Abstract
Objective: In this paper, we aim to show that the immunogenicity of the lyophilized human rabies vaccine (Vero cells) (investigational vaccine) developed by Dalian Aleph Biomedical Co., Ltd. in healthy participants aged 10–60 years old is non-inferior to the lyophilized PVRV (positive control) manufactured by Liaoning Chengda Biotechnology Co., Ltd. (Shenyang, China), and that its safety is clinically acceptable. Method: A total of 2776 participants were enrolled in this study and divided into four groups: a five-dose test group, a five-dose control group, a four-dose test group, and a four-dose control group. The patients in the four-dose groups (Zagreb) were vaccinated on Days 0 (two doses), 7 (one dose), and 21 (one dose), and those in the five-dose groups (Essen) were vaccinated on Days 0, 3, 7, 14, and 28 (one dose each). The rabies-virus-neutralizing antibody assay with the RFFIT was used to assess the immunogenicity, and the adverse events (AEs) and serious adverse events (SAEs) were identified and collated. Results: The positive seroconversion rate was up to 100% on Days 14 and 35/42 after vaccination following any procedures in pre-immunization antibody-negative participants, and the positive seroconversion rate and geometric mean concentration (GMC) of the test groups (Zagreb and Essen vaccination procedures) was not inferior to that of the control groups. On Day 7 after vaccination, the immunogenicity of the Zagreb procedure with two doses of the vaccine on Day 0 was superior to the Essen procedure with one dose of vaccine, that is, the former had a higher seroconversion rate and RVNA titer. The non-inferiority criterion of immunogenicity was met for the whole population, the population aged 10–18 years and ≥18 years, and the pre-immunization antibody-positive population. The incidences of all AEs, solicited AEs, and unsolicited AEs in both groups were not statistically significant, and no vaccination-related SAEs were observed. Conclusion: The investigated vaccine is safe, its immunogenicity is non-inferior to that of the control vaccine, and the efficacy of the Zagreb procedure is superior to that of the Essen procedure 7 days after the first dose.
Keywords: lyophilized human rabies vaccine (Vero cells), Essen and Zagreb procedures, immunogenicity, safety, non-inferiority
1. Introduction
Rabies is a zoonosis caused by a rabies virus infection mainly spread when an infected animal bites or scratches a person or licks a person’s skin [1]. Rabies was discovered more than 4000 years ago, affects 150 countries and regions worldwide, and causes an estimated annual loss of USD 8.6 billion globally [2]. According to statistics, nearly 59,000 people worldwide die of rabies every year [3]. Nowadays, 95% of rabies cases worldwide occur in Asia and Africa, and almost all rabies victims are bitten by dogs [4,5,6]. China is a country that faces a severe rabies threat. In 2007, China reported 3300 cases of rabies [6]. In China, effective measures to control rabies include strengthening epidemic surveillance, issuing norms and guidelines for rabies exposure disposal, promoting the inclusion of the rabies vaccine and passive immunization preparations in medical insurance, and standardizing the behavior of urban dog breeding [6]. However, there are still no treatments for the clinical symptoms of rabies, and death is virtually the only endpoint for a patient developing any clinical symptoms of rabies [7].
Rabies differs from other infectious diseases in humans because timely and effective vaccination against rabies can prevent its onset, even after exposure to the virus. In China, with the increase in immunization with rabies vaccines, the incidence of rabies decreased from 0.047/100,000 people in 2016 to 0.014/100,000 people in 2020 [8]. Cell-based rabies vaccines, such as the purified Vero cell rabies vaccine (PVRV) approved in the mid-1980s, have been proven to be safe and effective for vaccinating millions of people worldwide for more than 40 years [9]. In addition, compared to the risks associated with the existing PVRVs, the biosafety risks related to the use of serum (contamination by bacteria, fungi, mycoplasma, and bovine viruses, as well as the induction of hypersensitivity) can be avoided during the manufacture of lyophilized PVRVs, which are expected to improve vaccination safety and reduce adverse side effects [10]. Lyophilized PVRVs have been successfully manufactured in China. For example, the lyophilized PVRVs manufactured by Liaoning Chengda Biotechnology Co., Ltd. (Shenyang, China) and Liaoning Yisheng Biopharm Co., Ltd. (Shenyang, China) have been approved by the National Health Commission of the People’s Republic of China and the former State Food and Drug Administration (SFDA, now the National Medical Products Administration (NMPA)) and have been sold and widely used throughout the country [11,12]. Studies have shown that both vaccines are effective at the WHO-recommended dose of ≥2.5 IU per single intramuscular injection (IM), and the concentration of the induced serum rabies virus neutralizing antibodies (RVNA) is ≥0.5 IU/mL.
Rabies vaccines can be classified into pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) [13]. As recommended by the WHO, there are two IM regimens for PEP: the five-dose regimen (Essen, 1-1-1-1-1) and the four-dose regimen (Zagreb, 2-1-1) [13,14]. According to studies on other rabies vaccines, both regimens have performed well in terms of safety and immunogenicity [15]. The Zagreb procedure has been widely used in clinical practice due to the lower number of injections required, lower cost, higher patient compliance, and earlier establishment of protection [16,17,18]. In this study, a randomized, double-blind, phase III clinical trial was conducted to evaluate the safety and immunogenicity of a lyophilized PVRV developed and manufactured by Dalian Aleph Biomedical Co., Ltd., and it was compared with the lyophilized PVRV from Liaoning Chengda Biotechnology Co., Ltd., which is widely used in China. This study aimed to show that the investigated vaccine had immunogenicity that is non-inferior to the control vaccine, has clinically acceptable safety, and that the early (Day 7) immunogenicity of the Zagreb procedure is superior to that of the Essen procedure. The goal of this clinical trial was to provide a basis for the implementation of the Zagreb procedure using the lyophilized vaccine.
2. Materials and Methods
2.1. Study Design
This study was designed to be a randomized, blinded, active control trial to evaluate the immunogenicity and safety of the lyophilized human PVRV developed by Dalian Aleph Biomedical Co., Ltd. in a population aged 10–60 years following a five-dose procedure and a four-dose procedure (China Clinical Trial ID: CTR20200042). The study was conducted from 4 August 2020 (date of enrollment of the first participant) to 17 September 2021 (date of the last visit of the last participant), with the data collected at the Sichuan Center for Disease Control and Prevention. No major changes to the test method were made after the start of the study: the endpoint indicators were not changed, and no interim analyses, interruptions, or discontinuations occurred. The contract research organization of this trial was Simoon Record Beijing Co., Ltd., and the testing institution for blood samples was the National Institutes for Food and Drug Control. The data management and statistical analyses were performed by Beijing Key Tech Statistical Consulting Co., Ltd. (Beijing, China).
2.2. Study Population
The eligibility criteria for participants were participants aged 10–60 years, with a legal identity certificate available, who voluntarily agreed to participate in the study and signed an informed consent form (participants or their guardians) and were able to understand the study procedures and take part in all planned visits. The main exclusion criteria were the following items: (1) participants who had a history of rabies vaccine immunization or use of passive immunization preparations against the rabies virus; (2) participants who were bitten (wounded skin) by animals susceptible to the rabies virus (such as dogs and cats) within 1 year before the first dose of the vaccine; (3) participants who had received any vaccines within 14 days before the first dose of the vaccine; (4) participants who had a history of severe allergy to any component of the investigational vaccine and any history of serious side effects caused by vaccines or drugs; (5) participants who were diagnosed with immunodeficiency or had received immunosuppressive therapy in the past 3 months; (6) participants who had received blood or blood-related products 3 months before enrollment; (7) participants who had a personal history or a family history of convulsions, epilepsy, encephalopathy, or psychosis; and (8) participants who had any conditions that might interfere with the assessment of the study objectives as considered by the investigator.
2.3. Randomization
In this study, 2776 participants were planned to be enrolled. A statistician first used SAS statistical software to randomize 2776 serial numbers via the stratified block randomization method. The stratified factor was the blood sampling scheme (blood sampling on days 0, 7, and 35/42, blood sampling on days 0, 14, and 35/42). The ratio of the 4-dose test group (T4), 4-dose control group (C4), 5-dose test group (T5), and 5-dose control group (C5) in each layer was 1:1:1:1. The vaccine samples were taken out from the original packaging and repackaged with a unified small box to achieve sample blinding. The statistician in charge of the random design and the relevant personnel loaded the research vaccine or control vaccine in each small box with a numbered label according to the random assignment table, and then transported these vaccines to each test site. The on-site researchers strictly assigned serial numbers to qualify the participants in the order of enrollment and then obtained and administered the corresponding vaccine sample based on the number.
