Abstract
Background
Severe positive sagittal malalignment can potentially lead to shortening and contracture of the psoas and joint capsule in a flexed spinopelvic position. The utilization of bilateral psoas release to supplement sagittal spinal deformity correction in the same hospitalization was not reported in the literature.
Case presentation
A 66-year-old patient presented with a 5-year history of severe global spinal deformity (sagittal vertical axis 220 mm, 60° spinopelvic mismatch) that did not improve on supine radiographs, and a modified Thomas test with more than 30° flexion contracture of bilateral hips. A 3-stage operation utilizing posterior spinal column osteotomies, anterior lumbar interbody fusion, and bilateral psoas releases was performed.
Outcome
Her postoperative alignment significantly improved and she was pleased with her new posture and the ability to stand up straight.
Conclusions
This report is the first to demonstrate safe and substantial correction of severe spinal deformities associated with bilateral hip flexion contracture in 1 hospitalization.
Keywords: Hip-spine syndrome, Adult spinal deformity, Sagittal spinal deformity, Flexion contracture, Psoas release, Thomas test
Introduction
Patients with severe and fixed sagittal spinal deformities are challenging to treat. There has been a growing recognition of lower extremity involvement in compensating for sagittal spinal deformity, which is especially problematic when flexion contracture of the hips or knees is present [1].
Hip-spine syndrome has been specially recognized in adult spinal deformity (ASD) as severe hip osteoarthritis (OA) often occurs in patients with spinal deformity [2], [3], [4], [5], [6]. When both spinal realignment and hip replacement are indicated, the order of the operations is challenging to determine [7], [8], [9], [10], with several studies supporting a spinal realignment first due to the impact of restoring lumbar lordosis on the acetabular version and cup placement which can lead to hip dislocation if spinal deformity is corrected second [11], [12], [13].
In some patients with longstanding sagittal spinal deformity, hip pathology may not manifest as OA, but rather can be limited to periarticular soft tissue contracture. Conceptually, severe positive sagittal malalignment driven by loss of lordosis can potentially lead to shortening of the psoas as distance from origin to insertion decreases due to a flexed spinopelvic position. Despite the significant interest in hip-spine syndrome, there is no case report in the literature addressing both spine and hip pathologies during the same admission as a planned and staged treatment.
Case report
A 66-year-old female with a past medical history significant for obesity (BMI 36) and controlled diabetes presented with a 5-year history of back and right lower extremity pain. During the past 2 years, she had significant difficulties maintaining an upright posture, requiring a walker for ambulation. Additionally, she had been unable to lay supine with her lower extremities flat and reported sleeping most comfortably on her side with bilateral knee flexion. She had undergone an extensive physical therapy regimen for her hip flexion contracture preoperatively and made virtually no progress as hip extension was extremely painful. Numerous epidural steroid injections were used as well and they provided temporary incomplete relief.
On exam, she could not stand upright and had a shuffling gait with limited ability to flex and extend her hips. Her sensory exam was normal. Her motor exam revealed 5/5 motor strength, except for bilateral L4 and L5 nerve roots, where strength was 3/5. Achilles and patellar reflexes were +1 bilaterally with negative upper motor signs, including clonus, and Babinski. Straight leg raise and FABER tests were negative. Her bilateral hips’ range of motion (ROM) was limited to 40°–110°, lacking 40° of hip extension on the modified Thomas test. Similarly, she had severely limited active ROM of her lumbar spine. The latter was examined clinically and using the difference between supine and standing lumbar alignment.
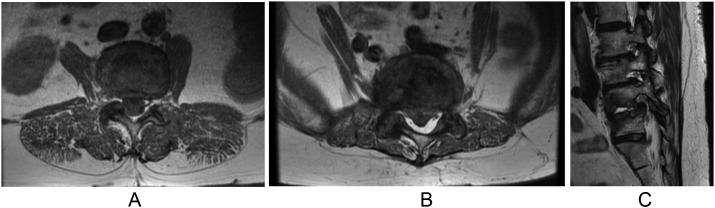
Full body radiographs revealed severe sagittal malalignment (Figure 1 A–B). To better understand this finding, a CT scan of the lumbar spine was ordered revealing a diffuse vacuum disc phenomenon and loss of disc space between L3 and S1. She had severe destruction of the L4–L5 disc with significant endplate bone loss. Noted was 6 nonrib bearing lumbar vertebral bodies with facet hypertrophy and auto-fusion, in addition to the L5–S1 disc osteophyte complex on the right side. MRI of the lumbar spine revealed multilevel spondylitic changes with bilateral neuroforaminal stenosis at L2–L3, L3–4, and L4–L5. There was mild central canal stenosis at L2–L3 and L4–L5 (Fig. 2 A–C). Then a DEXA scan revealed a T score of −0.4, thus osteoporosis was ruled out.
