Abstract
Simple Summary
This study explores the immunomodulatory properties of bovine colostrum (COL), the initial milk produced by lactating cows, on cytokine production in vitro and in a novel murine calf-fecal microbiota transplantation (FMT) model. The researchers utilized a Caco-2/THP-1 macrophage co-culture model stimulated with Phorbol 12-myristate 13-acetate (PMA) to investigate the effects of COL on cytokine production. The findings indicate that COL pretreatment significantly reduced IL-6 production while enhancing IL-10 production. The IL-6 suppressive effect was heat-sensitive and associated with components of higher molecular mass (100 kDa). Colostrum demonstrated decreased intestinal permeability, reduced immune cell infiltration, and suppressed IL-6 production during S. typhimurium infection. These results highlight the immunomodulatory potential of bovine colostrum and its prospective therapeutic applications in inflammatory disorders. Further research is necessary to elucidate the underlying mechanisms and corroborate the findings in bovine models.
Abstract
Bovine colostrum (COL), the first milk secreted by lactating cows postpartum, is a rich source of bioactive compounds that exert a significant role in the survival, growth, and immune development of neonatal calves. This study investigated the immunomodulatory effects of COL on cytokine production in vitro using a Caco-2/THP-1 macrophage co-culture model stimulated with Phorbol 12-myristate 13-acetate (PMA). COL pretreatment significantly reduced IL-6 (241.3 pg/mL) production induced by PMA (p < 0.05), while increasing IL-10 production (45.3 pg/mL), in comparison to PMA control (441.1 and 12.5 pg/mL, respectively). Further investigations revealed that the IL-6 suppressive effect of colostrum was heat-sensitive and associated with components of higher molecular mass (100 kDa). Moreover, colostrum primarily influenced THP-1 macrophages rather than Caco-2 epithelial cells. The effects of colostrum on IL-6 production were associated with reduced NF-κB activation in THP-1 macrophages. In calf-FMT transplanted C57BL/6 murine model, colostrum decreased intestinal permeability, reduced immune cell infiltration and intestinal score, and suppressed IL-6 (142.0 pg/mL) production during S. typhimurium infection, in comparison to control animals (215.2 pg/mL). These results suggest the immunomodulatory activity of bovine colostrum and its potential applications in inflammatory disorders. Further studies are needed to elucidate the underlying mechanisms and validate the findings in bovine models.
Keywords: bovine colostrum, cytokine production, immunomodulation, intestinal permeability, THP-1 macrophages, Caco-2 cells, NF-κB, S. typhimurium infection, inflammatory disorders
1. Introduction
Bovine colostrum (COL), the first milk produced by lactating cows postpartum, is a rich source of bioactive compounds that exert a significant role in the survival, growth, and immune development of neonatal calves [1,2,3]. Colostrum contains numerous bioactive constituents, including maternal immunoglobulins, growth factors, antimicrobial peptides, cytokines, and even immune cells [2,3,4]. These constituents collectively confer passive immunity against diverse pathogens, promoting intestinal microbiome development, maintaining anti-inflammatory homeostasis, and facilitating immune tolerance to microbiota [3,4].
Neonatal calves face notable challenges to their overall health, growth, and survival, particularly due to intestinal inflammation and infectious or non-infectious diarrheal diseases [5,6]. The gastrointestinal tract of neonates is highly susceptible to inflammatory and diarrheal conditions during this critical developmental period [6]. Early colonization of the intestinal tract by microbiota begins in the initial hours and continues through weeks and months of life [7,8]. The diversity of the microbial community is strongly influenced by the inflammatory status of the intestinal environment and could be further amplified by pathogenic microbiota and their secreted metabolites or factors. Additionally, several evidence suggests that dysregulation of innate immune responses in the gut, coupled with alterations in the intestinal microbiota composition, especially by the colonization of pathogenic flora, significantly contributes to the pathogenesis of inflammatory bowel diseases, leading to persistent diarrhea [8]. Therefore, gaining a comprehensive understanding of the molecular mechanisms underlying colostrum-mediated responses during inflammation and microbiota colonization holds substantial significance in improving calf health, preventing diseases, and developing novel host-directed therapeutic approaches.
Interleukin-6 (IL-6) represents a multifunctional pro-inflammatory cytokine that plays pivotal roles in numerous biological processes, including inflammation, immune regulation, and the interplay between innate and adaptive immune responses. Within the intestinal milieu, IL-6, previously shown to be crucial for the pathogenesis of inflammatory bowel diseases in humans, orchestrates acute inflammation, crucially contributes to immune cell recruitment, and modulates inflammatory processes [9]. Various cell types within the intestine, including macrophages, dendritic cells, T cells, and epithelial cells, produce IL-6 in response to diverse stimuli, such as infections, tissue damage, and inflammation [10]. Due to its highly deleterious nature, the production of IL-6 is tightly regulated; however, it can be chronically upregulated under inflammatory conditions, leading to tissue damage, compromised intestinal barrier function, and systemic infections [11].
Several studies have provided early evidence supporting the immunomodulatory activity of COL and milk derived from ruminant species. A study conducted by Kao et al. investigated the impact of goat milk supplementation in mice sensitized with ovalbumin to induce airway inflammation, revealing a significant increase in splenic CD3+ cell numbers [12]. The authors also showed that mice treated with goat milk demonstrated heightened production of interleukin-10 (IL-10) and interferon-gamma (IFNγ), suggesting an immune-modulating effect of goat milk on the cellular and cytokine responses [12]. Moreover, several distinct differences were observed in the composition of COL among different ruminant species [13]. Bovine COL exhibited higher levels of immunoglobulins IgG and IgA, while sheep colostrum displayed elevated IgG content compared to both goat and bovine counterparts [14,15]. Notably, goat COL demonstrated a unique composition of growth factors, small regulatory RNA, and cytokines, highlighting potential variances in immune system development and maturation [16]. Despite early insights into the differences in colostrum among ruminants, the detailed composition of colostrum as well as extensive comparisons between various ruminants remains poorly described.
Recent studies have demonstrated the immunomodulatory activity of bovine COL within the intestinal environment. Bovine COL comprises cytokines, growth factors, and maternal-derived immunoglobulins (Ig’s), such as IgG and IgA, which act synergistically to regulate immune responses both on mucosal and systemic levels [2,17,18]. Additionally, COL is enriched with immunomodulatory oligosaccharides, sialyloligosaccharides, as well as various glycoproteins and glycolipids, which further modulate immune function [18,19]. Throughout the lactation period, the concentrations of bioactive molecules in COL undergo intricate and dynamic changes, possibly tailored to meet the various needs of the growing calf [20]. As lactation progresses into the transitional phase, the composition of colostrum undergoes changes. The levels of maternal immunoglobulins, particularly IgG, begin to decline gradually [14,17]. Moreover, during this phase, the concentration of lactoferrin, antimicrobial peptides, and defensins increases, enhancing calf defense against pathogens [17].
It has been established that COL can augment the production of anti-inflammatory interleukin-10 (IL-10), thereby favorably shifting immune responses from pro-inflammatory Th1 responses towards anti-inflammatory Th2 responses [21]. Notably, administration of COL in a dextran sulfate-induced colitis model resulted in increased numbers of CD11b-positive leukocytes producing IL-10, ultimately leading to diminished intestinal inflammation via the Src–Akt pathway [22]. Furthermore, IL-10 can negatively regulate IL-6 production by potentially modulating nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) signaling pathways. However, the precise regulatory mechanisms underlying the interplay between IL-6 and IL-10 in response to colostrum within the intestine remain incompletely characterized.
Our previous studies have elucidated the critical impact of COL administration on the health and development of neonatal Holstein calves [23]. Notably, we observed heightened leukocyte numbers in colostrum-fed calves compared to the control group, providing preliminary evidence of the potential immunomodulatory activity of colostrum [23]. Building upon this, with our interest in comprehensively deciphering the molecular mechanisms underlying COL’s effects in calves, we aimed to employ advanced immunological, cellular biology, and in vivo animal models to delineate the immunomodulatory properties of COL. In this study, we present the development of novel calf-fecal microbiota transplantation (FMT) into convenient C57BL/6 mice models, in which mice harbor engrafted calf microbiota, as an approach to investigate the alterations induced by colostrum within the intestinal environment. Our findings provide experimental evidence regarding the interplay between IL-6 and IL-10 mediated by colostrum in both in vitro and in vivo models and propose the utility of colostrum as a preventative or therapeutic tool to mitigate intestinal inflammation in calves.
2. Materials and Methods
2.1. Ethics
The animal studies and all procedures involved were approved by the State Food and Veterinary Service (SFVS) animal care and welfare committee (approval number: No G2-164) in compliance with the rules of the National Institutes of Health Guide for Care and Use of Laboratory Animals guidelines.
2.2. Collection and Processing of Bovine Colostrum
Bovine colostrum (COL) was collected from randomly selected 10 healthy Holstein Friesian cows in their first or second parity from a farm (Lytagra Agriculture company, Naujieji Bernatoniai, Lithuania). Before COL collection, cows were evaluated by a veterinary doctor to ensure that there is no evidence of mastitis and that the animals are healthy. To ensure consistent experimental conditions and eliminate the potential influence of diet or animal management on the results, the colostrum was collected from the same farm where all the animals were managed in a uniform manner, including diet and circadian rhythm. The calves were born within a similar time frame (between 24–48 h). Right after the cows gave birth, they were milked every hour for 4 h to collect early-produced colostrum (10–25 mL). The colostrum samples obtained from different cows were promptly cooled on ice, pooled together, aliquoted into 10 mL portions, and then frozen at −80 °C until the day of the experiments.