2.4. Blinding
This trial was designed following the blinding method. The vaccine label was pasted at the designated position on the vaccine after being blinded by the statistician, who stored the blinded information and kept it confidential. The investigators and participants were blinded throughout the trial, and the randomization information of the trial was not accessible to the participants and/or their legal guardians, the investigators, or project members involved in any endpoint evaluation, data review, or data analysis of the study. The vaccinations were performed by investigators who were blinded.
2.5. Investigational Vaccine
The investigational vaccine was the Lyophilized Human Rabies Vaccine (Vero cells) manufactured by Dalian Aleph Biomedical Co., Ltd., which was prepared using Vero cells cultured on a sheet carrier by inoculating the fixed rabies virus CTN-1V strain (fixed strain) in a bioreactor following culture, harvest, concentration, virus inactivation, purification, and then lyophilization. Its active ingredient was inactivated rabies virus (CTN-1V strain), and its excipients were sodium chloride, potassium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate, sucrose, and human blood albumin. After reconstitution, each vial contained 0.5 mL of the vaccine, and the batch number was DG201908001. The control vaccine was the Lyophilized Human Rabies Vaccine (Vero cells) manufactured by Liaoning Chengda Biotechnology Co., Ltd., which was prepared using Vero cells cultured on a sheet carrier by inoculating the fixed rabies virus L. Pasteur PV2061 strain (fixed strain) in a bioreactor following culture, harvest, concentration, virus inactivation, purification, and then lyophilization. Its active ingredient was inactivated rabies virus, and its excipients were sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate, human blood albumin, and Dextran 40. The batch number was 201906167, and the strength and active ingredient were consistent with those of the investigational vaccine. After being synchronously detected by Dalian Aleph Biomedical Co., Ltd., the potencies of the investigational vaccine and the control vaccine were 5.2 IU/dose and 5.0 IU/dose, respectively.
2.6. Sample Size
In this study, a step-down strategy was used to sequentially evaluate the non-inferiority of the antibody-positive seroconversion rates and antibody geometric mean concentration (GMC) on Day 14 after the first dose of vaccination in T5 and C5 as well as in T4 and C4. With a test level of one-sided α = 0.025 and the power controlled at 85%, it was found that 295 cases were needed in each of the 4 groups, with a total of 1180 cases required. Considering that half of the participants were sampled on Day 7/14 after the first dose and the dropout rate was about 15%, the planned sample size for each group in the second period was [295/(1 − 0.15)] × 2 = 694 cases (“2” represents two blood-sampling schemes), with a total of 2776 cases planned for the 4 groups.
2.7. Study Population, Grouping, and Vaccination
A total of 2776 participants were enrolled in the study, with 694 cases each in T4 and C4, and they were administered two doses of the vaccine (test vaccine or control vaccine) on Day 0 and one dose each on Days 7 and 21. In addition, 694 participants were included in T5 and C5, who were administered one dose of the vaccine (test vaccine or control vaccine) each on Days 0, 3, 7, 14, and 28.
2.8. Study Endpoints
2.8.1. Primary Endpoints
Immunogenicity Endpoint: The positive seroconversion rates of antibodies and the GMC of antibodies in each group 14 days after the first vaccination dose.
Safety Endpoints: The incidence of solicited adverse events (AEs), including vaccination site (local) AEs and non-vaccination site (systemic) AEs, within 0–7 days after each dose; the incidence of unsolicited AEs within 0–30 days after each dose; and the incidence of all serious adverse events (SAEs) from the first dose to 6 months after the full course of immunization.
2.8.2. Secondary Endpoints
The secondary endpoints were the positive seroconversion rates of antibodies and the GMC of antibodies for all the participants 7 days after the first dose of vaccination and the positive seroconversion rates of antibodies and the GMC of antibodies for all the participants 14 days after the full course of immunization.
Here, the positive seroconversion rate of antibodies was defined as the percentage of participants with rabies-virus-neutralizing antibody (RVNA) before immunization < 0.5 IU/mL showing RVNA ≥ 0.5 IU/mL after immunization. The pre-immunization antibody-positive value was defined as RVNA ≥ 0.5 IU/mL before the first vaccination dose. The antibody-negative value was defined as rabies-virus-neutralizing antibody < 0.5 IU/mL before the first vaccination dose.
2.9. Statistical Analysis
The immunogenicity analysis was performed according to the per-protocol set (PPS) in the subgroups by age group, pre-immunization antibody-positive or antibody-negative populations, and populations aged 10–18 years or ≥18 years, without correction analyses. The Clopper–Pearson method was used to calculate the bilateral 95% CI of the positive seroconversion rate of antibodies, and the difference between groups was statistically tested using the Chi-square test/Fisher exact probability method. For the non-inferiority cut-off value of the positive seroconversion rate of antibodies, the lower 95% CI for the difference in the positive seroconversion rate of the neutralizing antibody (test group–control group) should be greater than −5%. For the superiority cut-off value of the positive seroconversion rate of antibodies, the lower 95% CI for the difference in the positive seroconversion rate of the neutralizing antibody (T4–T5) should be greater than 0. The two-sided 95% CIs of the antibody GMC, GMC fold change, and GMC ratio (test group/control group) after logarithmic transformation were statistically tested using analysis of covariance (ANCOVA) and paired t-tests. For the non-inferiority cut-off value of antibody GMC, the lower 95% CI of the neutralizing antibody GMC ratio (test group/control group) should be greater than 0.67.
Safety analysis was performed using the safety set (SS). AEs and SAEs were medically coded using MedDRA (Version: 24.1, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, ICH). The solicited AEs were statistically summarized as systemic AEs and local AEs. The cases and incidences of all AEs and SAEs in each group were calculated. Fisher’s exact test was used to identify the differences between the two dosing groups.
The statistical software SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. The difference was considered statistically significant at p < 0.05.
2.10. Serological Methods
All serum samples were collected by the National Institutes for Food and Drug Control in strict accordance with regulations and laboratory manuals using the rapid fluorescent focus inhibition test (RFFIT) to detect rabies-virus-neutralizing antibodies.
RFFIT is the standard method recommended by the WHO for the detection of rabies-virus-neutralizing antibodies (RVNAs). The rabies virus CVS strain was used as the challenge virus neutralizing the RVNAs in the serum samples. A suspension of susceptible cells containing viral residues was used to detect the viruses by fluorescent antibody staining. A fixed amount of CVS viruses was incubated with continuously diluted serum samples (neutralization in vitro), which were then added to the suspension of susceptible cells. After incubation for 24 h, the cells were fixed with a monolayer of acetone and stained with fluorescently labeled anti-NP antibodies to detect unneutralized viruses. The dilution required to reduce the concentration of the fixed amount of CVS viruses (FFU/mL) by 50% was calculated by comparing it with the viral control group, and the potency of neutralizing antibodies in each serum sample was determined through comparison with the reference serum with a known potency.
3. Results
3.1. Study Population
A total of 2776 participants were included in the trial, of whom 2671 participants completed the trial and 105 cases dropped out: 2 cases due to SAEs, 11 cases due to non-SAEs, 6 cases due to protocol violations, 61 cases due to voluntary withdrawal without any AEs, 14 cases because they left the region of the study site, 3 cases due to loss of follow-up, and 8 cases due to other reasons (Table 1, Appendix A, Table A1 and Table A2). The FAS set included 694 participants from T5, 691 participants from C5, 688 participants from T4, and 691 participants from C4, and no changes were made to the groupings. In addition, the ages, genders, heights, and weights of the participants in each group were evenly distributed among all of the participants and between the age groups (Table 2 and Appendix A, Table A3).
Table 1.
