Abstract
Introduction
The benchmarking for medication incidents rate is not regarded as a recognized key performance measure or indicator in national or international organizations. The absence of benchmarking the medication incidents results in the loss of a self-governing perception of how well we performed compared to other peers.
Methodology and settings
This 600-bed tertiary, Joint Commission International (JCI) accredited hospital retrospective analysis looked at all medication management-related events from January-2018 to December-2021. The study design follows descriptive, cross-sectional, retrospective prevalence research.
Results
The rate of medication incidents that resulted in harm declined from Q3-2019 to Q4-2021. A significant increase in Pharmacy interventions/clarifications was recorded. Additionally, a significant increase in incidents reported with no-harm coupled with a significant reduction in incidences of serious events from 2019 to 2020. Finally, no-harm events were significantly reduced from 2020 to 2021.
Discussion
The Pharmacy's study analyzed every medication incident documented from 2019 to 2021. 99.7% of reported incidents were classified as no-harm (near misses). There was an exponential decrease from Q1-2020 to Q1-202. A significant increase in incidents fell in the category of (near misses) with no-harm and a significant reduction in serious events. Pharmacy interventions/clarifications saw a massive increase and impact from Q3-2018 to Q2-2019, compared to the same period in 2018. By preventing medical incidents, benchmarking, and analyzing incidents and the reporting system, the use of information technology could dramatically reduce the rate of drug incidents.
Conclusion
This study found that benchmarking medication incidents is valuable, as it can help identify areas where improvements can be made, implement strategies to improve safety, and track progress over time. The benchmark was recommended to be below 100 incidents for every 10,000 prescriptions/orders processed, and for E-I categories, below one incident for every 10,000 prescriptions/orders processed. This will help develop a worldwide standard with an absolved culture with non-punitive consequences.
Keywords: Medication incidents, Medication data, Error, Events, Pharmacy, Incident rate, Benchmarking, Reporting
1. Introduction
The pharmaceutical services department's goal at an organization is to provide the healthcare community with advice concerning drug safety, efficacy, and efficiency (Anna Legreid Dopp et al., 2020). Therefore, pharmaceutical services' major concern is eliminating medication-related incidents (Anna Legreid Dopp et al., 2020). Unfortunately, the benchmarking for medication incidents rate is not regarded as a recognized key performance measure or indicator in national or international organizations. Lack of benchmarking medication incidents leads to a loss of self-governing perception, widening performance gaps, and unnoticed improvements. It also hinders safety culture and non-punitive concepts in the organization.
Benchmarking is described as assessing processes and systems in one or more areas compared to pioneers in a specific field (Barbora Jetmarová, 2011). This is because benchmarking equips an organization with an analytical perception of its performance against peer facilities (Barbora Jetmarová, 2011, ISMP, 1998). In other words, benchmarking illustrates an organization's performance against a standard or peers' performance (Barbora Jetmarová, 2011, ISMP, 1998). In addition, benchmarking supports industries in recognizing gaps in areas, systems, or processes and improving and progressing toward the needed goal (Barbora Jetmarová, 2011). Such improvements might be progressive or require a change in the process and workflow (Barbora Jetmarová, 2011). Accordingly, competing and checking your level against rivals is good, but benchmarking has allowed scientific approaches, created a trustworthy technique, and allowed a comprehensive comparison (Barbora Jetmarová, 2011).
A 2021 systematic analysis of Medication Error Trends in Middle Eastern Nations revealed various categories of reported errors and incidents (Aidah et al., 2021). However, most studies on medication incidents in these countries were few or nonexistent; the analysis found a downward trend in pharmaceutical incidents in Saudi Arabia, Lebanon, and Bahrain, highlighting the need for literature on pharmaceutical services due to underreporting or inconclusive findings (Aidah et al., 2021).
The Institute for Safe Medication Practices (ISMP) defines a medication incident as a medication error or an issue that might lead to a medication error; either the error did occur or was prevented at any stage of the medication management process (ISMP Canada, 2021). In addition, ISMP indicates that benchmarking medication incidents and error rates may appear excellent; however, they stated that since the focus is on incident rates, the healthcare facility should question its logic for benchmarking for evaluating the medication management process (ISMP, 1998). ISMP disclosed that the actual rate of drug incidents varies and is determined mainly by how incidents are discovered and recorded (ISMP, 1998). Furthermore, the concept of benchmarking needs to be clarified for the assumption that quality personnel can assess the quality and safety of the medication management process merely by comparing error rates within and outside the healthcare facility (ISMP, 2021, ISMP, 1998).
Similarly, to NCC MERP, the ISMP questioned benchmarking of incident rates in 1998. However, seven years later, in 2005, they refrained by mentioning on their website that it is ' doable but with limitations ' (ISMP, 2021, ISMP, 1998). Thus, both ISMP and NCC MERP argue that there is no such thing as an acceptable rate of medication incidents and that it is pointless to compare healthcare institutions based on drug errors and incident rates (Bursua et al., 2016, ISMP, 2021, ISMP, 1998, NCC Merp, 2008).
On the other hand, over 21,000 studies and articles addressed benchmarking or pointed it out, but the national or international standardizing organizations still need to offer or request to implement such benchmarking. A study of Saudi Arabian hospitals found a high rate of medication errors (44.4%) (Almalki et al., 2021). It highlights the need for improved medication management systems to prevent patient harm and suggest that benchmarking the medication incidents in KSA could be a useful way to identify areas where improvements can be made (Almalki et al., 2021). Another study found that the rate of medication errors was similar for two large hospice organizations in the United Kingdom; this suggests that the rate of medication errors in specialist palliative care is similar across organizations (Taylor et al., 2010).