Fig. 1.
Anteroposterior (A) and lateral (B) full body X-rays with the different spinopelvic parameters.
Fig. 2.
Axial MRI cuts showing central stenosis at L2–L3 (A), L4–L5 (B) and Parasagittal MRI cuts showing foraminal stenosis (C) at L2–L3, L3–L4, and L4–L5.
The patient was deemed a surgical candidate secondary to severe spinal deformity with concurrent bilateral hip flexion contracture. The surgery was planned in 3 stages. The first stage would address the auto-fusion of the posterior lumbar elements with posterior spinal column osteotomies (PCO) from L3–S1 and instrumentation, then proceed with anterior lumbar interbody fusion (ALIF) at L3–L4, L4–L5, and, if possible, at L5–S1. In the second stage, the patient would undergo posterior lumbar instrumentation from L2-Pelvis with bilateral S2AI fixation. The third stage was to be determined based on her full spine standing radiographs following spinal realignment. The third stage would include bilateral psoas releases using the traditional direct anterior approach if deemed necessary.
The patient underwent a 3-stage procedure:
-
1.
Schwab Grade 2 PCO from L2–S1 with segmental instrumentation of L3–S1 followed by ALIF of L3–L4 and L4–5.
-
2.
Posterior spinal instrumentation L2-Ilium with bilateral S2AI pelvic fixation, transforaminal interbody fusion of L2–L3 and L5–S1, and posterior tethering of UIV+1, UIV+2, and UIV+3.
-
3.
Bilateral psoas releases via direct anterior approach to bilateral hips.
Following a standard midline posterior approach to the spine, the L2–S1 facet capsules were stripped in anticipation of fusion. Pedicle screws were placed at levels L3–S1 using freehand technique with superb purchase. Grade 2 PCO was done utilizing a combination of high-speed bur and osteotomes. For the anterior approach, a standard left-sided paramedian approach was utilized. With a scalpel, the disc base of L4–L5 was incised. Osteotomy was required to mobilize the disc. Osteotomes and distractors were used to break the osteophytes, then sequential distractors were used to crack through the fixed collapse and listhesis. After the osteotomy, the bone was more mobile. Exposure and preparation of the vertebral space was done similarly to L3–4. A 25-degree cage with 2 interfixated screws was placed to allow for lordosis correction. Then, the same procedure was performed with a 20-degree gauge at L3–4. The exploration of the L5–S1 level showed variant venous anatomy with a large venous vessel overlying the L5–S1 disc base, prohibiting safe mobilization. This concluded the first stage.
The patient returned to the operating room 2 days later for stage 2. Again, through a standard midline posterior approach to the spine, pedicle screws were placed in L2 using freehand technique, and bilateral S2 AI iliac screws were placed with excellent purchase since ALIF at L5–S1 was not possible at the first stage. Then, a right kickstand screw was placed in the right ilium. In the osteotomy site created in stage 1, a posterolateral interbody fusion cage was placed at L2–3 and L5–S1. Bilateral expandable cages were positioned at L5–S1 as well as a right-sided unilateral cage at L2–3; the cages were expanded for lordotic correction and confirmed appropriate positioning with lateral and AP radiographs. Custom precontoured longitudinal rods were placed first on the left side from L2 down to the ileum. To correct the deformity, put set screws, and perform final tightening, reduction towers were used. Radiographs showed an acceptable position of correction. The right-sided rod including the kickstand rod were positioned. Then, suture anchors were placed in the L1 pedicles with excellent purchase and confirmed appropriate positioning with AP and lateral fluoroscopy. Subcutaneous tethers were put in the upper instrumented vertebra +3/2 interspace and 3 bur holes in the upper instrumented vertebra +2. At this point, all the tethers were connected, which were weaved in a figure-of-8 fashion around the posterior elements into the connectors onto the rods, tensioned until taught, and finally tightened. Fig. 3 shows intraoperative images with lordotic correction.