Enrollment, completion of the trial, and inclusion in each statistical analysis dataset.
| Analysis Item | T5 | C5 | T4 | C4 |
|---|---|---|---|---|
| Screened | 3128 | |||
| Randomized | 694 | 694 | 694 | 694 |
| Vaccination | ||||
| First dose | 693 | 693 | 693 | 689 |
| Second dose | 674 | 674 | 693 | 689 |
| Third dose | 672 | 670 | 678 | 674 |
| Fourth dose | 672 | 666 | 674 | 673 |
| Fifth dose | 668 | 661 | / | / |
| Blood sampling | ||||
| Pre-immunization 1 | 694 | 691 | 688 | 691 |
| 7 days after vaccination of the first dose 2 | 333 | 325 | 332 | 332 |
| 14 days after vaccination of the first dose 2 | 334 | 337 | 343 | 342 |
| 14 days after the full vaccination 1 | 669 | 661 | 664 | 672 |
| Dropout | 25 | 35 | 23 | 20 |
| SAEs | 0 | 2 | 0 | 0 |
| Non-SAEs | 3 | 5 | 1 | 2 |
| Protocol violation | 0 | 1 | 3 | 2 |
| Voluntary withdrawal without AEs | 12 | 22 | 13 | 12 |
| Leaving the region of the study site | 6 | 2 | 4 | 2 |
| Loss to follow-up | 3 | 0 | 0 | 0 |
| Other | 1 | 3 | 2 | 2 |
| Completion of the trial | 668 | 659 | 671 | 673 |
| Number of participants included in each analysis dataset | ||||
| SS | 693 | 693 | 693 | 689 |
| FAS | 694 | 691 | 668 | 691 |
| PPS1 | 324 | 316 | 321 | 323 |
| PPS2 | 329 | 329 | 333 | 331 |
| PPS3 | 659 | 643 | 653 | 663 |
Note: 1: Blood sampling from all participants; 2: blood sampling from 50% of participants before the third dose and from the other 50% of participants before the fourth dose. FAS: full analysis set; SS: safety set; PPS1: per-protocol set 1, which includes all the participants who were administered the first two doses of the vaccine, completed immunogenicity blood sampling prior to vaccination and 7 days after the first dose, and had effective antibody testing values; PPS2: per-protocol set 2, which includes all the participants who were administered the first three doses of the vaccine, completed immunogenicity blood sampling prior to vaccination and 14 days after the first dose, and had effective antibody testing values; PPS3: per-protocol set 3, which includes all the participants who had completed the full course of immunization and immunogenicity blood sampling prior to vaccination and 14 days after the full course of immunization and had effective antibody testing values.
Table 2.
Demographic data and baseline clinical characteristics (FAS) of the study participants.
| Analysis Item | T5 (N = 694) |
C5 (N = 691) |
T4 (N = 688) |
C4 (N = 691) |
p Value |
|---|---|---|---|---|---|
| Age (year) | |||||
| Mean (SD) | 43.1 (12.3) | 43.0 (12.3) | 42.8 (12.7) | 43.4 (12.2) | 0.8857 |
| Gender | |||||
| Female, n (%) | 470 (67.72) | 465 (67.29) | 472 (68.60) | 473 (68.45) | |
| Height (cm) | |||||
| Mean (SD) | 156.1 (7.8) | 156.4 (8.2) | 156.0 (8.2) | 155.9 (7.9) | 0.6202 |
| Weight (kg) | |||||
| Mean (SD) | 60.51 (10.82) | 60.12 (10.95) | 59.90 (10.95) | 59.74 (11.23) | 0.5819 |
3.2. Immunogenicity
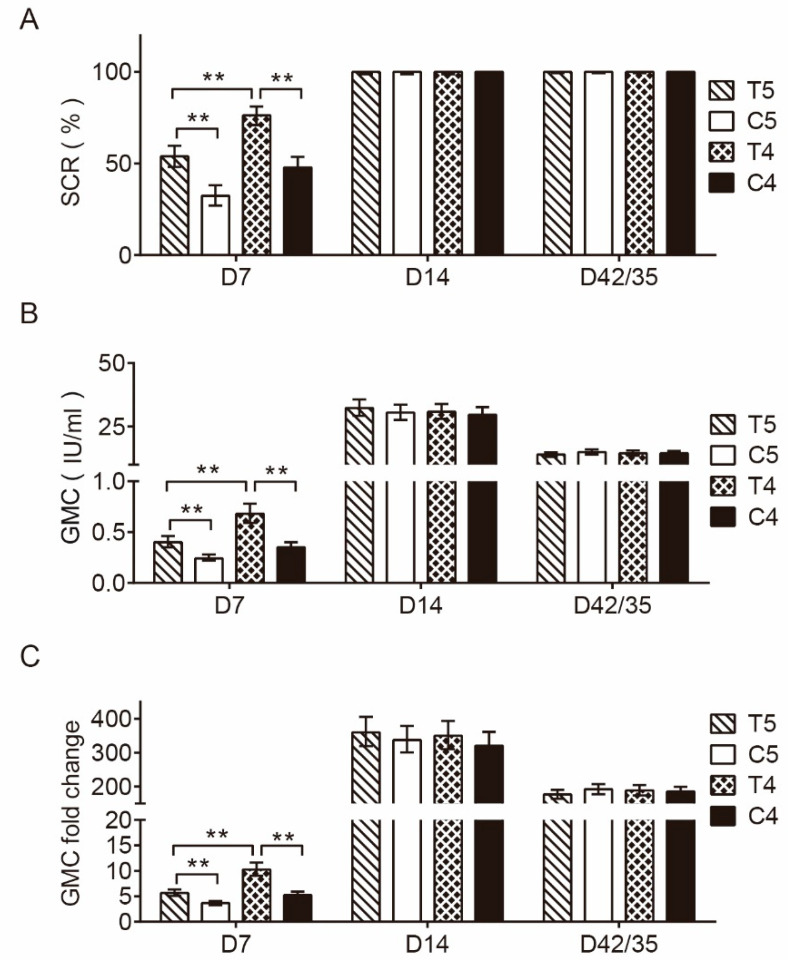
Figure 1A,B presents the positive seroconversion rates of neutralizing antibodies after immunization in the pre-immunization antibody-negative population and the GMC levels, respectively. The results show that the positive seroconversion rates and the GMC levels in T5 were higher than those in C5, and the positive seroconversion rates and the GMC levels in T4 were higher than those in C4 and T5 at 7 days after the first dose of immunization (PPS1); the RVNA ranges in T5, C5, T4, and C4 were (<0.1, 80.8), (<0.1, 72.4), (<0.1, 197.4), and (<0.1, 76.2), respectively.
Figure 1.
Neutralizing antibody SCR, GMC, and GMC fold change after immunization in the pre-immunization antibody-negative population. (A) Positive seroconversion rates of neutralizing antibodies in participants of each group. SCR = seroconversion rates—that is, the percentage of the number of participants with rabies-virus-neutralizing antibody (RVNA) ≥ 0.5 IU/mL after immunization among the total number of participants with RVNA < 0.5 IU/mL before immunization; or the percentage of participants with an RVNA titer that increased at least 4 times after immunization among the total number of participants with RVNA ≥ 0.5 IU/mL before immunization. **, p < 0.001. (B) GMC levels of neutralizing antibodies in participants of each group. GMC: geometric mean concentration. **, p < 0.001. (C) Neutralizing antibody GMC fold change in participants of each group. **, p < 0.001.
The positive seroconversion rates of neutralizing antibodies in T5, C5, T4, and C4 were 100.00% each. At 14 days after the first dose of immunization (PPS2) and at 14 days after the full course of immunization (PPS3), the differences in the positive seroconversion rates between T5 and C5 were 0.00% (95% CI: −1.30%, 1.28%) and 0.00% (95% CI: −0.63%, 0.66%), respectively, the differences between T4 and C4 were 0.00% (95% CI: −1.29%, 1.32%) and 0.00% (95% CI: −0.66%, 0.64%), respectively, and the differences between T4 and T5 were 0.00% (95% CI: −1.29%, 1.30%) and 0.00% (95% CI: −0.66%, 0.63%). The differences showed that the lower limits of all the 95% CIs of the positive seroconversion rates were greater than −5% (Figure 1A).
In addition, at 14 days after the first dose of vaccination (PPS2), the neutralizing antibody GMC levels in the serum were 32.30 IU/mL (95% CI: 29.25 IU/mL, 35.68 IU/mL) and 30.84 IU/mL (95% CI: 28.08 IU/mL, 33.88 IU/mL) in T5 and T4, respectively, increasing by 359.84 times and 320.46 times compared with those before vaccination, respectively; the RVNA ranges in T5, C5, T4, and C4 were (1.7, 1231.5), (2.6, 1231.5), (3.1, 326.4), and (1.5, 493), respectively. At 14 days after the full course of immunization (PPS3), the GMC levels were 13.97 IU/mL (95% CI: 13.09 IU/mL, 14.91 IU/mL) and 14.54 IU/mL (95% CI: 13.56 IU/mL, 15.58 IU/mL), increasing by 176.51 and 188.73 times compared with those before immunization, respectively (Figure 1B,C); and the RVNA ranges in T5, C5, T4, and C4 were (1.2, 493), (1.7, 333), (1.7, 999), and (0.9, 410.5), respectively.
Furthermore, at 14 days after the first dose of vaccination (PPS2) and 14 days after the full course of immunization (PPS3), the GMC ratios between T5 and C5 were 1.06 (95% CI: 0.92, 1.22) and 0.93 (95% CI: 0.85, 1.02), respectively, the GMC ratios between T4 and C4 were 1.04 (95% CI: 0.91, 1.19) and 1.00 (95% CI: 0.91, 1.10), respectively, and the GMC ratios between T4 and T5 were 0.95 (95% CI: 0.83, 1.09) and 1.04 (95% CI: 0.95, 1.14), showing that all the lower 95% CIs of the GMC ratios were greater than 0.67.
