Abstract
Fexuprazan is a potassium-competitive acid blocker approved for treating gastric-acid-related diseases. Although the effectiveness of the recent formulation fexuprazan 10 mg has been demonstrated in Phase 3 clinical trials, data on the pharmacokinetics (PKs) of administering fexuprazan 10 mg twice daily at a 12 h interval are lacking. Moreover, it is imperative to ensure the bioequivalence of the new formulation with the previously approved 40 mg formulation. This study evaluated the pharmacokinetics (PKs) of the single- and multiple-dose oral administration of fexuprazan 10 mg tablets in healthy participants (Part 1) and investigated their bioequivalence with 40 mg tablets (Part 2). Part 1 comprised a single- and multiple-dose, one-sequence, two-period design and eight participants, while Part 2 comprised a single-dose, 2 × 2 crossover design and 24 participants. In Part 1, in Periods 1 and 2, participants received single and multiple doses (twice daily) of fexuprazan 10 mg, respectively. The maximum plasma concentration (Cmax) area under the concentration–time curve from 0 to 12 h (AUC0–12h) of the multiple-dose participants was approximately double that of the single-dose participants. In Part 2, the geometric mean ratios (90% confidence intervals) for Cmax and AUC from zero to the time of the last quantifiable concentration (AUClast) of the use of four fexuprazan 10 mg tablets to those of one fexuprazan 40 mg tablet were 1.0290 (0.9352–1.1321) and 1.0290 (0.9476–1.1174), respectively, meeting the bioequivalence criteria. Favorable PKs were observed after single and multiple administrations of one fexuprazan 10 mg tablet, and four fexuprazan 10 mg tablets were pharmacokinetically equivalent to one fexuprazan 40 mg tablet.
Keywords: pharmacokinetics, bioequivalence, gastritis, multiple dose, fexuprazan
1. Introduction
Gastric-acid-related diseases (ARDs), which are primarily caused by the excessive production or imbalance of gastric acid in the stomach, mainly include gastroesophageal reflux disease (GERD), peptic ulcers, gastritis, and Zollinger–Ellison syndrome [1]. Among ARDs, gastritis is one of the most commonly clinically diagnosed diseases worldwide, and its prevalence is gradually increasing in Korea [2]. Gastritis manifests as the inflammation or irritation of the stomach lining and may be caused by many factors, including infection, alcohol, particular medications, and some allergy and immune conditions [3]. Treatments for ARDs are mainly designed to reduce gastric acid production, relieve symptoms, and promote tissue healing. Thus, antacids, such as proton pump inhibitors (PPIs) and H2 blockers, have been widely used to treat ARDs [4,5]. Although empirical treatment for ARDs primarily relies on conventional drugs, and the effects of H2-receptor antagonists and PPIs on the endoscopic improvement of acute and chronic gastritis have been reported [6], some patients may not respond well to these treatments [7,8,9]. Therefore, more effective medications are required to offer enhanced symptom relief for patients not experiencing sufficient symptom improvement with the existing therapeutic options.
Potassium-competitive acid blockers (P-CABs), a new class of acid suppressants, have recently been developed as an alternative treatment [10]. P-CABs, including vonoprazan, tegoprazan, and fexuprazan, are significantly more efficient than PPIs in suppressing gastric acid secretion [11]. As P-CABs do not require acid activation, they exhibit a faster onset than PPIs [12]. Additionally, they effectively reduce nocturnal acid breakthroughs owing to their longer action duration [13]. P-CABs are predominantly metabolized by cytochrome P450 (CYP) 3A4, which has little inter-individual variability. In contrast, many PPIs are mainly metabolized by CYP2C19 and may subsequently exhibit evident differences in drug exposure. In South Korea, the Ministry of Food and Drug Safety (MFDS) has recently approved fexuprazan, a P-CAB developed by Daewoong Pharmaceutical Co., Ltd. (Seoul, Korea), for use in two formulations of 10 [14] and 40 mg [15]. It is the first P-CAB to be approved for acute and chronic gastritis treatment in South Korea [16]. Fexuprazan 10 mg is a new formulation developed to improve patient medication compliance and market competitiveness by changing the shape of the tablet from round to oblong [17]. Fexuprazan 40 mg administered once daily is appropriate for therapeutic use in treating erosive GERD [18], whereas fexuprazan 10 mg administered twice daily is prescribed for ameliorating gastric mucosal lesions associated with acute and chronic gastritis [19]. Although the effectiveness of fexuprazan 10 mg (twice daily) has been demonstrated in Phase 3 clinical trials on patients with gastritis, data on the pharmacokinetics (PKs) of administering fexuprazan 10 mg twice daily at a 12 h interval are lacking. Therefore, further investigation is necessary to address this crucial information gap. Moreover, it is imperative to ensure the bioequivalence of the new formulation with the previously approved 40 mg formulation.
The present study aimed to evaluate the PKs of the new, recently approved, fexuprazan 10 mg formulation after single and multiple administrations (Part 1), and its bioequivalence to the previously approved 40 mg formulation was investigated (Part 2).
2. Results
2.1. Demographic Characteristics
Eight male participants were enrolled in and completed Part 1 of the study, and their PKs, safety, and demographics were evaluated. The mean (±standard deviation) age, height, weight, and BMI were 32.63 (±9.74) years, 176.58 (±7.44) cm, 74.76 (±7.50) kg, and 23.95 (±1.55) kg/m2, respectively. Twenty-four participants were enrolled in Part 2 and randomly assigned to Sequence A (nine male and three female participants) or B (ten male and two female participants). Of the participants in Part 2, one was excluded because of a failure to follow-up on 3d of Period 2, and 23 participants completed the clinical trial. Accordingly, the PK profiles of the 23 participants who completed the study and the safety profiles of the 24 participants who received at least one dose of IPs were analyzed. The mean (± standard deviation) age, height, weight, and body mass index (BMI) were 27.17 (±6.63) years, 172.24 (±8.50) cm, 72.48 (±10.90) kg, and 24.33 (±2.46) kg/m2, respectively. There were no significant differences between the sequence groups in terms of mean age, height, weight, or BMI (p-values = 0.1812, 0.7203, 0.4868, and 0.2141, respectively).
