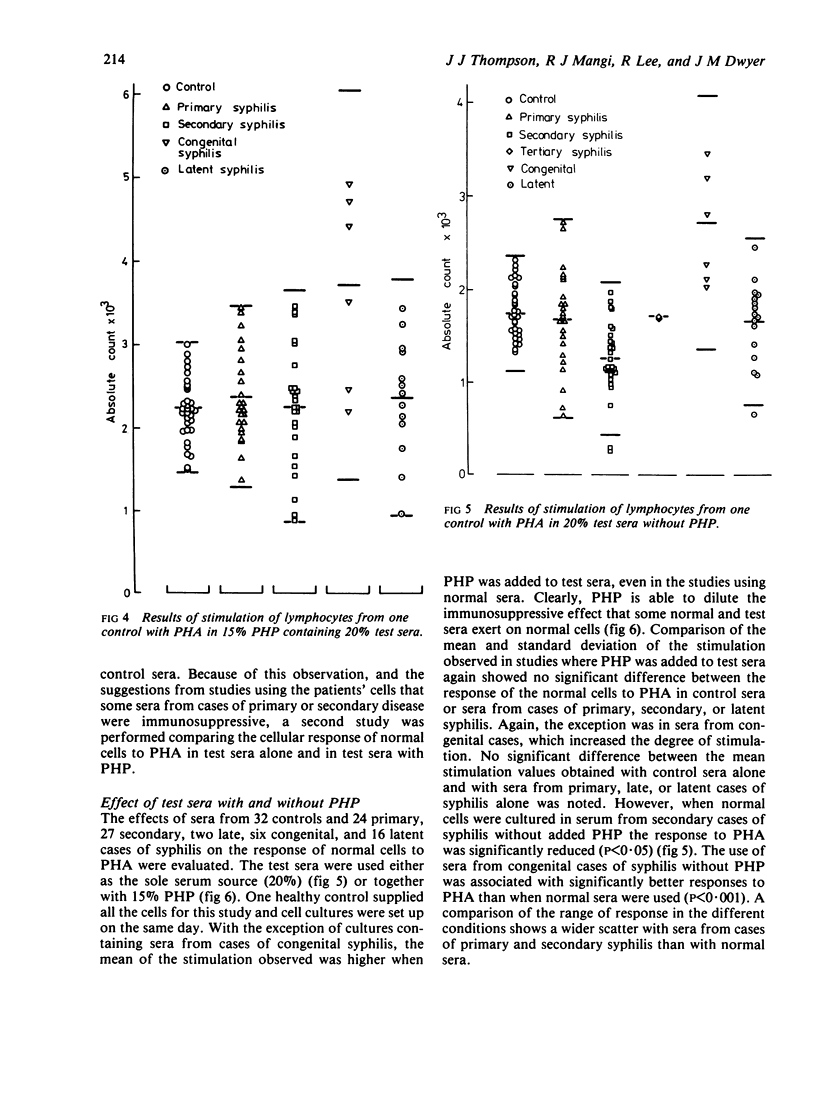
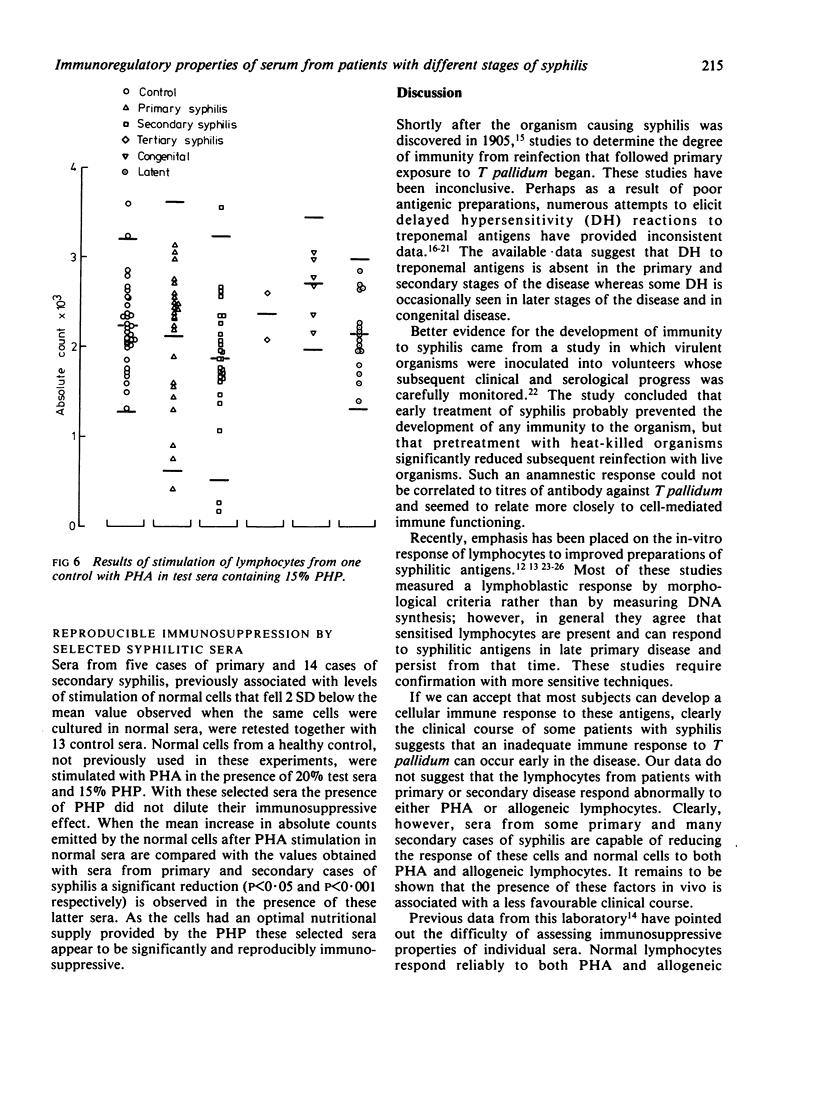
Stimulation of normal cells from one control in human plasma and 20% test serum showed that only in sera from congenital cases of syphilis was the mean response significantly different from the response seen in control sera; a significant increase in the response to stimulation occurred. The range of response to PHA with sera from cases of primary and secondary syphilis was wider than with normal sera. Sera from five (20%) cases of primary and 14 (44%) cases of secondary syphilis appeared to be immunosuppressive. When retested on another sample of normal cells, these sera were consistently immunosuppressive even in the presence of 15% pooled human plasma. Thus, in early syphilis antigenic stimulation may result in the release from suppressor cells of non-specific immunoregulators of cell-mediated immunity. Such phenomena may be a prelude to the development of tolerance to treponemal antigens. In congenital syphilis the development of suppressor cells may be impaired, resulting in the apparent immunostimulatory properties of serum from such cases.
Full text
PDF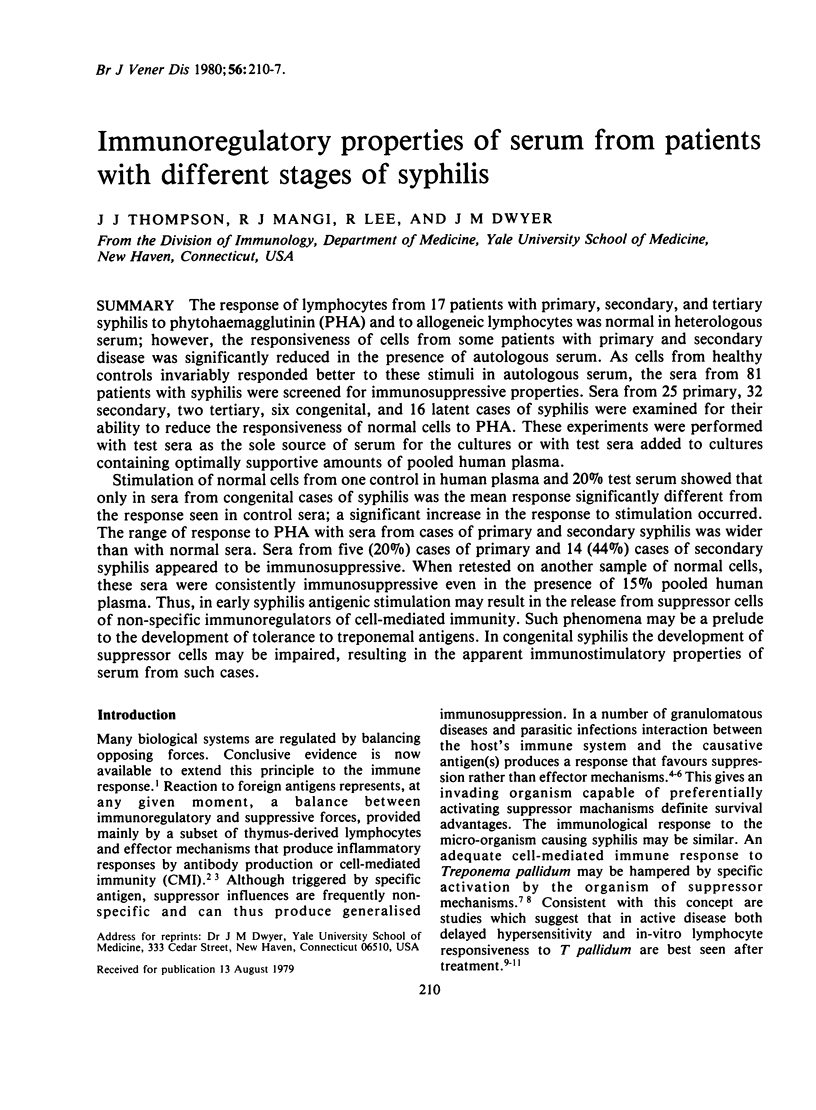



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basset A., Badanoiu A., Grosshans E., Heid B., Tardieu J. C., Ermolieff S., Maleville J. Studio sulla reattività immuno-cellulare nella lue con il test della trasformazione blastica dei linfociti in vitro (T.T.L. Minerva Med. 1972 Jul 28;63(56):3031–3036. [PubMed] [Google Scholar]