In terms of the total population, the population aged 10–18 years old, the population ≥ 18 years, and the pre-immunization antibody-negative population, the lower 95% CIs of the differences in the positive seroconversion rate between the five-dose groups, between the four-dose groups, and between T4 and T5 were all greater than −5%, and the lower 95% CIs of the GMC ratio were all greater than 0.67 (Appendix A, Table A4 and Table A5).
3.3. Safety
Table 3 presents the incidences of AEs in each group. The results show that the incidences of AEs were 36.80%, 35.41%, 37.52, and 34.34% and the incidences of AEs associated with vaccination were 33.77%, 31.35%, 33.19%, and 29.44% in T4, C4, T5, and C5, respectively. In addition, in T4, the incidence of systemic AEs was 11.40%, mainly characterized by headache (4.33%), and the incidence of local AEs was 28.72%, mainly characterized by pain at the vaccination site (27.13%). In T5, the incidence of systemic AEs was 12.41%, mainly characterized by vertigo (3.90%), and the incidence of local AEs was 28.14%, mainly characterized by pain at the vaccination site (26.98%). There was no statistical difference in the above-mentioned AEs between groups, with the exception that the incidences of local AEs and pain in T5 were slightly higher than those in C5. In addition, no vaccine-related SAEs were observed.
Table 3.
Incidences of adverse events in all participants.
| Adverse Event | T4 (N = 693) |
C4 (N = 689) |
T5 (N = 693) |
C5 (N = 693) |
* P1 | * P2 | * P3 |
|---|---|---|---|---|---|---|---|
| All adverse events | 36.80 | 35.41 | 37.52 | 34.34 | 0.2397 | 0.6144 | 0.8241 |
| Adverse events related to the investigational vaccine | 33.77 | 31.35 | 33.19 | 29.44 | 0.1476 | 0.3585 | 0.8644 |
| Solicited AEs | 33.48 | 31.06 | 32.76 | 29.29 | 0.1817 | 0.3573 | 0.8194 |
| Non-vaccination-site (systemic) AEs | 11.40 | 11.32 | 12.41 | 13.13 | 0.7476 | 1.0000 | 0.6188 |
| Fever | 2.74 | 2.76 | 0.14 | 2.45 | 0.8625 | 1.0000 | 1.0000 |
| Asthenia | 2.02 | 3.48 | 2.16 | 4.33 | 0.0618 | 0.1024 | 1.0000 |
| Headache | 4.33 | 3.63 | 3.32 | 4.18 | 0.4717 | 0.5825 | 0.4009 |
| Nausea | 1.30 | 0.87 | 1.44 | 2.60 | 0.2431 | 0.6053 | 1.0000 |
| Vomiting | 0.43 | 0.00 | 0.43 | 0.87 | 0.7258 | 0.2495 | 1.0000 |
| Vertigo | 2.60 | 3.05 | 3.90 | 4.62 | 0.6877 | 0.6301 | 0.2250 |
| Abdominal pain | 0.58 | 1.16 | 0.43 | 1.30 | 0.1442 | 0.2635 | 1.0000 |
| Arthralgia | 1.44 | 1.45 | 2.02 | 1.30 | 0.4009 | 1.0000 | 0.5377 |
| Muscle pain | 2.45 | 2.47 | 2.16 | 2.16 | 1.0000 | 1.0000 | 0.8584 |
| Acute allergic reaction | 0.14 | 0.44 | 0.72 | 0.14 | 0.3741 | 0.3730 | 0.2177 |
| Vaccination-site (local) AEs | 28.72 | 26.71 | 28.14 | 23.38 | 0.0493 | 0.4347 | 0.8582 |
| Pain | 27.13 | 25.40 | 26.98 | 22.08 | 0.0393 | 0.5014 | 1.0000 |
| Induration | 0.00 | 0.29 | 0.00 | 0.58 | 0.1245 | 0.2484 | 1.0000 |
| Redness | 0.72 | 0.00 | 0.87 | 0.72 | 1.0000 | 0.7258 | 1.0000 |
| Swelling | 1.30 | 0.87 | 1.44 | 0.87 | 0.4518 | 0.6053 | 1.0000 |
| Pruritus | 3.61 | 3.63 | 3.46 | 4.04 | 0.6719 | 1.0000 | 1.0000 |
| Rash | 0.00 | 0.15 | 0.00 | 0.14 | 1.0000 | 0.4986 | 1.0000 |
| Unsolicited AEs | 0.58 | 0.58 | 0.72 | 0.87 | 1.0000 | 1.0000 | 1.0000 |
| AEs not related to the investigational vaccine | 7.07 | 6.53 | 8.51 | 9.52 | 0.5738 | 0.7488 | 0.3672 |
| Grade 3 or higher AEs | 0.72 | 0.87 | 0.58 | 1.44 | 0.1774 | 0.7729 | 1.0000 |
Note: * P1: comparison between T5 and C5; P2: comparison between T4 and C4; P3: comparison between T4 and T5.
4. Discussion
In this study, the immunogenicity and safety of the lyophilized PVRV developed by Dalian Aleph Biomedical Co., Ltd. in a population aged 10–60 years following the Essen and Zagreb procedures were evaluated by comparing them with those of the lyophilized PVRV manufactured by Liaoning Chengda Biotechnology Co., Ltd. This study showed that the investigational vaccine was non-inferior to the positive control vaccine in both the Essen procedure and the Zagreb procedure. Moreover, the efficacy of the investigational vaccine administered according to the Zagreb procedure was superior to that of the Essen procedure shortly (Day 7) after vaccination, and the investigational vaccine showed good safety in both procedures.
Regarding immunogenicity, the alternative endpoints and evaluation criteria play an important role in evaluating rabies vaccines based on the RVNA-positive seroconversion rate and the GMC. In this study, as per the immunogenicity analysis of the pre-immunization antibody-negative population, the positive seroconversion rate of antibodies was 100.00% in both the five-dose group and the four-dose group 14 days after the first dose of vaccination and 14 days after the full course of immunization, which was consistent with the result that protective antibodies were rapidly generated following five-dose rabies vaccine immunization in a previous study [19], showing the reliability of this study in terms of the positive seroconversion rate of lyophilized PVRVs. In addition, the GMC in the participants gradually increased after vaccination and peaked on Day 14 (both greater than 28.08 IU/mL), much greater than the WHO specification for the effective protection capacity of rabies vaccines (RVNA ≥ 0.5 IU/mL). On Day 7 after vaccination, the GMC of the T4 group exceeded 0.68 IU/mL, which was higher than 0.40 IU/mL of T5 in this study. In combination with the similar GMC data in this study between the four-dose group and the five-dose group 14 days after the first dose of vaccination and the full course of immunization, we believe that the four-dose procedure in the early stage of immunization was superior to the five-dose procedure, which is consistent with the conclusion reported by Shen et al. [20]. Meanwhile, in the subgroup analysis between the populations aged 10–18 years and ≥18 years, it was found that the populations belonging to both age groups had high positive seroconversion rates of antibodies and GMC levels of antibodies after being administered the investigational vaccine, which was similar to the findings by Li et al. [21]. However, the positive rates and the GMC levels were higher in the population aged 10–18 years and ≥18 years old, different from the study by Fang et al. [22]. All these results indicate that the investigational lyophilized PVRV in this study had good immunogenicity that was not inferior to the control vaccine.
In terms of safety analysis, the incidence of AEs in each test group was similar to that in the control group, and the incidence of AEs in T5 was 37.52%, which was similar to the 36.8% reported by Shen et al. [20]. In addition, there was no significant difference in the incidences of local and systemic reactions among the groups. In this study, pain at the injection site was the most common local symptom, while headache was the most common systemic symptom, consistent with the studies by Wang et al. [23], Zhang et al. [15], and Pichon et al. [24]. Another common systemic adverse reaction was vertigo, of which the recovery was within 2 weeks in all cases. In addition, no vaccine-related SAEs were reported in this study, especially those observed in previous case reports, such as severe allergic reactions [22] and acute disseminated encephalomyelitis [25], indicating the good safety of the investigational vaccine.
In this comparative clinical trial involving both the Essen and Zagreb vaccination procedures and a control vaccine, participants of different age groups and the pre-immunization antibody-negative and -positive conditions were fully investigated, and multiple factors affecting the vaccination results were excluded. This clinical trial is a simulated exposure study in healthy participants, and the vaccine’s efficacy in the post-exposure population may be monitored after the vaccine is marketed.