2.2. PKs of Fexuprazan 10 mg after Single and Multiple Administration (Part 1)
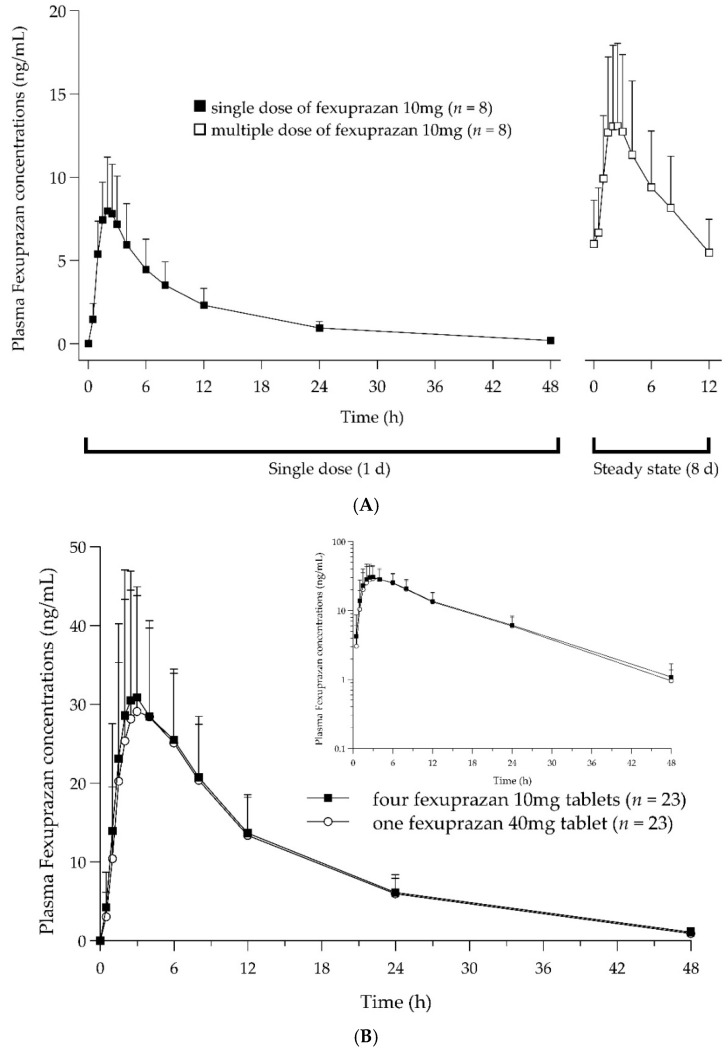
The PK parameters of single and multiple doses of fexuprazan are summarized in Table 1. During Period 1, after a single dose of fexuprazan 10 mg administered once daily, the plasma concentration of fexuprazan peaked at 2.25 h, and the mean maximum plasma concentration (Cmax) and AUC0–12h were 8.26 ng/mL and 53.47 h∙ng/mL, respectively. The plasma fexuprazan concentration showed rapid absorption, declining in a polyphasic manner (Figure 1A).
Table 1.
Comparison of the pharmacokinetic parameters of fexuprazan.
| Part 1: Single Dose vs. Multiple Doses | ||
|---|---|---|
| Parameter |
Single Dose Daily
1
(n = 8) |
Two Doses Daily
1
(n = 8) |
| Cmax or Cmax,ss (ng/mL) | 8.26 ± 2.96 | 13.85 ± 4.96 |
| Cmin,ss (ng/mL) | 5.36 ± 2.07 | |
| Tmax or Tmax,ss (h) | 2.25 (1.50–3.00) | 2.00 (1.50–3.00) |
| AUC0–12h or AUC0–12h,ss (h∙ng/mL) | 53.47 ± 20.83 | 109.73 ± 40.52 |
| AUClast (h∙ng/mL) | 84.91 ± 36.82 | |
| Accumulation ratio | 2.11 | |
| Part 2: Four 10 mg Tablets vs. One 40 mg Tablet | ||
| Parameter |
4 × fexuprazan 10 mg 2
(n = 23) |
1 × fexuprazan 40 mg 2
(n = 23) |
| Cmax (ng/mL) | 34.62 ± 15.44 | 33.87 ± 15.04 |
| Tmax (h) | 2.50 (1.50–6.00) | 3.00 (2.00–6.00) |
| AUClast (h∙ng/mL) | 463.19 ± 169.78 | 446.24 ± 152.94 |
| AUCinf (h∙ng/mL) | 479.88 ± 175.31 | 459.82 ± 154.55 |
| Vz/F (L) | 1372.91 ± 697.78 | 1341.60 ± 601.42 |
| t1/2 (h) | 9.91 ± 2.00 | 9.44 ± 1.65 |
| CL/F (L/h) | 94.60 ± 36.74 | 96.81 ± 32.69 |
1 single dose daily, a single dose of fexuprazan 10 mg once daily; two doses daily, multiple doses of fexuprazan 10 mg twice daily. 2 4 × fexuprazan 10 mg, single dose of four fexuprazan 10 mg tablets; 1 × fexuprazan 40 mg, single dose of one fexuprazan 40 mg tablet. All values are presented as arithmetic mean ± standard deviation (SD) except for Tmax, which is presented as median (minimum–maximum).