This study might be one of the few reports after Headford et al. and Gorbach et al. to quantify medication incident rates; however, we might be the first to use such data to benchmark and monitor rates and trends while implementing corrective action plans for every individual report overall process changing (Gorbach et al., 2015, Headford et al., 2001). Furthermore, Gorbach et al. quantitate the drug errors and incidents during the medication verification step only at the Pharmacy and identified the risk factors for errors and incidents at that stage alone (Gorbach et al., 2015). While Headford et al. emphasized that voluntary medication incident reporting is unreliable, they were able to create a new system, the Medication Incident Rate (MIR) Clinical Indicator, which was developed to make reporting more meaningful (Headford et al., 2001). This system has positively influenced the practice, provided a more accurate measure of medication errors, and identified areas for improvement (Headford et al., 2001).
Cheung et al. (2011) found that benchmarking medication incidents against national benchmarks can be valuable for identifying areas where improvements can be made (Cheung et al., 2011). They created the Central Medication Incident Reporting System (CMR) to provide a valuable resource for benchmarking medication incidents in the Netherlands (Cheung et al., 2011). They concluded that CMR data could be used to identify areas where medication incidents are more common and develop strategies to prevent them (Cheung et al., 2011).
In our study, we implemented the medication incident rates quantification and benchmarking at all medication management stages, from prescribing the drugs through validation, preparation, and dispensing to administration and disposal; it was forced all over the hospital.
2. Methodology and settings
Ethics approval: The study was approved on 29-November-2021 by the Institutional Review Board (Ref #21–467).
We used the medication incidents iceberg tip as an indicator. The tip indicates a broader hidden problem (Cambridge Dictionary, 2021). In other studies, their main concept was based mainly on the unseen part (submerged iceberg) of medication incidents and that unknowing it is considered a source of invalidated data to benchmark (Bursua et al., 2016, ISMP, 2021, ISMP, 1998, Jim Smith, 2004, NCC Merp, 2008). The submerged iceberg approach does not support benchmarking measuring for medication incidents (Bursua et al., 2016, Carolyn Robbins and Marti Wolf, 2016, ISMP, 2021, ISMP, 1998, NCC Merp, 2008).
obbiretet
Lo et al. discussed that benchmarking medication error rates in palliative care services are a valuable tool for improving patient safety(Lo et al., 2022). Still, it is challenging due to the variability in definitions, methods, and denominators used (Lo et al., 2022). However, it can be used to identify areas where improvements can be made and track progress over time; the study suggested some examples of denominators that can be used to measure medication error rates, such as (Lo et al., 2022):
-
•
The number of doses of medication administered.
-
•
The number of patients receiving medication.
-
•
The number of medication orders written.
-
•
The number of medication errors reported which we adopted in this study.
The results of our study, including the actual validated data, shall be used for medication safety benchmarking. Eventually, the results aim to guide healthcare leadership in identifying safety risk areas in hospital drug-related management and operations regarding medication incidents.
This retrospective analysis looked at all incidences that involved a patient's prescription from January-2018 to December-2021. The study design follows descriptive, cross-sectional, retrospective prevalence research. Using this study design, we gained an overview of the pattern of medication-related incidents in the Bahraini population. In addition, as a 600-bed tertiary hospital, all medication incidents reported during the three years between January-2019 and December-2021 were reviewed prospectively.
All patients in the hospital database were identified using the Hospital Information System (HIS). The patient's medical history, clinical state, and any reported medication incidents are listed as the primary data on their digital medical profile on HIS. To avoid errors, patient records were examined to ensure the report was due to a medication incident, complaint, or pharmacy intervention. The information was collected every quarter for three years. Information gleaned from patients' medical records and reporting technology of the HIS were recorded according to the type and number of medication incidents.
Starting in January-2019, the Pharmaceutical Services Department began to analyze the medication incident reports, as all previous data up to December-2018 was under the Quality and Patient Safety Department (QPS). The policy was there and written but was never implemented, and it required the QPS to do the classification, which could not do so. The change was due to the new Pharmacy Administration team and the Pharmacy Quality Head. From a Pharmacy quality point of view and standard of practice, the pharmacy team decided to do the study as implemented. The new team was assigned in 2019, declaring that the Pharmacy would not be responsible for anything before 2019; however, 2018 was our starting point in the study.
The Pharmacy Department worked to monitor and evaluate medication incidents and implement risk-reduction strategies to prevent incidents from happening again. As a result, QPS started to send all medication-related incidents to the Pharmacy, as well as the classification that included the type of incidents and the stage where the error occurred. These stages include but are not limited to receiving, storing, prescribing, dispensing, medication administration, and monitoring.
The NCC MERP recognized the necessity for a consistent error classification system (ISMP, 2021, ISMP Canada, 2021, NCC Merp, 2008). As a result, the NCC MERP adopted a Medication Error Index on 16-July-1996, categorizing incidents from A to I based on the severity of the outcome. The index is intended to assist healthcare providers and organizations track drug incidents consistently and systematically. The Pharmacy's action plan was to monitor the reporting rates through this index as a classification type and to incorporate it with the data gathering tool as per Appendix A (Droege et al., 2022, ISMP, 1998, NCC MERP, 2001, Parthasarathi et al., 2021). [Fig. 1] shows the Medication Error' Classes used based on the most updated version as per NCC MERP.
Fig. 1.
Edication errors' classes. adopted from the ncc merp index for categorizing medication errors, 2022.
The index considers whether the error reached the patient, whether or not the patient was affected, and to what extent (ISMP, 2021, ISMP Canada, 2021, NCC Merp, 2008). The Council encourages the index's use in all healthcare settings by researchers and software manufacturers tracking prescription incidents. This index has been implemented for usage in the ISMP Medication Errors Reporting Program's database. The report was assembled using the organizations newly adjusted HIS Occurrence Variance Report (OVR) system.