2.2.1. Colostrum Serum Preparation
For cell culture stimulation experiments, the colostrum was prepared following the method described by Zhang et al. with some slight modifications [24]. The pooled colostrum samples were thawed on ice and then centrifugated at 1500× g at 4 °C for 10 min. The resulting pellet, containing cellular components and precipitates, was discarded, and the supernatant was carefully transferred to screw-cap tubes 1.8 mL (JSHD, Jiangsu, China). Subsequently, the tubes were centrifugated at 25,000× g for 1 h at 4 °C. The upper fraction of colostrum, which contained fat, was aspirated and discarded, while the middle portion, consisting of colostrum serum, was carefully removed. The colostrum serum was then filtered through a 0.45 µm syringe filter and used for analysis in the experiments.
2.2.2. Colostrum Serum Protein Quantification
The Pierce Coomassie Bradford protein assay kit (ThermoFisher Scientific, #23236, Waltham, MA, USA) was utilized to quantify and normalize the colostrum samples for the cell culture stimulation experiments. The total protein content in the samples was determined following the instructions provided by the manufacturer. Bovine serum albumin (BSA) (ThermoFisher Scientific, Waltham, MA, USA), which was included in the kit, was used to prepare a standard curve and quantify the protein content of the colostrum samples.
2.2.3. Colostrum Inactivation by Heat
To generate heat-inactivated colostrum (HI-Col), samples of colostrum serum were subjected to incubation in a water bath at 70 °C for 30 min with constant agitation. The HI-Col was subsequently cooled on ice and then centrifuged at 3000× g for 10 min at 4 °C. The resulting supernatant was collected, and the total protein concentration was measured using the Bradford assay (ThermoFisher Scientific, #23236, Waltham, MA, USA). Finally, the HI-Col was utilized for the cell culture experiments.
2.2.4. Ultrafiltration of Colostrum
To generate 10, 50, and 100 kDa fractions of colostrum, Amicon-Ultra 15 filters (Sigma-Aldrich, #UFC9010, UFC9050, UFC90100, Schnelldorf, Germany) were filled with colostrum and subjected to centrifugation for 1 h at 7500× g, 4 °C. This centrifugation step allowed the separation of colostrum based on molecular size. The flow-through compartment of the filters flows through compartments containing defined molecular size fractions (10, 50, and 100 kDa) of colostrum. The total protein concentration in these fractions was quantified using the Bradford assay (ThermoFisher Scientific, #23236, Waltham, MA, USA). The protein content in each fraction was then used for the cell culture experiments.
2.3. Cells and Culture Conditions
Human Caco-2 intestinal epithelial cells (ATCC HTB-37) were obtained from American Type Culture Collection (ATCC) (https://www.atcc.org/products/htb-37, accessed on 9 September 2022) were cultured in DMEM/F12 supplemented with GlutaMAX (Gibco, #10565018, ThermoFisher Scientific, Waltham, MA, USA), PenStrep (Gibco, #10378016, ThermoFisher Scientific, Waltham, MA, USA), and 10% heat-inactivated FBS (Gibco, #A3160501, ThermoFisher Scientific, Waltham, MA, USA). Human THP-1 monocytes (ATCC TIB-202) were obtained from the ATCC (https://www.atcc.org/products/tib-202, accessed on 9 September 2022) and maintained in complete RPMI media (Gibco, #11875101, ThermoFisher Scientific, Waltham, MA, USA) supplemented with GlutaMAX (Gibco, #35050061, ThermoFisher Scientific, Waltham, MA, USA) and 10% heat-inactivated FBS (Gibco, #A3160501, ThermoFisher Scientific, Waltham, MA, USA). Both cell lines were cultured at 37 °C with 5% CO2. When differentiation of THP-1 cells into macrophages was required, the cells were incubated in complete RPMI media containing 200 ng/mL of Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, #P8139, Schnelldorf, Germany) for 48 h. Subsequently, the cells were rested in PMA-free RPMI media for an additional 48 h before being used in experiments.
2.4. Establishment of Caco-2/THP-1 Co-Culture System
The co-culture experiments were conducted following the methodology described elsewhere with some modifications [25]. Initially, Caco-2 cells were seeded onto permeable 0.4 µm inserts (Thermo Scientific, #141078, Waltham, MA, USA) and placed in 12-well carrier plates at a density of 2.5 × 105 cells per insert. The cells were then incubated for 12 days, with media changes carried out every two days. The formation of the epithelial layer was confirmed by measuring the transepithelial electric resistance (TEER) using an Evom instrument (NC2120402). The TEER value was considered to be approximately 250–300 Ω·cm2 in DMEM/F12 media, indicating the formation of the epithelium. Subsequently, THP-1-derived macrophages (2.5 × 105 cells/insert) were added to the Caco-2 cell inserts, and the co-culture system was further incubated for 48 h. During this period, the TEER of the co-culture system was approximately Ω·cm2, indicating the establishment of the Caco-2/THP-1 macrophage co-culture system. All experiments were performed in triplicate.
2.5. Stimulation of Caco-2/THP-1 Co-Culture with Colostrum
The Caco-2/THP-1 co-cultures were washed three times with DPBS (Gibco, #10010023, ThermoFisher Scientific, Waltham, MA, USA) to remove any residual substances. The colostrum was diluted in DMEM/F12 supplemented with 10% FBS to achieve a concentration of 250 µg/mL (based on total proteins), and the cells were incubated with the diluted colostrum for 1 h. After the incubation, PMA (10 µg/mL) was added to the basolateral compartment of the co-culture system, and the cells were further incubated for 24 h in the presence of PMA to induce cytokine production. The transepithelial electric resistance (TEER) was measured to assess the integrity of the epithelial barrier. Additionally, the conditioned media from the basolateral compartment were collected and used for cytokine ELISA and LDH assays to analyze the secreted cytokines and assess cell cytotoxicity, respectively. In cases where microscopy imaging was necessary, the cells were cultured in 12-well plates without transwell inserts to allow direct visualization. All experiments were performed in triplicate.
2.6. LDH Release Assay
According to the manufacturer’s protocol, LDH activity was measured using the CyQUANT LDH Cytotoxicity Assay Kit (ThermoFisher Scientific, #C20300, Waltham, MA, USA). In the beginning, 50 µL of conditioned media collected from each sample was utilized for LDH quantification. As a control for maximum LDH release, lysed Caco-2/THP-1 cells were prepared using the supplied lysis buffer. The absorbance of the samples was measured at 490 nm and 680 nm. LDH release was subsequently calculated based on the manufacturer’s instructions.
2.7. Immunoblot of NFκB Phosphorylation In Vitro
The THP-1 derived macrophages or Caco-2/THP-1 co-culture was treated with bovine colostrum and stimulated with PMA as described above. After the treatments, the cells were rinsed three times with ice-cold DPBS (Gibco, #10010023, ThermoFisher Scientific, Waltham, MA, USA) containing 1 mM sodium orthovanadate (NEB, # P0758S, New Engalnd Biolabs, Ipswich, England). The membrane from the transwell inserts was cut using forceps and placed in 300 µL of 1× cell lysis buffer (Invitrogen, # FNN0011, ThermoFisher Scientific, Waltham, MA, USA) for 10 min on ice. The membranes were sonicated, and samples were centrifuged at 5000× g for 10 min at 4 °C. The proteins were quantified using the Pierce BCA protein assay kit (ThermoFisher Scientific, #23227, Waltham, MA, USA) as described by the manufacturer. The protein samples were incubated at 70 °C in 1× NuPAGE LDS loading dye (Invitrogen, # NP0008, ThermoFisher Scientific, Waltham, MA, USA) with 1× Bolt Reducing agent (Invitrogen, # B0009, ThermoFisher Scientific, Waltham, MA, USA) for 10 min. The lysates were separated by SDS-PAGE, electrotransferred onto PVDF membrane, and blocked using Intercept TSB blocking buffer (Licor, #927-60001, LI-COR Biosciences, Lincoln, USA). Membranes were probed with primary rabbit anti-NF-κB p65 (D14E12) (Cell Signaling, #8242, ThermoFisher Scientific, Waltham, MA, USA) or rabbit-phosphor NF-κB p65 (93H1) (Ser 536) (Cell Signaling, #3033) antibodies, and mouse β-Actin (8H10D10) (Cell Signaling, #3700, ThermoFisher Scientific, Waltham, MA, USA). Membranes were then probed using secondary IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody (Licor, #926-32211, LI-COR Biosciences, Lincoln, USA) for NF-κB or IRDye® 800CW Goat anti-Mouse IgG (Licor, #926-32210) for β-Actin. Blots were visualized using the Licor system.
2.8. Fecal Microbiome Transplantation and Generation of Calf-FMT C57BL/6 Mice
The FMT transplantation (engrafting) and generation of convenient, non-gnotobiotic calf-FMT C57BL/6 mice, containing calf FMT were conducted as described by Wrzosek et al., with some modifications [26].
2.9. Depletion of C56BL/6 Mice Intestinal Microbiota
C57BL/6 mice, initially obtained from Jackson Laboratories, are currently maintained in the Animal Facility at the Lithuanian University of Health Sciences (LUHS). Animals were kept according to animal welfare recommendations, 12 h day and night cycle, room temperature at 21 °C, and 50% humidity.
Before the experiments, at 8 weeks old, C57BL/6 mice were randomly assigned to different experimental groups, with each group consisting of five mice (n = 5). To deplete the bacterial and fungal intestinal microbiota, the mice received penicillin-streptomycin (1 g/L) (PEN-STREP, Norbrook, Newry, UK, #9772) metronidazole (1 g/L) (SUPPLIN, 500 mg, SANDOZ pharma, Kundl, Austria, #3400), vancomycin (250 mg/L) (Vancosan 1000 mg, MIP pharma, Hamburg, Germany, J01XA01), and trimethoprim (500 mg/L) (TRIMETOP 100 mg, Vitabalans Oy, Hämeenlinna, Finland, 3246) in their drinking water for 2 weeks. Additionally, 30% PEG 4000 (Sigma-Aldrich, Taufkirchen, Germany) solution was administered via oral gavage (200 µL per animal), to avoid fungal microbiota fluconazole (12 mg/kg) (Fluconazole, Baxter, Utrecht, The Netherlands) and to cleanse the intestine mechanically. During the depletion period, the mice were provided with sterilized food to minimize the introduction of exogenous microbiota. The depletion of endogenous microbiota was monitored by conducting quantitative fecal cultures on Sheep Blood agar plates (Thermo Scientific, #R01200). This allowed the assessment of the reduction in microbial colony-forming units (log10CFU) in the fecal samples.
