Highlights
-
•
Features of plaque had prognostic value on stroke recurrence.
-
•
Inclusion of stroke subtypes in analysis.
-
•
Validation cohort verified the prediction.
Keywords: Ischemic stroke, Recurrence, Middle cerebral artery, Magnetic resonance imaging, Vessel wall
Abstract
Recurrence is a significant adverse outcome of ischemic stroke (IS), particularly in cases of intracranial arteriosclerosis (ICAS). In this study, we investigated the impact of imaging features of culprit plaque using high-resolution magnetic resonance vessel wall imaging (HR-MR-VWI) on the prediction of IS recurrence. A total of 86 patients diagnosed with ICAS-related IS within the middle cerebral artery (MCA) territory were included, of which 23.25% experienced recurrent IS within one year. Our findings revealed significant differences between the recurrence and non-recurrence groups in terms of age (p = 0.007), diabetes mellitus (p = 0.031), hyperhomocysteinemia (p = 0.021), artery-artery embolism (AAE) infarction (p = 0.019), prominent enhancement (p = 0.013), and surface irregularity of the culprit plaque (p = 0.009). Age (HR = 1.063, p = 0.005), AAE infarction (HR = 5.708, p = 0.008), and prominent enhancement of the culprit plaque (HR = 4.105, p = 0.025) were identified as independent risk factors for stroke recurrence. The areas under the receiver operating characteristic curve (AUCs) for predicting IS recurrence using clinical factors, conventional imaging findings, HR-MR-VWI plaque features, and a combination of clinical and conventional imaging models were 0.728, 0.645, 0.705, and 0.814, respectively. Notably, the combination model demonstrated superior predictive performance with an AUC of 0.870. Similarly, AUC of combination model for predicting IS recurrence in validation cohort which enrolled another 37 patients was 0.865. In conclusion, the presence of obvious enhancement in culprit plaque on HR-MR-VWI is a valuable factor in predicting IS recurrence in ICAS-related strokes within the MCA territory. Furthermore, our combination model, incorporating plaque features, exhibited improved prediction accuracy.
1. Introduction
Intracranial arteriosclerosis (ICAS) within the middle cerebral artery (MCA) territory is a predominant etiological subtype of ischemic stroke (IS) observed in African, Asian, and Hispanic populations, with Chinese individuals accounting for 33–50% of cases (Zhao et al., 2016). The high recurrence rate (10–24%) of ICAS stroke contributes to unfavorable outcomes among IS patients (Ran et al., 2020). Despite active treatment strategies aimed at mitigating vascular risk factors and implementing intensive antiplatelet therapy, the annual recurrence rate of ICAS stroke remains alarmingly high at 14.9% (Xu et al. 2022). Previous studies have indicated that the elevated recurrence rate of ICAS stroke within the MCA territory could be attributed to plaque rupture, plaque progression in the culprit vessel, and insufficient collateral circulation resulting from perforating vessel disease (Zhao et al., 2016, Song et al., 2020, Yang et al., 2022). Hence, identifying critical features of the culprit plaque associated with IS recurrence holds significant implications for risk stratification and precise treatment of ICAS.
Current stroke guidelines primarily rely on assessing the degree of stenosis (≥50%) in intracranial large arteries as a critical imaging factor for prognostic evaluation. However, the value of luminal assessment alone is limited, as conventional vascular imaging techniques fail to directly reveal the pathological characteristics of the culprit vessel wall. Consequently, the pathology of the culprit vessel wall is often overlooked, potentially contributing to treatment failure in certain IS patients (Ran et al., 2020). High-resolution magnetic resonance vascular wall imaging (HR-MR-VWI), a newly developed technique, has emerged as an effective means not only to clearly identify luminal stenosis but also to uncover plaque characteristics within the culprit vessel. Nonetheless, current studies, including investigations into the degree and incidence of enhancement of culprit plaques, have yet to establish a consensus on the association between plaque characteristics on HR-MR-VWI and stroke recurrence (Song et al., 2020, Yang et al., 2022). Several studies have recently demonstrated that the positive remodeling effect of the plaque characteristics in HR-MR-VWI of the culprit vessel on the prediction of IS recurrence (Ren et al., 2022, Song et al., 2020, Yang et al., 2022). However, few studies have compared the prediction efficacy between HR-MR-VWI and conventional MR features. These studies did not consider the etiology of IS, neither. However,stroke recurrence is influenced by the underlying etiology and the adoption of appropriate treatment strategies. Artery-to-artery embolism (AAE) subtype ICAS, characterized by unstable plaques within the culprit vessel, exhibits a particularly high recurrence rate (Kwee et al., 2019, Liu et al., 2022). Therefore, there is a need to further explore the relationship between plaque characteristics within the culprit vessel and stroke recurrence in patients with ICAS within the MCA territory. This study aimed to investigate whether plaque features observed on HR-MR-VWI serve as independent risk factors for stroke recurrence. Additionally, we aimed to assess the predictive value of culprit plaque features based on HR-MR-VWI in predicting the recurrence of IS in patients with ICAS subtype.
2. Materials and methods
2.1. Study population
This study was approved by the institutional review board, and informed consent was obtained from all participants. We conducted a retrospective analysis of patients diagnosed with ICAS who underwent HR-MR-VWI at our hospital between November 2019 and December 2021 (Fig. 1).
Fig. 1.
Flowchart of the study. ICAS, intracranial arteriosclerosis; HR-MR-VWI, high-resolution magnetic resonance vessel wall imaging; IS, ischemic stroke; ICA, internal carotid artery; MCA, middle cerebral artery.