2.1. Inclusion criteria
-
•
All medication incidents reported in the organization (from Q1-2018 till Q4-2021)
2.2. Exclusion criteria
-
•
Adverse drug reactions (ADR) that were non-preventable incidents upon investigation.
2.3. Materials, data collection methods, instruments used, and measurements
-
•The hospital voluntary reporting systems:
-
oPurchased OVR system and then the HIS OVR data (as a count of reported incidents and the count of incidents that resulted in harm to patients)
-
oCount of the near misses reported via the Pharmacy interventions/clarification system.
-
o
-
•
All healthcare providers' feedback, complaints concerning the medication management system, and incidents were reported through emails and official QPS channels involving patients.
-
•
All patients' complaints due to medication incidents were reported through emails and official QPS channels.
-
•
Total Pharmacy dispensations during the period of the study
2.4. In this study, we aim to
-
•Challenge organizations that believe benchmarking of medication incidents is of underrated value by introducing the effects of systematic interventions initiated by identifying the medication incidents rate on the following:
-
oIncidents rate, which may result in harm.
-
oRates reported by patient/healthcare providers' feedback or complaints.
-
oReflecting on the practice of Medication Management at the hospital compared to the dispensation magnitude done by the Pharmacy.
-
o
-
•
Identify the impact of the Pharmaceutical Services Department interventions and their results on incident reporting rates.
-
•
Explore the retrospective impact of implementing the benchmarking of medication incidents.
2.5. Data collection
-
•
In 2018, no data was collected or analyzed in detail.
-
•
In 2019, we changed things by gap analysis, checking where we stand internally and comparing to similar facilities internationally, along with validated data sources, making reporting and promoting the system easy, leading to an increase in 2020.
-
•
In 2020, we analyzed the data and created action plans from system changes to training and education. The Medication incident data collection sheet is shown in Appendix A.
-
•
Medication Incidents from Q1-2018 till Q4-2021 compared to total dispensations of the Pharmacy. Refer to [Table 1]
-
•
Then, all collected incidents and reports were categorized using the NCC MERP index classification, as shown in [Table 2].
Table 1.
Baseline Quarterly Raw Collected Data from 2018 till 2021.
| Q1-2018 | Q2-2018 | Q3-2018 | Q4-2018 | Q1-2019 | Q2-2019 | Q3-2019 | Q4-2019 | Q1-2020 | Q2-2020 | Q3-2020 | Q4-2020 | Q1-2021 | Q2-2021 | Q3-2021 | Q4-2021 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count of Medication incidents reported in the hospital through the voluntary reporting system (Ready Purchased System/HIS OVR system) | 2 | 2 | 2 | 3 | 43 | 53 | 94 | 127 | 102 | 95 | 85 | 111 | 96 | 96 | 84 | 82 |
| Pharmacy interventions/Clarification done and documented on the HIS system. | NA | NA | NA | NA | 542 | 1880 | 2400 | 2885 | 3488 | 4757 | 3901 | 2625 | 1832 | 1719 | 2484 | 2381 |
| Patient Complaints/Healthcare providers' complaints related to medication incidents sent by email or reported through official memos or meeting agendas | NA | NA | NA | NA | 1 | 16 | 3 | 1 | 0 | 4 | 1 | 1 | 0 | 2 | 5 | 3 |
| Total reported medication incidents | 2 | 2 | 2 | 3 | 586 | 1949 | 2497 | 3013 | 3590 | 4856 | 3987 | 2737 | 1928 | 1817 | 2573 | 2466 |
| Total Pharmacy dispensation (order-wise) | 6963 | 7627 | 4586 | 5367 | 269,683 | 295,404 | 177,641 | 207,878 | 271,468 | 301,389 | 379,063 | 430,396 | 426,720 | 1,412,650 | 1,396,076 | 1,179,200 |
| Count of medication incidents categorized between E-I in the hospital | 0 | 0 | 0 | 0 | 9 | 3 | 11 | 10 | 6 | 1 | 3 | 4 | 6 | 5 | 4 | 4 |
| (Internal Benchmark as target below 100) RATE of Medication incidents reported to every 10000-order/prescription processed | 2.87 | 2.62 | 4.36 | 5.59 | 21.73 | 65.98 | 140.56 | 144.94 | 132.24 | 161.12 | 105.18 | 63.59 | 45.18 | 12.86 | 18.43 | 20.91 |
| (Internal Benchmark as target below 1) RATE of Medication incidents (fill in category E-I) reported to every 10000-item order/prescription processed | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.10 | 0.62 | 0.48 | 0.22 | 0.03 | 0.08 | 0.09 | 0.14 | 0.04 | 0.03 | 0.03 |
Table 2.
Baseline Quarterly Raw Collected Data from 2019 till 2021 classified using the NCC MERP index.
| Q1-2018 | Q2-2018 | Q3-2018 | Q4-2018 | Q1-2019 | Q2-2019 | Q3-2019 | Q4-2019 | Q1-2020 | Q2-2020 | Q3-2020 | Q4-2020 | Q1-2021 | Q2-2021 | Q3-2021 | Q4-2021 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: No Error | NA | NA | NA | NA | 5 | 31 | 31 | 32 | 21 | 37 | 14 | 15 | 21 | 46 | 13 | 26 |
| B: Error, no-harm | NA | NA | NA | NA | 551 | 1888 | 2415 | 2943 | 3494 | 4798 | 3954 | 2700 | 1882 | 1752 | 2528 | 2419 |
| C: Error, no-harm | NA | NA | NA | NA | 7 | 19 | 6 | 2 | 3 | 17 | 6 | 5 | 9 | 3 | 23 | 10 |
| D: Error, no-harm | NA | NA | NA | NA | 14 | 8 | 34 | 26 | 66 | 3 | 10 | 13 | 10 | 11 | 5 | 7 |
| E: Error, harm | NA | NA | NA | NA | 9 | 3 | 11 | 10 | 6 | 0 | 3 | 4 | 6 | 5 | 3 | 4 |
| F: Error, harm | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| G: Error, harm | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H: Error, harm | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| I: Error, death | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sum Q by the incident | 586 | 1949 | 2497 | 3013 | 3590 | 4856 | 3987 | 2737 | 1928 | 1817 | 2573 | 2466 |
[Table 2] was recalculated to sum the whole years, as shown in [Table 3], using the NCC MERP index classification in [Table 2].