2.10. Calve Fecal Microbiota Transplant (FMT) Preparation
Fecal material was collected from randomly selected 7-day-old 3 male and 3 female calves, with a total of 6 calves included in the study. The calves were inspected for diarrhea and health status by a veterinary doctor. The collected fecal samples were kept refrigerated on ice during transportation to the laboratory to maintain sample integrity.
Upon arrival at the laboratory, the individual fecal samples obtained from different animals were combined and pooled together. The pooled fecal sample was then diluted in a preservation media (Brain-Heart Infusion broth (Sigma-Aldrich, Taufkirchen, Germany), 20% skim milk, 1.25 g L-cysteine (Sigma-Aldrich, Taufkirchen, Germany) preservation media medium) to achieve a concentration of 1 g/mL of FMT. To ensure homogeneity, the fecal sample was homogenized using the Stomacher 450 Bam homogenization system (Thomas Scientific, #1185V52) for 1 min at room temperature.
Following homogenization, the fecal microbial transplant (FMT) was divided into aliquots and transferred to sterile polypropylene screw-cap tubes (JSHD, Jiangsu, China). These tubes were then stored at −80 °C until the experiment to preserve the viability and stability of the microbial content within the FMT samples.
2.11. Calve-FMT Transplantation to C57BL/6 Mice
After the depletion period, the administration of antibiotics through the drinking water was discontinued, and the mice started receiving sterile drinking water for 3 days to allow for the clearance of antibiotics from their system before the administration of calf FMT.
The necessary number of vials containing calf FMT was defrosted at 10–15 min in a water bath at 37 °C and mixed by gently inverting the vials to ensure homogeneity. The calf FMT was then administered to the mice via oral gavage twice a day for 3 consecutive days. Following the last dose of calf FMT administration, the mice were referred to as calf-FMT C57BL/6 mice. These mice then underwent feeding studies or further experiments related to the specific research objectives.
2.12. Calf-FMT C57BL/6 Mice Feeding Studies
The calf-FMT C57BL/6 mice were divided into different experimental groups and subjected to feeding with either colostrum (COL) or normal saline (NS), which served as a control.
To administer colostrum, the frozen samples were thawed at room temperature and given to the calf-FMT C57BL/6 mice via oral gavage at a dose of 20 mL/kg. The colostrum was administered every 8 h twice a day for 5 days.
For the control group, normal saline was administered to the mice at a dose of 20 mL/kg, also via oral gavage. The normal saline was given every 8 h twice a day for 5 days. After the feeding period, the mice were euthanized by cervical dislocation. Blood samples were collected via cardiac puncture, and the blood was then separated into serum by centrifugation at 10,000× g for 10 min at 4 °C. Furthermore, the intestines of the mice were dissected and used for histological analysis, which involved examining the tissue microscopically to evaluate any changes or effects.
2.13. Lethal Challenge with S. typhimurium ATCC 14028 Strain
The Salmonella typhimurium ATCC 14028 (S. typhimurium ATCC 14028) strain was cultured on Tryptic-Soy agar (Sigma-Aldrich, Schnelldorf, Germany) plates overnight to obtain single colonies. The bacterial mass was then suspended in sterile saline, and the inoculum size was quantified spectrophotometrically to achieve approximately 1.5 × 108 CFU/mL. Each animal received a volume of 200 µL of the S. typhimurium ATCC 14028 suspension, resulting in an infectious dose of approximately 3.0 × 107 CFU/animal. The accuracy of the inoculum size was confirmed by performing serial dilutions and plating on Tryptic-Soy agar (Sigma-Aldrich, Schnelldorf, Germany) plates (BD Difco, #236920, ThermoFisher Scientific, Waltham, MA, USA).
After 24 h after infection, the calf-FMT C57BL/6 mice received either colostrum (20 mL/kg) or normal saline (20 mL/kg) at regular intervals of 8 h twice a day for 5 days. The dosage of COL or NS was selected based on the maximum tolerable quantity that could be delivered to C57BL/6 mice via oral gavage. In the survival studies, the administration of colostrum or normal saline continued until the experimental endpoint was reached, which was defined as a 20% loss of body weight or a morbid appearance. The animals were euthanized by cervical dislocation. Blood samples were collected via cardiac puncture, and the blood serum was prepared by centrifugation. The bacterial burden, expressed as log10 CFU/mL, was determined by performing serial dilutions and plating on MacConkey agar plates (BD Difco, #212123, ThermoFisher Scientific, Waltham, MA, USA). Additionally, the fecal burden of S. typhimurium ATCC 14028 was calculated by serially diluting fecal material collected from the cecum and plating it on Xylose Lysine Deoxycholate agar (XLD) (Biomerieux, # CM0469B, ThermoFisher Scientific, Waltham, MA, USA) to differentiate from fecal flora. Sections of the intestine were collected for histological analysis.
2.14. Cytokine Quantification by ELISA
THP-1-derived macrophages or Caco-2/THP-1 co-cultures were utilized for the cell culture experiments. Following PMA stimulation, the conditioned cell culture media (50–100 µL per assay) collected from the basolateral compartment were subjected to quantification of various cytokines. Specifically, IL-1β (R&D Systems, #DLB50, Sigma-Aldrich, Schnelldorf, Germany), IL-6 (Invitrogen, #88-7066-88), IL-8 (Invitrogen, #KHC0081, ThermoFisher Scientific, Waltham, MA, USA), IL-10 (Invitrogen, #EHIL10, ThermoFisher Scientific, Waltham, MA, USA), IL-12p70 (Invitrogen, #BMS238, ThermoFisher Scientific, Waltham, MA, USA), TNFα (Invitrogen, #88-7346-88, ThermoFisher Scientific, Waltham, MA, USA), and INFγ (Invitrogen, #KHC402, ThermoFisher Scientific, Waltham, MA, USA) were measured using commercially available ELISA kits. As necessary, 96-well plates were coated with capture antibodies, and the assays were performed according to the manufacturer’s instructions.
For the colostrum feeding experiments in calf-FMT C57BL/6, cytokine quantification was performed using 1:5 diluted mouse blood serum. The following cytokines were measured: IL-6 (Invitrogen, #88-7064-88, ThermoFisher Scientific, Waltham, MA, USA), IL-10 (Invitrogen, #88-7105-88, ThermoFisher Scientific, Waltham, MA, USA), IL-12p70 (Invitrogen, #88-7121-88, ThermoFisher Scientific, Waltham, MA, USA), and INFγ (Invitrogen, #88-7314-22, ThermoFisher Scientific, Waltham, MA, USA). The quantification was carried out following the manufacturer’s instructions.
2.15. Evans Blue Intestinal Permeability Assay
An Evans blue (EB) solution was prepared by preparing 10 mg/mL of Evans blue (Sigma-Aldrich, #E2129, Schnelldorf, Germany) in saline. The solution was then sterilized by passing it through a 0.4 µm filter to ensure sterility.
For the experimental feeding or S. typhimurium ATCC 14028 infection studies involving Calf-FMT C57BL/6 mice receiving colostrum or normal saline, the EB solution was administered to the mice at a dose of 200 mg/kg via intraperitoneal (i.p.) route. This administration took place 12 h prior to the scheduled euthanasia.
To collect samples for EB analysis, the Calf-FMT C57BL/6 mice were euthanized, and their whole intestines were carefully dissected and separated into four sections: duodenum, jejunum, ileum, and colon. Each section was weighed and homogenized in 500 µL formamide (Sigma-Aldrich, Taufkirchen, Germany, #200-842-0). The EB present in the tissue samples was extracted by incubating the samples at 55 °C for 24–48 h. After incubation, the extracts were clarified by centrifugation at 10,000× g for 10 min.
The concentration of EB in the tissue samples was determined spectrophotometrically by measuring the absorbance at 610 nm using a spectrophotometer (Bio-Tek Synergy HT, Santa Clara, CA, USA). The tissue concentration of EB was calculated by using known concentrations of EB in formamide as a reference.
2.16. Histological Analysis
The calf-FMT C57BL/6 mouse intestines were processed by using the Swiss roll technique as described by [27]. Right after euthanasia, the intestines were dissected and placed in ice-cold PBS (Gibco, # 10010031). The cecum was dissected and discarded, and the small intestine was flushed by ice-cold PBS, following cold modified Bouin fixative (5% acetic acid, 50% ethanol in deionized water) [23,27]. The intestinal rolls were then formed as described by Grigaleviciute et al., and tissues were placed in tissue processing cassettes, fixed overnight in 10% neutral formaldehyde (Fisher Scientific, # SF98-4, Waltham, MA, USA), processed and embedded by an automatic tissue processor. Tissue sections were then stained with Hematoxylin-Eosin (HE) stain to characterize tissue architecture and Alcian blue (AB) staining to visualize mucins [23]. Tissue sections were then evaluated, and the inflammation as well as tissue architecture was scored by a pathologist using the method described by Erben et al. [28].
2.17. Statistical Analysis
The collected experimental data were analyzed using GraphPad Prism (GraphPad Software 8.0.1, San Diego, CA, USA). The in vitro cell culture experiments consisted of at least three experimental replicas, and data are shown as mean ± standard deviation (SD). The statistical significance was analyzed Mann–Whitney U test, where the mean of experimental values was compared with the mean of control values-tests. Data were considered significant when p < 0.05.