Fig. 3.
Intraoperative image showing correction of the lordosis.
On day 2 postoperatively, full spine scoliosis radiographs showed residual positive sagittal malalignment (Fig. 4 A–B). A radiographic modified Thomas test revealed flexion contracture bilaterally with an angle of 32° when the left leg was down and 28.4° when the right leg was down (Fig. 5 A–B), confirming the indication for stage 3—bilateral psoas releases. The patient underwent right-sided psoas release 3 days postoperatively using a standard direct anterior approach. Details of this approach are: a longitudinal incision was made 2 fingerbreadths lateral and 2 fingerbreadths distal to the anterior superior iliac spine. The incision was then performed down through the subcutaneous fat to the fascia and divided the fascia in line with the incision. Caution was taken to protect the lateral femoral cutaneous nerve. Deep retractors were placed, then identified and protected the circumflex femoral vessels. Making an anatomic window distal to the circumflex vessels, the psoas tenotomy began. The psoas tendon was palpated and was tight with both strong tendons. The lesser trochanter was identified, a Schnidt was placed directly on the psoas tendon, and verified an appropriate location with an AP X-ray. Next, the Cobra retractor was placed medial to the tendon to protect the neurovascular bundle and tenotomy scissors were used to release the psoas tendon. The hip flexion contracture was significantly improved, so the wound was thoroughly irrigated and closed. The same procedure for the left psoas was performed 2 days later. Radiographic imaging was obtained after each procedure (Fig. 6 A–B).
Fig. 4.
Postoperative full body lateral X-rays with the patient standing straight (A) and relaxed (B) showing positive residual malalignment.
Fig. 5.
X-rays of the patient during the modified Thomas test with the left leg down (A) and the right leg down (B).
Fig. 6.
Lateral full body X-rays after release of the right psoas (A) and after bilateral release (B).
The patient tolerated each procedure well without complication, and her pain was well controlled. Physical therapy sessions began after releasing her psoas bilaterally, as the patient could not maintain an erect posture before that. A multidisciplinary discussion was held with the physical therapy team outlining treatment goals, including the utmost necessity for aggressive range of motion of the hips to reduce the possibility of flexion contracture recurrence. The patient was also educated on reversing the old habits of sleeping sideways in bed with her knees flexed. She was recommended to look in the mirror daily, especially in the acute postoperative period, to improve her neurosensorial input and help accommodate new alignment. She had an unremarkable postoperative course and was discharged to inpatient rehabilitation on postoperative day 12 for ambulation training, strengthening, and training for activities of daily living. Six months postoperatively (Fig. 7 A–B), she was seen in the office and doing extremely well. Her postoperative alignment significantly improved from SVA of 232mm to 77mm (Table). Despite residual malalignment, the neurological exam of the lower limbs normal with 5/5 strength and she was pleased with her new posture and the ability to stand up straight for the first time in 5 years.
Fig. 7.
Anteroposterior (A) and lateral (B) full body X-rays after a 6 months follow-up.
Table.
Preoperative and 6 months postoperative spinopelvic parameters showing significant improvements.
