Graphical abstract

Keywords: Aedes aegypti, Larval selection, Spinosad, Bacilod, Blood meal digestion, Reproductive potential
Abstract
A laboratory strain of Aedes aegypti (L) was subjected repeatedly to larval selection pressure with two bacterial insecticides, spinosad (Saccharopolyspora spinosa) and bacilod (Bacillus thuringiensis israelensis). The results indicated that the mosquito Ae. aegypti acquired low resistance to spinosad and bacilod by about 3.1 and 2.4-fold, respectively, due to selection pressure for fifteen successive generations. The slope values of the selected strains were increased gradually from one generation to the next, indicating moderate homogeneity between individuals in their response to the test bio-insecticide. Moreover, larval selection with current bacterial bioinsecticides prolonged the time required to digest a blood meal. It showed an evident decrease in the reproductive potential of adult mosquitoes surviving selected larvae.
1. Introduction
Vector-borne arbovirus diseases (dengue, chikungunya, and Zika) continue to emerge worldwide and have been reported as global public health problems in the last five decades. Outbreaks have been endemic to the Americas, Africa, Asia-Pacific, and the Middle East, specifically the Arabian Peninsula. In the Kingdom of Saudi Arabia (KSA), dengue fever was first recorded in 1994 (Fakeeh and Zaki, 2003).
Insecticide spraying is the primary strategy for controlling dengue fever and other vector-borne diseases. These approaches rely heavily on using pyrethroids and organophosphates; however, they lead to developing pesticide resistance in the field. Thus, it poses a major threat to these control programs. In the case of Saudi Arabia, pyrethroid resistance was reported in Jazan, Al-Quoz, Jeddah, Makkah, and Madinah (Algamdi and Mahyoub, 2022, Mashlawi et al., 2022). Insecticide resistance is a significant evolutionary phenomenon when insects develop the ability to survive exposure to insecticides that would typically kill them (Naqqash et al., 2016). This resistance can arise through various mechanisms, including changes in the insect's metabolic pathways, target site insensitivity, and reduced insecticide penetration. Overall, developing mosquito resistance to pesticides is a complex process that depends on multiple factors, including genetic, physiological, behavioral, and ecological factors (Zhu et al., 2016). Moreover, Mosquitoes possess inherent characteristics that make them particularly prone to developing insecticide resistance. One of the key factors is their short life cycle, which allows rapid genetic changes to occur over multiple generations. This means that mosquitoes can quickly evolve resistance to insecticides that are repeatedly used against them (Liu, 2015). Understanding these factors is important for developing effective strategies to control mosquito-borne diseases and reduce mosquito populations and spread. The development of resistance by mosquitoes to the chemical compounds used against them as larvicides and adulticides was first observed in 1949 when some species of Aedes began to show resistance to DDT in Florida (Brown, 1986). Recently, resistance to chemical insecticides has been documented in mosquito vectors for several classes of insecticides, including cyclodiene, organophosphates, carbamates, pyrethroids, and insect growth regulators (Karunaratne et al., 2018). Moreover, the excessive use of synthetic insecticides to control mosquitoes not only leads to the development of mosquito resistance but also causes environmental pollution (Tang et al., 2021), prompting researchers to search for environmentally safe and effective nontraditional insecticides for mosquito control, such as bioinsecticides. Several authors (Rodcharoen and Mulla, 1994, Batra et al., 2005, Hertlein et al., 2010, Almadiy et al., 2014, Virgillito et al., 2022) have reported that formulations of bacterial agents have biological activity properties against mosquito larvae, indicating their potential as safe alternatives in mosquito control. Few published works have examined the development of mosquito resistance to bacterial insecticides in regions around the world (Rodcharoen and Mulla, 1994, Su and Cheng, 2014); however, no local attempts have been made. Therefore, this study focuses on two points: (i) investigating the potential development of resistance to Ae. aegypti mosquitoes, the primary vector of dengue virus in Saudi Arabia, as a result of larval selection for 15 successive generations with two bioinsecticides and (ii) studying the delayed effects of larval selection using the test bioinsecticides on some biological features of adult survivors.