The inclusion criteria for patient enrollment were as follows: (1) first onset of stroke within 4 weeks of HR-MR-VWI (Ran et al., 2020, Jiang et al., 2022); (2) acute IS within the unilateral MCA territory as observed on diffusion-weighted imaging (DWI); (3) presence of at least one cardiovascular disease risk factor: hypertension, diabetes mellitus, hyperlipidemia, hyperhomocysteinemia, smoking and/or drinking habits (Song et al., 2020, Jiang et al., 2022); (4) age of 18 years or older. The exclusion criteria were as follows: (1) presence of chronic IS or transient ischemic attack (TIA); (2) culprit vessel other than the ipsilateral MCA; (3) ipsilateral carotid artery stenosis ≥ 50% (Song et al., 2020, Xu et al., 2022); (4) patients with infarcts in multiple artery territories beyond MCA (Li et al., 2021). (5) stroke due to other causes, such as arteritis, moyamoya disease, or cerebral artery dissection (Xu et al. 2022), embolic stroke of undetermined source (ESUS), cryptogenic strokes (Wang et al. 2022); (6) presence of risk factors for cardiogenic stroke, including patent foramen ovale, atrial fibrillation, acute myocardial infarction, cardiomyopathy, and valvular heart disease (Liu et al., 2022, Yang et al., 2022); (7) poor quality of HR-MR-VWI imaging or inadequate clinical information.
We collected general data of the enrolled patients, including gender, age, risk factors for atherosclerosis (hypertension, diabetes mellitus, hyperlipidemia, hyperhomocysteinemia, tobacco and alcohol use), National Institutes of Health Stroke Scale (NIHSS) score, and modified Rankin Score (mRS) at 90 days (Shi et al., 2021). Follow-up assessments were conducted in outpatient clinic to two specialist physicians at 3, 6, and 12 months after discharge, and annually thereafter (Yang et al., 2022). Neurological function outcomes and stroke recurrence were evaluated by two physicians with 6 and 10 years of experience in neurology, respectively. The NIHSS score was used to assess the degree of neurological impairment and short-term outcome. Stroke recurrence was diagnosed when a new lesion exhibiting hyperintensity on DWI and hypointensity on apparent diffusion coefficient (ADC) images within the MCA territory was observed on follow-up MR examination, accompanied by corresponding neurological dysfunction (Jiang et al., 2022). The patient with transit stroke symptoms without new infarction lesion on MRI was diagnosed as TIA instead of IS recurrence. Treatment strategies included anticoagulation, antiplatelet therapy, lipid-lowering and plaque-fixing, and circulation improvement therapy.
2.2. HR-MR-VWI image acquisition
The HR-MR-VWI imaging was conducted using a 3.0 T MR imaging system (Philips Achieva 3.0 T) with an 8-channel head coil. The following sequences and corresponding parameters were employed: (1) Fluid-attenuated inversion recovery (FLAIR): repetition time (TR), 9000 ms; echo time (TE), 140 ms; inversion time (TI), 2600 ms; field of view (FOV), 230 × 230 mm; slice thickness, 5.0 mm. (2) DWI: b = 1000 s/mm2, TR 4500 ms, TE 72.5 ms, slice thickness, 5.0 mm. (3) Time-of-flight MRA (TOF-MRA): TR 20 ms, TE 3.5 ms, slice thickness 0.6 mm. (4) HR-MR-VWI: TR 800 ms, TE 20 ms, slice thickness 0.3 mm,FOV 200 mm × 181 mm, matrix 332 × 334, spatial resolution 0.6 mm × 0.6 mm × 0.3 mm, recon voxel size 0.3 mm, reconstruction matrix 672 × 672, total acquisition time 9min51s. We performed interpolation before multiplanar reformation (MPR) and increased slice thickness to 1 mm for better signal to noise ratio (SNR) of MPR images. For enhanced scanning, repeated HR-MR-VWI was performed 5 min after intravenous injection of gadolinium glutamate (Bayer Schering Pharma AG, Berlin, Germany) at a dose of 0.1 mmol/kg.
2.3. Imaging analysis
All images were independently reviewed by two radiologists with 5 and 15 years of experience in neuroimaging, respectively.
The patients were classified into two groups: artery-artery embolism (AAE) type and non-AAE type, based on the findings from DWI (Wu et al., 2018). AAE type IS was defined as the presence of scattered (≥2) high signal intensity lesions in the cerebral cortex, subcortical, or internal watershed regions on DWI. Non-AAE type IS was defined as the presence of hyperintensity lesions in the territory of perforating arteries on DWI, including the putamen, globus pallidus, internal capsule, and caudate head (Chimowitz et al., 1995).