Table 3.
Baseline Raw Collected Data from 2019 to 2021 classified using the NCC MERP index.
| 2019 | 2020 | 2021 | Overall | |
|---|---|---|---|---|
| A: No Error | 99 | 87 | 106 | 292 |
| B: Error, no-harm | 7797 | 14,946 | 8581 | 31,324 |
| C: Error, no-harm | 34 | 31 | 45 | 110 |
| D: Error, no-harm | 82 | 92 | 33 | 207 |
| E: Error, harm | 33 | 13 | 18 | 64 |
| F: Error, harm | 0 | 1 | 1 | 2 |
| G: Error, harm | 0 | 0 | 0 | 0 |
| H: Error, harm | 0 | 0 | 0 | 0 |
| I: Error, death | 0 | 0 | 0 | 0 |
| Sum by year | 8045 | 15,170 | 8784 | 31,999 |
3. Statistical methods
All descriptive and comparative analyses were performed using the Statistical Package for the Social Sciences (Version 20.0, SPSS Inc., Chicago, IL, USA). The aim of the analysis was to evaluate the impact of benchmarking on medication incident rates, and a significance level of P < 0.05 was established. To accomplish this objective, the study utilized a range of statistical analysis techniques, including exploratory data analysis, descriptive statistics, regression analysis, and time series analysis.
4. Results
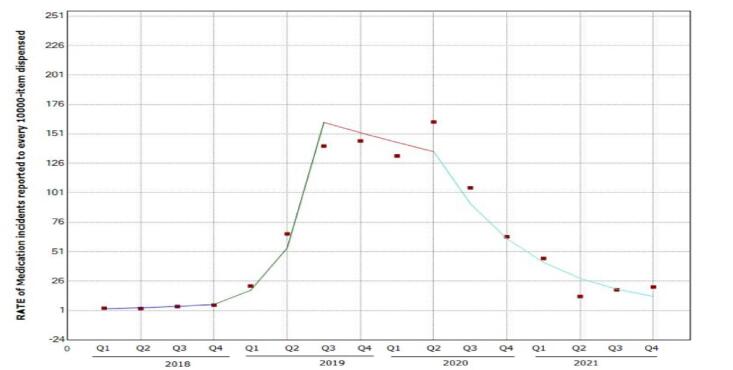
Analysis of the Rate of Medication Incidents reported to every 10,000-item order/prescription processed quarterly, refer to [Fig. 2] and [Table 4].
Fig. 2.
Quarterly Rate of Medication incidents reported for every 10,000-item order/prescription processed.
Table 4.
Quarterly P-value of Medication incidents reported for every 10,000-item order/prescription processed.
| Segment | Average Percentage change | Lower CI | Upper CI | Test Statistic (t) | P-value |
|---|---|---|---|---|---|
| Q1 2018 – Q4 2018 | 35.9 | −38.0 | 197.9 | 1.0 | 0.4 |
| Q4 2018 – Q3 2019 | 197.4 | −38.1 | 1329.1 | 1.8 | 0.1 |
| Q3 2019 – Q2 2020 | −5.4 | −80.3 | 354.4 | −0.1 | 0.9 |
| Q2 2020 – Q4 2021 | –32.5* | −48.2 | −12.0 | −3.8 | 0.00 |
*A significant decrease in the rate was seen from Q2-2020 – to Q4-2021, with an average decrease of 32.5 %.
(CI: −48.2 %- − 12.0%, p value = 0.00).
Analysis of the RATE of Medication incidents (fill in Category E-I) reported to every 10,000-item order/prescription processed quarterly [Fig. 3].
Fig. 3.
Quarterly Rate of Medication incidents (fill in Category E-I) reported for every 10,000-item order/prescription processed.
The medication incidents (Category E-I) rate declined from Q3-2019 to Q2-2020 and remained relatively stable until Q4-2021. The differences were, however, not statistically significant.
Analysis of the Pharmacy interventions/Clarification done and documented on the HIS system, refer to [Fig. 4] and [Table 5].
Fig. 4.
Quarterly Pharmacy interventions/Clarification reported for every 10,000-item order/prescription processed.
Table 5.
Quarterly P-value of Pharmacy interventions/Clarification reported for every 10,000-item order/prescription processed.
| Segment | Average Percentage change | Lower CI | Upper CI | Test Statistic (t) | P value |
|---|---|---|---|---|---|
| Q1 2018 – Q3 2018 | 16.6 | −56.4 | 211.7 | 0.4 | 0.7 |
| Q3 2018 – Q2 2019 | 1113.1* | 353.7 | 3143.5 | 5.9 | 0.0 |
| Q2 2019 – Q4 2021 | −2.0 | −9.2 | 5.8 | −0.6 | 0.6 |
*A significant increase in Pharmacy interventions/clarifications was seen from Q3-2018 to Q2-2019 with an average increase of 1113.1 % (CI: 353.7 % − 3143.5%, p value = 0.00).
Analysis of the Medication incidents classified using the NCC MERP index through the three years [Fig. 5].
Fig. 5.