2. Methods
2.1. Mosquito strain
A colony of Ae. aegypti was established from eggs obtained from the Public Health Pest Laboratory in Jeddah, which is the most reliable source in Saudi Arabia (Aljameeli, 2023, Alyahya, 2023). The original strain was collected from wild mosquito larvae spread in stagnant ponds in Jeddah city and maintained in the laboratory under favorable conditions (27 ± 1 °C and 70 ± 5% R. H) for more than 231 generations (generation > F231) away from any insecticides and maintained under a 14:10 (light: dark) cycle. Mosquito Ae. aegypti was bred under suitable conditions to generate individuals of similar size, as this is a common practice in mosquito research. This is because mosquito size can affect their susceptibility to insecticides, as larger mosquitoes may be able to metabolize insecticides more efficiently than smaller ones. Adult mosquitoes were housed in aluminum-base cages measuring 30 × 30 × 30 cm. The size of the cage allows enough space for mosquitoes to fly and move about, while the aluminum base provides durability and easy cleaning. The larvae were kept in enamel pans and provided with appropriate food (fish food) to allow standardization of larval rearing conditions in order to reduce experimental variability and ensure the accuracy and reproducibility of research results. Larvae were kept in enamel pans and provided with appropriate food (fish food) to allow standardization of larval rearing conditions to reduce experimental variability and ensure the accuracy and reproducibility of research findings. Providing an appropriate amount of food commensurate with the age of the larvae is also an important aspect of rearing mosquito larvae. This helps ensure the larvae get the nutrients needed for their development and growth. Emerging pupae (which follow the larval stage and precede the adult stage) were transferred to plastic containers containing potable water and housed in 30 cm3 cages. Plastic cups provide a suitable environment for pupae to complete development and emerge as adult mosquitoes, while cages prevent adult mosquitoes from escaping and allow eggs to be collected for further breeding. The Emerging mosquitoes were fed a 10% glucose solution, as the glucose solution serves as an energy source for them and helps ensure their survival.
2.2. Bioinsecticides tested
Spinosad (Saccharopolyspora spinosa, 24%) and bacilod (Bacillus thuringiensis israelensis, Bti) are naturally occurring bacterial insecticides commonly used for mosquito control. Dow Agro Science, UK provides Spinosad; another (bacilod WP 1200) is supplied by LOD, Ltd. The bacterial bioinsecticides, a wettable powder formulation kindly supplied by Dr. Jazem A. Mahyoub, Fac. of Science, King Abdulaziz Univ.
2.3. Experiments
Dosage–mortality tests (WHO, 2003) were carried out using fourth-instar larvae of the parental strain Ae. aegypti in preparation for the experiments to be conducted in this study. Larval selection was applied using the dipping method. The experiment involved exposing approximately 4,000 early fourth-instar mosquito larvae to a single dose of either spinosad or bacilod for a whole day, which was determined based on the toxicity lines, which caused death in 90% of the larvae (LC90). The experiment requires breeding mosquito larvae through multiple generations. Live larvae from each generation were washed thoroughly, placed in trays of tap water, fed their usual food, and raised to become the next generation's parents. The process of exposure of mosquito larvae to insecticides continued through multiple generations until the F15 generation. The experiment determines LC50 values for spinosad and basilode for selected mosquito strains over multiple successive generations. This was performed every five successive generations (F1, F5, F10, and F15) and compared with the LC50 of the parental strain to determine the magnitude of acquired resistance. The resistance ratio (RR) measures the resistance level developed in the tested mosquito strains. In this experiment, the RR for the two tested insecticides (spinosad and bacilod) was calculated for each generation by dividing the LC50 of the selected strain by the LC50 of the parental strain.