The evaluation of plaques was conducted by two neuroradiologists using a workstation (Siemens Syngo.viaVB20) without knowledge of the patients' clinical information. Atherosclerotic plaque was defined as focal wall thickening observed on pre-contrast HR-MR-VWI. The culprit plaque was defined as the plaque located at the most severe stenosis segment of the culprit vessel when multiple plaques were present, or as the only plaque within the territory of the stroke lesion. After magnifying the images to a 400% scale, centered on the focus of the culprit plaque, the outer wall area (OWA) and lumen area (LA) of the stenosis segment and the proximal normal segment were manually delineated on post-contrast HR-MR-VWI images perpendicular to the long axis of the culprit vessel (Fig. 2) (Wu et al., 2018). The following metrics of the most stenotic segment of the culprit vessel at the site of the culprit plaque were calculated according to the method used in the previous studies (Chimowitz et al., 1995, Liu et al., 2022): (1) Wall area (WA) = OWA - LA; (2) Plaque area (PA) = WA - WAreference (3) Plaque burden (PB) = WA / OWA; (4) Remodeling index (RI) = OWA / OWAreference, where positive remodeling was defined as RI ≥ 1.05 and negative remodeling as RI ≤ 0.95; (5) Degree of stenosis (DS) = (1 - LA / LAreference) × 100%. The distribution of plaques was categorized on cross-sectional images into ventral, dorsal, superior, and inferior quadrants (Li et al., 2021). Focal plaque was defined as the distribution across ≤ 2 quadrants, while diffuse plaque was defined as distribution across ≥ 3 quadrants (Liu et al., 2022). The presence of mild enhancement of the plaque was considered when the signal intensity of the plaque was lower than that of the pituitary stalk after contrast medium injection. Mild and obvious enhancement were defined when the signal intensity of the culprit plaque were weaker, and equal to or higher than that of the pituitary stalk on post-contrast HR-MR-VWI separately (Jiang et al., 2022, Ren et al., 2023). For the cases with IPH, a subtraction imaging between post- contrast and non-contrast HR-MR-VWI was made for judging true enhancement (Fig. 3). Intraplaque hemorrhage was defined as high signal intensity within the plaque that was more than 150% of the adjacent muscle signal intensity on pre-contrast HR-MR-VWI (Jiang et al., 2022). The presence of a discontinuous surface of the plaque adjacent to the lumen was considered an irregularity of the plaque (Wu et al., 2018).
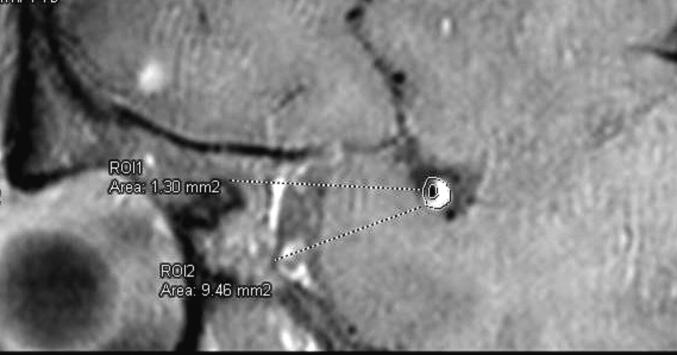
Fig. 2.
The vessel area (ROI 2) and lumen area (ROI 1) were measured on the image which was perpendicular to the long axis of the culprit vessel.
Fig. 3.

Intraplaque hemorrhage (IPH) and subtraction images. (A) Pre-contrast HR-MR-VWI in axial plane showed IPH (black arrow). (B) Post-contrast HR-MR-VWI in axial plane showed that both obvious enhancement of plaque (white arrow) and IPH (black arrow) were hyperintensity. (C) Subtraction image showed that the obvious enhancement of plaque was still hyperintensity (white arrow), and IPH (black arrow) were hypointensity.
2.4. Statistical analysis
Statistical analysis in this study was performed using the software SPSS 25.0 (IBM Corp., Armonk, NY, USA). Quantitative variables that followed a normal distribution were expressed as mean ± standard deviation (X ± S) and analyzed using the Mann-Whitney U test or independent t-test. Quantitative variables that did not follow a normal distribution were expressed as median (m) and interquartile range (P25, P75) and analyzed using the chi-square test. The reproducibility of continuous variables between the two radiologists was assessed using the intraclass coefficient (ICC) and the Kappa value with 95% confidence intervals (CIs). Univariate and multivariate Cox proportional hazards regression models were used to identify independent risk factors (p < 0.05) for stroke recurrence. These independent factors were then used to construct various prediction models for stroke recurrence, including clinical, conventional imaging features, plaque feature, clinical + conventional imaging features, and combination models. Receiver operating characteristic (ROC) curves were employed to evaluate the diagnostic efficiency of these models. A p-value<0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical parameters
Among the initial 177 IS patients who underwent HR-MR-VWI examinations, a total of 91 patients were excluded for various reasons. This included patients with other diseases such as arteritis (n = 8), Moyamoya disease (n = 4), and brain tumors (n = 2), patients with risk factors of cardiogenic stroke (n = 15), patients with poor HR-MR-VWI imaging (n = 3), patients with inadequate clinical information (n = 2), patients with chronic IS and TIA (n = 6), patients with the culprit vessel other than the ipsilateral middle cerebral artery (n = 38), and patients with ipsilateral internal carotid artery stenosis ≥ 50% (n = 3). The final study cohort consisted of 86 patients with ICAS type IS, with ages ranging from 30 to 81 years old. Among them, 57 (66.3%) were male (Fig. 1). The median follow-up duration was 19 months (95% CI, 1–35.65 months). The enrolled patients were divided into two groups: the recurrence group (n = 20, 23.25%) and the non-recurrence group (n = 66, 76.75%). A comparison between the two groups revealed that patients in the recurrence group were older (median age 57.50 years vs. 50 years, p = 0.007), had a higher rate of diabetes mellitus (35.0% vs. 13.6%, p = 0.031), and a lower rate of hyperhomocysteinemia (5.0% vs. 30.3%, p = 0.021) (Table 1).
Table 1.
