Abstract
An international multicenter study was performed to evaluate a new, automated human immunodeficiency virus (HIV) third-generation antibody assay. The Enzymun-Test Anti-HIV 1 + 2 + Subtyp O showed 100% sensitivity and 99.8% specificity among 11,172 samples from hospitalized patients and blood donors. For early HIV antibody detection in seroconversion panels, Enzymun-Test showed a sensitivity equivalent to that of the Abbott Recomb. HIV-1/HIV-2 3rd Gen. assay.
Worldwide, there are more than 100 tests from more than 40 commercial companies available for testing antibodies to the human immunodeficiency virus (HIV). Since the first screening enzyme immunoassays (EIA) and Western blots came onto the market in 1985, considerable effort has been made to improve the quality of screening and confirmatory assays (3, 4, 6–12). The continued need for optimizing tests is dictated by several aspects of HIV infection. One focus is the problem of the window phase during early infection (15). The second focus, which is becoming increasingly important, is the variability of the virus (i.e., the detection of emerging new subtypes) (5). In addition, a high reliability of the results with low risk of false connection between sample donor and test result is extremely important. For this purpose, automated analyzers have been introduced in blood banks and routine laboratories (7, 9–11).
In an international multicenter study, the new automated Enzymun-Test Anti-HIV 1 + 2 + Subtyp O was compared to several currently available second- and third-generation assays. The aim of the present study was to evaluate the accuracy of the new assay by testing a large collective of samples originating from different geographical regions and clinical settings (i.e., blood banks and clinical diagnostic laboratories).
A total of 45 laboratories from 15 countries participated in the multicenter study, which was performed from September to December 1995.
The Accurun Multi-Marker Run Control (Boston Biomedica, Inc. [BBI], West Bridgewater, Mass.), diluted 1:5 and 1:10 in HIV-negative serum, was given to all of the participants in the study for quality control and in order to evaluate the reproducibility of the assay. Each dilution of the control was tested in single measurements in three different assay runs. Only laboratories experienced with Enzymun-System EIA ES 300 and ES 700 processors participated in the present study. Prior to the beginning of the study, the technical performance of the ES 300 and ES 700 processors was controlled. In order to guarantee the integrity of the data, only results presented on the original ES 300 and ES 700 report forms were considered.
The Enzymun-Test Anti-HIV 1 + 2 + Subtyp O is a double-antigen sandwich enzyme-linked immunosorbent assay ELISA which uses the fully automated ES 300 or ES 700 processor with the universal streptavidin solid phase. In the first incubation step, sample antibodies react with biotinylated antigens and digoxigenin-labelled antigens (recombinant antigens and peptides of HIV-1, HIV-2, and HIV-1 subtype O). The resulting immune complexes bind to the streptavidin solid phase. After washing, the immune complex is detected by an antidigoxigenin antibody-peroxidase conjugate. Following a second washing step, the peroxidase is detected with the substrate di-ammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS). The assay can be performed at 25 or 37°C in a total assay time of 4 h. Samples giving absorbencies greater than or equal to the cut-off value (0.09 × signal of the positive control + signal of the negative control) should be regarded as HIV-1 or HIV-2 positive. Test results within the range of 90 to 100% of the cut-off should be regarded borderline. The ES 300 processor was used by 44 laboratories, and the ES 600 and ES 700 processors were used by 3 and 13 participants, respectively. All participants with the exception of four performed the assay at 25°C.
Alternative assays are listed in Table 1. Each EIA reactive sample was tested by Western blotting (LAV BLOT 1 and 2; Fujirebio, Tokyo, Japan; New LAV BLOT 1 and 2, Sanofi Pasteur Diagnostics, Marnes la Coquette, France; and NovaPath immunoblot assay; Nippon Bio-Rad Laboratories K.K., Tokyo, Japan). LAV Blot 1 and 2 and New LAV Blot 1 and 2 results were interpreted according to World Health Organization criteria (14), while American Red Cross criteria (2) were applied for the NovaPath Immunoblot assay. Samples presenting Western blot banding patterns corresponding to World Health Organization or ARC criteria were considered as true positives. Samples were regarded as true negatives in the absence of any Western blot reactivity or in the case of an indeterminate Western blot result if HIV infection had been excluded by follow-up investigations and/or by alternative methods (i.e., PCR and antigen detection).