Adult mosquitoes of the selected strain F15 that survived larval selection using spinosad or bacilod were separated and placed in special, clean cages. After three days, the female mosquitoes that emerged from the pupal stage were fed a blood meal using the Hemotek Membrane Feeding System. This system is commonly used to simulate mosquitoes' feeding behavior. Then, each engorged female mosquito was left with a male mosquito in a small glass beaker half filled with water and covered with a cheesecloth. The pairs of mosquitoes were fed a food source of a pre-prepared sucrose solution with a concentration of 10%. The mosquitoes' blood meal digestion time and reproductive ability for the first gonotrophic cycle were recorded. The time to digest a blood meal is defined as the period between feeding and egg laying, which can vary depending on various factors, including the size of the blood meal, environmental conditions, and the physiological state of the mosquito. This time is important because it represents the period during which the mosquito develops and prepares to lay her eggs. On the other hand, reproductive ability is typically measured based on the number of eggs laid by the female mosquito and their hatchability. The hatchability of the eggs is also an important factor, as it determines the number of viable offspring that can be produced.
2.4. Statistical analysis
Logarithms of concentration (LC) – probability regression (P) lines were plotted for the tested insecticides, and statistical parameters were calculated using LDP software probit analyses according to (Litchfield and Wilcoxon, 1949, Bakr, 2005). The resistance ratio (RR) rating based on (Mazzarri and Georghiou 1995) was as follows: low (RR < 5); moderate (5 < RR < 10) and high (RR greater than 10). The T-test was used to analyze significant differences between the parental and selected mosquito strains regarding the time taken to digest a blood meal or produce eggs.
3. Results
Our study showed that the susceptibility levels of Ae. aegypti larvae could change dramatically following continuous larval selection pressure with the bacterial insecticides spinosad and Bacilod over multiple generations, as listed in Table 1, Table 2 and illustrated in Fig. 1, Fig. 2. The selection of Ae. aegypti larvae with the LC90 of spinosad resulted in a slight decrease in susceptibility levels for the F1 generation, with a resistance ratio (RR) of 1.4-fold (Table 1).
Table 1.
Susceptibility level of Ae. aegypti larvae following selection pressure with LC90 of the bacterial insecticide spinosad for 15 successive generations.
| Generation | Effective concentration (ppm) | Larval mortality a (%) | LC50 (ppm) | 95%Confidence limits of LC50 | LC90(ppm) | slope | RR(fold |
|---|---|---|---|---|---|---|---|
| P* | 0.07–0.16 | 14–92 | 0.11 | 0.102–0.116 | 0.19 | 2.2 | |
| F1 | 0.09–0.21 | 16–90 | 0.16 | 0.149–0.166 | 0.25 | 2.4 | 1.45 |
| F5 | 0.12–0.29 | 14–89 | 0.18 | 0.172–0.191 | 0.28 | 2.8 | 1.63 |
| F10 | 0.17–0.38 | 15–91 | 0.27 | 0.257–0.280 | 0.40 | 3.2 | 2.46 |
| F15 | 0.21–0.45 | 17–88 | 0.34 | 0.323–0.351 | 0.49 | 3.4 | 3.10 |
*parental strain.
a: Five replicates, 20 larvae each.
RR: Resistance ratio (Mazzarri and Georghiou,1995).
Table 2.
Susceptibility level of Ae. aegypti larvae following selection pressure with LC90 of the bacterial insecticide bacilod for successive generations Table 2.
| Generation | Effective concentration (ppm) | Larval mortality a (%) | LC50 (ppm) | 95%Confidence limits of LC50 | LC90 (ppm) | slope | RRb(fold) |
|---|---|---|---|---|---|---|---|
| P* | 0.04–0.24 | 15–94 | 0.13 | 0.119–0.135 | 0.22 | 1.8 | |
| F1 | 0.11–0.27 | 13–90 | 0.16 | 0.151–0.170 | 0.27 | 1.9 | 1.2 |
| F5 | 0.14–0.31 | 16–92 | 0.19 | 0.182–0.204 | 0.32 | 2.2 | 1.5 |
| F10 | 0.16–0. 36 | 13–90 | 0.25 | 0.243–0.265 | 0.37 | 2.5 | 1.9 |
| F15 | 0.21–0.44 | 14–93 | 0.32 | 0.311–0.330 | 0.43 | 2.9 | 2.5 |
*parental strain.
a: Five replicates, 20 larvae each.