5. Conclusions
This study has demonstrated that the lyophilized PVRV manufactured by Dalian Aleph Biomedical Co., Ltd. is not inferior to the lyophilized PVRV manufactured by Liaoning Chengda Biotechnology Co., Ltd. in terms of immunogenicity, with safety similar to that of the vaccine. In addition, the groups undergoing the Zagreb vaccination procedure showed higher GMC levels 7 days after the first vaccination dose than those undergoing the Essen vaccination procedure. In short, the results of this study indicate that the investigational vaccine administered following the Essen and Zagreb vaccination procedures is safe, with good immunogenicity, and the Zagreb vaccination procedure produces protection earlier than the Essen vaccination procedure, providing a valuable alternative for the prevention of rabies.
Appendix A
Table A1.
SAEs leading to dropouts.
| SAE Description | System Organ Class | Preferred Term | Start Date | End Date | Duration | Days from Vaccination | Correlation | SAE Leading to | Outcome | Leading to Dropouts? |
|---|---|---|---|---|---|---|---|---|---|---|
| Participant Serial Number: 0733; Age Group: 18–60-year-old group; Group: C5; Age: 53 years; Gender: male; First Dose Date: 13 November 2020 | ||||||||||
| Acute brain stem infarction: unconsciousness, weakness in the right limb, distortion of commissure, slurred speech, and gatism. Head CT: cavitary hard foci in the right cerebellar hemisphere; a few cavitary hard foci in the bilateral basal ganglia and right cerebellar hemisphere, and bone density shadow of nodule in the left ethmoid sinus; foci of brain stem infarction and inflammation in the bilateral ethmoid sinuses; foci of brain stem infarction; and inflammatory changes in the bilateral ethmoid sinuses and sphenoid sinuses. | Nervous system disorders | Brain stem infarction | 16 February 2021 | 21 March 2021 | 34 | 96 | Not related | Hospitalization or prolonged hospitalization | Death | Yes |
| Participant Serial Number: 2349; Age Group: 18–60-year-old group; Group: C5; Age: 52 years; Gender: female; First Dose Date: 18 November 2020 | ||||||||||
| Gallstones with chronic cholecystitis: sudden upper right quadrant pain for 1+ days, pain and discomfort in the upper abdomen, persistent dull pain, paroxysmal exacerbation, nausea and retching, obvious tenderness in the upper right quadrant, slight rebound tenderness, slight percussion pain in the liver region, coarse hepatic parenchymal echoes, cholecystitis, gallstones, gallbladder enlargement and effusion, gastrointestinal pneumatosis, neutrophil ratio 89.2% ↑, total bilirubin 28.75 umol/L ↑ * , direct bilirubin 15.87 umol/L ↑, amylase 225.80 U/L ↑, glutamic–pyruvate transaminase 173.10 U/L ↑, glutamic–oxaloacetic transferase 163 U/L ↑, glutamyltransferase 113.40 U/L ↑, alkaline phosphatase 135.5 U/L ↑, and amylase in urine 616.60 U/L ↑. | Hepatobiliary disorders | Cholecystitis, chronic | 25 November 2020 | 4 December 2020 | 10 | 8 | Unlikely | Hospitalization or prolonged hospitalization | Recovered | Yes |
| Gallstones with chronic cholecystitis: sudden upper right quadrant pain for 1+ days, pain and discomfort in the upper abdomen, persistent dull pain, paroxysmal exacerbation, nausea and retching, obvious tenderness in the upper right quadrant, slight rebound tenderness, slight percussion pain in the liver region, coarse hepatic parenchymal echoes, cholecystitis, gallstones, gallbladder enlargement and effusion, gastrointestinal pneumatosis, neutrophil ratio 89.2% ↑, total bilirubin 28.75 umol/L ↑, direct bilirubin 15.87 umol/L ↑, amylase 225.80 U/L ↑, glutamic–pyruvate transaminase 173.10 U/L ↑, glutamic–oxaloacetic transferase 163 U/L ↑, glutamyltransferase 113.40 U/L ↑, alkaline phosphatase 135.5 U/L ↑, and amylase in urine 616.60 U/L ↑. | Hepatobiliary disorders | Cholelithiasis | 25 November 2020 | 4 December 2020 | 10 | 8 | Unlikely | Hospitalization or prolonged hospitalization | Recovered | Yes |
Note: * The upward arrow “↑” indicates an increase.
Table A2.
Non-SAEs leading to dropouts.
| Adverse Event Description | System Organ Classes | Preferred Term | Types of Adverse Events | Number of Vaccinations | Start Date | End Date | Date of Vaccination | Duration Days | Days from Vaccination | Is it within 30 min? | Severity | Treatment Situation | Correlation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant Serial Number: 0147, Age Group: 18–60 years, Group: C4, Age: 40 years, Gender: Male | |||||||||||||
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
1/2 | 23 October 2020 | 27 October 2020 | 23 October 2020 | 5 | 0 | No | Grade 2 | Without medication | May be related |
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
1/2 | 23 October 2020 | 27 October 2020 | 23 October 2020 | 5 | 0 | No | Grade 2 | Without medication | May be related |
| Participant Serial Number: 0185, Age Group: 18–60 years, Group: C5, Age: 47 years, Gender: Female | |||||||||||||
| Vomiting | Gastrointestinal system diseases | Vomiting |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 6 November 2020 | 6 November 2020 | 6 November 2020 | 1 | 0 | No | Grade 2 | Outpatient medication treatment | May be related |
| Nausea | Gastrointestinal system diseases | Nausea |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 6 November 2020 | 6 November 2020 | 6 November 2020 | 1 | 0 | No | Grade 2 | Outpatient medication treatment | May be related |
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
4 | 6 November 2020 | 9 November 2020 | 6 November 2020 | 4 | 0 | No | Grade 1 | Self-medication treatment | May be related |
| Rash | Systemic diseases and various reactions at the administration site | Inoculation site rash |
Solicited //Adverse events at the inoculation site (local) |
4 | 6 November 2020 | 11 November 2020 | 6 November 2020 | 6 | 0 | No | Grade 3 | Self-medication treatment | May be related |
| Indurate | Systemic diseases and various reactions at the administration site | Inoculation site hardening |
Solicited //Adverse events at the inoculation site (local) |
4 | 6 November 2020 | 9 November 2020 | 6 November 2020 | 4 | 0 | No | Grade 2 | Self-medication treatment | May be related |
| Flush | Systemic diseases and various reactions at the administration site | Vaccination site erythema |
Solicited //Adverse events at the inoculation site (local) |
4 | 6 November 2020 | 9 November 2020 | 6 November 2020 | 4 | 0 | No | Grade 3 | Self-medication treatment | May be related |
| Swelling | Systemic diseases and various reactions at the administration site | Swelling of the vaccination site |
Solicited //Adverse events at the inoculation site (local) |
4 | 6 November 2020 | 11 November 2020 | 6 November 2020 | 6 | 0 | No | Grade 3 | Self-medication treatment | May be related |
| Abdominal pain | Gastrointestinal system diseases | Abdominal pain |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 6 November 2020 | 6 November 2020 | 6 November 2020 | 1 | 0 | No | Grade 2 | Outpatient medication treatment | May be related |
| Acute gastroenteritis | Infection and infectious diseases | Gastroenteritis | Unsolicited | 4 | 6 November 2020 | 7 November 2020 | 6 November 2020 | 2 | 0 | No | Grade 3 | Outpatient medication treatment | May be unrelated |
| Participant Serial Number: 0199, Age Group: 18–60 years, Group: C5, Age: 50 years, Gender: Male | |||||||||||||
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
2 | 26 October 2020 | 31 October 2020 | 26 October 2020 | 6 | 0 | No | Grade 2 | Without medication | May be related |
| Participant Serial Number: 1999, Age Group: 18–60 years, Group: C4, Age: 43 years, Gender: Female | |||||||||||||
| Erythema | Skin and subcutaneous tissue diseases | Contact dermatitis | Unsolicited | 1/2 | 14 December 2020 | 19 December 2020 | 8 December 2020 | 6 | 6 | No | Grade 2 | Without medication | May be unrelated |
| Participant Serial Number: 2108, Age Group: 18–60 years, Group: T5, Age: 46 years, Gender: Female | |||||||||||||
| Breast nodules | Reproductive system and breast diseases | Breast lump | Unsolicited | 1 | 2 November 2020 | 15 July 2021 | 31 October 2020 | 256 | 2 | No | Grade 2 | Outpatient medication treatment | May be unrelated |