TABLE 1.
Comparative HIV-1 and HIV-2 screening assays used in the multicenter study
| Assay | Manufacturer | Test principle | No. of samples tested | No. of centers using the assay |
|---|---|---|---|---|
| Abbott Recomb. HIV-1/HIV-2 3rd Gen. EIA | Abbott, North Chicago, Ill. | Antigen sandwich EIA | 895 | 9 |
| Abbott HIV-1/HIV-2 3rd Gen. Plus EIA | Abbott, North Chicago, Ill. | Antigen sandwich EIA | 1,169 | 4 |
| IMx HIV-1/HIV-2 3rd Gen. | Abbott, North Chicago, Ill. | Antigen sandwich MEIA | 1,393 | 9 |
| IMx HIV-1/HIV-2 3rd Gen. Plus | Abbott, North Chicago, Ill. | Antigen sandwich MEIA | 670 | 4 |
| Axsym HIV-1/HIV-2 | Abbott, North Chicago, Ill. | Antigen sandwich MEIA | 458 | 2 |
| Enzygnost Anti-HIV 1/2 Plus | Behringwerke, Marburg, Germany | Antigen sandwich EIA | 2,222 | 6 |
| Bioelisa HIV-1+2 | Biokit S.A., Barcelona, Spain | Antigen sandwich EIA | 100 | 1a |
| Biotest Anti-HIV-1/2 Recombinant | Biotest, Dreieich, Germany | Indirect EIA | 99 | 1a |
| Serodia-HIV-1/2 Type 2 | Fujirebio, Inc., Tokyo, Japan | Particle agglutination assay | 165 | 2a |
| Serodia-HIV | Fujirebio, Inc., Tokyo, Japan | Particle agglutination assay | 248 | 2 |
| Wellcozyme HIV Recombinant | Murex, Dartford, England | Competitive EIA | 1,205 | 8 |
| Vironostika HIV Uni-Form II | Organon Technika BV, Boxteel, Holland | Antigen sandwich EIA | 443 | 2 |
| Genelavia Mixt | Sanofi Pasteur, Marnes la Coquette, France | Antigen sandwich EIA | 882 | 7 |
| Anti HIV-1/HIV-2 EIA DAGS Cobas Core | Hoffmann La Roche, Basel, Switzerland | Antigen sandwich EIA | 152 | 1a |
| Anti HIV-1/HIV-2 EIA | Hoffmann La Roche, Basel, Switzerland | Antigen sandwich EIA | 105 | 1a |
| ETI-AB-HIV-1/2 K | Sorin Biomedica, Saluggia, Italy | Antigen sandwich EIA | 109 | 1a |
| Micro Trak II HIV-1/HIV-2 EIA | Syva, Palo Alto, Calif. | Indirect EIA | 297 | 2a |
Results are not shown in the present study, since the low sample number tested with this assay allows no statistically valid conclusion.
The specificity evaluation was performed with 5,332 HIV-negative samples from hospitalized patients and 3,950 negative samples from blood donors (Table 2).
TABLE 2.