3. Results
3.1. Effects of Bovine Colostrum on PMA-Induced Cytokine Production and Caco-2/THP-1-Derived Macrophage Co-Culture Model
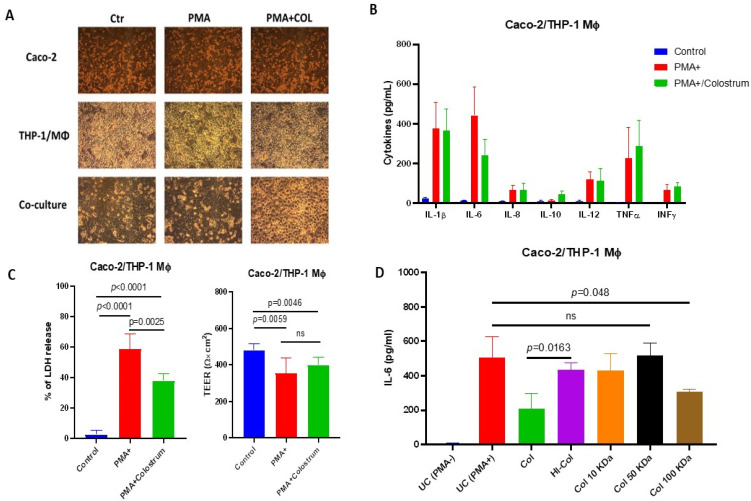
To investigate the immunomodulatory effect of COL on cytokine production in vitro, we utilized a well-established, polarized Caco-2/THP-1 macrophage co-culture model. The cells were stimulated with phorbol 12-myristate 13-acetate (PMA) that acted as broad-spectrum mitogen inducing various cytokines. Initially, the Caco-2/THP-1 system was pretreated with COL at a fixed concentration of 250 µg/mL for 1 h. Subsequently, PMA was added at a concentration of 10 µg/mL in the presence of COL, and cell cultures were then incubated for 24 h to induce cytokine secretion to the culture media without noticeable phenotype change in cells (Figure 1A).
Figure 1.
Bovine colostrum exerts immunomodulatory and chemoprotective activity on the Caco-1/THP-1 macrophage co-culture system. Panel (A) shows the morphological changes of Caco-2, THP-1, and co-cultures during the stimulation with Phorbol 12-myristate 13-acetate (PMA) and treatment with bovine colostrum (COL). Scale bar represents 100 µm. Panel (B) shows the effects of bovine colostrum on PMA-induced cytokine production by Caco-2/THP-1 macrophage co-culture. Panel (C) shows the effects of bovine colostrum on PMA-induced LDH release and transepithelial electric resistance (TEER) changes. Panel (D) demonstrates the effect of various treatments of bovine colostrum on the ability to suppress IL-6 production by Caco-2/THP-1 cells. Data are shown as mean ± standard deviation (SD). ns—non-significant.
Our results demonstrated that PMA stimulation significantly increased the production of cytokines, such as IL-1b, IL-6, IL-8, IL-12, TNFα, and INFγ, compared to the unstimulated control (Figure 1B). However, pretreatment of the Caco-2/THP-1 cells with COL resulted in a significant decrease in IL-6 production compared to the cells treated with PMA alone (p < 0.05). It suggests that COL exerts an inhibitory effect on IL-6 production in response to PMA stimulation (Figure 1B). Furthermore, our study revealed that pretreatment with COL prior to PMA stimulation enhanced the production of IL-10 compared to both the control group and the cells stimulated with PMA alone. These findings indicate that COL has the ability to augment the production of IL-10, an anti-inflammatory cytokine (Figure 1B).
In addition to assessing cytokine production, we evaluated the effects of COL on Caco-2/THP-1 viability by measuring the release of lactate dehydrogenase (LDH) (Figure 1C). Interestingly, we observed that pretreatment with COL prior to PMA stimulation significantly reduced the release of LDH compared to PMA alone. Moreover, PMA stimulation alone or in combination with COL pretreatment reduced the transepithelial electrical resistance (TEER), indicating increased permeability of the Caco-2/THP-1 epithelium (Figure 1C). However, COL pretreatment failed to significantly restore the loss of TEER induced by PMA.
After demonstrating the immunomodulatory activity of COL on IL-6 production using a PMA-stimulated Caco-2/THP-1 cell culture model, we aimed to characterize the types of colostrum constituents responsible for IL-6 inhibition COL (Figure 1D). To do so, we performed a series of experiments where we used a heat inactivation or ultrafiltration, to prepare different types of colostrum for the cell culture experiments. Our findings indicated that the IL-6 suppressive effect of COL is influenced by temperature-sensitive factors. Heat inactivation significantly attenuated the colostrum’s ability to inhibit IL-6 production in the Caco-2/THP-1 cell culture model, suggesting the involvement of heat-sensitive components (Figure 1D). To further investigate the factors responsible for the observed IL-6 suppression, we fractionated COL based on molecular size. The fractions with molecular weights of 10 kDa and 50 kDa did not show significant IL-6 inhibitory activity. However, the 100 kDa fraction of COL restored the IL-6 suppressive effect, indicating the presence of immunomodulatory factors with a higher molecular mass (Figure 1D).
These data suggest that COL possesses chemoprotective and immunomodulatory activity by suppressing the PMA-induced IL-6 production and cell death by Caco-2/THP-1 derived macrophage model.
3.2. Bovine Colostrum Is Able to Suppress PMA-Induced IL-6 Production in THP-1-Derived Macrophages via NF-κB-Dependent and Independent Manner
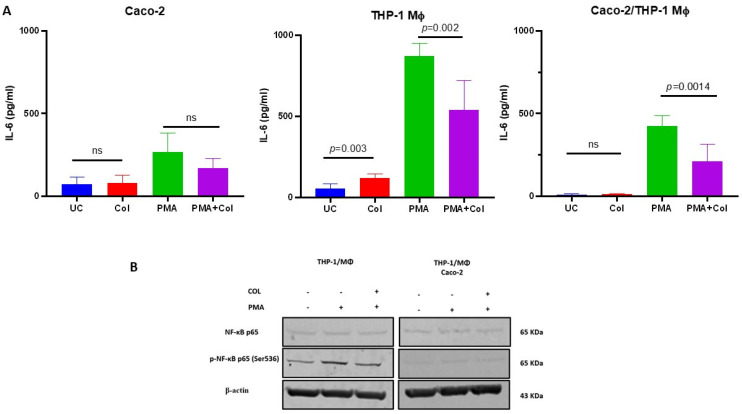
After demonstrating the suppressive effect of COL on IL-6 production and its chemoprotective activity, our next objective was to identify whether Caco-2 epithelial cells or THP-1 derived macrophages are mediating the IL-6 supresory phenotype in response to COL. To investigate this, we individually pre-treated Caco-2 cells and THP-1 macrophages with colostrum and performed co-culture experiments, followed by stimulation with PMA to induce cytokine production, and compared the findings to the co-culture experiment.
Interestingly, we observed that COL did not significantly decrease IL-6 production in Caco-2 intestinal epithelial cells alone. However, when THP-1 macrophages treated with COL were stimulated with PMA, a significant reduction in IL-6 was observed. Similar results were obtained when cells in the co-culture system were treated with colostrum and then stimulated with PMA (Figure 2). These findings suggest that the IL-6 suppressive activity of colostrum may be primarily mediated through the THP-1 macrophage axis rather than the Caco-2 epithelial cells (Figure 2A).
Figure 2.
Bovine colostrum is able to reduce IL-6 production via THP-1-derived macrophage modulation. Panel (A) shows the effects of COL treatment on PMA-induced IL-6 production by Caco-2, THP-1 derived macrophages or their co-cultures. Panel (B) shows Western blots for NF-κB from THP-1-derived macrophages and Caco-2 co-culture. Data are shown as mean ± standard deviation (SD).
To further support our observations, we investigated the activation of the NF-κB signaling cascade by measuring the phosphorylation of p65 at Ser536 in THP-1 macrophages and the Caco-2/THP-1 macrophage co-culture after pre-treatment with colostrum followed by PMA stimulation. PMA stimulation, both in monoculture and co-culture conditions, led to increased phosphorylation of p65 at Ser536, indicating NF-κB activation compared to unstimulated controls. However, pre-treatment with colostrum resulted in decreased phosphorylation of p65 at Ser536, specifically in THP-1 macrophages (Figure 2B, Figures S1–S4). In contrast, no significant reduction in phosphorylation was observed when THP-1 macrophages were co-cultured with Caco-2 cells, despite the observed decrease in IL-6 protein production in colostrum-treated co-cultures.
These findings provide additional evidence that the IL-6 inhibitory effects of COL are likely mediated through the THP-1 macrophage population, as indicated by the reduction in IL-6 production and decreased phosphorylation of p65 at Ser536. However, the lack of significant reduction in p65 phosphorylation in the co-culture system suggests that other mechanisms may contribute to the observed IL-6 suppression in Caco-2/THP-1 co-cultures treated with colostrum.
3.3. Colostrum Reduces the Intestinal Permeability in Calf-FMT Mice
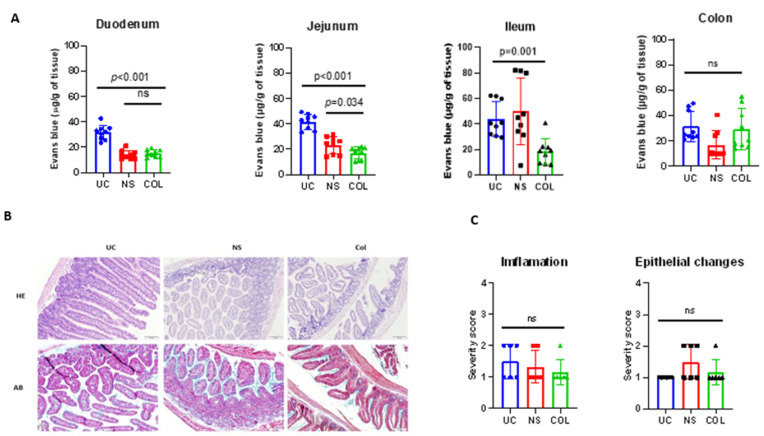
It is widely accepted that the interplay between gut microbiota and the host plays a crucial role in mucosal immune responses and intestinal homeostasis. We first sought to investigate further the histological changes and intestinal inflammation associated with colostrum feeding in calf-FMT transplanted C57BL6 mice. Due to a lack of bovine experimental models and well-established murine models, we conducted experiments using calf fecal microbiota transplantation (FMT) in conventional C57BL/6 mice (calf-FMT C57BL/6).