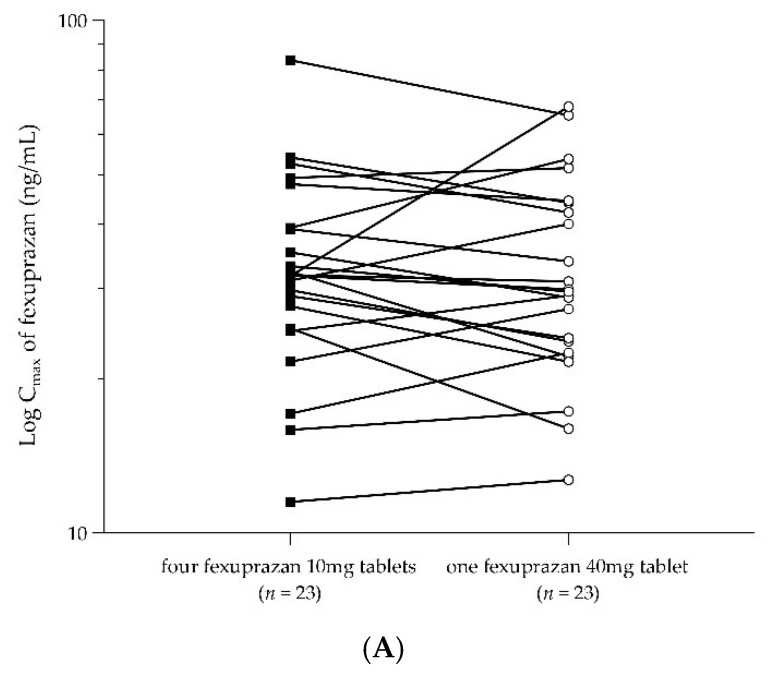
Figure 1.
Plasma concentration–time curve. (A) After administration of a single dose (black square) and multiple doses (white square) of fexuprazan 10 mg. (B) After administration of four fexuprazan 10mg tablets (T1, black square) and one fexuprazan 40 mg tablet (T2, white circle).
During Period 2, following multiple doses of fexuprazan 10 mg administered twice daily, the plasma fexuprazan concentration peaked at 2 h, and the mean Cmax,ss, AUC0–12h,ss, and Cmin,ss were 13.85 ng/mL, 109.73 h∙ng/mL, and 5.36 ng/mL, respectively. Following the repeated administration of fexuprazan 10 mg twice daily, its plasma concentrations at 7d 12 h, 8d 0 h, and 8d 12 h were compared to the slope (β1 = 0) using a regression equation to determine whether a steady state had been reached. At this point, the concentration gradient (90% CI) was −0.0004 (−0.0168–0.0160), indicating that a steady state had been reached. Multiple doses accumulated twice as much as a single dose, and the calculated mean accumulation ratio was 2.11. After administering multiple doses of fexuprazan 10 mg twice daily, a steady state was achieved. Moreover, rapid absorption was observed up to the peak, followed by a rapid decline in the plasma concentration–time curves for up to 12 h.
2.3. PKs of a Single Dose of Four Fexuprazan 10 mg Tablets and a Single Dose of One Fexuprazan 40 mg Tablet (Part 2)
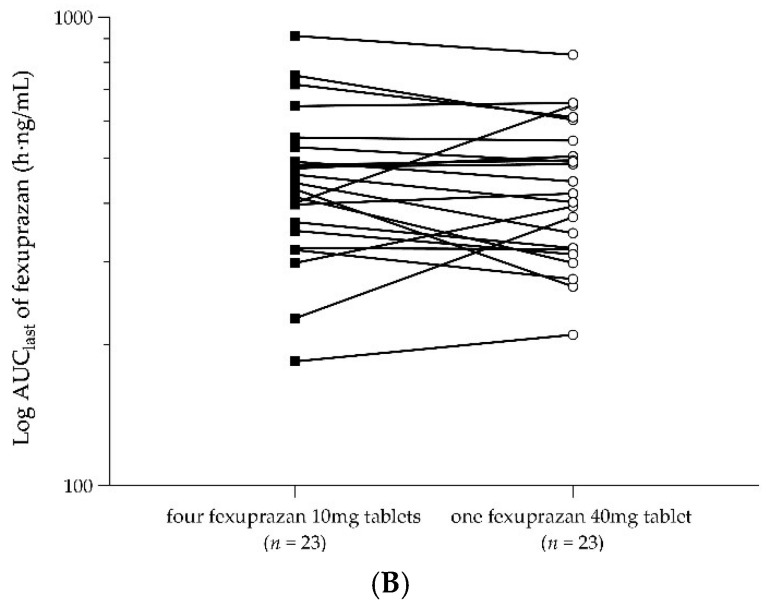
The PK parameters of fexuprazan after a single dose of four fexuprazan 10 mg (T1) tablets and after a single dose of one fexuprazan 40 mg (T2) tablet are summarized in Table 1. An overlap was observed in the mean plasma concentration–time curves between T1 and T2 (Figure 1B). The rapid absorption of T1 and T2 was detected for up to 2.50 and 3.00 h, respectively. After reaching Cmax (T1, 34.62 ng/mL; T2, 33.87 ng/mL), the plasma concentration–time curves exhibited rapid declines for up to 12 h, followed by slow declines for up to 48 h, indicating a similar half-life for T1 and T2 (T1, 9.91 h; T2, 9.44 h). The mean AUClast and AUCinf values for T1 were 463.19 and 479.88 h∙ng/mL, respectively, and those for T2 were 446.24 and 459.82 h∙ng/mL, respectively. When administered with T1 and T2, the primary PK parameters, Cmax and AUClast, were comparable. Moreover, the point estimates and 90% CIs of the geometric mean ratios (GMRs) (GMR = T1/T2) of Cmax and AUClast were 1.0290 (0.9352–1.1321) and 1.0290 (0.9476–1.1174), respectively (Table 2). The 90% CIs of the analyzed PK parameters were within the bioequivalence standard range of 0.80–1.25. The intra-subject CV (%), calculated using Equation (1), was <19% for both Cmax and AUClast. There was no noticeable within-subject difference between Cmax and AUClast for the reference or test formulations (Figure 2).
| (1) |
Table 2.