Results and calculation of specificity and sensitivity of HIV antibody EIAs with samples from hospitalized patients and blood donors
| Assay | No. of samples with result
|
% Sensitivity | % Specificity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalized patientsa
|
Blood donors
|
|||||||||||
| Positive | Negative | False positive | False negative | Total | Positive | Negative | False positive | False negative | Total | |||
| Enzymun-Test Anti-HIV 1 + 2 + Subtyp O | 1,887 | 5,332 | 10 | 0 | 7,219 | 3 | 3,950 | 9 | 0 | 3,953 | 100 | 99.8 |
| Abbott Recomb. HIV-1/HIV-2 3rd Gen. EIA | 257 | 491 | 29 | 0 | 748 | 0 | 190 | 1 | 0 | 191 | 100 | 95.8 |
| Abbott HIV-1/HIV-2 3rd Gen. Plus EIA | 134 | 618 | 4 | 0 | 752 | 0 | 153 | 0 | 0 | 153 | 100 | 99.5 |
| IMx HIV-1/HIV-2 3rd Gen. | 275 | 910 | 3 | 0 | 1,185 | 0 | 0 | 0 | 0 | 0 | 100 | 99.7 |
| IMx HIV-1/HIV-2 3rd Gen. Plus | 113 | 457 | 0 | 0 | 570 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Axsym HIV-1/HIV-2 | 47 | 411 | 0 | 0 | 458 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Enzygnost Anti-HIV 1/2 Plus | 199 | 467 | 0 | 0 | 666 | 2 | 158 | 0 | 0 | 160 | 100 | 100 |
| Wellcozyme HIV Recombinant | 157 | 506 | 19 | 0 | 663 | 0 | 234 | 1 | 0 | 234 | 100 | 97.4 |
| Genelavia Mixt | 153 | 308 | 4 | 0 | 461 | 0 | 225 | 0 | 0 | 225 | 99.3 | 100 |
These samples include 268 potentially interfering samples obtained from patients with autoimmune diseases (n = 116), patients who are rheumatoid factor positive (n = 35), and patients with acute viral illness caused by cytomegalovirus (n = 24), Epstein-Barr virus (n = 7), hepatitis B (n = 26) and C (n = 40) viruses, and human T-cell leukemia virus type 1 (n = 20).
For the evaluation of the sensitivity of the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O in comparison to that of the alternative assays, 1,887 HIV-positive samples from hospitalized patients and 3 positive samples from blood donors were tested along with 39 longitudinal HIV-1 seroconversion panels provided by North American Biologicals (NABI panels, Table 3) and from BBI (BBI panels, Table 3). Seroconversion panels with time intervals between the different blood sampling times not exceeding 4 weeks were included for the study (Table 3). Furthermore, 65 confirmed HIV-2-positive samples, 109 late-phase HIV-1 samples (including 1 rapidly progressive infection), and 16 follow-ups of children from HIV-1-infected mothers (Table 2) were measured with the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O. A variable number of these samples was measured with the alternative assays (Table 1).
TABLE 3.
Detection of HIV antibody in seroconversion panels
| Seroconversion panel | No. of samples tested | Day of blood donation with first positive HIV antibody result (sample no.)
|
||
|---|---|---|---|---|
| Enzymun-Test Anti-HIV 1 + 2 + Subtyp O | Abbott bead EIA 3rd Gen. | Genelavia Mixt | ||
| BBI D | 5 | 92 (4) | 92 (4) | |
| BBI E | 10 | 126 (10) | 126 (10) | |
| BBI H | 6 | 28 (6) | 28 (6) | |
| BBI I | 8 | 7 (2) | 7 (2) | |
| BBI J | 7 | 26 (3) | 26 (3) | |
| BBI K | 8 | 13 (4)a | 15 (5)a | |
| BBI L | 6 | 0 (1) | 0 (1) | |
| BBI M | 2 | 22 (2) | 22 (2) | |
| BBI N | 5 | 0 (1) | 0 (1) | |
| BBI P | 6 | 30 (5) | 30 (5) | |
| BBI Q | 7 | 65 (4) | 65 (4) | |
| BBI R | 6 | 7 (3) | 7 (3) | |
| BBI S | 3 | 9 (2) | 9 (2) | |
| BBI U | 6 | 0 (1)a | 3 (2)a | |
| BBI V | 4 | 4 (2) | 4 (2) | |