- Beurey J., Weber M., Janot C., Grandidier M., Pupil P. L'allergie au cours de la syphilis. Intérêt du test de transformation lymphoblastique. Ann Dermatol Syphiligr (Paris) 1971;98(5):536–538. [PubMed] [Google Scholar]
- Bullock W. W., Katz D. H., Benacerraf B. Induction of T-lymphocyte responses to a small molecular weight antigen. II. specific tolerance induced in azebenzenearsonate (ABA)-specific T cells in Guniea pigs by administration of low doses of an ABA conjugate of chloroacetyl tyrosine in incomplete Freund's adjuvant. J Exp Med. 1975 Aug 1;142(2):261–274. doi: 10.1084/jem.142.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieregato G. C., Faldarini G., Pagnes P. La stimolazione linfocitaria in vitro in soggetti luetici. Minerva Dermatol. 1967 Aug;42(8):406–407. [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Modern concepts of antigen binding receptors on lymphocytes: their nature and role in immune responses. Rev Eur Etud Clin Biol. 1971 Oct;16(8):743–751. [PubMed] [Google Scholar]
- Dwyer J. M., Mangi R. J., Gee B., Kantor F. S. Comparison of the anergy of sarcoidosis with experimentally induced anergy in guinea pigs. Ann N Y Acad Sci. 1976;278:29–35. doi: 10.1111/j.1749-6632.1976.tb47013.x. [DOI] [PubMed] [Google Scholar]
- Feighery C., Whelan C. A., Weir D. G., Greally J. F. In vitro studies of suppressor cell function in human peripheral blood mononuclear cells. Clin Exp Immunol. 1978 Jun;32(3):459–465. [PMC free article] [PubMed] [Google Scholar]