Characteristics of ICAS IS in MCA territory based on stroke recurrence (n = 86).
| Recurrence (n = 20) | Non-recurrence (n = 66) | p Value | |
|---|---|---|---|
| Gender (male) | 10(50.0%) | 47(72.3%) | 0.063 |
| Age, years | 57.50(45.25,66.75) | 50.00(40.75,55.00) | 0.007* |
| Risk factors of atherosclerosis | |||
| Hypertension | 15(75.0%) | 43(65.2%) | 0.410 |
| Diabetes mellitus | 7(35.0%) | 9(13.6%) | 0.031* |
| Hyperlipidemia | 1(5.0%) | 6(9.1%) | 0.558 |
| Hyperhomocysteinemia | 1(5.0%) | 20(30.3%) | 0.021* |
| Drink | 5v25.0%) | 29(43.9%) | 0.129 |
| Smoking | 7(35.0%) | 30(45.5%) | 0.408 |
| Neurological function score | |||
| NIHSS at admission | 3(2,4) | 2(1,6) | 0.282 |
| mRS at 90 days | 3(1,4) | 2(1,4) | 0.759 |
| Conventional imaging findings | |||
| Largest area of infarction (mm2) | 2.30(1.08,5.58) | 2.52(0.98,5.54) | 0.906 |
| AAE infarction | 17(85.0%) | 37(56.1%) | 0.019* |
| Characteristics of culprit plaque | |||
| Area of plaque | 2.00(1.17,2.61) | 1.88(1.18,2.36) | 0.653 |
| Load of plaque | 0.90(0.85,0.94) | 0.87(0.82,0.92) | 0.144 |
| Enhancement of plaque | |||
| No enhancement | 2 (10.00%) | 12 (18.20%) | 0.420 |
| Mild enhancement | 2(10.00%) | 22(33.33 %) | 0.042 |
| Obviuos enhancement | 16(80.0%) | 32(48.5%) | 0.013* |
| Distribution of plaque | 0.588 | ||
| Ventral | 9(45.0%) | 40(60.6%) | |
| Dorsal | 2(10.0%) | 7(10.6%) | |
| Superior | 5(25.0%) | 10(15.2%) | |
| Inferior | 4(20.0%) | 9(13.6%) | |
| Focal plaque | 11(55.0%) | 49(74.2%) | 0.101 |
| Hyperintensity on T1WI | 4(20.0%) | 13(19.7%) | 0.976 |
| Surface irregularity of plaque | 8(40.0%) | 9(13.6%) | 0.009* |
| Vessel lumen at the plaque | |||
| Remodeling index | 1.01(0.99,1.04) | 1.00(0.98,1.05) | 0.591 |
| Stenosis > 50% | 16(80.0%) | 42(63.6%) | 0.171 |
Abbreviations: ICAS, intracranial arteriosclerosis; IS, ischemic stroke; MCA, middle cerebral artery; NIHSS, national institute of health stroke scale; mRs, Modified Rankin score; AAE, artery-artery embolism; T1WI, T1-weighted imaging.
3.2. Imaging features and stroke recurrence
In the recurrence group, the rate of AAE infarction was significantly higher compared to the non-recurrence group (85.0% vs. 56.1%, p = 0.019). However, there was no significant difference in the infarct area between the recurrence and non-recurrence groups (2.30 cm2 vs. 2.52 cm2, p = 0.906) (Table 1).
When comparing the recurrence group to the non-recurrence group, the patients in the recurrence group had a higher rate of mild enhancement of plaque (33.33% vs. 10.00%, p = 0.042), obvious enhancement of plaque (80.0% vs. 48.5%, p = 0.013) and surface irregularity of plaque (40.0% vs. 13.6%, p = 0.009). However, there were no significant differences between the two groups in terms of plaque area (p = 0.653), plaque burden (p = 0.144), plaque location (p = 0.588), plaque distribution (p = 0.101), intra-plaque T1WI hyperintensity (p = 0.976), frequency of plaque enhancement (p = 0.385), remodeling index (p = 0.591), and stenosis degree (p = 0.171) of the culprit vessel (Table 1).
In the comparison between patients with AAE stroke and patients with non-AAE stroke, there were no significant differences in T1WI hyperintensity (p = 0.134), plaque surface irregularity (p = 0.855), enhancement degree of plaque (p = 0.615), incidence of plaque enhancement (p = 0.633), and obvious enhancement of plaque (p = 0.609) (Table 2).
Table 2.
Comparison of the characteristics of culprit plaque between patients with AAE and non-AAE infarction.
| AAE infarction(n = 54) | Non-AAE infarction(n = 32) | p Value | |
|---|---|---|---|
| Hyperintensity on T1WI | 8(14.8%) | 9(28.1%) | 0.134 |
| Surface irregularity of plaque | 11(20.4%) | 6(18.8%) | 0.855 |
| Enhancement | 0.615 | ||
| None(grade 0) | 8(14.8%) | 6(18.7%) | |
| Mild(grade 1) | 17(31.5%) | 7(21.9%) | |
| Obvious(grade 2) | 29(53.7%) | 19(59.4%) | |
| Frequency of enhancement | 46(85.2%) | 26(81.3%) | 0.633 |
Abbreviations: AAE, artery-artery embolism; T1WI, T1-weighted imaging.
Representative DWI and HR-MR-VWI images of ICAS patients are shown in Fig. 4A and Fig. 4B, respectively.
Fig. 4A.

Representative DWI and HR-MR-VWI of a ICAS patients with AAE infarction and without recurrence. A 48-year-old male patient with right limb weakness 3 days. The 90-day mRs was 2. There was no stroke recurrence in the follow-up period of 30 months. Axial DWI showed one lesion in left lenticular nucleus with high signal intensity. MRA showed stenosed M2 of left middle cerebral artery (arrow). Axial and saggital pre-contrast HR-MR-VWI showed atherosclerotic plaque and mild lumen stenosis of the left MCA. Axial and saggital postcontrast HR-MR-VWI showed no enhancement of the plaque.
Fig. 4B.

Representative DWI and HR-MR-VWI of a ICAS patients with AAE infarction and with recurrence. A51-year-old male patient with left limb weakness and aphasia 1 day. The 90-day mRs was 4. There was stroke recurrence 75 days after first onset. Axial DWI showed multiple lesions in right cerebral hemisphere with high signal intensity on DWI. MRA showed stenosed M1-2 of right middle cerebral artery (arrow). Axial and saggital pre-contrast HR-MR-VWI showed irregular atherosclerotic plaque and lumen stenosis of right MCA (arrow). Axial and saggital postcontrast HR-MR-VWI showed obvious enhancement of the plaque (arrow).