| BBI W | 13 | 84 (9) | 84 (9) | |
| BBI X | 8 | 33 (6) | 33 (6) | |
| BBI Y | 6 | 44 (5) | 44 (5) | |
| BBI Z | 6 | 27 (5) | 27 (5) | |
| BBI AB | 5 | 33 (3) | 33 (3) | |
| BBI AC | 5 | 111 (2) | 111 (2) | |
| NABI SV-0021 | 4 | 10 (3)a | 3 (2)a | 10 (3)a |
| NABI SV-0031 | 14 | 46 (8) | 46 (8) | 46 (8) |
| NABI SV-0051 | 6 | 9 (3)a | 7 (2)a | 14 (4)a |
| NABI SV-0061 | 9 | 7 (3) | 7 (3) | 7 (3) |
| NABI SV-0071 | 5 | 17 (3)a | 17 (3)a | 22 (4)a |
| NABI SV-0081 | 6 | 48 (4)a | 23 (3)a | 48 (4)a |
| NABI SV-0091 | 9 | 6 (3) | 6 (3) | 6 (3) |
| NABI SV-0111 | 7 | 7 (3) | 7 (3) | 7 (3) |
| NABI SV-0161 | 6 | 12 (4) | 12 (4) | 12 (4) |
| NABI 11444 | 6 | 7 (3)a | 3 (2)a | 3 (2)a |
| 38A | 3 | 3 (2) | 10 (3) | |
| 38B | 3 | 4 (2) | 4 (2) | |
| 38C | 2 | 4 (2) | 4 (2) | |
| 38D | 2 | 1 (0) | 1 (0) | |
| Dy 135/791 | 2 | 11 (2) | 0 (1) | |
| 21945 | 2 | 0 (1) | 21 (2) | |
| 4731 | 2 | 27 (2) | 27 (2) | |
| 4742 | 5 | 0 (1) | 20 (3) | 20 (3) |
Difference in test performance from other assays.
All samples were stored at −20°C until tested.
The interassay reproducibility of Enzymun-Test Anti-HIV 1 + 2 + Subtyp O was evaluated by the testing in five different runs of the positive and negative controls and positive serum samples with different index values (ratio of the sample signal to cutoff value). The interassay coefficients of variation (CVs) ranged from 1.7% (positive control) to 6.8% (negative control).
The intralaboratory variability of each participant was determined by testing in three independent runs two dilutions (1:5 and 1:10) of the Accurun Control. For the 1:5 and 1:10 dilutions, the CVs varied between 0.6 and 22% and 0.5 and 27%, respectively.
The interlaboratory variability was determined by calculating the CV of the mean value obtained for each of the two dilutions of the Accurun Control in each of three independent test runs in 45 laboratories. The interlaboratory CVs for the Accurun 1:5 and 1:10 dilutions were 11.5 and 13.0%, respectively.
Among 5,332 HIV-negative samples from hospitalized patients and 3,950 HIV-negative samples from blood donors, 10 and 9 false-positive results, respectively, were observed with the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O, giving a specificity of 99.8% in both negative sample collectives. With the alternative assays used with different parts of the total number of negative samples in the study, specificities of nominally 99.3 to 100% were observed, but in most cases, the total number of samples measured with one method was too low to get a statistically reliable result for specificity (Table 2).
Among 1,887 HIV-1-positive samples from hospitalized patients and 3 HIV-1-positive samples from blood donors, no false-negative result was observed with the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O or the alternative assays used for different parts of this sample collection (Table 2).
Results from HIV-1 antibody detection in seroconversion panels are shown in Table 3. All 65 of the HIV-2-positive serum samples gave concordant positive results with Enzymun-Test Anti-HIV 1 + 2 + Subtyp O and the different comparative assays.
Two of 109 late-phase HIV-1 infection samples were concordant negative in Behring Enzygnost and Enzymun-Test Anti-HIV 1 + 2 + Subtyp O. These samples were taken few days before the death of the patients and showed strongly reduced banding on an HIV-1 Western blot (i.e., an indeterminate result).