Medication incidents analysis classified using the NCC MERP index through the three years.
A significant increase in incident A-D and a significant reduction in incidences of serious events from 2019 to 2020 (p = 0.000). However, A-D was significantly reduced from 2020 to 2021 (p = 0.012).
5. Discussion
The Pharmacy's study analyzed every medication incident documented from 2019 to 2021 using all reporting channels as a data source (after the results of 2018, which indicated a clear underreporting). Benchmarking can reflect the organization's cultures, including the Safety, Just, or Punitive cultures (Barbora Jetmarová, 2011, Carolyn Robbins and Marti Wolf, 2016). Our approach was unconventional, and it had accepted results and significant changes in the medication management process at our organization.
The first 6-month reporting period (January-2019 to June-2019) was sufficient to gather important data. The number of reported incidents indicated a culture of openness to incident reporting operating throughout the hospital and assigning benchmarks. 99.7% of reported incidents were classified as no-harm (near misses). Here, the Pharmacy campaigned with the slogan [RR: Report it; to Resolve it] to encourage reporting and gather more information and data.
Appendix A was used by the Pharmacy to collect the data in detail and to classify the incidents using the NCC MERP index classification algorithm and other types of classification of incidents such as medication management stage where the incident occurred, the staff category involved, the type of error, the risk grade of it, the potentiality rate and the class of drug involved in the incident, and this allowed the Pharmacy to have a full picture of the incidents' trends and causes. The data of the incidents that are not classified as incidents as per the NCC MERP index (e.g., non-preventable adverse drug reactions) were excluded from this study and from the report presented to the Drug and Therapeutic (D&T) committee using the same tool in Appendix A. All incidents analyzed were medication incidents that were expected to cause harm or did harm to the patient. Most incidents were categorized as no-harm, especially in category B; as shown in [Fig. 5], the D&T committee undertook the severity rating to exclude any bias by the reporter. In addition, the D&T committee considered the error's severity and consequences; this was this study's strength. The standardized reporting criteria would facilitate the development of targeted interventions to reduce medication incidents. In addition to the main NCC MERP index classification, other rankings of the incidents were done according to a variety of parameters, such as incident types, preventability, severity, stage of the process, and staff category; this helped to find the root cause and trend the events where applicable, refer to Appendix A. Adverse drug reaction detecting and reporting programs also help identify preventable ADRs and anticipatory surveillance for high-risk drugs or patients. The Pharmacy used the Naranjo tool to evaluate the significance of ADRs reported, resulting in action plans that improved the safety of the Medication Module at the HIS, noting that ADRs fell in category A and were excluded from this study and the medication incidents report.
Overall results have shown that 67,722,111 orders were processed for dispensation during these three years (2019–2021), with a prevalence of 0.047%. In addition, 31,999 medication incidents occurred during the same three years; analysis occurred every quarter. Therefore, the total increase between Q1-2018 and Q1-2019 is 86.8%. Further, the increase between Q1-2019 and Q1-2020 is 83.6%. However, there was an exponential decrease from Q1-2020 to Q1-2021 by 192.7%. The data were reviewed and corrected, and action plans were initiated accordingly.
The benchmarking part took place in Q3-2019, as the results were taken to the D&T committee by the Pharmacy with a proposal to set a benchmark as [100 to every 10,000 prescription/orders processed] and for the E-I categories to be set [below 1 to every 10,000 prescription/orders processed]. The D&T reviewed the proposal and found that neither a national nor international benchmark was known and agreed to create the internal benchmarks as proposed by the Pharmacy.
Starting in Q3-2019, data was analyzed, categorized, and trended, leading the Pharmaceutical Services Department and the D&T committee to stabilize an internal benchmark by Q4-2019; this supported the calculations of the rate of incidents for every 10000-item order/prescription processed. Therefore, the rate of Medication incidents reported to every 10000-item order/prescription processed shall be below 100, and the RATE of Medication incidents (fill in Category E-I) reported to every 10000-item order/prescription processed, is to be below 1, this including all cases shared with all related committees across the organization. As a result, a significant increase in the rate of Medication Incidents reported for every 10,000-item order/prescription processed quarterly was seen from Q4 2018 – to Q3 2019, with an average decrease of 32.5 %; then, a significant decrease in the rate was seen from Q2 2020 – to Q4 2021, with an average decrease of 32.5 % with a P-value = 0.
The findings of this study provide evidence that the reporting system and processes at this GCC hospital were effective. Of the reports extracted, neither duplicate reports nor reports for incidents not classified as medication incidents were found. Noting that a significant increase in incidents fell in category (A-D) with no-harm and a significant reduction in incidences of serious events from 2019 to 2020 (p = 0.000). However, A-D was significantly reduced from 2020 to 2021 (p = 0.012).
In previous research, Bursua et al. supported voluntary reporting of medication mishaps and emphasized risk factors for such incidents to improve patient safety (Bursua et al., 2016). However, this study disagrees with their core concept that comparing drug incident rates within hospital operations is pointless (Bursua et al., 2016). Bursa et al. based their study on the ISMP and National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) recommendations. NCC MERP stated in a statement published on their website in 2002, quoting: 'Use of Medication Error Rates to Compare Health Care Organizations is of “No Value” '(Bursua et al., 2016, ISMP, 2021, ISMP, 1998, NCC Merp, 2008). Nevertheless, in the same NCC MERP statement, they referenced it as a [non-recommended] method, which varies from the term “of no value” (NCC MERP, 2008).