The C57BL/6 mice were initially treated with a combination of antibiotics and antifungal drugs, followed by PEG 4000 cleansing to deplete the intestinal microbiota. The calf FMT was then engrafted into the conditioned mice via oral gavage. The animals were divided into two groups: one receiving colostrum (COL) (20 mL/kg, q8 h two times a day) and the other receiving normal saline (20 mL/kg, q8 h two times a day) for 5 days. To quantify the feeding-associated changes, we performed experiments using COL and normal saline (NS) feeding and employed the Evans blue assay to assess tissue permeability, which indicates cellular junction integrity. Our findings revealed that COL significantly (p = 0.0395) reduced the accumulation of Evans blue as a marker of intestinal permeability in the jejunum and ileum of the calf-FMT C57BL/6 mouse model, as compared to the untreated control (UC) (49.2 and 62.3 μg/g of tissue) or NS feeding (31.2 and 82.1 μg/g of tissue) (Figure 3A). Moreover, feeding with COL was associated with a lower pathological score of inflammation (2 severity score) and noticeable changes in tissue architecture (Figure 3C). Specifically, mice that received colostrum exhibited reduced numbers of tissue-infiltrating neutrophils and macrophages, as compared to those in the UC or NS groups, while mucus production and the localization of Goblet cells were similar (Figure 3B).
Figure 3.
Bovine colostrum decreases intestinal permeability and infiltration with immune cells in calf-FMT C57BL/6 mice. Panel (A) demonstrates the intestinal permeability and inflammation measured by Evans blue assay. Panel (B) shows representative photomicrographs of the mouse intestine stained with hematoxylin/eosin (HE) and Alcian blue (AB). Panel (C) shows the pathological scoring of calf-FMT C57BL6 mice undergoing feeding with normal saline (NS) and colostrum (COL). Data are shown as mean ± standard deviation (SD).
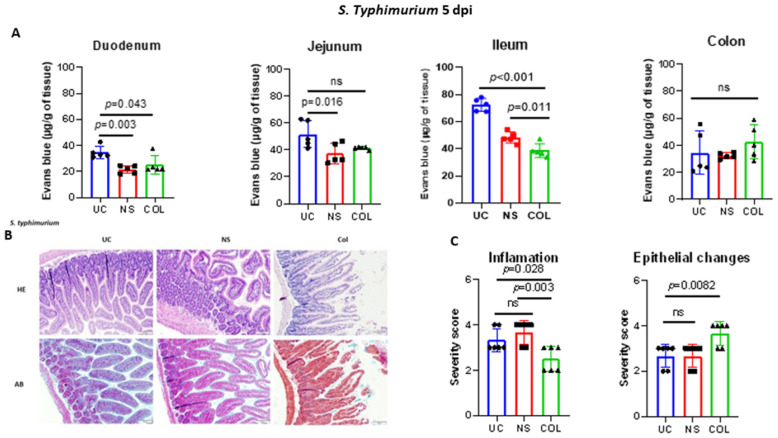
Additionally, when the mice were challenged with S. typhimurium, a significant (p = 0.0113) reduction in Evans blue accumulation was observed in the ileum of COL-treated animals in comparison to the UC or NS groups. However, no significant differences were observed in the jejunum, suggesting that the anti-inflammatory and modulatory effects of COL are primarily directed in the ileum. Furthermore, the administration of COL in S. typhimurium-infected mice resulted in a decreased histological score of inflammation and an enhanced epithelial changes score, suggesting possible pathogen-mediated tissue damage (Figure 4A–C).
Figure 4.
Bovine colostrum reduces intestinal inflammation in calf-FMT C57BL/6 mice lethally challenged with S. typhimurium ATCC 14028 strain. Panel (A) demonstrates the intestinal permeability and inflammation measured by Evans blue assay followed by lethal challenge with S. typhimurium ATCC 14028. Panel (B) shows representative photomicrographs of S. typhimurium infected calf-FMT C57BL/6 mouse intestine stained with hematoxilin/eosin (HE) and Alcian blue (AB). Panel (C) shows the pathological scoring of Salmonella-infected calf-FMT C57BL6 mice undergoing feeding with normal saline (NS) and colostrum (COL). Data are shown as mean ± standard deviation (SD).
Collectively, these data suggest that COL modulates the infiltration of immune cells into the gut environment as well as reduces the inflammation-mediated damage during lethal S. typhimurium challenge.
3.4. Colostrum Exerts Immunomodulatory Activity in Calf FMT-Transplanted Mice
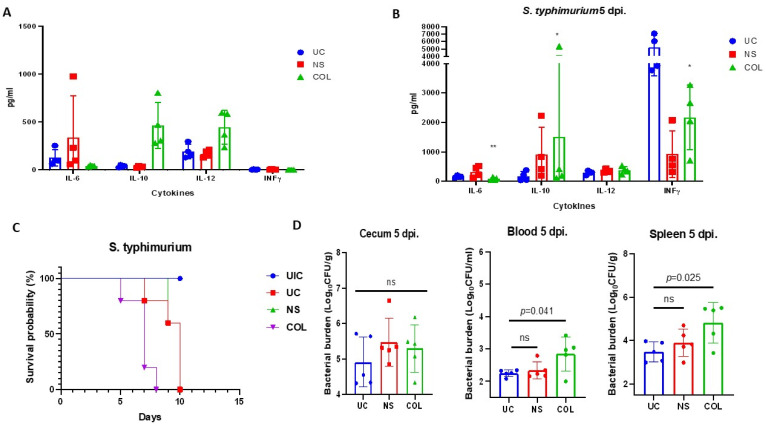
Surprisingly, the mice fed with COL exhibited significantly decreased production of IL-6 (42.3 pg/mL) compared to the untreated control (UC) or the group receiving normal saline (NS) (Figure 5A). Additionally, we observed an enhanced production of IL-10, indicating that colostrum can modulate the production of cytokines in our calf-FMT C57BL/6 mice (Figure 5A). Building upon this observation, we investigated whether colostrum feeding could modulate inflammatory immune responses in the gut during the challenge with S. typhimurium ATCC 14028 (Figure 5B).
Figure 5.
Bovine colostrum exhibits IL-6-targeted anti-inflammatory effects and mitigates S. typhimurium ATCC 14028 infection in calf-FMT C57BL/6 mice. Panel (A) illustrates cytokine production in calf-FMT C57BL/6 mice subjected to colostrum feeding. Panel (B) displays cytokine production in response to a lethal challenge with the S. typhimurium ATCC 14028 strain. Panel (C) presents survival data of calf-FMT C57BL/6 mice following a lethal challenge with S. typhimurium ATCC 14028, with interventions involving normal saline (NS), colostrum (COL), untreated Salmonella-challenged control, or calf-FMT transplanted and non-infected mice. Panel (D) demonstrates the Salmonella bacterial burden subsequent to the lethal challenge and feeding with NS or COL. Data are shown as mean ± standard deviation (SD). * p < 0.05; ** p < 0.001.
To examine this, we infected calf-FMT C57BL/6 mice with S. typhimurium ATCC 14028 12 h before administration of colostrum. The administration of colostrum (COL) (20 mL/kg, q8 h two times a day) or normal saline (NS) (20 mL/kg, q8 h two times a day) was continued during the experiment for 5 days. We found that colostrum administration during S. typhimurium infection resulted in reduced production of IL-6 and INFγ compared to the untreated control as well as increased production of IL-10 (Figure 5B). However, when a survival study was conducted, the colostrum-treated animals showed decreased survival percentage during the lethal challenge, suggesting an altered intestinal immune defense against S. typhimurium and subsequent systemic dissemination (Figure 5C). To confirm this hypothesis, we measured the bacterial burden in the feces (cecum), blood, and spleen (Figure 5D).
Notably, there were no significant changes in cecal S. typhimurium burden between infected and treated animals, indicating that COL or NS feeding was not associated with increased bacterial proliferation in the cecum (Figure 5D). Conversely, when assessing systemic dissemination, the bacterial burden in the blood was significantly higher in COL-treated animals (3.4 log10 CFU/mL) compared to the UC (2.3 log10 CFU/mL) or NS group (2.7 log10 CFU/mL). Similar results were observed when quantifying S. typhimurium in the spleen of COL-treated animals (5.5 log10 CFU/mL), suggesting that colostrum feeding provides immunomodulatory activity in the calf-FMT-transplanted C57BL/6 mice by modulating IL-6 and IL-10 responses (Figure 5D).
4. Discussion
Colostrum (COL), as the initial milk produced by cows after parturition, plays a pivotal role in the systemic and gut immune development of neonate calves, contributing to their enhanced survival and optimal growth during this critical period [29,30]. The rich composition of biologically active substances in COL, including vital nutrients, growth factors, and immunoglobulins, orchestrates the maturation of the intestinal mucosa and shapes the immunological milieu at both local and systemic levels [30]. Notably, COL has been found to influence the microbiome of calves, suggesting a potential interplay between colostrum, the microbiome, and mucosal immune responses that contribute to intestinal homeostasis, tolerance, and protection against diarrheal diseases [31]. Despite the well-documented observations regarding the beneficial effects of COL on calf health and development, there remains a significant knowledge gap concerning the specific molecular pathways regulated by colostrum that underlie its beneficial effects on the intestinal environment and innate immune responses [32,33,34].