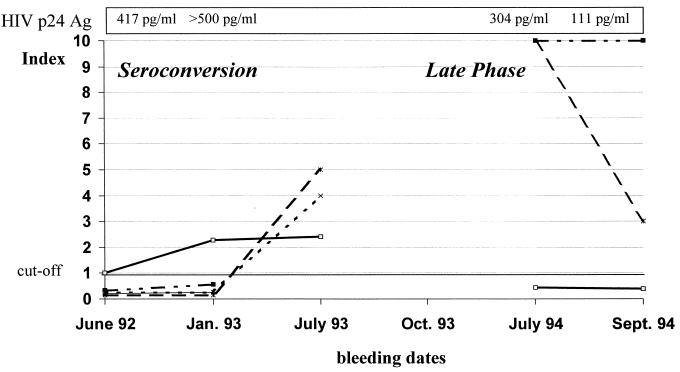
Two last blood samples from a patient who seroconverted in June 1992 (Table 3 and Fig. 1 [seroconversion panel 4742]) and who showed a rapid progressive HIV-1 infection with full-blown AIDS already 1 year after primary infection and finally died in October 1994 (very rapid progressor) displayed discordant results between the methods used. The samples of the seroconversion phase were found to be positive two blood samples earlier by the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O than by the alternative assays, whereas the two blood samples taken during the final stage of disease were found negative by the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O, but were still positive with Abbott 3rd Gen. and Vidas HIV 1/2 New. These late-phase blood samples showed strongly reduced Western blot reactivity (p31 positive, gp120 weakly positive, and gp160 positive) and were HIV antigen positive (Fig. 1).
FIG. 1.
Follow-up investigations in a patient with a very rapid progressive HIV-1 infection. Bleeding dates refer to the times blood samples were taken. □, Enzymun-Test Anti-HIV 1 + 2 + Subtyp O; ■, Abbott Recomb. HIV-1/HIV-2 3rd Gen. EIA; ▴, Enzygnost Anti HIV 1/2 Plus; ×, Wellcozyme HIV Recombination; ∗, Vidas HIV 1/2 New.
Samples from consequential blood samplings of 16 children born to HIV-1-infected mothers over ranges of 1 to 5 years were measured with the Boehringer Mannheim assay in comparison to Pasteur Genelavia Mixt, Abbott 3rd Gen., and Behring Enzygnost Anti-HIV 1/2 Plus. All results were in agreement. Children not infected by their mothers could be found to revert to seronegativity at the same blood sampling date in all methods used.
The results of the international multicenter study demonstrate that the fully automated antigen sandwich EIA Enzymun-Test Anti-HIV 1 + 2 + Subtyp O is suited very well for routine serologic diagnosis of HIV infection and screening of blood donors. It shows very good interassay and interlaboratory precision and a high specificity of 99.8% (with a 95% confidence interval of 99.7 to 99.9%) with both blood donor samples and samples from hospitalized patients. This is important, since a previous study (13) showed that it remains difficult to combine high specificity and very sensitive detection of early seroconversion in anti-HIV-1 and anti-HIV-2 screening assays. In this study, several of the methods showed lower specificity in collections of samples from hospitalized patients than in those from blood donors. The specificity of second- and third-generation screening EIAs may even fall under 90% when potentially cross-reactive serum samples are tested (4, 10, 11). Immunological disturbances in organ transplant recipients and during pregnancy, anti-HLA positivity, and rheumatoid factor are a frequent cause of false-positive reactions in HIV antibody screening assays (4, 6).
In a total of over 35 seroconversion panels, there was no significant difference in early detection of seroconversions between the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O and the Abbott 3rd Gen. EIA. Third-generation sandwich EIAs detect seroconversion an average of 5 days earlier than second-generation assays (15). As demonstrated recently, the sensitivity of third-generation assays can further be improved by using different antigen presentation (i.e., microparticles) (9).
There is a potential risk that samples from the very late phase of infection may not be detected by current screening EIAs. With serum samples drawn a few days before death and displaying an incomplete Western blot banding, negative results were observed with different screening EIAs. Two blood samples from a patient with rapid progressive infection were highly HIV antigen positive, which indicates the presence of immune complexes. Immune complexes are predominant in the late stage of disease and lead to reduced Western blot banding and ELISA reactivity (1).