Aidah et al. reported that about 18 scientific articles associated with medication incidents did not represent all the areas examined in this study and did not consider the data sources as this study overcovered (Aidah et al., 2021). Most of these scientific articles showed no conclusive medication incidents reported in Middle-eastern countries, noting that several diverse types of reported incidents in the Middle Eastern countries were relatively few or nonexistent (Aidah et al., 2021). In addition, some of the articles' quality was weak (Aidah et al., 2021). Although we agree that there needs to be more reporting in the Middle East and the Gulf Cooperation Council (GCC), we believe that fundamental improvements in the healthcare sector are necessary (Aidah et al., 2021). Furthermore, most of the studies used data sources like incidents per 1,000 admissions, incidents per 100 prescriptions, or incidents per 1000 patient days which restrict any direct evaluations (Lewis et al., 2009, Wolfe et al., 2018).
In our study, the hospital in 2018 was using incidents for 1000 patients' days which was not found to be objective and had some logistics faults and miscalculations. Therefore, although at this point, the Pharmaceutical Services Department requested to calculate it as incidents per 100 prescriptions in Jan 2019, it was adjusted to be incidents per 10,000 prescriptions to enable analysis.
The rate of medication incidents was low (0.047 per 100 medication orders) compared to numbers from a previous comprehensive analysis, which showed that the medication incidents average was 28.18 per 100 prescription orders (Thomas et al., 2021). Several reasons could be attributed to these differences, including but not limited to incident definitions, procedural methodologies, and outcome processes (Khatri et al., 2009); this demonstrates the necessity for facilities to agree on a specified procedure and reporting criteria to promote data pooling, comparison, and learning from best practice (Lewis et al., 2009). In addition, such advancements would provide a standardized benchmark for assessing the effectiveness of any treatments (Lewis et al., 2009).
Furthermore, these limitations raised by other studies were avoided in our study by computerized order input, prescription validation systems by pharmacists and nurses, and health care provider training, all critical components we focused on.
The decrease in our medication incidents observed is due to avoiding human slips as much as possible and systemizing the process. Since the prescription process is highly human-dependent, we characterized it by automatic prescription filling and mandatory requirements to fulfill them. In addition, drug processing is verified in more detail with ancillary validation procedures.
Yet, the study did have its limitations; underreporting is a major issue, as healthcare workers may not report all incidents due to fear of retribution, lack of time, or knowledge of the reporting process leading to inaccurate data and skewed analysis (Aidah et al., 2021, Catchpole et al., 2020, Tchijevitch et al., 2023). Variability in reporting across different healthcare workers can also make comparing data across departments difficult; cause such lack of standardization in medication incident reporting makes it difficult to compare data across different hospitals or regions and benchmark against other hospitals (Aidah et al., 2021, Tchijevitch et al., 2023). Limited data analysis may not provide a complete picture of the medication administration process, as factors like staffing levels, workload, and training may also contribute to medication errors (Aidah et al., 2021, Catchpole et al., 2020, Tchijevitch et al., 2023). Bias in benchmarking can occur if there are differences in reporting practices or patient populations, making it difficult to accurately compare performance and identify areas for improvement (Lewis et al., 2009). In conclusion, medication incident reporting is essential for identifying and addressing errors in hospitals, but it is crucial to recognize its limitations in analysis and benchmarking. Hospitals should strive to improve reporting practices and standardize reporting systems to ensure accurate data and benchmarking (Aidah et al., 2021, Catchpole et al., 2020).
Our study considered overcoming complications reported by different studies in the field via interpreting incidence data using Appendix A, resulting in not compromising the validity of the collected data because all medication incidents were identified and promptly reported (Thomas et al., 2021). The main obstacle was to eliminate fear and worry that reporting an incident might lead to additional inquiry, worries about the influence on working relationships, and the possible loss of concealment (Thomas et al., 2021) after intensive campaigns and changes in HIS to ensure a Just Culture (Khatri et al., 2009) [a shared accountability approach wherein corporations are responsible for the mechanisms they have created and for appropriately handling employee behavior. It acknowledges that individual practitioners should not be held liable for systemic flaws they have no influence over] (Khatri et al., 2009); the incidence data derived from this study can therefore be considered an estimate of the actual incidence of medication incidents at the organization. It was found that most of the medication incident reports were submitted by pharmacists and nurses, proving a considerable under-reporting by physicians (Thomas et al., 2021). In addition, we encouraged to overcome limitations in medication incident reporting by increasing awareness and education among healthcare workers, encouraging a reporting culture, standardizing reporting systems, expanding data collection, and using benchmarking data with caution, which lead to improving data comparability and benchmarking, ultimately improving patient safety. By addressing differences in reporting practices or patient populations, we tried to avoid bias in benchmarking and ensure accurate comparisons.
This study has several strengths, including analyzing data collected over three years and applying a practical framework within the study that could be tailored to any organization. Employees' positive attitudes are improved because of the electronic system and the training campaign, which improved the safety and quality of the services. By preventing medical incidents and analyzing incidents and the care system, using various forms of information technology may dramatically reduce the rate of drug incidents and enhance the quality of services offered to patients. The benchmark for medication incidents is also possible, especially since it was proven successful in benchmarking prescribing incidents in the pediatric intensive care unit among nine children's hospitals in the US (Cimino et al., 2004). Another strength of this study is the prospective data analysis of incidents and the retrospective process. The latter contained the risk of missing information and could have provided an incomplete picture to the D&T committee, which will review all the captured data. The involvement of D&T provided a less biased evaluation of the incidents, not considering the reporter's point of view of the incident classification.
The continuous and detailed analysis identified that the leading cause of medication incidents was miscommunication between different parties, lack of compliance with the double-checking protocol, lack of training, and non-compliance to agreed policies and procedures.
As such, it was reported that differences were founded among various studies due to better health systems and lower ratios of physicians or prescribers to patients in middle- and high-income countries compared to those in low-income countries (Papanicolas et al., 2019); hence this shall be considered a major contributing factor while comparing studies to ours.