| Breast tenderness | Reproductive system and breast diseases | Breast pain | Unsolicited | 1 | 2 November 2020 | 5 January 2021 | 31 October 2020 | 75 | 2 | No | Grade 2 | Outpatient medication treatment | May be unrelated |
| Participant Serial Number: 2138, Age Group: 18–60 years, Group: T5, Age: 36 years, Gender: Female | |||||||||||||
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
1 | 2 November 2020 | 6 November 2021 | 31 October 2020 | 5 | 2 | No | Grade 1 | Without medication | May be related |
| Participant Serial Number: 2188, Age Group: 18–60 years, Group: C5, Age: 44 years, Gender: Female | |||||||||||||
| Fever: 39.3 °C | Systemic diseases and various reactions at the administration site | Fever |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 15 November 2020 | 17 November 2020 | 15 November 2020 | 3 | 0 | No | Grade 3 | Outpatient medication treatment | May be related |
| Headache | Various nervous system disease | Headache |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 15 November 2020 | 15 November 2020 | 15 November 2020 | 1 | 0 | No | Grade 3 | Outpatient medication treatment | May be related |
| Nausea | Gastrointestinal system diseases | Nausea |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 15 November 2020 | 15 November 2020 | 15 November 2020 | 1 | 0 | No | Grade 1 | Outpatient medication treatment | May be related |
| Participant Serial Number: 2265, Age Group: 18–60 years, Group: C5, Age: 51 years, Gender: Female | |||||||||||||
| Acute gastroenteritis | Infection and infectious diseases | Gastroenteritis | Unsolicited | 1 | 2 November 2020 | 3 November 2020 | 1 November 2020 | 2 | 1 | No | Grade 3 | Outpatient medication treatment | May be unrelated |
| Participant Serial Number: 2405, Age Group: 18–60 years, Group: T5, Age: 48 years, Gender: Female | |||||||||||||
|
Acute allergic reaction Other: rash with itching, redness, and swelling |
Immune system diseases | Hypersensitivity |
Solicited //Non-inoculation site (systemic) adverse events |
4 | 6 December 2020 | 13 December 2020 | 5 December 2020 | 8 | 1 | No | Grade 2 | Outpatient medication treatment | May be related |
| Participant Serial Number: 2467, Age Group: 18–60 years, Group: C5, Age: 54 years, Gender: Male | |||||||||||||
| Asthenia | Systemic diseases and various reactions at the administration site | Asthenia |
Solicited //Non-inoculation site (systemic) adverse events |
3 | 11 December 2020 | 20 December 2020 | 8 December 2020 | 10 | 3 | No | Grade 2 | Without medication | May be related |
| Participant Serial Number: 2540, Age Group: 18–60 years, Group: T4, Age: 52 years, Gender: Female | |||||||||||||
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
1/2 | 1 December 2020 | 4 December 2020 | 1 December 2020 | 4 | 0 | No | Grade 1 | Without medication | May be related |
| Pain | Systemic diseases and various reactions at the administration site | Pain at the inoculation site |
Solicited //Adverse events at the inoculation site (local) |
1/2 | 1 December 2020 | 4 December 2020 | 1 December 2020 | 4 | 0 | No | Grade 1 | Without medication | May be related |
Table A3.
Demographic data and baseline clinical characteristics (FAS) of each age group.
| Analysis Item | Participants Aged 10–17 Years | Participants Aged 18–60 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T5 (N = 47) |
C5 (N = 53) |
C4 (N = 47) |
T4 (N = 53) |
p-Value | T5 (N = 47) |
C5 (N = 53) |
C4 (N = 47) |
T4 (N = 53) |
p-Value | |
| Age (years) | ||||||||||
| Mean (SD) | 12.1 (1.4) | 12.7 (2.0) | 12.0 (1.9) | 12.5 (2.0) | 0.1964 | 45.3 (9.3) | 45.5 (8.9) | 45.4 (9.5) | 45.7 (9.1) | 0.9087 |
| Gender | ||||||||||
| Female, n (%) | 22 (46.81) | 27 (50.94) | 22 (46.81) | 24 (45.28) | 448 (69.24) | 438 (68.65) | 448 (70.55) | 451 (70.03) | ||
| Height (cm) | ||||||||||
| Mean (SD) | 153.4 (10.1) | 152.4 (11.0) | 148.4 (11.4) | 151.8 (12.5) | 0.1615 | 156.3 (7.6) | 156.8 (7.9) | 156.3 (7.6) | 156.4 (7.3) | 0.7025 |
| Weight (kg) | ||||||||||
| Mean (SD) | 49.52 (14.22) | 44.86 (8.91) | 43.13 (12.32) | 45.58 (12.06) | 0.0678 | 61.31 (10.09) | 61.39 (10.13) | 61.09 (9.97) | 60.95 (10.14) | 0.8540 |
Table A4.
Comparison of positive seroconversion (four-fold growth) rates of neutralizing antibodies in the different subgroup populations.
| Population | Blood Sampling Time (Analysis Set) |
T5 | C5 | T4 | C4 (95% CI) | Between T5s | Between T4s | Between T4 and T5s | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Positive Seroconversion (Four-Fold Growth) Rate (95% CI) | N | Positive Seroconversion (Four-Fold Growth) Rate (95% CI) | N | Positive Seroconversion (Four-Fold Growth) Rate (95% CI) | N | Positive Seroconversion (Four-Fold Growth) Rate (95% CI) | Ratio Difference (95% CI) | p | Ratio Difference (95% CI) | p | Ratio Difference (95% CI) | p | ||
| Total population | 7 days after the first dose of vaccine (PPS1) |
324 | 53.70 (48.11, 59.23) | 316 | 32.59 (27.45, 38.07 | 321 | 74.14 (68.99, 78.85) | 323 | 49.23 (43.65, 54.82 | 21.11 (13.50, 28.47) | <0.0001 | 24.92 (17.54, 32.03) | <0.0001 | 41.55 (34.27, 48.34) | <0.0001 |
| 14 days after the first dose of vaccine (PPS2) |
329 | 99.70 (98.32, 99.99) | 329 | 99.70 (98.32, 99.99) | 333 | 99.70 (98.34, 99.99) | 331 | 99.70 (98.33, 99.99) | 0.00 (−1.42, 1.42) | 1.0000 | 0.00 (−1.40, 1.42) | 1.0000 | 0.00 (−1.40, 1.43) | 1.0000 | |
| 14 days after the full course of immunization (PPS3) |
659 | 99.70 (98.91, 99.96) | 643 | 99.38 (98.41, 99.83) | 653 | 99.54 (98.66, 99.91) | 663 | 99.55 (98.68, 99.91) | 0.32 (−0.55, 1.32) | 0.4466 | −0.01 (−0.94, 0.91) | 1.0000 | 0.16 (−0.79, 1.18) | 0.7239 | |
| Pre-immunization antibody-positive population | 7 days after the first dose of vaccine (PPS1) |
22 | 50.00 (28.22, 71.78) | 29 | 34.48 (17.94, 54.33) | 29 | 51.72 (32.53, 70.55) | 22 | 68.18 (45.13, 86.14) | 15.52 (−11.73, 41.06) | 0.2648 | −16.46 (−41.10, 11.09) | 0.2369 | 1.72 (−25.27, 28.52) | 0.9029 |
| 14 days after the first dose of vaccine (PPS2) |
36 | 97.22 (85.47, 99.93) | 32 | 96.88 (83.78, 99.92) | 39 | 97.44 (86.52, 99.94) | 44 | 97.73 (87.98, 99.94) | 0.35 (−11.58, 13.44) | 1.0000 | −0.29 (−11.26, 9.65) | 1.0000 | 0.21 (−10.86, 12.02) | 1.0000 | |
| 14 days after the full course of immunization (PPS3) |
56 | 96.43 (87.69, 99.56) | 62 | 93.55 (84.30, 98.21) | 70 | 95.71 (87.98, 99.11) | 66 | 95.45 (87.29, 99.05) | 2.88 (−6.52, 12.47) | 0.6818 | 0.26 (−7.99, 8.85) | 1.0000 | −0.71 (−8.88, 8.37) | 1.0000 | |
| 10–18 years | 7 days after the first dose of vaccine (PPS1) |
19 | 89.47 (66.86, 98.70) | 14 | 28.57 (8.39, 58.10) | 18 | 94.44 (72.71, 99.86) | 18 | 94.44 (72.71, 99.86) | 60.90 (28.40, 81.57) | 0.0003 | 0.00 (−21.53, 21.53) | 1.0000 | 4.97 (−17.34, 27.29) | 1.0000 |
| 14 days after the first dose of vaccine (PPS2) |
25 | 100.00 (86.28, 100.00) | 37 | 100.00 (90.51, 100.00) | 31 | 100.00 (88.78, 100.00) | 26 | 100.00 (86.77, 100.00) | 0.00 (−13.51, 9.55) | 1.0000 | 0.00 (−11.20, 13.07) | 1.0000 | 0.00 (−11.20, 13.53) | 1.0000 | |
| 14 days after the full course of immunization (PPS3) |
44 | 100.00 (91.96, 100.00) | 51 | 100.00 (93.02, 100.00) | 50 | 100.00 (92.89, 100.00) | 45 | 100.00 (92.13, 100.00) | 0.00 (−8.11, 7.07) | 1.0000 | 0.00 (−7.21, 7.94) | 1.0000 | 0.00 (−7.21, 8.11) | 1.0000 | |
| ≥18 years | 7 days after the first dose of vaccine (PPS1) |
305 | 51.48 (45.71, 57.21) | 302 | 32.78 (27.51, 38.39) | 303 | 72.94 (67.56, 77.86) | 305 | 46.56 (40.85, 52.33) | 18.69 (10.88, 26.28) | <0.0001 | 26.38 (18.73, 33.72) | <0.0001 | 21.46 (13.84, 28.85) | <0.0001 |
| 14 days after the first dose of vaccine (PPS2) |
304 | 99.67 (98.18, 99.99) | 292 | 99.66 (98.11, 99.99) | 302 | 99.67 (98.17, 99.99) | 305 | 99.67 (98.19, 99.99) | 0.01 (−1.53, 1.61) | 1.0000 | −0.00 (−1.55, 1.53) | 1.0000 | −0.00 (−1.55, 1.54) | 1.0000 | |
| 14 days after the full course of immunization (PPS3) |
615 | 99.67 (98.83, 99.96) | 592 | 99.32 (98.28, 99.82) | 603 | 99.50 (98.55, 99.90) | 618 | 99.51 (98.59, 99.90) | 0.35 (−0.58, 1.44) | 0.4436 | −0.01 (−1.02, 0.98) | 1.0000 | −0.17 (−1.16, 0.73) | 0.6840 | |
Note: Positive conversion (4-fold increase) rate: the percentage of participants with RVNA titer that increased at least 4 times after immunization among the total number of participants with RVNA ≥ 0.5 IU/mL before immunization.