3.3. Prediction of stroke recurrence
The univariate logistic regression analysis revealed that age [hazard ratio (HR) = 1.048, 95 %CI: 1.007–1.091, p = 0.021], diabetes mellitus (HR = 2.552, 95 %CI: 1.014–6.423, p = 0.047), AAE infarction (HR = 0.165, 95 %CI: 0.045–0.603, p = 0.006), surface irregularity of plaque (HR = 3.461, 95 %CI: 1.406–8.521, p = 0.007), mild enhancement of plaque (HR = 0.274, 95 %CI: 0.064,1.183, p = 0.083), and obvious enhancement of plaque (HR = 3.646, 95 %CI: 1.216–10.930, p = 0.021) were identified as risk factors for stroke recurrence. However, hyperhomocysteinemia (HR = 0.148, 95 %CI: 0.020–1.109, p = 0.063) was found to be a protective factor against stroke recurrence.
In the multivariate logistic regression analysis, age (HR = 1.063, 95 %CI: 1.018, 1.109, p = 0.005), AAE infarction (HR = 5.708, 95 %CI: 1.589, 20.498, p = 0.008), and obvious enhancement of plaque (HR = 4.105, 95 %CI: 1.193, 14.121, p = 0.025) remained as independent risk factors for stroke recurrence. The risk of stroke recurrence increased by 0.055-fold with each year increase in age. The risk of recurrence in patients with AAE infarction was 0.165 times higher compared to patients without AAE infarction. Additionally, the recurrence risk of IS was 4.404 times higher in patients with significant plaque enhancement compared to those without obvious plaque enhancement (Table 3).
Table 3.
Univariate and Multivariate analysis of factors associated with stroke recurrence.
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR(95 %CI) | p Value | HR(95 %CI) | p Value | |
| Age | 1.048(1.007,1.091) | 0.021* | 1.063(1.018,1.109) | 0.005 |
| Diabetes mellitus | 2.552(1.014,6.423) | 0.047* | ||
| hyperhomocysteinemia | 0.148(0.020,1.109) | 0.063 | ||
| AAE infarction | 3.939v1.150,13.488) | 0.029* | 5.708(1.589,2.498) | 0.008 |
| Surface irregularity of plaque | 3.461(1.406,8.521) | 0.007* | ||
| Mild enhancement of plaque | 0.274 (0.064, 1.183) | 0.083 | ||
| Obvious enhancement of plaque | 3.646(1.216,10.930) | 0.021* | 4.105(1.193,14.121) | 0.025 |
Abbreviations: HR, Hazard ratio; CI, confidence interval; AAE, artery-artery embolism.
The AUCs for the different models predicting stroke recurrence were as follows: 0.728 for the clinical model (age), 0.645 for the conventional imaging model (AAE infarction), 0.705 for the plaque feature model (obvious enhancement of plaque), and 0.814 for the clinical + conventional imaging model (age, AAE infarction). After adding the plaque feature, the AUC of the combination model (all independent risk factors for stroke recurrence) for predicting stroke recurrence increased to 0.870 (Fig. 5). The sensitivity, specificity, and Youden index for predicting recurrence were 0.850, 0.773, and 0.623, respectively.
Fig. 5.
ROC curves of different models in predicting stroke recurrence. Con MRI, conventional magnetic resonance imaging.
3.4. Consistency test for imaging evaluation
The ICCs between the two radiologists for evaluating various imaging findings were as follows: 0.997 (95 %CI: 0.996–0.998, p < 0.001) for the largest area of infarct, 0.935 (95 %CI: 0.900–0.957, p < 0.001) for plaque area, 0.960 (95 %CI: 0.940–0.974, p < 0.001) for plaque load, 0.974 (95 %CI: 0.960–0.983, p < 0.001) for remodeling index, and 0.948 (95 %CI: 0.920–0.966, p < 0.001) for vessel stenosis. The ICCs between the two physicians for evaluating NIHSS at admission and 90-day mRs were 0.991 (95 %CI: 0.986–0.994, p < 0.001) and 0.959 (95 %CI: 0.938–0.973, p < 0.001), respectively. The Kappa values between the two radiologists for evaluating AAE infarction, enhancement grading of plaque, plaque location, plaque distribution, identifying T1WI hyperintensity in plaque, and irregular surface of plaque were 0.853, 0.856, 0.845, 0.838, 0.888, and 0.846, respectively (p < 0.001 for all these features).
To verify the predictive efficacy of the models for IS recurrence, consecutive 37 patients with ICAS IS in MCA territory who had HR-MR-VWI during January 2022 to June 2022 were enrolled for validation. AUCs for IS recurrence prediction in the validation cohort were as follows: 0.750 for the clinical model, 0.588 for the conventional imaging model, 0.638 for the plaque feature model, 0.803 for the clinical + conventional imaging model, and 0.865 for the combination model.
4. Discussion
In this retrospective observational cohort study, we confirmed that the plaque features of the culprit vessel on HR-MR-VWI can be utilized to predict the recurrence of ICAS type IS in the MCA territory. The study identified age, AAE type IS, and obvious enhancement of the culprit plaque as independent risk factors for stroke recurrence. The obvious enhancement of the culprit plaque appears to be a valuable imaging biomarker for predicting the recurrence of ICAS stroke. Furthermore, when the plaque features of the culprit vessels were incorporated into the clinical-conventional imaging model, the prediction efficiency for IS recurrence improved. These findings suggest that considering plaque characteristics in addition to clinical and conventional imaging factors can enhance the prediction of stroke recurrence in ICAS patients.