Bioequivalence comparison between four fexuprazan 10 mg tablets and one fexuprazan 40 mg tablet in Part 2.
| Pharmacokinetic Parameters |
No. | Geometric LS Mean | Geometric LS Mean Ratio (T1/T2) | IntraCV(%) | ||
|---|---|---|---|---|---|---|
| T1 1 | T2 1 | Point Estimate |
90% CI | |||
| Cmax (ng/mL) | 23 | 31.46 | 30.57 | 1.0290 | 0.9352–1.1321 | 19.0 |
| AUClast (h∙ng/mL) | 23 | 431.30 | 419.15 | 1.0290 | 0.9476–1.1174 | 16.3 |
1 T1, four fexuprazan 10 mg tablets; T2, one fexuprazan 40 mg tablet. LS, least squares; CV, coefficient of variance.
Figure 2.
Participant profiles. (A) Cmax and (B) AUClast after administration of four fexuprazan 10 mg tablets (T1, black square) or one fexuprazan 40 mg tablet (T2, white circle).
2.4. Safety Assessment
In Part 1, one participant experienced two TEAEs, both of which were headaches. One treatment-emergent adverse event (TEAE) occurred with a single fexuprazan 10 mg dose, whereas the other occurred after multiple fexuprazan 10 mg doses. Two TEAEs were assessed as adverse drug reactions (ADRs) but were mild and resolved over time without any action.
In Part 2, five participants experienced eight TEAEs; three TEAEs were reported by two participants in T1, and five TEAEs were reported by four participants in T2. The details of the TEAEs are summarized in Table 3. The safety analysis revealed no significant differences in TEAE incidence between the two treatment groups (p = 0.3827). Seven TEAEs (T1, three TEAEs reported by two participants; T2, four TEAEs reported by three participants) reported by four participants were assessed as ADRs. The reported ADRs included abdominal pain (two TEAEs in two participants), diarrhea (one TEAE in one participant), hematochezia (one TEAE in one participant), nausea (one TEAE in one participant), and headache (one TEAE in one participant). All TEAEs were mild and resolved spontaneously within a few hours or days. No TEAE leading to investigational product (IP) withdrawal, serious adverse events, or death was observed. No clinically significant findings were reported in terms of physical examination, vital signs, or 12-lead electrocardiogram (ECG). Safety data, including TEAEs, laboratory tests, vital signs, and ECG, confirmed that T1 was as safe and tolerable as T2 in healthy adults.
Table 3.
Treatment-emergent adverse events following oral administration of four fexuprazan 10 mg tablets or one fexuprazan 40 mg tablet in Part 2.
| Adverse Events | 4 × Fexuprazan 10 mg 1 (n = 24) | 1 × Fexuprazan 40 mg 1 (n = 24) | Total (n = 24) |
|---|---|---|---|
| Participants with TEAEs | 2 (8.3) (3) | 4 (16.7) (5) | 5 (20.8) (8) |
| Abdominal pain | 1 (4.2) (1) | 1 (4.2) (1) | 2 (8.3) (2) |
| Diarrhea | 1 (4.2) (1) | 1 (4.2) (1) | 1 (4.2) (2) |
| Haematochezia | 1 (4.2) (1) | 1 (4.2) (1) | |
| Nausea | 1 (4.2) (1) | 1 (4.2) (1) | |
| Oedema peripheral | 1 (4.2) (1) | 1 (4.2) (1) | |
| Headache | 1 (12.5) (1) | 1 (4.2) (1) | 1 (4.2) (2) |
1 4 × fexuprazan 10 mg, four fexuprazan 10 mg tablets; 1 × fexuprazan 40 mg, one fexuprazan 40 mg tablet. Values are presented as the number of participants (percentage of subject) (number of TEAEs). TEAEs are presented by preferred term (PT) according to the MedDRA version 24.0.
3. Discussion
We successfully conducted a two-part study according to the objectives of our research. In Part 1, the safety and PK profiles after single and multiple administrations of fexuprazan 10 mg were evaluated in eight participants. After a single administration, PKs were evaluated for 3 d, with twice daily administration following a 4-day washout period. Considering the observed terminal half-life of approximately 9 h after the single administration, the PKs after multiple administrations were assessed under steady-state conditions. The observed AUC0–12,ss after administering fexuprazan 10 mg at 12 h intervals was 109.73 h∙ng/mL in the present study, corresponding to half of the AUC0–24,ss (223.7 h∙ng/mL) after the once-daily administration of fexuprazan 20 mg that was reported in a first-in-human study [20]. This result suggests that administering fexuprazan 10 mg twice daily and fexuprazan 20 mg once daily result in similar exposure over 24 h. After multiple fexuprazan dose administrations in the first-in-human study, a significant correlation was observed between gastric pH parameters and doses in the range of 20–160 mg [20]. Thus, the comparable values of AUC0–24,ss after administering fexuprazan 10 mg twice daily and fexuprazan 20 mg once daily suggest similar gastric pH parameters, including the percentage of time that the gastric pH was ≥4.0. Our findings report therapeutic effects similar to those observed in the Phase 3 clinical trial conducted on patients with acute and chronic gastritis, indicating that both the once-daily dose of 20 mg and the twice-daily dose of 10 mg exhibited superior efficacy compared to the placebo [19].