Table A5.
Comparison of neutralizing antibody GMC levels in different the subgroup populations.
| Population | Blood Sampling Time | Analysis Set | T5 | C5 | T4 | C4 | Between T5s | Between T4s | Between T4 and T5s | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GMC (95% CI) | N | GMC (95% CI) | N | GMC (95% CI) | N | GMC (95% CI) | Ratio (95% CI) | P | Ratio (95% CI) | P | Ratio (95% CI) | P | |||
| Total population | 7 days after the first dose of vaccine | PPS1 | 324 | 0.48 (0.41,0.55) | 316 | 0.30 (0.26, 0.35) | 321 | 0.85 (0.73, 0.99) | 323 | 0.43 (0.37, 0.50) | 1.58 (1.29, 1.94) | <0.0001 | 1.97 (1.58, 2.47) | <0.0001 | 1.78 (1.43, 2.20) | <0.0001 |
| 14 days after the first dose of vaccine | PPS2 | 329 | 33.04 (30.04, 36.34) | 329 | 30.86 (28.13, 33.86) | 333 | 32.67 (29.71, 35.92) | 331 | 30.99 (28.21, 34.03) | 1.07 (0.94, 1.22) | 0.3142 | 1.05 (0.92, 1.20) | 0.4364 | 0.99 (0.86, 1.13) | 0.8691 | |
| 14 days after the full course of immunization | PPS3 | 659 | 14.45 (13.56, 15.41) | 643 | 15.51 (14.54, 16.54) | 653 | 15.37 (14.34, 16.48) | 663 | 15.42 (14.44, 16.46) | 0.93 (0.85, 1.02) | 0.1263 | 1.00 (0.91, 1.10) | 0.9514 | 1.06 (0.97, 1.17) | 0.1982 | |
| Pre-immunization antibody-positive population | 7 days after the first dose of vaccine | PPS1 | 22 | 4.99 (2.44, 10.21) | 29 | 2.42 (1.52, 3.85) | 29 | 7.48 (4.04, 13.85) | 22 | 7.76 (3.59, 16.78) | 2.07 (0.93, 4.59) | 0.0739 | 0.96 (0.37, 2.49) | 0.9383 | 1.50 (0.60, 3.75) | 0.3801 |
| 14 days after the first dose of vaccine | PPS2 | 36 | 39.69 (28.48, 55.31) | 32 | 34.77 (25.24, 47.89) | 39 | 50.36 (34.30, 73.94) | 44 | 41.49 (30.70, 56.07) | 1.14 (0.72, 1.80) | 0.5635 | 1.21 (0.75, 1.95) | 0.4197 | 1.27 (0.77, 2.10) | 0.3485 | |
| 14 days after the full course of immunization | PPS3 | 56 | 20.84 (15.96, 27.22) | 62 | 21.45 (17.22, 26.71) | 70 | 24.54 (18.66, 32.26) | 66 | 27.16 (20.17, 36.58) | 0.97 (0.69, 1.36) | 0.8678 | 0.90 (0.61, 1.35) | 0.6164 | 1.18 (0.80, 1.73) | 0.4022 | |
| 10–18 years | 7 days after the first dose of vaccine | PPS1 | 19 | 1.17 (0.62, 2.21) | 14 | 0.37 (0.21, 0.63) | 18 | 1.33 (0.82, 2.17) | 18 | 1.02 (0.51, 2.04) | 3.20 (1.38, 7.40) | 0.0083 | 1.31 (0.58, 2.97) | 0.5076 | 1.14 (0.52, 2.48) | 0.7398 |
| 14 days after the first dose of vaccine | PPS2 | 25 | 44.10 (30.62, 63.51) | 37 | 63.07 (48.08, 82.73) | 31 | 53.75 (39.57, 73.02) | 26 | 53.35 (39.48, 72.11) | 0.70 (0.45, 1.08) | 0.1061 | 1.01 (0.66, 1.54) | 0.9719 | 1.22 (0.77, 1.93) | 0.3940 | |
| 14 days after the full course of immunization | PPS3 | 44 | 23.33 (18.49, 29.44) | 51 | 24.28 (20.99, 28.08) | 50 | 26.99 (21.64, 33.65) | 45 | 24.33 (19.06, 31.06) | 0.96 (0.74, 1.25) | 0.7637 | 1.11 (0.80, 1.53) | 0.5275 | 1.16 (0.84, 1.59) | 0.3631 | |
| ≥18 years | 7 days after the first dose of vaccine | PPS1 | 305 | 0.45 (0.39, 0.52) | 302 | 0.30 (0.26, 0.35) | 303 | 0.83 (0.70, 0.97) | 305 | 0.41 (0.35, 0.48) | 1.51 (1.23, 1.86) | 0.0001 | 2.02 (1.61, 2.55) | <0.0001 | 1.83 (1.47, 2.28) | <0.0001 |
| 14 days after the first dose of vaccine | PPS2 | 304 | 32.26 (29.23, 35.61) | 292 | 28.19 (25.66, 30.97) | 302 | 31.04 (28.12, 34.26) | 305 | 29.58 (26.84, 32.60) | 1.14 (1.00, 1.31) | 0.0526 | 1.05 (0.91, 1.20) | 0.4949 | 0.96 (0.84, 1.11) | 0.5862 | |
| 14 days after the full course of immunization | PPS3 | 615 | 13.97 (13.08, 14.91) | 592 | 14.92 (13.94, 15.97) | 603 | 14.67 (13.66, 15.77) | 618 | 14.92 (13.95, 15.96) | 0.94 (0.85, 1.03) | 0.1685 | 0.98 (0.89, 1.09) | 0.7436 | 1.05 (0.95, 1.16) | 0.3186 | |
Author Contributions
Conceptualization: L.Z., J.C. (Jianmin Chen), J.C. (Jing Chai), Y.Z., Y.L. (Yuhua Li), X.W., J.L. and Q.M.; methodology: Y.L. (Yuan Lin), J.C. (Jianmin Chen), Y.L. (Yuhua Li), Y.W. (Yanan Wang), P.H., L.Z., J.L., X.W., Z.J., J.C. (Jing Chai), Y.W. (Yunpeng Wang) and D.Z.; validation: Q.M., D.Z., Y.Z., Y.L. (Yuhua Li), Z.J., J.C. (Jianmin Chen), J.L., L.Z. and Q.M.; formal analysis: X.W., J.L., Y.L. (Yuan Lin), Y.Z., Y.W. (Yanan Wang), P.H., H.G., Q.M. and L.Z.; investigation: X.W., J.L., Y.L. (Yuhua Li), J.Y., H.W., Y.W. (Yunpeng Wang), J.C. (Jianmin Chen), Z.J., H.G. and D.Z.; resources: J.C. (Jianmin Chen), H.W., J.Y., Z.J., Y.W. (Yunpeng Wang), X.W., J.L., J.C. (Jing Chai) and N.C.; data curation: J.C. (Jing Chai), H.G., N.C., J.Y., Y.W. (Yanan Wang), P.H., X.W., J.L. and Q.M.; writing—original draft: Y.W. (Yanan Wang), Y.L. (Yuan Lin), H.W., J.Y., J.C. (Jing Chai) and H.G.; writing—review and editing: Z.J., Y.W. (Yunpeng Wang), Y.Z., N.C., L.Z., Q.M., D.Z., J.C. (Jianmin Chen), J.L. and Y.L. (Yuhua Li); visualization: Y.L. (Yuan Lin), J.C. (Jing Chai), H.W., Y.W. (Yunpeng Wang), X.W., J.C. (Jianmin Chen), D.Z. and N.C.; supervision: Y.L. (Yuhua Li), Y.Z., L.Z., Z.J. and P.H.; project administration: Y.W. (Yanan Wang), P.H., Z.J., H.G., X.W. and L.Z.; funding acquisition: Y.Z. and L.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Vaccine Clinical Trial Ethics Committee of the Sichuan Provincial Center for Disease Control and Prevention (Protocol code: LC201904-DG, approval No.: SC-0820190601, approval Date: 30 December 2019).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request. The data are not publicly available due to privacy and ethical considerations.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was fully funded by Dalian Aleph Biomedical Co., Ltd.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization WHO News Item: World Rabies Day 2020. 28 September 2020. [(accessed on 27 October 2022)]. Available online: https://www.who.int/news/item/28-09-2020-world-rabies-day-2020.