Determining the factors associated with stroke recurrence is crucial for optimizing the clinical management and prevention of future stroke events in patients. In line with previous research (Ran et al., 2020, Jiang et al., 2022), our preliminary results highlight the significant prognostic value of obvious enhancement of plaque on HR-VWI for the recurrence of IS, with a hazard ratio (HR) of 4.105. The enhancement of plaque, which reflects the instability of the lesion, may be attributed to various factors such as inflammation, endothelial dysfunction of the culprit vessel, and neovascularization within the plaque (Huang et al., 2023, Tian et al., 2023). Previous studies have demonstrated that unstable plaques undergo an inflammatory process, leading to plaque rupture, thrombosis, and ultimately the onset of IS (Kwee et al., 2019, Tian et al., 2023). These findings suggest that the assessment of plaque enhancement can provide valuable insights into the pathophysiology and prognosis of IS recurrence.
When exploring the impact of plaque enhancement on the onset of IS recurrence, two aspects should be considered: the incidence and degree of enhancement. In this study, although the incidence of enhancement was slightly higher in the recurrence group (90.0%) compared to the non-recurrence group (83.3%), there was no significant difference between the two groups (p = 0.385). This phenomenon can be explained by the fact that the IS patients included in this study were within 4 weeks of stroke onset, which corresponds to the acute and subacute stages. Previous studies have demonstrated that the degree of enhancement of culprit plaques tends to diminish over time, with more obvious enhancement observed in the acute and subacute phases of IS (Liang et al., 2019, Yang et al., 2020). However, in the chronic stage of IS, the degree and frequency of plaque enhancement tend to decrease (Lyu et al., 2019, Lu et al., 2021).
The degree of enhancement of culprit plaque has been shown to be a more valuable predictor of IS recurrence compared to the frequency of enhancement in previous studies (Kwee et al., 2019, Liu et al., 2022). In the present study, we also found that obvious enhancement of plaque was an independent risk factor for IS recurrence (p = 0.025). The mechanism underlying obvious plaque enhancement involves increased endothelial permeability and extracellular volume due to inflammation and neovascularization within the plaque. These processes are pathological markers of unstable plaques (Song et al., 2020, Tang et al., 2022). However, it is worth noting that a few studies did not find a significant association between obvious plaque enhancement and stroke recurrence (Liang et al., 2019, Jiang et al., 2022). This discrepancy may be explained by differences in the inclusion criteria between studies. In Jiang et al.'s study (Jiang et al., 2022), which did not identify obvious plaque enhancement as an independent risk factor for stroke recurrence, the enrolled patients included 52 individuals with TIA among the 206 patients with symptomatic intracranial atherosclerotic stenosis. The vessel stenosis leading to TIA symptoms may be caused by plaque calcification or fibrosis at a later stage of arteriosclerosis. As a result, the plaques in TIA patients may be relatively more stable and exhibit a lower rate of enhancement compared to other individuals (Liang et al., 2019, Xiao et al., 2021).
In our study, we identified AAE infarction as an independent risk factor for stroke recurrence. Several reasons may contribute to AAE infarction being a predictor of stroke recurrence, including hyperintense plaques on T1WI, plaque surface irregularity, obvious enhancement of plaque, and high-on-aspirin platelet reactivity (Wu et al., 2018, Noh et al., 2022). It is important to note that all patients in our study were prescribed antiplatelet agents. However, we did not find a significant difference in T1WI hyperintensity and plaque surface irregularity between patients with AAE infarction and those with non-AAE infarction. This discrepancy may be attributed to the recruitment of symptomatic patients in each group, which introduced complexity and heterogeneity into the analysis of these factors.
Although the individual role of factors such as age, diabetes mellitus, AAE infarction, plaque surface irregularity, and obvious enhancement of plaque in predicting stroke recurrence is limited according to the univariate analysis in our study, their contribution is influenced by the complex nature of ICAS stroke. Therefore, we incorporated the imaging features of plaque on HR-MR-VWI into a combination model with clinical and conventional imaging factors to enhance the predictive value of stroke recurrence. Other studies have also confirmed the contribution of clinical and conventional imaging factors, including age, diabetes, and AAE infarction, to stroke recurrence, as these factors can exacerbate plaque instability (Tyrrell and Goldstein, 2021, Ambreen et al., 2022, Wu et al., 2023). The combination of clinical, imaging features, and plaque characteristics has a synergistic effect on predicting the risk of stroke recurrence. Therefore, it is recommended to comprehensively evaluate clinical, conventional imaging factors, and plaque characteristics when assessing the risk of stroke recurrence.