In one subgroup analyses, regardless of Helicobacter pylori infection, the group receiving fexuprazan 10 mg twice daily showed a significantly higher erosion improvement rate than the placebo group [19]. Specifically, in patients with H. pylori infection, members of the group receiving a twice-daily dose of fexuprazan 10 mg showed a significantly higher erosion improvement rate than in those without H. pylori infection (81.8% vs. 61.3%, respectively). In addition, in patients with chronic gastritis, the twice-daily fexuprazan 10 mg group showed significantly higher erosion improvement rates than the once-daily fexuprazan 20 mg group (66.7% (10 mg b.i.d.) vs. 43.3% (20 mg q.d.)) [19]. Therefore, these previous findings and ours suggest that fexuprazan can be effectively used for various ARDs based on comparable exposure and efficacy using either a once-daily dose of 20 mg or a twice-daily dosage regimen.
Part 2 was conducted to compare the PK and safety profiles of the two formulations containing fexuprazan 40 mg; The test formulation (four tablets of fexuprazan 10 mg) had PK characteristics bioequivalent to those of the reference formulation (one tablet of fexuprazan 40 mg). This conclusion was supported by the finding that the Cmax and AUClast GMRs (90% CI) were within the conventional bioequivalence criteria following the administration of each formulation. In addition, the mean plasma concentration–time profiles of the two formulations were superimposable from pre-dosing to 48 h after dosing, and their safety profiles were similar. These results indicate that the test formulation of fexuprazan can be used as an alternative to the reference formulation without significant differences in systemic exposure and safety. Moreover, the sample size and design of this study were appropriate for evaluating the bioequivalence and safety profiles of the two drugs. In this study, to attain an intra-subject variability of Cmax of 19% for fexuprazan 40 mg, the minimum sample size required was 18 participants. Twenty-four participants were enrolled, and 23 completed the study. The number of participants was sufficient to minimize β-errors (type II), and the randomization of the study groups was sufficiently balanced to avoid bias associated with sequence allocation. The PK sampling time points were well established to observe the Tmax and systemic exposure, which was supported by the fact that the AUCextra (%) was <5% in both the test and reference formulations.
Among the P-CAB class of drugs, fexuprazan is the only drug approved in South Korea for gastritis. Fexuprazan has a longer half-life than other P-CABs, such as vonoprazan (6.95 h) and tegoprazan (3.65–5.39 h) [21], and in preclinical studies, gastric acid secretion was found to be similar to or greater than that of drugs of the same class [20]. Therefore, efforts are being made to develop different formulations and strengths of fexuprazan to enhance clinical practice, expand indications, and improve medication convenience. The 10 mg formulation used in this study is a newly developed formulation that differs from the formulation used in the first-in-human study [20]; it was changed from a white, round, film-coated tablet weighing 156 mg to an orange, oblong, film-coated tablet weighing 157.5 mg (Table S1, Supplementary Materials). Oblong-shaped tablets are easier to swallow and have faster transit times in the esophagus than round-shaped tablets of the same weight [17]. Considering that chronic ARDs can cause frequent irritation to the esophagus, leading to complications, such as dysphagia [22,23], formulations of changed shapes might help improve medication compliance in patients with gastritis.
This study had several limitations. First, PKs, safety, and tolerability were evaluated in relatively few healthy volunteers. Nevertheless, the bioequivalent drug exposure and pharmacokinetic–pharmacodynamic (PK/PD) relationships suggest favorable outcomes, effectiveness, and better compliance in a large number of patients with gastritis. Second, this study was conducted only on healthy Korean participants. However, Hwang et al. showed that PK profiles, PK/PD relationships, gastric acid suppression, and safety profiles were similar among Korean, Caucasian, and Japanese participants after the administration of single and multiple doses of fexuprazan [24].
4. Materials and Methods
4.1. Participants and Study Design
This study was conducted at the Global Clinical Trials Center of the CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea, with strict adherence to the key ethical principles outlined in the Declaration of Helsinki, the Good Clinical Practice Guidelines of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, and local laws and regulations. The study protocol was reviewed and approved by the MFDS and Institutional Review Board (IRB) of CHA University (IRB no. CHAMC 2021-06-021). Furthermore, the study has been registered and can be found on ClinicalTrials.gov (https://clinicaltrials.gov, accessed on 8 December 2021) under the identifier NCT05149274.
Before initiating the clinical trial, the investigators provided all participants with the study information and relevant details. Screening procedures were conducted exclusively for individuals who voluntarily consented to participate in the clinical trial. The inclusion criteria were healthy adults aged 19–45 years, assessed for eligibility based on medical history, physical examination, vital signs, clinical laboratory tests, and a 12-lead ECG. The exclusion criteria included individuals with a clinically significant medical history, those who had participated in another clinical trial within six months preceding the screening, and those taking prescribed medications that could not be temporarily discontinued for at least two weeks before the screening.