RR: Resistance ratio (Mazzarri and Georghiou,1995).
Fig. 1.
Concentration – mortality relationships of Ae. aegypti for the parental strain (P) and F1, F5, F10 & F15 generations after larval selection with spinosad and bacilod for 15 successive generations.
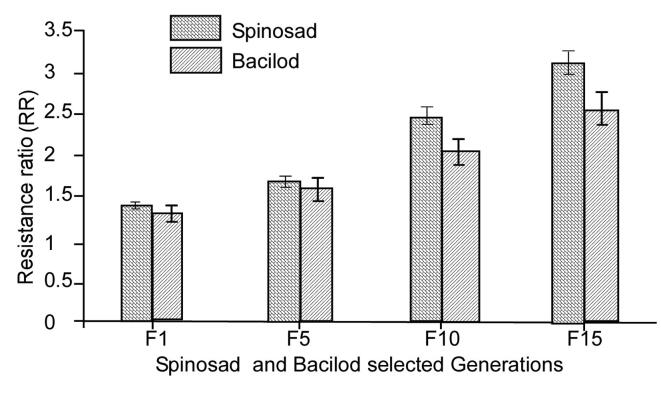
Fig. 2.
Gradual increase of spinosad and bacilod resistances in Ae. aegypti after larval selection with LC90 values of spinosad for 15 successive generations.
This was highly evident according to the LC50 values obtained for the first selected (F1) generation (0.16 ppm) and the parental strain (0.11 ppm). Starting from the F5 generation, the selected spinosad strain exhibited a lower tolerance, approximately 1.63-fold lower than the previous generations. With the continuous selection, the LC50 values for the selected strain of spinosad gradually increased in successive generations, reaching 0.26 ppm in the F10 generation and 0.34 ppm in the F15 generation compared to 0.11 ppm for the parental strain. The results also revealed that the selected strain of Ae. aegypti gained a significant spinosad tolerance level with an RR of 3.1-fold due to fifteen generations of larval selection pressure. On the other hand, according to the LC50 values given in Table 2, larval selection with bacilod caused a slight reduction in the susceptibility of the F1 generation (0.16 ppm) by approximately 1.2-fold compared with the parental strain (0.13 ppm). The estimated LC50 values for the F5 and F10 generations were recorded with respect to 0.19 ppm and 0.25 ppm with RRs of 1.5-fold and 1.9-fold, respectively. By the F15 generation, the susceptibility status of the selected strains was fairly decreased to bacilod, and an LC50 of 0.32 ppm was recorded compared to the parental strains. The records show that after 15 generations of larval selection, the selected strain of Ae. aegypti acquired a moderate tolerance to B. thuringiensis, approximately 2.4-fold higher than its original tolerance level.
Table 3 shows the potential effect of larval selection with the present bacterial insecticides for fifteen successive generations on the time of blood digestion and the reproductive potential of surviving Ae. aegypti females. The records showed that the average time taken by females to digest the ingested blood meal, which emerged from larval selection with spinosad and bacilod, ranged between 4.6 days and 4.8 days compared to 3.5 days in unselected females. These results indicated that larval selection with spinosad and bacilod for 15 generations caused a prolongation of the blood digestion time in the selected female Ae. aegypti mosquitoes by approximately 31.4% and 37.1%, respectively, compared to the unselected mosquitoes. Furthermore, Table 3 reveals that the selected spinosad and Bacillus thuringiensis strains exhibited an average of 36.1 and 41.7 eggs per female, respectively, during their first gonotrophic cycle, compared to 62.2 eggs in the parental strain. The results indicate that larval selection with spinosad and Bacillus thuringiensis led to a significant reduction (of approximately 42% and 33%, respectively) in the egg-laying capacity of the surviving female mosquitoes. The statistical analysis revealed significant differences in the average number of eggs between the parental and the selected strains. The hatchability percentage was 86.3% and 90.1% in the selected strains with spinosad and bacilod, respectively, compared to 93.2% in the parental strain. Thus, these records indicate a slight decrease in hatching levels in mosquitoes selected with spinosad by 7.4% and bacilod by 3.3%.