The present study has several limitations that should be acknowledged. Firstly, it is important to note that this study is cross-sectional and retrospective, conducted in a single center with a relatively small sample size. Therefore, the results obtained from this study would benefit from validation in larger-scale longitudinal studies in the future. However, it is worth mentioning that a validation analysis was conducted in the present study using another 37 patients, which confirmed the predictive efficacy of the combination model that included plaque features. In contrast, previous studies with a larger sample sizes (Jiang et al., 2022; Ren et al., 2022) have demonstrated that plaque characteristics, including plaque burden, plaque intensification, and T1WI hyperintensity, were the independent risk factors for recurrence of IS. However, no distinction was made between anterior and posterior circulation and etiology of stroke, and TIA cases were included. There was certain difference of the plaque features between anterior and posterior circulations. Thus, the exploration of certain artery territories would be more valuable for accurate diagnosis and prevention of IS recurrence. Secondly, one of the challenges in this study is the determination of the true significance of imaging features of plaque on HR-MR-VWI, as histopathological examination of the plaques was not performed. Further studies incorporating histopathological examination would provide more insight into the interpretation and meaning of these imaging features. Thirdly, the study population included only patients with infarction associated with MCA stenosis caused by atherosclerotic plaques. Therefore, the generalizability of the results to patients with infarction in other intracranial artery territories with atherosclerotic plaques, as well as those with TIA, may be limited. There was certain difference of plaque feature of cerebral arteries between anterior and posterior circulation, including the higher incidence of plaque enhancement, and hyperintensity on T1WI in posterior circulation vessels (Tang et al., 2022). However, it is important to note that intracranial atherosclerosis of the MCA is a major cause and an important location of IS (Zhao et al., 2016). In our another study which enrolled 166 IS cases, including 123 cases with anterior circulation IS and 43 cases with posterior circulation IS, we also demonstrated that obvious plaque enhancement (HR = 3.080) and IPH (HR = 5.109) of culprit vessel were an independent risk factor for IS recurrence. Therefore, the elucidation of mechanisms related to the imaging features of culprit plaques and their impact on stroke recurrence may have significant clinical implications for the treatment strategies of ICAS stroke in the MCA territory.
In conclusion, our preliminary study demonstrated that age, AAE infarction, and obvious enhancement of culprit plaques on HR-MR-VWI were independently associated with stroke recurrence in patients with ICAS in the MCA territory. The obvious enhancement of culprit plaque holds promise as a valuable predictive factor for stroke recurrence.
5. Data availability
Analytical methods such as the Univariate and multivariate Cox proportional hazards regression and output files of the analyses will be made available on request from the corresponding author.
Funding
This work is funded by Hebei Natural Science Foundation (H2022206191).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Ambreen S., Fatima S., Elwakiel A., Rana R., Singh K., Gupta A., Gupta D., Khawaja H., Manoharan J., Besler C., Laufs U., Kohli S., Isermann B., Shahzad K. Hypercoagulability impairs plaque stability in diabetes-induced atherosclerosis. Nutrients. 2022;14(10):1991. doi: 10.3390/nu14101991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimowitz M.I., Kokkinos J., Strong J., Brown M.B., Levine S.R., Silliman S., Pessin M.S., Weichel E., Sila C.A., Furlan A.J., Kargman D.E., Sacco R.L., Wityk R.J., Ford G., Fayad P.B. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995;45(8):1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- Huang L.-X., Wu X.-B., Liu Y.-A., Guo X., Ye J.-S., Cai W.-Q., Wang S.-W., Luo B. Qualitative and quantitative plaque enhancement on high‐resolution vessel wall imaging predicts symptomatic intracranial atherosclerotic stenosis. Brain Behav. 2023;13(6) doi: 10.1002/brb3.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ren K., Li T., Qian C., Gong S., Wang T., Zhu L.i. Correlation of the characteristics of symptomatic intracranial atherosclerotic plaques with stroke types and risk of stroke recurrence: a cohort study. Ann. Transl. Med. 2022;10(12):658. doi: 10.21037/atm-22-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee R.M., Qiao Y.e., Liu L.i., Zeiler S.R., Wasserman B.A. Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI. Neuroradiology. 2019;61(6):651–657. doi: 10.1007/s00234-019-02190-4. [DOI] [PubMed] [Google Scholar]
- Li S., Song X., Hu Q., Zhao J., Du H., Yan Y., Wang G., Chen X., Wang Q. Association of Plaque Features with Infarct Patterns in Patients with Acutely Symptomatic Middle Cerebral Artery Atherosclerotic Disease. J. Stroke Cerebrovasc. Dis. 2021;30(5):105724. doi: 10.1016/j.jstrokecerebrovasdis.2021.105724. [DOI] [PubMed] [Google Scholar]
- Li J., Zheng L., Yang W.J., Sze-To C.Y., Leung T.W., Chen X.Y. Plaque Wall Distribution Pattern of the Atherosclerotic Middle Cerebral Artery Associates With the Circle of Willis Completeness. Front. Neurol. 2021;11 doi: 10.3389/fneur.2020.599459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Guo J., Liu D., Shi C., Luo L. Application of High-Resolution CUBE Sequence in Exploring Stroke Mechanisms of Atherosclerotic Stenosis of Middle Cerebral Artery. J. Stroke Cerebrovasc. Dis. 2019;28(1):156–162. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhong F., Xie Y.u., Lu X., Hou B., Ouyang K., Fang J., Liao M., Liu Y. A Predictive Model for the Risk of Posterior Circulation Stroke in Patients with Intracranial Atherosclerosis Based on High Resolution MRI. Diagnostics (Basel, Switzerland) 2022;12(4):812. doi: 10.3390/diagnostics12040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Ye M.-F., Zhao J.-j., Diao S.-S., Li T., Ding D.-X., Zhang L.-l., Yao F.-R., Kong Y., Xu Z. Gadolinium enhancement of atherosclerotic plaque in the intracranial artery. Neurol. Res. 2021;43(12):1040–1049. doi: 10.1080/01616412.2021.1949682. [DOI] [PubMed] [Google Scholar]