- 2.World Health Organization WHO Fact Sheet: Rabies. 19 January 2023. [(accessed on 30 June 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies.
- 3.World Health Organization WHO Feature Stories: Rabies. Education Is Vital to Prevent Rabies Deaths. 26 September 2018. [(accessed on 24 October 2022)]. Available online: https://www.who.int/zh/news-room/feature-stories/detail/education-is-vital-to-prevent-rabies-deaths.
- 4.Koprowski H. Rabies in the face of the 21st century. Zoonoses Public Health. 2009;56:258–261. doi: 10.1111/j.1863-2378.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 5.Pantha S., Subedi D., Poudel U., Subedi S., Kaphle K., Dhakal S. Review of rabies in Nepal. One Health. 2020;10:100155. doi: 10.1016/j.onehlt.2020.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health and Family Planning Commission of the People’s Republic of China. [(accessed on 6 July 2023)]; Available online: http://www.nhc.gov.cn/wjw/jiany/202007/31bf2cc4b89b4d40baf09117211f52bd.shtml.
- 7.Rupprecht C., Kuzmin I., Meslin F. Lyssaviruses and rabies: Current conundrums, concerns, contradictions and controversies. F1000 Res. 2017;6:184. doi: 10.12688/f1000research.10416.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z., Liu M., Tao X., Zhu W. Epidemic Characteristics of Human Rabies—China, 2016–2020. China CDC Wkly. 2021;3:819–821. doi: 10.46234/ccdcw2021.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulenat T., Petit C., Bosch Castells V., Houillon G. Purified Vero Cell Rabies Vaccine (PVRV, Verorab®): A Systematic Review of Intradermal Use Between 1985 and 2019. Trop. Med. Infect. Dis. 2020;5:40. doi: 10.3390/tropicalmed5010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa W.A., Cunha R.S., Bolzan V.L., Silva Ade C., Caporale G.M., Chaves L.B., Oselka G.W., Junqueira D.A., Panacho M.R., Dias R.A., et al. Immunogenicity and safety of a new Vero cell rabies vaccine produced using serum-free medium. Vaccine. 2007;25:8140–8145. doi: 10.1016/j.vaccine.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Zhang X., Song Q., Tang K. Promising rabies vaccine for postexposure prophylaxis in developing countries, a purified vero cell vaccine produced in China. Clin. Vaccine Immunol. 2010;17:688–690. doi: 10.1128/CVI.00433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalimuddin S., Wijaya L., Chan Y.F.Z., Wong A.W.L., Oh H.M.L., Wang L.F., Kassim J.A., Zhao J., Shi Z., Low J.G. A phase II randomized study to determine the safety and immunogenicity of the novel PIKA rabies vaccine containing the PIKA adjuvant using an accelerated regimen. Vaccine. 2017;35:7127–7132. doi: 10.1016/j.vaccine.2017.10.097. [DOI] [PubMed] [Google Scholar]
- 13.Rupprecht C.E., Briggs D., Brown C.M., Franka R., Katz S.L., Kerr H.D., Lett S.M., Levis R., Meltzer M.I., Schaffner W., et al. Centers for Disease Control and Prevention (CDC). Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 2010, 59, 1–9; Erratum in MMWR Recomm. Rep. 2010;59:493. [PubMed] [Google Scholar]
- 14.World Health Organization Rabies vaccines: WHO position paper, April 2018—Recommendations. Vaccine. 2018;36:5500–5503. doi: 10.1016/j.vaccine.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Huang S., Cai L., Zhu Z., Chen J., Lu S., Zhu Z., Zhang M., Fang Y., Hu Q. Safety, immunogenicity of lyophilized purified vero cell cultured rabies vaccine administered in Zagreb and Essen regimen in post-exposure participants: A post-marketing, parallel control clinical trial. Hum. Vaccine Immunother. 2021;17:2547–2553. doi: 10.1080/21645515.2021.1880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J., Yao L., Sun J., Gong Z. Zagreb regimen, an abbreviated intramuscular schedule for rabies vaccination. Clin. Vaccine Immunol. 2015;22:1–5. doi: 10.1128/CVI.00531-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H., Huang G., Tang Q., Li J., Cao S., Fu C., Cao Q., Liu B., Pan H., Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum. Vaccine. 2011;7:220–224. doi: 10.4161/hv.7.2.14003. [DOI] [PubMed] [Google Scholar]
- 18.Hu Q., Liu M.Q., Zhu Z.G., Zhu Z.R., Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum. Vaccines Immunother. 2014;10:1645–1649. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheiermann N., Baer J., Hilfenhaus J., Marcus I., Zoulek G. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC)-rabies vaccine in man. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. Ser. A: Med. Microbiol. Infect. Dis. Virol. Parasitol. 1987;265:439–450. doi: 10.1016/S0176-6724(87)80263-8. [DOI] [PubMed] [Google Scholar]
- 20.Shen H., Wang Z., Yang B., Cai K., Jiang C., Xie R., Xiao H., Ren Q., Qi Z., Li J., et al. Immunogenicity and safety of purified vero cell-cultured rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in the healthy Chinese participants: A randomized, double-blind, positive controlled phase 3 clinical trial. Hum. Vaccine Immunother. 2021;17:351–357. doi: 10.1080/21645515.2020.1778408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li R., Huang L., Li J., Mo Z., He B., Wang Y., Wu X., Minutello M., Guinet-Morlot F., Pichon S. A next-generation, serum-free, highly purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab® when administered according to a post-exposure regimen in healthy children and adults in China. Vaccine. 2013;31:5940–5947. doi: 10.1016/j.vaccine.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Fang Y., Liu M.Q., Chen L., Zhu Z.G., Zhu Z.R., Hu Q. Rabies post-exposure prophylaxis for a child with severe allergic reaction to rabies vaccine. Hum. Vaccine Immunother. 2016;12:1802–1804. doi: 10.1080/21645515.2016.1143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Luo F., Feng Z., Li L., Bai Y., Ai X., Ma J., Zhang Z., Shi N. Immunogenicity and safety of purified vero cell rabies vaccine (PVRV) produced by Liaoning Cheng Da Co. under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese adults aged 50 and above. Hum. Vaccine Immunother. 2017;13:144–150. doi: 10.1080/21645515.2016.1230260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichon S., Moureau A., Petit C., Chu L., Essink B., Muse D., Saleh J., Guinet-Morlot F., Minutello A.M. Safety and immunogenicity of a serum-free purified Vero rabies vaccine in healthy adults: A randomised phase II pre-exposure prophylaxis study. Vaccine. 2022;40:4780–4787. doi: 10.1016/j.vaccine.2022.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Peng J., Chen L., Zhu Z.G., Zhu Z.R., Hu Q., Fang Y. Effect of Corticosteroids on RVNA production of a patient with acute disseminated encephalomyelitis following rabies vaccination as well as administration of HRIG. Hum. Vaccine Immunother. 2014;10:3622–3626. doi: 10.4161/21645515.2014.979621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request. The data are not publicly available due to privacy and ethical considerations.