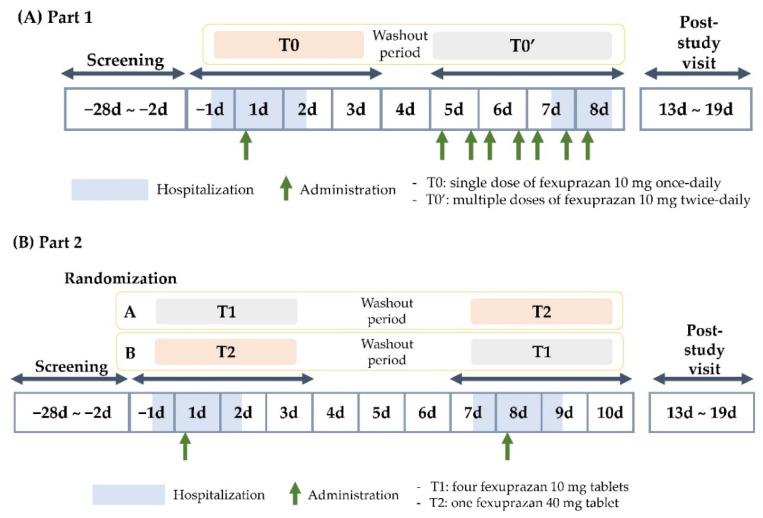
This study included two parts (Figure 3). Part 1 followed an open-label, single- or multiple-dose, 1-sequence, and 2-period design and aimed to evaluate the PKs of the single-dose and multiple-dose oral administration of fexuprazan 10 mg tablets in healthy participants. It involved eight participants and aimed to explore their PK characteristics after single- and multiple-dose fexuprazan 10 mg administration. The number of participants was determined based on the ascending dose cohort in the first-in-human study on fexuprazan [20]. The participants were admitted to the clinical trial center a day before the first administration and were instructed to keep fasting state for at least 10 h prior. The next day (1d), at around 9 am, the participants received a single dose of fexuprazan 10 mg (T0) with 150 mL of water, and blood samples (6 mL) were collected at various time points, including pre-dosing (0 h) and post-dosing (after 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h). After a 4-day washout period, the participants visited the clinical trial center and were administered multiple doses of fexuprazan 10 mg twice daily (T0′) with 150 mL of water at approximately 9 am and 9 pm for 4 days (D5–7: approximately 9 am and 9 pm; D8: approximately 9 am). Upon reaching a steady state (D8), blood sampling was conducted at the same time points as in Period 1, up to 12 h, to determine the area under the concentration–time curve (AUC) from time zero to the end of the dosing interval.
Figure 3.
Study schema.
Part 2 employed a randomized, open-label, single-dose, 2-sequence, 2-period, crossover design and aimed to confirm the bioequivalence between a single dose of four fexuprazan 10 mg tablets (T1) and a single dose of one fexuprazan 40 mg tablet (T2). The participants were randomly assigned to Sequence A or B. Sequence A participants received T1 in Period 1 and T2 in Period 2. Sequence B participants received T2 in Period 1 and T1 in Period 2. Blood samples (6 mL) were collected at pre-dosing (0 h) and post-dosing (0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h) during each period. According to a previous study on fexuprazan, the intra-subject CVs for Cmax and AUC from zero to the time of the last quantifiable concentration (AUClast) were approximately 19.8% and 16.8%, respectively [12]. To assume an actual (four fexuprazan 10 mg tablets)/(one fexuprazan 40 mg tablet) ratio of 1.1 and an equivalence range of 0.8–1.25, a minimum of 18 participants would be required to achieve a statistical power of at least 80% at a significance level of 0.05. Therefore, considering a dropout rate of 25%, the target sample size was set at 24 participants.
The fasted study participants were administered IPs (either fexuprazan 10 mg or 40 mg) with 150 mL of water. The IPs used in this study were developed by Daewoong Pharmaceutical Co., Ltd. Blood samples were promptly collected in heparinized tubes at each blood sampling time point, and the plasma was separated through centrifugation at 1900× g for 10 min at 4 °C. The collected plasma samples were then transferred to 2 Eppendorf tubes at a volume of approximately ≥1.0 mL and stored in a freezer at ≤−70 °C until further analysis.
Participants were admitted the day before the administration of the IPs and discharged the day after dosing. Throughout their hospital stay, all meals were provided, except for breakfast on the day of dosing, and participants were only allowed to consume the meals and beverages provided by the clinical trial center. The decision to participate in the clinical trial was entirely voluntary for the participants, and declining to participate had no consequences. Furthermore, participants retained the right to withdraw from the trial at any point, without facing any disadvantages regarding their future medical care. During the duration of the clinical trial, the researchers prioritized the safety of the participants, taking all necessary precautions to promptly and appropriately minimize any potential adverse effects, if they were to occur. Insurance coverage was obtained to cover any injuries that participants might sustain during the clinical trial procedures.
4.2. Determination of Plasma Fexuprazan Concentration
Human plasma was analyzed using validated liquid chromatography–tandem mass spectrometry (LC-MS/MS). The chromatographic separation of fexuprazan and internal standard (IS) was achieved using an ACE C18, 2.1 × 50 mm, and a 3 μm column (Aberdeen, Scotland) under isocratic conditions with a flow rate of 0.5 mL/min. Fexuprazan and IS were detected using an AB SCIEX API 5000 mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA) using multiple reaction monitoring in the positive electrospray ionization mode. The mass transitions for fexuprazan and IS were m/z 411.3 → 380.0 and m/z 414.3 → 380.2, respectively, and the method was validated over a concentration range of 0.1 to 100 ng/mL. The lower limit of quantitation was 0.1 ng/mL. The within-run precision and accuracy were 1.0 to 5.7% and −7.3 to 5.0%, respectively, and the between-run precision and accuracy were 1.0 to 6.4% and −1.0 to 1.0%, respectively.