The same patient was detected as anti-HIV antibody positive during seroconversion several months earlier by the Enzymun-Test Anti-HIV 1 + 2 + Subtyp O than by Abbott 3rd Gen. and Vidas HIV 1/2 New tests, which is more important in practice than the detection of final-stage anti-HIV seropositivity, because patients with AIDS will normally be known to be infected by HIV at that stage and also are not likely to be accepted to donate blood.
Third-generation assays have achieved a high degree of reliability, and major improvements of the antigen sandwich EIA technique are not to be expected in the future. The next test generation which is based on the combined detection of antigen and antibody in one assay run will substantially reduce the diagnostic window period in comparison to that of third-generation EIAs.
Acknowledgments
I acknowledge the participants of the 1995 International Enzymun-Test Anti-HIV 1 + 2 + Subtyp O Study Group: Australia: E. Dax and F. Rae (Fairfield); Austria: U. Michl, A. Mühlbacher, W. Patsch and I. Schmid (Salzburg) and L. Pokierser and G. Dorfinger-Gyurjacs (Vienna); Denmark: P. Staun-Olsen and T. E. Jessen (Holbaek) and C. Bohn-Christiansen (Copenhagen); France: M. Baccard (Grenoble), J.-C. Tardy (Lyon), J. Cottalorda (Nice), and S. Rogez (Limoges); Germany: W. Ehret and I. W. Renk (Augsburg); R. Fitzner, K. Hensel-Wiegel, E. Köttgen, and C. Müller (Berlin); N. Holland-Moritz (Dessau); O. Adams and P. Nemes (Düsseldorf); A. Eberhard and F. Kissing (Dortmund); B. Weber (Frankfurt); T. Talaska (Frankfurt/Oder); O. Bätz (Geesthacht); G. Töpfer and F. Hornig (Görlitz); P. Haase (Halle); T. Fenner and E. Otzipka (Hamburg); M. Aulmann and M. Holfelder (Heidelberg); K. Döerner and S. Schulze (Kiel); J. Hofmann (Leipzig); I. Page (Magdeburg); W. Bieger and W. F. Tiller (Munich); K. P. Kohse (Oldenburg); H. Emmerich (Remscheid); and B. Flehmig (Tübingen); Japan: J. Matsuda and K. Fukutake (Tokyo); India: A. Kalathiveetil (Co Chin) and A. Lal (New Delhi); Malaysia: G. Duraisamy, Z. Hassan, M. Sinniah, and B. Vijayamalar (Kuala Lumpur); The Netherlands: G. J. J. van Doornum and M. Buimer (Amsterdam) and A. M. van Loon and A. G. M. Komen (Utrecht); Portugal: J. Bicó, M. J. Silvestre, F. Ladeira, V. Loureiro, I. Teixeira, J. Pereira, I. Neves, M. J. Alpoim, F. Fernandes, C. Pereira, A. Paiva, M. Lurdes Correira, and M. Suzete Oubinà (Lisbon); Spain: V. Ausina, L. Matas, and M. Gimenez (Barcelona); A. Agulla (El Ferrol); A. Gimeno (Huelva); R. Pérez Vicente and L. Moris (Leon); J. Ramón Dominguez and I. Bermejo (Madrid); J. Llaneza and A. González (Oviedo); J. Dorronsoro and C. Fernández (Pamplona); R. Villaescusa and A. Eiras (Santiago); M. Sabaté (Tarragona); and R. Benito (Zaragoza); Sweden: G. Hellberg (Stockholm); Turkey: E. Palaoglu (Istanbul); South Africa: K. King (Johannesburg) and R. Eraman (Durban); and Republic of Ireland: S. Dooley (Dublin).