- Lyu J., Ma N., Tian C., Xu F., Shao H., Zhou X., Ma L., Lou X. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc. Neurol. 2019;4(3):129–134. doi: 10.1136/svn-2018-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh K.C., Choi H.-Y., Woo H.G., Chang J.Y., Heo S.H., Chang D.-i., Kim B.J. High-on-Aspirin Platelet Reactivity Differs Between Recurrent Ischemic Stroke Associated With Extracranial and Intracranial Atherosclerosis. J. Clin. Neurol. (Seoul, Korea) 2022;18(4):421. doi: 10.3988/jcn.2022.18.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran Y., Wang Y., Zhu M., Wu X., Malhotra A., Lei X., Zhang F., Wang X., Xie S., Zhou J., Zhu J., Cheng J., Zhu C. Higher Plaque Burden of Middle Cerebral Artery Is Associated With Recurrent Ischemic Stroke: A Quantitative Magnetic Resonance Imaging Study. Stroke. 2020;51(2):659–662. doi: 10.1161/STROKEAHA.119.028405. [DOI] [PubMed] [Google Scholar]
- Ren, K., Jiang, H., Li, T., Qian, C., Zhu, L., Wang, T., 2023. Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence. Diagnostics (Basel, Switzerland) 13, 804. [DOI] [PMC free article] [PubMed]
- Ren K., Jiang H., Li T., Qian C., Gong S., Wang T., Zhu L.i. Predictive value of the combination between the intracranial arterial culprit plaque characteristics and the Essen Stroke Risk Score for short-term stroke recurrence. J. Stroke Cerebrovasc. Dis. 2022;31(9):106624. doi: 10.1016/j.jstrokecerebrovasdis.2022.106624. [DOI] [PubMed] [Google Scholar]
- Shi Z., Zhao M., Li J., Meddings Z., Shi Y., Jiang T., et al. Association of Hypertension With Both Occurrence and Outcome of Symptomatic Patients With Mild Intracranial Atherosclerotic Stenosis: A Prospective Higher Resolution Magnetic Resonance Imaging Study. J. Magnetic Resonance Imaging: JMRI. 2021;54:76–88. doi: 10.1002/jmri.27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Zhao X., Liebeskind D.S., Wang L., Xu W., Xu Y., Hou D., Zheng Z., Wu J. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology. 2020;62(9):1123–1131. doi: 10.1007/s00234-020-02418-8. [DOI] [PubMed] [Google Scholar]
- Tang M., Gao J., Ma N., Yan X., Zhang X., Hu J., Zhuo Z., Shi X., Li L., Lei X., Zhang X. Radiomics Nomogram for Predicting Stroke Recurrence in Symptomatic Intracranial Atherosclerotic Stenosis. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.851353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Zhu C., Tian X., Kang Q., Shao C., Mossa-Basha M., Lu J., Saloner D.A. Baseline vessel wall magnetic resonance imaging characteristics associated with in-stent restenosis for intracranial atherosclerotic stenosis. J. Neurointervent. Surg. 2023;15(3):288–291. doi: 10.1136/neurintsurg-2021-018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.J., Goldstein D.R. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021;18(1):58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang L., Shen Y., Gong X., Ju Y. Relationship Between Carotid Artery Angle and Plaque Morphology in Acute Cerebral Infarction Patients. Neurologist. 2022;27:240–244. doi: 10.1097/NRL.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Kong Q., Huang H., Xu S., Qu W., Zhang P., Yu Z., Luo X. Effect of PCSK9 inhibition in combination with statin therapy on intracranial atherosclerotic stenosis: A high-resolution MRI study. Front. Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1127534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Song H., Ma Q., Xiao J., Jiang T., Huang X., Bi X., Guo X., Li D., Yang Q.i., Ji X., Fan Z., Yu H., Cui B., Sun J., Sun B., Xia S., Han T., Cheng J. Hyperintense Plaque on Intracranial Vessel Wall Magnetic Resonance Imaging as a Predictor of Artery-to-Artery Embolic Infarction. Stroke. 2018;49(4):905–911. doi: 10.1161/STROKEAHA.117.020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Padrick M.M., Jiang T., Xia S., Wu F., Guo Y.u., Gonzalez N.R., Li S., Schlick K.H., Dumitrascu O.M., Maya M.M., Diniz M.A., Song S.S., Lyden P.D., Li D., Yang Q.i., Fan Z. Acute ischemic stroke versus transient ischemic attack: Differential plaque morphological features in symptomatic intracranial atherosclerotic lesions. Atherosclerosis. 2021;319:72–78. doi: 10.1016/j.atherosclerosis.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Qin J., Yu J., Sun Y., Hu D., Wu G., Li Y. Association of plaque enhancement on vessel wall MRI and the phosphodiesterase 4D variant with stroke recurrence in patients with symptomatic intracranial atherosclerosis. Neuroradiology. 2022;64(9):1781–1794. doi: 10.1007/s00234-022-02948-3. [DOI] [PubMed] [Google Scholar]
- Yang W.-J., Abrigo J., Soo Y.-Y., Wong S., Wong K.-S., Leung T.-H., Chu W.-W., Chen X.-Y. Regression of Plaque Enhancement Within Symptomatic Middle Cerebral Artery Atherosclerosis: A High-Resolution MRI Study. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Liu J., Yao W., Huang K., Zhou C., Bi J., Cheng X., Ma M., Zhu W., Zhang J., Zhang L., Cai J., Teng Z., Du J., Liu X. The MRI enhancement ratio and plaque steepness may be more accurate for predicting recurrent ischemic cerebrovascular events in patients with intracranial atherosclerosis. Eur. Radiol. 2022;32(10):7004–7013. doi: 10.1007/s00330-022-08893-2. [DOI] [PubMed] [Google Scholar]
- Zhao D.-L., Deng G., Xie B.o., Gao B.o., Peng C.-Y., Nie F., Yang M., Ju S., Teng G.-J. Wall characteristics and mechanisms of ischaemic stroke in patients with atherosclerotic middle cerebral artery stenosis: a high-resolution MRI study. Neurol. Res. 2016;38(7):606–613. doi: 10.1179/1743132815Y.0000000088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analytical methods such as the Univariate and multivariate Cox proportional hazards regression and output files of the analyses will be made available on request from the corresponding author.
Data will be made available on request.