4.3. PK Assessment
Non-compartmental analysis (NCA) was performed using the Phoenix WinNonlin software version 8.3 (Certara Co., Princeton, NJ, USA) to determine the PK parameters of fexuprazan. In Part 1, the PK parameters were Cmax, Cmax at steady state (Cmax,ss), the minimum observed concentration at steady state during the dosing interval (Cmin,ss), time to reach Cmax (Tmax), time to reach Cmax,ss (Tmax,ss), AUC from 0 to 12 h (AUC0–12h), AUC0–12h at steady state (AUC0–12h,ss), and AUClast. The accumulation ratio based on AUC0–12h was calculated as AUC0–12,ss multiple dose/AUC0–12 after a single dose.
In Part 2, the PK parameters included Cmax, Tmax, AUClast, the AUC from zero to infinity (AUCinf), apparent volume of distribution (Vz/F), elimination half-life (t1/2), and apparent clearance (CL/F); of these, Cmax, Cmax,ss, Tmax, Tmax,ss, and Cmin,ss were the actual observed values. AUC0–12h, AUC0–12h,ss, AUClast, and AUCinf were calculated using the linear trapezoidal rule. The elimination rate constant (ke) was estimated by the linear regression of the terminal declining phase in the logarithmic plasma concentration–time profile, and t1/2 was calculated from the ratio of the natural logarithm of 2 to ke. AUCinf was determined by adding AUClast to the extrapolated area beyond the last observed concentration (Clast), AUClast + Clast/ke. CL/F was calculated as Dose/AUCinf, and Vz/F was calculated as (CL/F)/ke.
4.4. Safety and Tolerability Assessment
Safety was evaluated based on TEAEs, physical examinations, vital signs, 12-lead ECGs, and clinical laboratory tests. TEAEs were either spontaneously reported by participants or identified through data collected during scheduled interviews throughout the study period. All TEAEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 and summarized by treatment, severity, and relationships with IPs.
4.5. Statistical Analysis
Descriptive statistics were used to summarize baseline demographics, such as age, weight, height, and BMI. The PK parameters and safety profiles were evaluated using descriptive statistics. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). In Part 1, the method used to determine whether the curve is flat involved stepwise testing for linear trends to assess the steady state. This assessment utilized pre-dose samples obtained on the day before the PK assessment day and on the PK assessment day [25]. For the analysis, plasma concentrations at 7d 12 h, 8d 0 h, and 8d 12 h were considered.
In Part 2, the primary PK endpoints (Cmax and AUClast) were log-transformed to develop a mixed-effect model, with treatment effects as fixed effects and participant effects as random effects. GMRs with 90% confidence intervals (Cis) of the primary PK parameters between the two treatment groups (T1 vs. T2) were estimated to evaluate bioequivalence. Bioequivalence for T1 vs. T2 was determined by assessing whether the 90% CI of the GMRs of the primary PK parameters were within the bioequivalence range of 0.8 to 1.25. Numerical data, such as demographic characteristics and PK parameters between the two treatments, were compared using an independent t-test or the Wilcoxon rank-sum test. Categorical data, such as TEAE incidence, were compared using the chi-square or Fisher’s exact test.
5. Conclusions
The new fexuprazan 10 mg formulation demonstrated favorable safety and tolerability when administered in single or multiple doses and exhibited bioequivalence compared to fexuprazan 40 mg. Therefore, this new formulation can be effectively used in developing dosing regimens for various gastric ARDs, including acute and chronic gastritis.
Acknowledgments
The authors would like to express their gratitude to all the patients and staff who participated in the study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16081141/s1, Table S1: Comparison between a newly developed fexuprazan 10 mg and the formulation used in a first-in-human study.
Author Contributions
Conceptualization, W.S., A.-Y.Y. and A.K.; methodology, W.S. and A.K.; validation, H.P., H.L. and A.K.; formal analysis, W.S. and A.-Y.Y.; investigation, W.S., A.-Y.Y., H.Y. and A.K.; resources, A.K.; data curation, W.S. and A.-Y.Y.; writing—original draft preparation, W.S. and A.-Y.Y.; writing—review and editing, W.S., A.-Y.Y., H.P., H.L., H.Y. and A.K.; visualization, W.S. and A.-Y.Y.; supervision, H.P. and H.L.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice (ICH-GCP), and the Korean Good Clinical Practice guidelines and was approved by the Institutional Review Board of CHA University, Bundang Medical Center, located in Seongnam, Korea (IRB No. CHAMC 2021-06-021).
Informed Consent Statement
Informed consent was obtained from all participants prior to participation.
Data Availability Statement
The data are not publicly available due to confidentiality reasons.