REFERENCES
- 1.Ascher D P, Roberts C, Fowler A. Acidification modified p24 antigen capture assay in HIV seropositives. J Acquired Immune Defic Syndr. 1992;5:1080–1083. [PubMed] [Google Scholar]
- 2.Centers for Disease Control. 1989. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. Morbid. Mortal. Weekly Rep. 38(Suppl. S-7):1–7.
- 3.Constantine N T. Serologic tests for the retroviruses: approaching a decade of evolution. AIDS. 1993;7:1–13. doi: 10.1097/00002030-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Enzensberger R, Hühn S, Kauk U, Doerr H W. Sensitivity and specificity of HIV antibody tests: evaluation of a proficiency test performed by German laboratories. AIDS-Forsch. 1988;11:622–628. [Google Scholar]
- 5.Gürtler L G, Hauser P H, Eberle J, von Brunn A, Knapp S, Zekeng L, Tsague J M, Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994;68:1581–1585. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess G, Rossol S, Weber K C, Meyer zum Büschenfelde K H. Diagnose der HIV-1-Infektion: Vergleich von fünf kommerziellen Enzymimmunoassays zum Nachweis von anti-HIV-1. Lab Med. 1988;11:361–364. [Google Scholar]
- 7.Matter L, Germann D. Detection of human immunodeficiency virus (HIV) type 1 antibodies by a new automated microparticle enzyme immunoassay for HIV types 1 and 2. J Clin Microbiol. 1995;33:2338–2341. doi: 10.1128/jcm.33.9.2338-2341.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAlpine L, Gandhi J, Parry J V, Mortimer P P. Thirteen current anti-HIV-1/HIV-2 enzyme immunoassays: how accurate are they? J Med Virol. 1994;42:115–118. doi: 10.1002/jmv.1890420203. [DOI] [PubMed] [Google Scholar]
- 9.Weber B, Behrens N, Doerr H W. Detection of human immunodeficiency virus type 1 and type 2 antibodies by a new automated microparticle immunoassay. J Virol Methods. 1997;63:137–143. doi: 10.1016/s0166-0934(96)02122-2. [DOI] [PubMed] [Google Scholar]
- 10.Weber B, Doerr H W. Evaluation of the automated VIDAS system for the detection of anti-HIV-1 and anti-HIV-2 antibodies. J Virol Methods. 1993;42:63–74. doi: 10.1016/0166-0934(93)90177-s. [DOI] [PubMed] [Google Scholar]
- 11.Weber B, Hess G, Koberstein R, Doerr H W. Evaluation of the automated “Enzymun-Test Anti HIV 1 + 2” and “Enzymun-Test Anti-HIV1/2 selective” for the combined detection and differentiation of anti-HIV-1 and anti-HIV-2 antibodies. J Virol Methods. 1993;44:251–260. doi: 10.1016/0166-0934(93)90060-5. [DOI] [PubMed] [Google Scholar]
- 12.Weber B, Hess G, Enzensberger R, Harms F, Evans C J, Hamann A, Doerr H W. Multicenter evaluation of the novel ABN Western blot (immunoblot) system in comparison with an enzyme-linked immunosorbent assay and a different Western blot. J Clin Microbiol. 1992;30:691–697. doi: 10.1128/jcm.30.3.691-697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber B, Moshtaghi-Boronjeni M, Brunner M, Preiser W, Breiner M, Doerr H W. Evaluation of the reliability of 6 current anti-HIV-1/HIV-2 enzyme immunoassays. J Virol Methods. 1995;55:97–104. doi: 10.1016/0166-0934(95)00048-y. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Acquired immunodeficiency syndrome (AIDS). Proposed WHO criteria for interpreting results from Western blot assays for HIV-1, HIV-2 and HTLV-I/II. Weekly Epidemiol Rec. 1990;65:281–283. [PubMed] [Google Scholar]
- 15.Zaaijer H L, Exel-Oehlers P, Kraaijeveld T, Altena E, Lelie P N. Early detection of antibodies to HIV-1 by third generation assays. Lancet. 1992;340:770–772. doi: 10.1016/0140-6736(92)92303-w. [DOI] [PubMed] [Google Scholar]