| Spino-pelvic parameters | Preoperatively | At 6 months postoperatively |
|---|---|---|
| Pelvic tilt (PT, °) | 24.4 | 31.2 |
| Pelvic incidence (PI, °) | 69.1 | 72.9 |
| Sacral slope (SS, °) | 41.8 | 48 |
| Lumbar lordosis (LL, °) | 1.8 | −52.7 |
| L1–L4 (°) | −4.2 | −16.1 |
| L4–S1 (°) | 6.5 | −37.2 |
| PI–LL (°) | 67.9 | 26.5 |
| L1–S1 (mm) | 144.1 | 151.1 |
| Thoracic kyphosis (TK, °) | −15.2 | 25.1 |
| T1 spino-pelvic inclination (SPi, °) | 25.8 | 2.0 |
| T9 SPi (°) | 26.4 | −2.4 |
| T1 slope (°) | 42.7 | 36.2 |
| T1 pelvic angle (TPA, °) | 50.2 | 33.2 |
| Sagittal vertical axis (SVA, mm) | 232.0 | 77.9 |
Discussion
Patients with hip-spine syndrome represent a unique treatment challenge with special considerations. In fact, in severe spinal deformities such as in this case, vertebral column resection using osteotomies constitute the standard management [14] which can be supplemented by anterior column support using ALIF [15] or TLIF to restore lordosis [16]. While some hip or spine pathologies have little impact on treatments of either condition, hip flexion contracture with or without hip osteoarthritis resistant to physical therapy has significant implications on spinal realignment in the setting of sagittal deformity. In a stepwise fashion, this case report reveals the drastic impact of addressing hip flexion contracture on spinal realignment. Despite adequate intraoperative lordosis, our patient had a residual sagittal plane deformity and malalignment secondary to severe and longstanding bilateral hip flexion contractures that were resistant to physical therapy. Moreover, the pelvic incidence changed at 6 months follow-up and was increased by around 4° which is within the range of measurements error. Higher changes of PI were described previously in the literature without any consequences on postoperative outcomes and may be due to the pelvic fixation using S2-alar-ilac screws [17,18]. However, Rizkallah et al. [19] compared S2-alar-ilac screws and iliac screws, and reported that the pelvic fixation itself instead of its type is what drives postoperative change in pelvic incidence.
While the benefit of psoas release is evident in this case report, there is a paucity of literature detailing the long-term impact bilateral psoas release has on hip flexion strength [20]. In fact, a study by Chen et al. [20] tried to reach a consensus regarding this management technique. Indications for this surgery were recorded to be internal snapping, followed by iliopsoas impingement, and tendonitis [20]. None of the surveyed surgeons included sagittal malalignment causing hip flexion contracture as an indication for this surgery. As for postoperative adverse outcomes, the most commonly reported one was hip flexion weakness, followed by hip instability, and pain. When it comes to this postoperative weakness, the literature is still contradictive [21], [22], [23], [24], [25], [26]. Furthermore, Bradenburg et al. [27] quantified this weakness demonstrating that psoas tenotomy led to a 20% decrease in hip flexion strength while seated and no significant difference while supine. However, this study has limitations due to its small sample size, a wide range of patient ages, and analysis in the setting of a unilateral release [27]. Moreover, flexion contracture constituted an indication for psoas release mainly in patients with cerebral palsy where complications such as postoperative weakness remained a contradictive finding in ambulatory children [28,29]. Thus, the long-term effect of releasing the bilateral psoas must be carefully studied to establish safety and efficacy and achieve external validation.
Our report does not encourage routine bilateral psoas releases in hip-spine syndrome patients but does highlight the importance of diagnosing hip flexion contracture with clinical or radiographic modified Thomas test and the potential value to psoas release in patients with dual spinal deformity and hip flexion contracture. Once identified, contractures should be treated with extensive preoperative physical therapy to avoid their impact of tethering the lumbar spine downward to the lesser trochanters and blocking an erect posture.
Conclusion
This is the first case report addressing both spine and hip pathologies in the same admission in a patient presenting with a hip-spine syndrome. This patient received 3 staged procedures including a Schwab Grade 2 PCO with segmental instrumentation and ALIF, posterior spinal instrumentation with bilateral pelvic fixation and transforaminal interbody fusion with posterior tethering of UIV+1, UIV+2, and UIV+3, and finally to address the psoas contractures, bilateral psoas releases. Nevertheless, before proceeding with the third surgery, hip flexion contracture should be diagnosed with a clinical and radiographic Thomas test or a modified Thomas test if the contracture was bilateral. There is a paucity of the literature regarding such reports which is why more high-quality studies are needed to further study this management technique.
Patient informed consent
Complete written informed consent was obtained from the patient for the publication of this study and accompanying images.
Declarations of competing interests
One or more authors declare potential competing financial interests or personal relationships as specified on required ICMJE-NASSJ Disclosure Forms.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: BGD: Consulting: Spinevision, Clariance (B). MBC: Nothing to disclose. MD: Nothing to disclose. AHD: Royalties: Stryker (D); Royalties: Spineart (F); Medicrea/Medtronic (A); Consulting: Orthofix (B); Consulting: Stryker (B); Consulting: Medtronic (B); Research Support(Investigator Salary, Staff/Materials): Orthofix (D); Fellowship Support: Medtronic (F).