Table 3.
The delayed effect of larval selection with two bacterial insecticides spinosad and bacilod for 15 successive generations on the time of blood meal digestion and reproductive potential of Ae. aegypti female survivors.
| Strain | Time of blood meal digestion (in days) |
Egg production |
Hatchability of eggs |
|||
|---|---|---|---|---|---|---|
| Range | Mean*±S.E | Total | Mean*±S.E | Total of larvae hatched | % | |
| Parental St. | 3.7–4.1 | 3.5 ± 0.31a | 1243 | 62.2 ± 7.1a | 1158 | 93.2 |
| Spinosad-Selected St. | 3.9–5.6 | 4.6 ± 0.38b | 721 | 36.1 ± 6.6b | 622 | 86.3 |
| Bacilod-selected St. | 4.6–6.1 | 4.8 ± 0.42b | 833 | 41.7 ± 7.4b | 751 | 90.1 |
* Mean of 20 engorged females;means followed different superscript are significantly different.
4. Discussion
As mentioned above, our results indicate that the mosquito Ae. aegypti acquired a low level of resistance to the bacterial insecticides spinosad (S. spiosa) and bacilod (Bti) due to larval selection for 15 successive generations. Several authors have conducted similar trials to investigate the development of resistance to bioinsecticides in laboratory mosquito populations. Unfortunately, studies on developing resistance to spinosad in mosquito vectors are rare. The first attempt was made in C. quinquefasciatus by Su and Cheng, who found that the susceptibility of third- and fourth-instar larvae to spinosad after selection pressure at levels of LC70-LC90 gradually declined from G1 to G35 (Su and Cheng, 2014). However, the spiosad-resistant C. quinquefasciatus was not cross-resistant to Bti or B. sphaericus (Su and Cheng, 2014). On the other hand, our results and other studies indicated that the selection of mosquito larvae with the bacterial agent Bti has not led to high resistance levels. Early studies with Bti suggested that resistance to this toxin develops slowly and at lower levels. This was attributed to Bti's ability to produce complex parasporal crystals containing diverse toxins that may act on different receptors, thereby making the development of resistance a challenging process (Wirth et al., 1998). Georghio et al. documented an 11-fold reduction in susceptibility for some mosquito species, specifically C. quinquefasciatus, after 32 generations of selection with the LC90 of Bti (Georghiou et al. 1983). Goldman et al. reported a twofold increase in resistance to Bti at the LC50 level in Ae. aegypti mosquitoes after 14 generations of selection pressure (Goldman et al., 1986). Saleh reported that approximately 1.8-fold and 1.7-fold increases in resistance to Bti and B. sphaericus were induced in Ae. aegypti due to eight generations of selection (Saleh, 1987). In another study, C. pipiens larvae were selected using Bti for 20 generations at the LC90. The study observed a maximum resistance of 2.8-fold, but the selected strain lost approximately 58% of its resistance after cessation of selection for three generations (Saleh et al., 2003). Mosquito larvae of C. pipiens showed 2–3-fold resistance after 20 generations of laboratory selection with Bti (Mittal et al., 2005). However, the toxicity lines of the present two bioinsecticides showed that the slope values of the selected strain increased progressively from each generation to the next one compared to the parental strain. The increase in the slope indicates moderate homogeneity among individuals of the selected strain in their response to the test bioinsecticide (Xu et al., 2005). Our results indicate that larval selection with the LC90 of spinosad and bacilod caused an apparent prolongation of the time required to digest the blood meal by the selected female Ae. aegypti. These results are consistent with what has already been observed by Mebrahtu et al. on delayed oviposition of resistant Ae. aegypti compared to susceptible females (Mebrahtu et al., 1997). It has been proposed that the application of insecticides in larval selection may result in midgut damage that persists into adulthood, thereby affecting the physiological status of