Conflicts of Interest
Hyung Park and Hyejung Lee are employees of Daewoong Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
Funding Statement
This study was funded by Daewoong Pharmaceutical Co., Ltd. (grant no. DWP14012107).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Welage L.S., Berardi R.R. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J. Am. Pharm. Assoc. 2000;40:52–123. doi: 10.1016/S1086-5802(16)31036-1. [DOI] [PubMed] [Google Scholar]
- 2.Kim M.K., Ko B.J., Kim E.Y., Han B.D., Cho K.H. Fast Eating Speed Increases the Risk of Endoscopic Erosive Gastritis in Korean Adults. Korean J. Fam. Med. 2015;36:300–304. doi: 10.4082/kjfm.2015.36.6.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azer S.A., Akhondi H. Gastritis. StatPearls Publishing; Treasure Island, FL, USA: 2023. [PubMed] [Google Scholar]
- 4.Baldwin C.M., Keam S.J. Rabeprazole: A review of its use in the management of gastric acid-related diseases in adults. Drugs. 2009;69:1373–1401. doi: 10.2165/00003495-200969100-00007. [DOI] [PubMed] [Google Scholar]
- 5.Shin J.M., Vagin O., Munson K., Kidd M., Modlin I.M., Sachs G. Molecular mechanisms in therapy of acid-related diseases. Cell Mol. Life Sci. 2008;65:264–281. doi: 10.1007/s00018-007-7249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Hollander W.J., Kuipers E.J. Current pharmacotherapy options for gastritis. Expert Opin. Pharmacother. 2012;13:2625–2636. doi: 10.1517/14656566.2012.747510. [DOI] [PubMed] [Google Scholar]
- 7.Megha R., Farooq U., Lopez P.P. Stress-Induced Gastritis. StatPearls Publishing; Treasure Island, FL, USA: 2023. [PubMed] [Google Scholar]
- 8.Hanafy A.S., Seleem W.M. Refractory Helicobacter pylori gastritis: The hidden predictors of resistance. J. Glob. Antimicrob. Resist. 2019;19:194–200. doi: 10.1016/j.jgar.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwagi H. Ulcers and gastritis. Endoscopy. 2005;37:110–115. doi: 10.1055/s-2004-826147. [DOI] [PubMed] [Google Scholar]
- 10.Oshima T., Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J. Neurogastroenterol. Motil. 2018;24:334–344. doi: 10.5056/jnm18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachs G., Shin J.M., Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr. Gastroenterol. Rep. 2010;12:437–447. doi: 10.1007/s11894-010-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Yoo H., Shin W., Lee S., Lee H., Kim E., Kim A. Size-reduced fexuprazan 20 mg demonstrated the optimal bioavailability and bioequivalence with the reference formulation. Transl. Clin. Pharmacol. 2023;31:40–48. doi: 10.12793/tcp.2023.31.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzka D.A., Kahrilas P.J. Potassium-Competitive Acid Blocker Suppression of Gastric Acid in Erosive Esophagitis: Is Stronger and Longer Better? Gastroenterology. 2023;164:14–15. doi: 10.1053/j.gastro.2022.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Food and Drug Safety 2022 Drug Approval Report. 2023. [(accessed on 29 June 2023)]. Available online: https://www.mfds.go.kr/eng/brd/m_19/view.do?seq=70438&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1.
- 15.Ministry of Food and Drug Safety 2021 Drug Approval Report. 2022. [(accessed on 30 June 2022)]. Available online: https://www.mfds.go.kr/eng/brd/m_19/down.do?brd_id=eng0004&seq=70437&data_tp=A&file_seq=1.
- 16.Daewoong Pharmaceutical’s ‘Fexuclue’: First Domestic P-CAB Agent to Obtain Indication for Gastritis Korea Biomedical Review. 2022. [(accessed on 20 August 2022)]. Available online: http://www.docdocdoc.co.kr/news/articleView.html?idxno=2026314.
- 17.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Food Drug Adminsitration Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules Guidance for Industry. [(accessed on 3 October 2022)];2022 Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/size-shape-and-other-physical-attributes-generic-tablets-and-capsules.
- 18.Lee K.N., Lee O.Y., Chun H.J., Kim J.I., Kim S.K., Lee S.W., Park K.S., Lee K.L., Choi S.C., Jang J.Y., et al. Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J. Gastroenterol. 2022;28:6294–6309. doi: 10.3748/wjg.v28.i44.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim G.H., Choi M.G., Kim J.I., Lee S.T., Chun H.J., Lee K.L., Choi S.C., Jang J.Y., Lee Y.C., Kim J.G., et al. Efficacy and Safety of Fexuprazan in Patients with Acute or Chronic Gastritis. Gut Liver. 2023 doi: 10.5009/gnl220457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunwoo J., Oh J., Moon S.J., Ji S.C., Lee S.H., Yu K.S., Kim H.S., Lee A., Jang I.J. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium-competitive acid blocker, in healthy male participants. Aliment Pharmacol. Ther. 2018;48:206–218. doi: 10.1111/apt.14818. [DOI] [PubMed] [Google Scholar]
- 21.Shibli F., Kitayama Y., Fass R. Novel Therapies for Gastroesophageal Reflux Disease: Beyond Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2020;22:16. doi: 10.1007/s11894-020-0753-y. [DOI] [PubMed] [Google Scholar]
- 22.Vakil N.B., Traxler B., Levine D. Dysphagia in patients with erosive esophagitis: Prevalence, severity, and response to proton pump inhibitor treatment. Clin. Gastroenterol. Hepatol. 2004;2:665–668. doi: 10.1016/S1542-3565(04)00289-7. [DOI] [PubMed] [Google Scholar]
- 23.Chiocca J.C., Olmos J.A., Salis G.B., Soifer L.O., Higa R., Marcolongo M., Argentinean Gastro-Oesophageal Reflux Study Group Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: A nationwide population-based study. Aliment Pharmacol. Ther. 2005;22:331–342. doi: 10.1111/j.1365-2036.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 24.Hwang J.G., Jeon I., Park S.A., Lee A., Yu K.S., Jang I.J., Lee S. Pharmacodynamics and pharmacokinetics of DWP14012 (fexuprazan) in healthy subjects with different ethnicities. Aliment Pharmacol. Ther. 2020;52:1648–1657. doi: 10.1111/apt.16131. [DOI] [PubMed] [Google Scholar]
- 25.Maganti L., Panebianco D.L., Maes A.L. Evaluation of methods for estimating time to steady state with examples from phase 1 studies. AAPS J. 2008;10:141–147. doi: 10.1208/s12248-008-9014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available due to confidentiality reasons.