Intestinal inflammation and diarrhea are prevalent and severe conditions in neonate calves, contributing to high morbidity and mortality rates [35]. Various infectious and non-infectious factors can trigger diarrhea, leading to intestinal and systemic inflammation, compromised intestinal barrier integrity, dysbiosis of the intestinal microbiota, impaired growth patterns, and increased mortality [36,37]. The dysregulated production of pro-inflammatory cytokines in the gut is responsible for initiating and perpetuating the inflammatory processes within the gut leading to systemic inflammation and potential autoimmune disorders [38,39,40]. A study conducted by Fisher et al. highlighted the critical role of interleukin-6 (IL-6) in bovine diarrhetic diseases, suggesting its potential as a prognostic marker [41]. In addition to IL-6, interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNFα) have been implicated in the acute phase responses observed in neonate calves, underscoring the central role of these cytokines in intestinal inflammation [39].
However, due to the limited availability of molecular reagents and established bovine cell lines and intestinal models, it is difficult to accurately recapitulate the bovine intestinal environment in vitro. Therefore, we employed a well-established Caco-2/THP-1 intestinal model to investigate the innate immune responses to bovine colostrum [42]. To induce inflammatory processes, we utilized phorbol 12-myristate 13-acetate (PMA), a non-specific mitogen that acts through protein kinase C (PKC), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and mito-gen-activated protein kinase (MAPK) signaling pathways, ultimately resulting in the production of pro-inflammatory cytokines across various cell lines, including Caco-2/THP-1 co-culture [30,43,44].
In our experimental in vitro model, pretreatment with COL before PMA stimulation resulted in a decreased production of the pro-inflammatory cytokine IL-6. Interestingly, we observed that COL also exhibited immunomodulatory properties by inducing the production of the anti-inflammatory cytokine IL-10 in response to PMA stimulation, suggesting its potential to regulate the inflammatory response in Caco-2/THP-1 cells. Furthermore, colostrum demonstrated the ability to restore cellular viability by reducing lactate dehydrogenase (LDH) production induced by PMA, indicating its potential cytoprotective effects [45].
Early investigations have provided valuable insights into the potential immunomodulatory activity of COL. For instance, Biswas et al. demonstrated that exposure of human peripheral blood mononuclear cells (PBMCs) to bovine colostrum led to the polarization of immune responses towards Th1 responses [46]. This suggests that COL has the ability to modulate the balance of immune cell subsets and their associated cytokine profiles. A comprehensive review conducted by Menchetti highlighted the diverse activities of COL on human innate and adaptive immune responses, indicating its potential therapeutic applications. Colostrum contains high quantities of maternal immunoglobulins, cytokines, growth factors, and immunomodulatory milk oligosaccharides [47]. These components have been shown to regulate intestinal mucosal responses through interactions with cytokine and growth factor receptors, as well as with various pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [48]. This suggests that COL can exert its immunomodulatory effects by influencing the signaling pathways involved in immune cell activation and regulation. The presence of maternal immunoglobulins in colostrum provides passive immunity to newborns, offering protection against various pathogens [1,49,50]. As non-protein-derived constituents, immunomodulatory milk oligosaccharides found in colostrum have been shown to interact with the gut microbiota, influencing its composition and function, which in turn can impact immune responses and overall gut health [51].
In our study, we observed that the ability of COL to suppress PMA-induced IL-6 production is influenced by heat, indicating a temperature sensitivity, which supports the hypothesis of the possible involvement of proteinaceous matter. Furthermore, our subsequent experiments involving ultrafiltration demonstrated that the constituents of COL, capable of passing through 10 kDa molecular cutoff filters, possess the ability to mediate this activity. In contrast to the findings reported by Ann et al., who demonstrated that the inhibition of pro-inflammatory cytokine expression by colostrum occurs through the regulation of epithelial NF-κB, our study reveals that the production of IL-6 induced by PMA in a co-cultured Caco-2 and THP-1 cells probably does not fully rely on the activation of NF-κB [52].
Intestinal inflammation often leads to impaired intestinal barrier function and increased gut permeability, which can exacerbate immune responses, disrupt the gut microbiota, and potentially result in systemic infections and mortality, particularly in neonate calves [53,54]. Additionally, colostrum is rich in beneficial probiotic bacteria, such as Lactobacillus and Bifidobacterium, which have been shown to, directly and indirectly, impact intestinal health [55,56]. These probiotics can modulate mucus production, maintain tight junctions between epithelial cells, and influence immunological mucosal tolerance through the IL-10/regulatory T cell axis [57]. Thus, the interplay between colostrum and the intestinal microbiota emerges as a critical factor in maintaining intestinal homeostasis.
To validate our in vitro findings regarding the IL-6/IL-10-directed immunomodulatory activity of colostrum, we utilized murine models with engrafted calf fecal microbiota to replicate the intestinal environment of calves in a well-established organism with defined immunological and histological properties. We chose the C57BL/6 background mice due to their wild-type Th1 responses, both at mucosal and systemic levels, and the similar strategy described by Wrzosek et al. [26]. To simulate acutely inflamed gastrointestinal conditions, we employed a lethal challenge with Salmonella typhimurium ATCC 14028 to investigate the effects of colostrum on the host’s innate immune responses to this intestinal pathogen [58]. As S. typhimurium induces robust Th1 inflammatory responses in the intestine, characterized by immune cell recruitment, cytokine production, and increased gut permeability, we hypothesized that the immunomodulatory and anti-inflammatory effects of COL would enhance the S. typhimurium infection, resulting in enhanced lethality due to an inability to mount pro-inflammatory cytokine responses necessary for immune cell recruitment [59].
In our FMT murine model, we observed that COL significantly reduced intestinal permeability in the duodenum, jejunum, and ileum, while having no effect on the colon, as evidenced by reduced Evans blue accumulation in calf-FMT C57BL/6 mice. Surprisingly, we demonstrated decreased intestinal infiltration of neutrophils and macrophages in colostrum-treated animals compared to untreated controls or those receiving normal saline. Following the S. typhimurium ATCC 14028 challenge, we observed decreased intestinal inflammation and leakiness in the ileum of colostrum-fed calf-FMT C57BL/6 mice, along with reduced tissue-infiltrating immune cells. These findings suggest the immunomodulatory and anti-inflammatory activity of COL in the calf-FMT C57BL/6 mouse model. Similar insights into the anti-inflammatory properties of colostrum were observed by Menchetti et al., although the exact mechanism was not proposed [47]. Furthermore, we unexpectedly observed that calf-FMT C57BL/6 mice undergoing colostrum feeding exhibited decreased IL-6 production and enhanced IL-10 and IL-12 production compared to untreated calf-FMT C57BL/6 mice or those receiving saline feeding. This finding demonstrates the in vivo relevant immunomodulatory and anti-inflammatory activity of COL. When mice were challenged with S. typhimurium, colostrum-treated mice displayed an IL-6 suppressive phenotype and increased IL-10 production. This indicates that the anti-inflammatory activity of COL is directed toward the IL-6/IL-10 axis, even in a highly inflammatory environment, as anticipated, leading to early mortality and systemic bacterial dissemination, through regulation of IL-6/IL-10 production without significant activity on other inflammatory cytokines.
Despite using multiple in vitro approaches and validating our findings in novel murine models, our study has several limitations. Firstly, the use of the Caco-2/THP-1 model cannot guarantee that similar phenotypes would be exhibited by bovine epithelium. Moreover, in vitro model using Caco-2/THP-1 cell co-culture resulted in a high variability of cytokine production, suggesting that the effects observed in this model could differ if other epithelial cells or macrophages would be used. However, due to the lack of established bovine cell lines and intestinal models, as well as the limited availability of immunological reagents, conducting comprehensive in vitro studies to evaluate the effects on bovine epithelial models would be challenging. Secondly, calves possess a unique ruminant intestinal system with potentially bovine-specific enzymatic and metabolic properties that differ significantly from rodents. Since the intestinal microbiota is known to influence mucosal immune responses, we performed bovine fecal microbiota transplantation (FMT) into mice models with wild-type responses to generate an intestinal environment similar to bovines. Lastly, we understand that our findings in cell culture models and calf-FMT C57BL/6 mice need further validation using bovine models to fully confirm the colostrum-mediated immunomodulatory effects.
5. Conclusions
Our study provided an important insight into the immunomodulatory activity of COL in the intestinal environment. The findings described in this study suggest that COL has the capacity to modulate the inflammatory response by potentially acting on the IL-6 and IL-10 axis in cell culture-based models. Furthermore, in our in vivo model using calf-FMT C57BL/6 mice, COL feeding further confirmed effects observed in in vitro models, where COL feeding resulted in decreased production of IL-6 and enhanced production of IL-10 and IL-12 compared to untreated mice. Moreover, feeding with COL was able to suppress IL-6 production in S. typhimurium-infected animals, demonstrating powerful immunomodulatory activity during acute inflammation and infection. This confirms the in vivo relevance of the immunomodulatory activity of COL and its potential ability to shift the cytokine profile towards anti-inflammatory responses both in tissue culture models and mice. Taken together, our data provide valuable insights into the potential anti-inflammatory applications of COL via interaction with the IL-6/IL-10 axis. Further studies are needed to fully elucidate the underlying molecular mechanisms and validate these findings in bovine models, with the long-term aim of potentially developing COL-based interventions for modulating intestinal inflammatory conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci10080519/s1, Figure S1. The full-length Western blot image demonstrating human total NF-κB and beta-actin in THP-1-derived macrophages as described in the materials and methods section of the manuscript. Figure S2. The full-length Western blot image demonstrating human total NF-κB and beta-actin in Caco-2/THP-1 co-culture as described in the materials and methods section of the manuscript. Figure S3. The full-length Western blot image demonstrates human phosphor-NF-κB (Ser536) and beta-actin in THP-1 macrophages as described in the materials and methods section of the manuscript. Figure S4. The full-length Western blot image demonstrating human phosphor-NF-κB (Ser536) and beta-actin in Caco-2/THP-1 co-culture as described in the materials and methods section of the manuscript.