This report shows safe and substantial correction of a severe spinal deformity by supplementing sagittal spinal deformity with bilateral psoas release.
References
- 1.Ferrero E, Liabaud B, Challier V, et al. Role of pelvic translation and lower-extremity compensation to maintain gravity line position in spinal deformity. Deformity. 2016;24:436–446. doi: 10.3171/2015.5.SPINE14989.436. [DOI] [PubMed] [Google Scholar]
- 2.Offierski CMCM, MacNab I. Hip-spine syndrome. Spine (Phila Pa 1976) 1983;8:316–321. doi: 10.1097/00007632-198304000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Diebo BG, Shah NV, Boachie-Adjei O, et al. Adult spinal deformity. Lancet. 2019;394:160–172. doi: 10.1016/S0140-6736(19)31125-0. [DOI] [PubMed] [Google Scholar]
- 4.Day LM, DelSole EM, Beaubrun BM, et al. Radiological severity of hip osteoarthritis in patients with adult spinal deformity: the effect on spinopelvic and lower extremity compensatory mechanisms. Eur Spine J. 2018;27:2294–2302. doi: 10.1007/s00586-018-5509-0. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto T, Kobayashi T, Tsukamoto M, et al. Hip-spine syndrome: a focus on the pelvic incidence in hip disorders. J Clin Med. 2023;12 doi: 10.3390/jcm12052034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain M, Mohapatra A, Tripathy SK, Mohakud S, Das A, Sethy SS. Do spinopelvic parameters relate with secondary hip spine syndrome in secondary hip arthritis? Indian J Orthop. 2022;56:1937–1943. doi: 10.1007/s43465-022-00741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SE, Anatone AJ, Figgie MP, Long WJ, Della Valle AG, Lee G-C. Spine or hip first? Outcomes in patients undergoing sequential lumbar spine or hip surgery. J Arthroplasty. 2023;38:114–118. doi: 10.1016/j.arth.2023.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Zandi R, Manafi-Rasi A, Talebi S, Ehsani A, Salarzadeh-Jenatabadi H. Spinopelvic imbalances are associated with worse postoperative functional outcomes in patients undergoing total hip arthroplasty. Eur J Orthop Surg Traumatol. 2023 doi: 10.1007/s00590-023-03600-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Kim BI, Schwartz AM, et al. Does order of operation matter in patients who have concomitant hip and spine pathology? J Arthroplasty. 2023;38:106–113. doi: 10.1016/j.arth.2023.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Clarius M, Farweez M, Innmann MM. Battle: total hip arthroplasty or spine surgery first in patients with hip-spine-syndrome? The arthroplasty surgeon's point of view. Orthopade. 2020;49:899–904. doi: 10.1007/s00132-020-03974-w. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz J, Yeroushalmi D, Hepinstall M, Buckland AJ, Schwarzkopf R, Meftah M. Effect of pelvic sagittal tilt and axial rotation on functional acetabular orientation. Orthopedics. 2023;46:e27–e30. doi: 10.3928/01477447-20221003-04. [DOI] [PubMed] [Google Scholar]
- 12.Wiznia DH, Buchalter DB, Kirby DJ, Buckland AJ, Long WJ, Schwarzkopf R. Applying the hip-spine relationship in total hip arthroplasty. Hip Int. 2021;31:144–153. doi: 10.1177/1120700020949837. [DOI] [PubMed] [Google Scholar]
- 13.Buckland AJ, Vigdorchik J, Schwab FJ, et al. Acetabular anteversion changes due to spinal deformity correction: bridging the gap between hip and spine surgeons. J Bone Joint Surg Am. 2015;97:1913–1920. doi: 10.2106/JBJS.O.00276. [DOI] [PubMed] [Google Scholar]
- 14.Saifi C, Laratta JL, Petridis P, Shillingford JN, Lehman RA, Lenke LG. Vertebral column resection for rigid spinal deformity. Glob Spine J. 2017;7:280–290. doi: 10.1177/2192568217699203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi Z, Chou D, Mummaneni PV, et al. Anterior lumbar compared to oblique lumbar interbody approaches for multilevel fusions to the sacrum in adults with spinal deformity and degeneration. J Neurosurg Spine. 2020;33:1–10. doi: 10.3171/2020.4.SPINE20198. [DOI] [PubMed] [Google Scholar]