- From E., Thestrup-Pedersen K., Thulin H. Reactivity of lymphocytes from patients with syphilis towards T. pallidum antigen in the leucocyte migration and lymphocyte transformation tests. Br J Vener Dis. 1976 Aug;52(4):224–229. doi: 10.1136/sti.52.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Janot C., Grandidier M., Pupil P., Thomas J. L., Beurey J., de Lavergne E. Le test de transformation lymphoblastique au cours de la syphilis. Presse Med. 1971 Oct 16;79(43):1901–1904. [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Laird S. M., Thorburn A. L. Assessment of the "luotest" in late syphillis. Br J Vener Dis. 1966 Jun;42(2):119–121. doi: 10.1136/sti.42.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMAS E. W., OLANSKY S., KAPLAN B. I., DE MELLO L., CUTLER J. C. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956 Feb;35(1):33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- Mangi R. J., Dwyer J. M., Kantor F. S. The effect of plasma upon lymphocyte response in vitro. Demonstration of a humoral inhibitor in patients with sarcoidosis. Clin Exp Immunol. 1974 Dec;18(4):519–528. [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIVOLET J., SIMERAY A., ROLLAND M., CHALLUT F. Etude de l'intradermoréaction aux suspensions de Tréponèmes formolées souche Nichols pathogène chez les syphilitiques et les sujets normaux. Ann Inst Pasteur (Paris) 1953 Jul;85(1):23–33. [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