female mosquitoes and causing difficulties in the digestion of blood meals (Fernandes et al., 2019). However, identifying the gonotrophic cycle is important in estimating the chances of acquiring and transmitting pathogens. Sy et al., WHO reported that the longer gonotrophic cycle observed in resistant mosquito strains might indicate a decrease in the biting frequencies and, thus, the possibility of reducing disease transmission (Sy et al., 2019, WHO, 1975). Moreover, our results indicate a clear decrease in egg production with a slight decrease in hatching levels of eggs in female mosquitoes selected with spinosad and bacilod. Another study indicated that the decline in reproductive capacity might be due to continued larval selection with present bioinsecticides affecting larval gonads and thus, adult fertility, in addition to the fact that some of the engorged females of selected strains failed to oviposit (Saleh and Wright, 1990). Similar findings were reported by Thomas, who found that selection of C. gelidus at LC50 values of DDT did indeed result in small adults with fewer eggs (Thomas, 1962). The increase in the resistance of the C. pipiens mosquito to DDT and malathion after selection at LC90 levels for five generations led to a decrease in egg production (Gaaboub and Dawood, 1974). Several authors (Belinato et al., 2009, Fernandes et al., 2019, Saleh et al., 2013) have shown that using slow-release spinosad tablets against Ae. aegypti larvae for several weeks negatively affects the reproductive capacity of adults.
5. Conclusion
Our study suggests that bacterial insecticides such as spinosad and bacilod can be used in field control for long before high resistance levels appear in wild mosquito populations. In addition to their larvicidal effectiveness, adult survivors may be expected to have a reduced vectorial capacity. However, it is necessary to conduct long-term follow-up experiments to investigate the potential delayed effects of larval selection pressure with bioinsecticides on various biological and behavioral aspects of adult mosquito survivors.
CRediT authorship contribution statement
Jazem A. Mahyoub: Conceptualization, Methodology, Writing – original draft. Abdullah G. Algamdi: Sample collection, manuscript review. Mohammad M. Aljameeli: Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are deeply grateful to Dr. Esam Al-Wesabi, Director of the Public Health Pest Operation and Development Project, for his assistance in providing the eggs of the susceptible mosquito strain used in this study.
Funding
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 1322-130-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103776.
Contributor Information
Jazem A. Mahyoub, Email: jabdulrahman@kau.edu.sa.
Abdullah G. Algamdi, Email: Agalghamdi1@kau.edu.sa.
Mohammad M. Aljameeli, Email: MOHAMMAD.ALJAMEELI@NBU.EDU.SA.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Algamdi A.G., Mahyoub J.A. Detection of insecticide detoxification enzymes activities in Aedes aegyptimosquito, the vector of dengue fever in Saudi Arabia. Main Gr. Chem. 2022:1–11. [Google Scholar]
- Aljameeli M. Larvicidal effects of some essential oils against Aedes aegypti (L.), the vector of dengue fever in Saudi Arabia. Saudi. J. Biol. Sci. 2023;30 doi: 10.1016/j.sjbs.2022.103552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almadiy A.A., Saleh M.S., Alsagaf A.A. Larvicidal activity of some bacterial insecticides and insect growth regulators against mosquito larvae of Aedes aegypti (L.) Alexandria Sci. Exch. J. 2014;35:256–262. [Google Scholar]
- Alyahya H.S. Comparative study of three herbal formulations against dengue vectorsAedes aegypti. Saudi J. Biol. Sci. 2023 doi: 10.1016/j.sjbs.2023.103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakr, E., 2005. LDP Line Software, to calculate probit analyses according to Finney (1971), Which is used to illustrate the relation between stimulus and response in toxicological and biological studies. Illus. dose-response Regres. line.