Author Contributions
Conceptualization, R.G. and P.K.; methodology, P.K. and R.G.; software, R.G. and P.K.; validation, R.G. and P.K.; formal analysis, R.G. and P.K.; investigation, R.G. and P.K.; resources, R.G. and P.K.; data curation, R.G. and P.K.; writing—original draft preparation, P.K. and R.G.; writing—review and editing, R.P., E.R.-V., A.B., A.Ž., A.Z., V.Z., A.K., P.M. and R.S.; visualization, P.K. and R.G.; supervision, P.K. and P.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the State Food and Veterinary Service (SFVS) animal care and welfare committee (approval number: No G2-164).
Informed Consent Statement
Informed consent to collect samples from farm animals were obtained from the representative of the farm. All experiments involving animal subjects were approved State Food and Veterinary Service (SFVS) animal care and welfare committee (approval number: No G2-164).
Data Availability Statement
All data generated during this study is disclosed within the manuscript. The biological samples obtained during this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The research was partly funded by the Science Foundation of the Lithuanian University of Health Sciences (No. 119-05).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Poonia A. Shiva Bioactive Compounds, Nutritional Profile and Health Benefits of Colostrum: A Review. Food Prod. Process. Nutr. 2022;4:26. doi: 10.1186/s43014-022-00104-1. [DOI] [Google Scholar]
- 2.Puppel K., Gołębiewski M., Grodkowski G., Slósarz J., Kunowska-Slósarz M., Solarczyk P., Łukasiewicz M., Balcerak M., Przysucha T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals. 2019;9:1070. doi: 10.3390/ani9121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Playford R.J., Weiser M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients. 2021;13:165. doi: 10.3390/nu13010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Struff W.G., Sprotte G. Bovine Colostrum as a Biologic in Clinical Medicine: A Review-Part II: Clinical Studies. Int. J. Clin. Pharmacol. Ther. 2008;46:211–225. doi: 10.5414/CPP46211. [DOI] [PubMed] [Google Scholar]
- 5.Radostits O.M. Treatment and Control of Neonatal Diarrhea in Calves. J. Dairy Sci. 1975;58:464–470. doi: 10.3168/jds.S0022-0302(75)84589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinwald M., Creutzinger K., Keunen A., Winder C.B., Haley D., Renaud D.L. Predictors of Diarrhea, Mortality, and Weight Gain in Male Dairy Calves. J. Dairy Sci. 2022;105:5296–5309. doi: 10.3168/jds.2021-21667. [DOI] [PubMed] [Google Scholar]
- 7.He Z., Ma Y., Yang S., Zhang S., Liu S., Xiao J., Wang Y., Wang W., Yang H., Li S., et al. Gut Microbiota-Derived Ursodeoxycholic Acid from Neonatal Dairy Calves Improves Intestinal Homeostasis and Colitis to Attenuate Extended-Spectrum β-Lactamase-Producing Enteroaggregative Escherichia Coli Infection. Microbiome. 2022;10:79. doi: 10.1186/s40168-022-01269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H.S., Whon T.W., Sung H., Jeong Y.S., Jung E.S., Shin N.R., Hyun D.W., Kim P.S., Lee J.Y., Lee C.H., et al. Longitudinal Evaluation of Fecal Microbiota Transplantation for Ameliorating Calf Diarrhea and Improving Growth Performance. Nat. Commun. 2021;12:161. doi: 10.1038/s41467-020-20389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X., Li W., Wang S., Zhang P., Wang Q., Xiao J., Zhang C., Zheng X., Xu X., Xue S., et al. YAP Aggravates Inflammatory Bowel Disease by Regulating M1/M2 Macrophage Polarization and Gut Microbial Homeostasis. Cell Rep. 2019;27:1176–1189.e5. doi: 10.1016/j.celrep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Wu X., Wei S., Chen M., Li J., Wei Y., Zhang J., Dong W. P2RY13 Exacerbates Intestinal Inflammation by Damaging the Intestinal Mucosal Barrier via Activating IL-6/STAT3 Pathway. Int. J. Biol. Sci. 2022;18:5056–5069. doi: 10.7150/ijbs.74304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y., Wang B., Wang T., Gao L., Yang Z.J., Wang F.F., Shang H.W., Hua R., Xu J.D. Biological Characteristics of Il-6 and Related Intestinal Diseases. Int. J. Biol. Sci. 2020;17:204–219. doi: 10.7150/ijbs.51362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao H.F., Wang Y.C., Tseng H.Y., Wu L.S.H., Tsai H.J., Hsieh M.H., Chen P.C., Kuo W.S., Liu L.F., Liu Z.G., et al. Goat Milk Consumption Enhances Innate and Adaptive Immunities and Alleviates Allergen-Induced Airway Inflammation in Offspring Mice. Front. Immunol. 2020;11:184. doi: 10.3389/fimmu.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polidori P., Rapaccetti R., Klimanova Y., Zhang J.J., Santini G., Vincenzetti S. Nutritional Parameters in Colostrum of Different Mammalian Species. Beverages. 2022;8:54. doi: 10.3390/beverages8030054. [DOI] [Google Scholar]
- 14.Moore M., Tyler J.W., Chigerwe M., Dawes M.E., Middleton J.R. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 2005;226:1375–1377. doi: 10.2460/javma.2005.226.1375. [DOI] [PubMed] [Google Scholar]
- 15.Kessler E.C., Bruckmaier R.M., Gross J.J. Immunoglobulin G Content and Colostrum Composition of Different Goat and Sheep Breeds in Switzerland and Germany. J. Dairy Sci. 2019;102:5542–5549. doi: 10.3168/jds.2018-16235. [DOI] [PubMed] [Google Scholar]
- 16.Mecocci S., Pietrucci D., Milanesi M., Pascucci L., Filippi S., Rosato V., Chillemi G., Capomaccio S., Cappelli K. Transcriptomic Characterization of Cow, Donkey and Goat Milk Extracellular Vesicles Reveals Their Anti-inflammatory and Immunomodulatory Potential. Int. J. Mol. Sci. 2021;22:12759. doi: 10.3390/ijms222312759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulfman L.H., Leusen J.H.W., Savelkoul H.F.J., Warner J.O., van Neerven R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018;5:52. doi: 10.3389/fnut.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimori S., Shimaoka H., Furukawa J.I., Yamashita T., Amano M., Fujitani N., Takegawa Y., Hammarström L., Kacskovics I., Shinohara Y., et al. Alteration of the N-Glycome of Bovine Milk Glycoproteins during Early Lactation. FEBS J. 2011;278:3769–3781. doi: 10.1111/j.1742-4658.2011.08299.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T., Kawase H., Kimura K., Watanabe Y., Ohtani M., Arai I., Urashima T. Concentrations of Sialyloligosaccharides in Bovine Colostrum and Milk during the Prepartum and Early Lactation. J. Dairy Sci. 2003;86:1315–1320. doi: 10.3168/jds.S0022-0302(03)73715-1. [DOI] [PubMed] [Google Scholar]
- 20.Gazi I., Reiding K.R., Groeneveld A., Bastiaans J., Huppertz T., Heck A.J.R. Key Changes in Bovine Milk Immunoglobulin G during Lactation: NeuAc Sialylation Is a Hallmark of Colostrum Immunoglobulin G N -Glycosylation. Glycobiology. 2023;33:115–125. doi: 10.1093/glycob/cwad001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip W.K.E., Hoshi N., Shouval D.S., Snapper S., Medzhitov R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Hartog G., Savelkoul H.F.J., Schoemaker R., Tijhaar E., Westphal A.H., de Ruiter T., van de Weg-Schrijver E., van Neerven R.J.J. Modulation of Human Immune Responses by Bovine Interleukin-10. PLoS ONE. 2011;6:e18188. doi: 10.1371/journal.pone.0018188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigaleviciute R., Planciuniene R., Prikockyte I., Radzeviciute-Valciuke E., Baleviciute A., Zelvys A., Zinkeviciene A., Zigmantaite V., Kucinskas A., Matusevicius P., et al. The Influence of Feeding with Colostrum and Colostrum Replacer on Major Blood Biomarkers and Growth Performance in Dairy Calves. Vet. Sci. 2023;10:128. doi: 10.3390/vetsci10020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Boeren S., Hageman J.A., Van Hooijdonk T., Vervoort J., Hettinga K. Bovine Milk Proteome in the First 9 Days: Protein Interactions in Maturation of the Immune and Digestive System of the Newborn. PLoS ONE. 2015;10:e0116710. doi: 10.1371/journal.pone.0116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makon-Sébastien N., Francis F., Eric S., Henri V.P., François L.J., Laurent P., Yves B., Serge C. Lycopene Modulates THP1 and Caco2 Cells Inflammatory State through Transcriptional and Nontranscriptional Processes. Mediat. Inflamm. 2014;2014:507272. doi: 10.1155/2014/507272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrzosek L., Ciocan D., Borentain P., Spatz M., Puchois V., Hugot C., Ferrere G., Mayeur C., Perlemuter G., Cassard A.M. Transplantation of Human Microbiota into Conventional Mice Durably Reshapes the Gut Microbiota. Sci. Rep. 2018;8:6854. doi: 10.1038/s41598-018-25300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Naour J., Montégut L., Joseph A., Garbin K., Vacchelli E., Kroemer G., Pol J.G., Maiuri M.C. Improved Swiss-Rolling Method for Histological Analyses of Colon Tissue. MethodsX. 2022;9:101630. doi: 10.1016/j.mex.2022.101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erben U., Loddenkemper C., Doerfel K., Spieckermann S., Haller D., Heimesaat M.M., Zeitz M., Siegmund B., Kühl A.A. Original Article A Guide to Histomorphological Evaluation of Intestinal Inflammation in Mouse Models. Int. J. Clin. Exp. Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 29.Godden S.M., Lombard J.E., Woolums A.R. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019;35:535–556. doi: 10.1016/j.cvfa.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacoma R., Gelsinger S.L., Lam Y.W., Scuderi R.A., Ebenstein D.B., Heinrichs A.J., Greenwood S.L. Exploration of the Bovine Colostrum Proteome and Effects of Heat Treatment Time on Colostrum Protein Profile. J. Dairy Sci. 2017;100:9392–9401. doi: 10.3168/jds.2017-13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asbjornsdottir B., Miranda-Ribera A., Fiorentino M., Konno T., Cetinbas M., Lan J., Sadreyev R.I., Gudmundsson L.S., Gottfredsson M., Lauth B., et al. Prophylactic Effect of Bovine Colostrum on Intestinal Microbiota and Behavior in Wild-Type and Zonulin Transgenic Mice. Biomedicines. 2023;11:91. doi: 10.3390/biomedicines11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamsjäger L., Cirone K.M., Schluessel S., Campsall M., Herik A., Lahiri P., Young D., Dufour A., Sapountzis P., Otani S., et al. Host Innate Immune Responses and Microbiome Profile of Neonatal Calves Challenged with Cryptosporidium Parvum and the Effect of Bovine Colostrum Supplementation. Front. Cell Infect. Microbiol. 2023;13:1165312. doi: 10.3389/fcimb.2023.1165312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.J. Immunomodulatory Effects of Human Colostrum and Milk. Pediatr. Gastroenterol. Hepatol. Nutr. 2021;24:337–345. doi: 10.5223/pghn.2021.24.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh S., Iacucci M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients. 2021;13:3798. doi: 10.3390/nu13113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakandalage R., Guan L.L., Malmuthuge N. Microbial Interventions to Improve Neonatal Gut Health. Microorganisms. 2023;11:1328. doi: 10.3390/microorganisms11051328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corapi W.V., French T.W., Dubovi E.J. Severe Thrombocytopenia in Young Calves Experimentally Infected with Noncytopathic Bovine Viral Diarrhea Virus. J. Virol. 1989;63:3934–3943. doi: 10.1128/jvi.63.9.3934-3943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoonderwoerd M., Clarke R.C., Van Dreumel A.A., Rawluk S.A. N1G 3W4 (Clarke) and Veterinary Laboratory Services, Ontario Ministry of Agriculture and Food. Can. J. Vet. Res. = Rev. Can. Rech. Vétérinaire. 1988;52:484–487. [PMC free article] [PubMed] [Google Scholar]
- 38.Brujeni N. Correlation between Neonatal Calf Diarrhea and the Level of Maternally Derived Antibodies. Iran. J. Vet. Res. 2018;19:3–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Beheshtipour J., Raeeszadeh M. Evaluation of Interleukin-10 and pro-Inflammatory Cytokine Profile in Calves Naturally Infected with Neonatal Calf Diarrhea Syndrome. Arch. Razi Inst. 2020;75:213–218. doi: 10.22092/ARI.2018.124058.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osorio J.S. Gut Health, Stress, and Immunity in Neonatal Dairy Calves: The Host Side of Host-Pathogen Interactions. J. Anim. Sci. Biotechnol. 2020;11:105. doi: 10.1186/s40104-020-00509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer S., Bauerfeind R., Czerny C.P., Neumann S. Serum Interleukin-6 as a Prognostic Marker in Neonatal Calf Diarrhea. J. Dairy Sci. 2016;99:6563–6571. doi: 10.3168/jds.2015-10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kordulewska N.K., Topa J., Tańska M., Cieślińska A., Fiedorowicz E., Savelkoul H.F.J., Jarmołowska B. Modulatory Effects of Osthole on Lipopolysaccharides-Induced Inflammation in Caco-2 Cell Monolayer and Co-Cultures with THP-1 and THP-1-Derived Macrophages. Nutrients. 2021;13:123. doi: 10.3390/nu13010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue R., Zhao Y., Chen P. Involvement of PKCα in PMA-Induced Facilitation of Exocytosis and Vesicle Fusion in PC12 Cells. Biochem. Biophys. Res. Commun. 2009;38:371–376. doi: 10.1016/j.bbrc.2009.01.105. [DOI] [PubMed] [Google Scholar]
- 44.Le N.P.K., Altenburger M.J., Lamy E. Development of an Inflammation-Triggered In Vitro “Leaky Gut” Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages. Int. J. Mol. Sci. 2023;24:7427. doi: 10.3390/ijms24087427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann S., Curone G., Chandler T.L., Sipka A., Cha J., Bhawal R., Zhang S. Heat Treatment of Bovine Colostrum: II. Effects on Calf Serum Immunoglobulin, Insulin, and IGF-I Concentrations, and the Serum Proteome. J. Dairy Sci. 2020;103:9384–9406. doi: 10.3168/jds.2020-18619. [DOI] [PubMed] [Google Scholar]
- 46.Biswas P., Vecchi A., Mantegani P., Mantelli B., Fortis C., Lazzarin A. Immunomodulatory Effects of Bovine Colostrum in Human Peripheral Blood Mononuclear Cells. New Microbiol. 2007;30:447–454. [PubMed] [Google Scholar]
- 47.Menchetti L., Traina G., Tomasello G., Casagrande-Proietti P., Leonardi L., Barbato O., Brecchia G. Insulin-Like Growth Factors I and II (IGF-I and IGF-II) 2016;Volume 8 [Google Scholar]
- 48.Wong E.B., Mallet J.F., Duarte J., Matar C., Ritz B.W. Bovine Colostrum Enhances Natural Killer Cell Activity and Immune Response in a Mouse Model of Influenza Infection and Mediates Intestinal Immunity through Toll-like Receptors 2 and 4. Nutr. Res. 2014;34:318–325. doi: 10.1016/j.nutres.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Costa A., Franzoi M., Visentin G., Goi A., De Marchi M., Penasa M. The Concentrations of Immunoglobulins in Bovine Colostrum Determined by the Gold Standard Method Are Genetically Correlated with Their Near-Infrared Prediction. Genet. Sel. Evol. 2021;53:87. doi: 10.1186/s12711-021-00681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Hese I., Goossens K., Vandaele L., Opsomer G. Invited Review: MicroRNAs in Bovine Colostrum—Focus on Their Origin and Potential Health Benefits for the Calf. J. Dairy Sci. 2020;103:1–15. doi: 10.3168/jds.2019-16959. [DOI] [PubMed] [Google Scholar]
- 51.Järvinen K.M., Martin H., Oyoshi M.K. Immunomodulatory Effects of Breast Milk on Food Allergy. Ann. Allergy Asthma Immunol. 2019;123:133–143. doi: 10.1016/j.anai.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An M.J., Cheon J.H., Kim S.W., Park J.J., Moon C.M., Han S.Y., Kim E.S., Kim T.I., Kim W.H. Bovine Colostrum Inhibits Nuclear Factor ΚB-Mediated Proinflammatory Cytokine Expression in Intestinal Epithelial Cells. Nutr. Res. 2009;29:275–280. doi: 10.1016/j.nutres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Mecocci S., Ottaviani A., Razzuoli E., Fiorani P., Pietrucci D., De Ciucis C.G., Giudici S.D., Franzoni G., Chillemi G., Cappelli K. Cow Milk Extracellular Vesicle Effects on an In Vitro Model of Intestinal Inflammation. Biomedicines. 2022;10:570. doi: 10.3390/biomedicines10030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du W., Wang X., Hu M., Hou J., Du Y., Si W., Yang L., Xu L., Xu Q. Modulating Gastrointestinal Microbiota to Alleviate Diarrhea in Calves. Front. Microbiol. 2023;14:1181545. doi: 10.3389/fmicb.2023.1181545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arshad M.A., Hassan F.-u., Rehman M.S., Huws S.A., Cheng Y., Din A.U. Gut Microbiome Colonization and Development in Neonatal Ruminants: Strategies, Prospects, and Opportunities. Anim. Nutr. 2021;7:883–895. doi: 10.1016/j.aninu.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schäfer T.V., Ivnitsky J.J., Rejniuk V.L. Permeability of Intestinal and Blood—Tissue Barriers in Rats for Evans Blue Dye under Conditions of Acute Intoxication with Cyclophosphamide. Bull. Exp. Biol. Med. 2019;168:38–40. doi: 10.1007/s10517-019-04640-8. [DOI] [PubMed] [Google Scholar]
- 57.Hu J., Deng F., Zhao B., Lin Z., Sun Q., Yang X., Wu M., Qiu S., Chen Y., Yan Z., et al. Lactobacillus Murinus Alleviate Intestinal Ischemia/Reperfusion Injury through Promoting the Release of Interleukin-10 from M2 Macrophages via Toll-like Receptor 2 Signaling. Microbiome. 2022;10:38. doi: 10.1186/s40168-022-01227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X., Dai Q., Jia R., Zhu D., Liu M., Wang M., Chen S., Sun K., Yang Q., Wu Y., et al. Two Novel Salmonella Bivalent Vaccines Confer Dual Protection against Two Salmonella Serovars in Mice. Front. Cell Infect. Microbiol. 2017;7:391. doi: 10.3389/fcimb.2017.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui W.W., Emerson L.E., Clapp B., Sheppe A.E., Sharma J., del Castillo J., Ou M., Maegawa G.H.B., Hoffman C., Larkin J., III, et al. Antigen-Encapsulating Host Extracellular Vesicles Derived from Salmonella-Infected Cells Stimulate Pathogen-Specific Th1-Type Responses In Vivo. PLoS Pathog. 2021;17:e1009465. doi: 10.1371/journal.ppat.1009465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study is disclosed within the manuscript. The biological samples obtained during this study are available upon reasonable request from the corresponding author.