Our initial target was to catch the error with no-harm, the best-in-class B, and the overall decrease in error is the ultimate goal. However, considering that the count is not the aim (although it supports Just culture as the number of reports did increase), the rate for every 10,000 orders processed by the Pharmacy was considered.
The start was with the purchased-ready system used by QPS till 2018. The system's user feedback stated that it needed to be more user-friendly and cover our institute's needs. This resulted in building our own HIS reporting system, part of which is voluntary and anonymous, also known as the OVR system, and the rest was after an actual intervention that stopped a near miss. These interventions were based on the medication order level, capturing the full details of the incident and the actual correction. The newly designed indigenous OVR system was used starting Mar-2020.
The pharmacy interventions documented the prescribing incidents (which could be errors if not caught by the Pharmacist, AKA' near miss), and they were not captured through the OVR system (even if captured, it was with a minimal non-significant percentage). Pharmacists' interventions (clarification data) showed the pharmacists' critical impact in the hospital with prescribing incidents. A significant increase in Pharmacy interventions/clarifications was seen from Q3-2018 to Q2-2019, with an average increase of 1113.1 % (P value = 0.00).
The Pharmaceutical Services Department has been sharing this data since April 2019 quarterly with the D&T committee, the chief medical officer and the heads of medical departments, the director of nurses, and the Risk Management Committee of the hospital, which has resulted in action plans, including many HIS modifications on pharmacy screens and the Medication Management Module. In addition, the Pharmacy Quality Department initiated and conducted extensive training by educating, training, tutoring, and coaching the Pharmacy team and all the involved healthcare providers. The training course took five months and involved presentations, surveys, quizzes with prizes, and visiting all operational departments involved in the changes to train on the ground with life examples and scenarios. Moreover, the medical departments were addressed during their training and since September 2019 with the total number of orders prescribed by physicians classified by their name & specialty to compare the percentage of incidents done per prescriber to their overall orders in their department; such data is essential for the Ongoing Professional Practice Evaluation (OPPE) for each legible prescriber at our organization, such sharing of data impacted the quality of services and enabled the management of trends and decreasing them per each medical department.
The overall trends were Duplication of therapy. The Duplication of therapy was the second trend in Q3-2020 after incomplete/unclear order. The Pharmaceutical Services Department had fixed this by making prescription fields mandatory to fulfill a complete order (-85%, from 1170 to 171 only in Q 1–2021). Furthermore, even the data by specialty, applying the PARETO rule to target 20% of departments with 80% impact was implemented. In addition, every specialty's in-house training was conducted across the organization, resulting in improvements. Even targeting by staff involved: Prescribers then Nurses were dominant among the staff involved in the incidents, resulting in action plans tailored to each incident scenario (Dorothy et al., 2021).
Comparing medication error rates across various healthcare organizations is a technique known as benchmarking medication incidents; this can be done to recognize improvement areas and monitor development over time. The benchmarking of medication incidents presents an array of challenges. The fact that different organizations may have different definitions of pharmaceutical errors presents one difficulty. Another difficulty is that several ways might be employed to gather information on medication errors. Due to this, comparing medication error rates across various institutions may be challenging. Despite these obstacles, benchmarking medication events can still be useful for enhancing patient safety. Healthcare organizations can pinpoint areas for improvement by benchmarking medication error rates, as our study proved.
6. Conclusion
This study proved the necessity of benchmarking pharmaceutical incidents by going through various stages. First, we identified medication incidents and devised multiple mechanisms for gathering data on medication occurrences. We then compared those events to internal benchmarks to identify opportunities for improvement, which lead us to establish safety measures and track their progress over time.
Analyzing every medication incident documented from 2019 to 2021 using all reporting channels as a data source showed that:
-
•
99.7% of reported incidents were classified as no-harm (near misses).
-
•
The decrease in our medication incidents observed is due to avoiding human slips as much as possible and systemizing the process.
-
•
It was found that most of the medication incident reports were submitted by pharmacists and nurses, proving a considerable under-reporting by physicians.
-
•
The leading cause of medication incidents was miscommunication between different parties.
-
•
A significant rise in Pharmacy interventions/clarifications was seen from Q3-2018 to Q2-2019; these pharmacists' interventions (clarification data) showed the pharmacists' critical impact in the hospital with prescribing incidents.
The study showed that benchmarking medication occurrences serves in identifying areas for improvement and adopting safety procedures and contributed to enhancing patient safety via systematic actions made by the Pharmaceutical Services Department.
Furthermore, the study observed that benchmarking medication incidents is beneficial in identifying the medication incident rate that may cause harm, the medication incident rate reported by patients, and healthcare providers' feedback. It reflected the hospital practice in Medication Management compared to the Pharmacy's dispensation magnitude by the number of orders processed. We found that medication incident benchmarking is useful by introducing the impacts of systematic actions launched by the Pharmaceutical Services Department and assessed by the D&T committee (to ensure objectivity).
We recommend benchmarking medication incidents at a given organization to develop a worldwide standard with an absolved culture with non-punitive consequences. Our suggested benchmark is below 100 incidents for every 10,000 prescriptions/orders processed and for E-I categories to be below one incident for every 10,000 prescriptions/orders processed.
7. Authors' contributions
All authors have contributed to preparing, writing, and reviewing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.101726.
Contributor Information
May Hassan ElLithy, Email: ellithymay@gmail.com.
Hager Salah, Email: hager.salah@rocketmail.com.
Lamyaa Samir Abdelghani, Email: Lamyaa_ghani81@yahoo.com.
Walid Assar, Email: walid.assar@khuh.org.bh.