- Batra C.P., Mittal P.K., Adak T., Ansari M.A. Efficacy of IGR compound Starycide 480 SC (Triflumuron) against mosquito larvae in clear and polluted water. J. Vector Borne Diseases. 2005;42:109. [PubMed] [Google Scholar]
- Belinato T.A., Martins A.J., Lima J.B.P., de Lima-Camara T.N., Peixoto A.A., Valle D. Effect of the chitin synthesis inhibitor triflumuron on the development, viability and reproduction of Aedes aegypti. Memórias do Instituto Oswaldo Cruz. 2009;104:43–47. doi: 10.1590/s0074-02762009000100007. [DOI] [PubMed] [Google Scholar]
- Brown A.W. Insecticide resistance in mosquitoes: a pragmatic review. J. Am. Mosquito Control Assoc. 1986;2:123–140. [PubMed] [Google Scholar]
- Fakeeh M., Zaki A.M. Dengue in Jeddah, Saudi Arabia, 1994–2002. Dengue Bull. 2003;27:13–18. [Google Scholar]
- Fernandes K.M., Tomé H.V.V., Miranda F.R., Gonçalves W.G., Pascini T.V., Serrão J.E., Martins G.F. Aedes aegypti larvae treated with spinosad produce adults with damaged midgut and reduced fecundity. Chemosphere. 2019;221:464–470. doi: 10.1016/j.chemosphere.2019.01.068. [DOI] [PubMed] [Google Scholar]
- Gaaboub I.A., Dawood M.R. Effects of sublethal concentrations of DDT and malathion on the fecundity and reproduction of Culex pipiens L. Zeitschrift für Angew. Entomol. 1974;75:435–443. [Google Scholar]
- Goldman I.F., Arnold J., Carlton B.C. Selection for resistance to Bacillus thuringiensis subspecies israelensis in field and laboratory populations of the mosquito Aedes aegypti. J. Invertebr. Pathol. 1986;47:317–324. doi: 10.1016/0022-2011(86)90102-3. [DOI] [PubMed] [Google Scholar]
- Hertlein M.B., Mavrotas C., Jousseaume C., Lysandrou M., Thompson G.D., Jany W., Ritchie S.A. A review of spinosad as a natural product for larval mosquito control. J. Am. Mosquito Control Assoc. 2010;26:67–87. doi: 10.2987/09-5936.1. [DOI] [PubMed] [Google Scholar]
- Karunaratne S., De Silva W., Weeraratne T.C., Surendran S.N. Insecticide resistance in mosquitoes: development, mechanisms and monitoring. Ceylon J. Sci. 2018;47:299–309. [Google Scholar]
- Litchfield, J.T. jnr, Wilcoxon, F., 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96, 99–113. [PubMed]
- Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Ann. Rev. Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- Mashlawi A.M., Al-Nazawi A.M., Noureldin E.M., Alqahtani H., Mahyoub J.A., Saingamsook J., Debboun M., Kaddumukasa M., Al-Mekhlafi H.M., Walton C. Molecular analysis of knockdown resistance (kdr) mutations in the voltage-gated sodium channel gene of Aedes aegypti populations from Saudi Arabia. Parasit. Vectors. 2022;15:1–13. doi: 10.1186/s13071-022-05525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebrahtu Y.B., Norem J., Taylor M. Inheritance of larval resistance to permethrin in Aedes aegypti and association with sex ratio distortion and life history variation. Am. J. Trop. Med. Hygiene. 1997;56:456–465. doi: 10.4269/ajtmh.1997.56.456. [DOI] [PubMed] [Google Scholar]
- Mittal P.K., Adak T., Subbarao S.K. Laboratory selection to investigate the development of resistance to Bacillus thuringiensis var. israelensis H-14 in Culex quinquefasciatus Say (Diptera: Culicidae) National Acad. Sci. Lett. 2005;28:281–283. [Google Scholar]