- 16.Han N, Pratt N, Usmani MF, et al. Anterior longitudinal ligament release from a posterior approach: an alternative to three-column osteotomy. Eur Spine J. 2022;31:2196–2203. doi: 10.1007/s00586-021-07100-y. [DOI] [PubMed] [Google Scholar]
- 17.Wei C, Zuckerman SL, Cerpa M, et al. Can pelvic incidence change after spinal deformity correction to the pelvis with S2-alar-iliac screws? Eur Spine J. 2021;30:2486–2494. doi: 10.1007/s00586-020-06658-3. [DOI] [PubMed] [Google Scholar]
- 18.Tseng C, Liu Z, Bao H, et al. Long fusion to the pelvis with S2-alar-iliac screws can induce changes in pelvic incidence in adult spinal deformity patients: analysis of predictive factors in a retrospective cohort. Eur Spine J. 2019;28:138–145. doi: 10.1007/s00586-018-5738-2. [DOI] [PubMed] [Google Scholar]
- 19.Rizkallah M, Shen J, Phan P, et al. Can pelvic incidence change after lumbo-pelvic fixation for adult spine deformity and would the change be affected by the type of pelvic fixation? Spine (Phila Pa 1976) 2023 doi: 10.1097/EDE.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 20.Chen AW, Steffes MJ, Laseter JR, et al. How has arthroscopic management of the iliopsoas evolved, and why? A survey of high-volume arthroscopic hip surgeons. J Hip Preserv Surg. 2020;7:322–328. doi: 10.1093/jhps/hnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilizaliturri VM, Villalobos FE, Chaidez PA, Valero FS, Aguilera JM. Internal snapping hip syndrome: treatment by endoscopic release of the iliopsoas tendon. Arthroscopy. 2005;21:1375–1380. doi: 10.1016/j.arthro.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Contreras MEK, Dani WS, Endges WK, De Araujo LCT, Berral FJ. Arthroscopic treatment of the snapping iliopsoas tendon through the central compartment of the hip: a pilot study. J Bone Joint Surg Br. 2010;92:777–780. doi: 10.1302/0301-620X.92B6.22797. [DOI] [PubMed] [Google Scholar]
- 23.Nelson IR, Keene JS. Results of labral-level arthroscopic iliopsoas tenotomies for the treatment of labral impingement. Arthroscopy. 2014;30:688–694. doi: 10.1016/j.arthro.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Wettstein M, Jung J, Dienst M. Arthroscopic psoas tenotomy. Arthroscopy. 2006;22:907.e1–907.e4. doi: 10.1016/j.arthro.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 25.Flanum ME, Keene JS, Blankenbaker DG, Desmet AA. Arthroscopic treatment of the painful “internal” snapping hip: results of a new endoscopic technique and imaging protocol. Am J Sports Med. 2007;35:770–779. doi: 10.1177/0363546506298580. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SA, Keene JS. Results of arthroscopic iliopsoas tendon release in competitive and recreational athletes. Am J Sports Med. 2008;36:2363–2371. doi: 10.1177/0363546508322130. [DOI] [PubMed] [Google Scholar]
- 27.Brandenburg JB, Kapron AL, Wylie JD, et al. The Functional and structural outcomes of arthroscopic iliopsoas release. Am J Sports Med. 2016;44:1286–1291. doi: 10.1177/0363546515626173. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland DH, Zilberfarb JL, Kaufman KR, Wyatt MP, Chambers HG. Psoas release at the pelvic brim in ambulatory patients with cerebral palsy: operative technique and functional outcome. J Pediatr Orthop. 1997;17:563–570. doi: 10.1097/00004694-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bialik GM, Pierce R, Dorociak R, Lee TS, Aiona MD, Sussman MD. Iliopsoas tenotomy at the lesser trochanter versus at the pelvic brim in ambulatory children with cerebral palsy. J Pediatr Orthop. 2009;29:251–255. doi: 10.1097/BPO.0b013e31819c4041. [DOI] [PubMed] [Google Scholar]