- Naqqash M.N., Gökçe A., Bakhsh A., Salim M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016;115:1363–1373. doi: 10.1007/s00436-015-4898-9. [DOI] [PubMed] [Google Scholar]
- Rodcharoen J., Mulla M.S. Resistance development in Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus. J. Econ. Entomol. 1994;87:1133–1140. [Google Scholar]
- Saleh M.S. Effect of larval selection with two bioinsecticides on susceptibility levels and reproductive capacity of Aedes aegypti(L.) Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz. 1987;60:55–57. [Google Scholar]
- Saleh M.S., El-Meniawi F.A., Kelada N.L., Zahran H.M. Resistance development in mosquito larvae Culex pipiens to the bacterial agent Bacillus thuringiensis var. israelensis. J. Appl. Entomol. 2003;127:29–32. [Google Scholar]
- Saleh M.S., Abuzinadah O.A., Al-Ghamdi K.M., Alsagaf A.A., Mahyoub J.A. Effectiveness of slow-release tablet formulations of the IGR diflubenzuron and the bioinsecticide spinosad against Larvae of Aedes aegypti (L.) African Entomol. 2013;21:349–353. [Google Scholar]
- Saleh M.S., Wright R.E. Evaluation of the IGR cyromazine as a feed-through treatment against Culex pipiens and Aedes epacticus (Diptera, Culicidae) J. Appl. Entomol. 1990;109:247–250. [Google Scholar]
- Su T., Cheng M.-L. Cross resistances in spinosad-resistant Culex quinquefasciatus (Diptera: Culicidae) J. Med. Entomol. 2014;51:428–435. doi: 10.1603/me13207. [DOI] [PubMed] [Google Scholar]
- Sy F.A., Faye O., Diallo M., Dia I. Effects of insecticide resistance on the reproductive potential of two sub-strains of the malaria vector Anopheles coluzzii. J. Vector Borne Diseases. 2019;56:207. doi: 10.4103/0972-9062.289401. [DOI] [PubMed] [Google Scholar]
- Tang F.H.M., Lenzen M., McBratney A., Maggi F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021;14:206–210. doi: 10.1038/s41561-021-00712-5. [DOI] [Google Scholar]
- Thomas V. DDT susceptibility and selection of the larvae of a laboratory-colony of Culex gelidus theobald. Indian J. Malariol. 1962;16:203. [PubMed] [Google Scholar]
- Virgillito C., Manica M., Marini G., Rosà R., Torre A.D., Martini S., Drago A., Baseggio A., Caputo B. Evaluation of Bacillus Thuringiensis Subsp. Israelensis and Bacillus Sphaericus combination against Culex Pipiens in highly vegetated ditches. J. Am. Mosquito Control Assoc. 2022;38:40–45. doi: 10.2987/21-7024. [DOI] [PubMed] [Google Scholar]
- WHO, 1975. WHO_OFFSET_13_(part2).pdf.
- WHO, 2003. Pencegahan dan Penanggulangan Penyakit Demam Dengue dan Demam Berdarah Dengue : Terjemahan dari WHO Regional Publication Searo No. 29, “Prevention and Control of Dengue and Dengue Haemorrhagic Fever” 1–93.
- Wirth M.C., Delécluse A., Federici B.A., Walton W.E. Variable cross-resistance to Cry11B from Bacillus thuringiensis subsp. jegathesan in Culex quinquefasciatus (Diptera: Culicidae) resistant to single or multiple toxins of Bacillus thuringienisis subsp. israelensis. Appl. Environ. Microbiol. 1998;64:4174–4179. doi: 10.1128/aem.64.11.4174-4179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.-C., Sun H., Chen J.-F., Bian Q., Qian J., Song L., Wang X.-R. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Zhu F., Lavine L., O’Neal S., Lavine M., Foss C., Walsh D. Insecticide resistance and management strategies in urban ecosystems. Insects. 2016;7:1–26. doi: 10.3390/insects7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.