Martin Corbally, Email: martin.corbally@khuh.org.bh.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aidah S., Gillani S.W., Alderazi A., Abdulazeez F. Medication error trends in Middle Eastern countries: A systematic review on healthcare services. J. Educ. Health Promot. 2021;10:227. doi: 10.4103/jehp.jehp_1549_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almalki Z.S., Alqahtani N., Salway N.T., Alharbi M.M., Alqahtani A., Alotaibi N., Alotaibi T.M., Alshammari T. Evaluation of medication error rates in Saudi Arabia. Medicine. 2021;100:e24956. doi: 10.1097/MD.0000000000024956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna Legreid Dopp, Kendall K. Hall, Eleanor Fitall, 2020. Pharmacist Role in Patient Safety [WWW Document]. ANNUAL PERSPECTIVE, The PSNet Collection. URL https://psnet.ahrq.gov/perspective/pharmacist-role-patient-safety (accessed 5.10.21).
- Barbora Jetmarová, 2011. BENCHMARKING – METHODS OF RAISING COMPANY EFFICIENCY BY LEARNING FROM THE BEST-IN-CLASS. EKONOMIKA A MANAGEMENT.
- Bursua A., Hartke P.L., Larson C.M. Benchmarking and medication error rates. Am. J. Health Syst. Pharm. 2016;73:744–745. doi: 10.2146/ajhp150803. [DOI] [PubMed] [Google Scholar]
- Cambridge Dictionary, 2021. THE TIP OF THE ICEBERG - Cambridge English Dictionary [WWW Document]. Cambridge English Dictionary. URL https://dictionary.cambridge.org/dictionary/english/tip-of-the-iceberg (accessed 5.20.21).
- Catchpole K., Abernathy J., Neyens D., Sutcliffe K. Understanding the limitations of incident reporting in medication errors. Br. J. Anaesth. 2020;125:e343–e344. doi: 10.1016/j.bja.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.-C., van den Bemt P.M.L.A., Bouvy M.L., Wensing M., De Smet P.A.G.M. A nationwide medication incidents reporting system in The Netherlands. J. Am. Med. Inform. Assoc. 2011;18:799–804. doi: 10.1136/amiajnl-2011-000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino M.A., Kirschbaum M.S., Brodsky L., Shaha S.H. Assessing medication prescribing errors in pediatric intensive care units*. Pediatr. Crit. Care Med. 2004;5:124–132. doi: 10.1097/01.PCC.0000112371.26138.E8. [DOI] [PubMed] [Google Scholar]
- Dorothy A., Yadesa T.M., Atukunda E. Prevalence of medication errors and the associated factors: A prospective observational study among cancer patients at Mbarara Regional Referral Hospital. Cancer Manag. Res. 2021;13:3739–3748. doi: 10.2147/CMAR.S307001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droege, M.E., Stacy, E.S., Guido, M.R., Alhayani, T., Keegan, S.P., Miller, A.M., McKinney, K.C., Morris, P.A., Parrish, N.J., Reinstatler, K.M., Takieddine, S.C., Vora, A.M., Warner, A.R., Waters, G.E., Mueller, E.W., 2022. Characterization and evaluation of a pharmacy morbidity, mortality, and improvement case conference for drug safety optimization: An 8‐year experience. JACCP: JOURNAL OF THE AMERICAN COLLEGE OF CLINICAL PHARMACY 5, 428–435. https://doi.org/10.1002/jac5.1588.
- Gorbach C., Blanton L., Lukawski B.A., Varkey A.C., Pitman E.P., Garey K.W. Frequency of and risk factors for medication errors by pharmacists during order verification in a tertiary care medical center. Am. J. Health Syst. Pharm. 2015;72:1471–1474. doi: 10.2146/ajhp140673. [DOI] [PubMed] [Google Scholar]
- Headford C., McGowan S., Clifford R. Analysis of medication incidents and development of a medication incident rate clinical indicator. Collegian. 2001;8:26–31. doi: 10.1016/S1322-7696(08)60019-0. [DOI] [PubMed] [Google Scholar]
- ISMP Canada, 2021. ISMP Canada Definitions of Terms [WWW Document]. ISMP Canada. URL https://www.ismp-canada.org/definitions.htm (accessed 5.2.21).
- ISMP, 1998. Benchmarking - When is it Dangerous? [WWW Document]. ISMP. URL https://www.ismp.org/resources/benchmarking-when-it-dangerous (accessed 5.1.21).
- ISMP, 2021. Measuring Up to Medication Safety [WWW Document]. Institute For Safe Medication Practices. URL https://www.ismp.org/resources/measuring-medication-safety (accessed 5.2.21).
- Khatri N., Brown G.D., Hicks L.L. From a blame culture to a just culture in health care. Health Care Manage. Rev. 2009;34:312–322. doi: 10.1097/HMR.0b013e3181a3b709. [DOI] [PubMed] [Google Scholar]
- Lewis P.J., Dornan T., Taylor D., Tully M.P., Wass V., Ashcroft D.M. Prevalence, incidence and nature of prescribing errors in hospital inpatients. Drug Saf. 2009;32:379–389. doi: 10.2165/00002018-200932050-00002. [DOI] [PubMed] [Google Scholar]
- Lo T.J., Tan S., Fong S.Y., Wong Y.Y., Soh T.L.G. Benchmarking medication error rates in palliative care services: Not as simple as it seems. Am. J. Hosp. Palliat. Med.®. 2022;39:1484–1490. doi: 10.1177/10499091221083019. [DOI] [PubMed] [Google Scholar]
- NCC MERP, 2001. Types of Medication Errors [WWW Document]. NCC MERP. URL http://www.nccmerp.org/types-medication-errors (accessed 9.23.22).
- NCC MERP, 2008. Statement on Medication Error Rates [WWW Document]. National Coordinating Council for Medication Error Reporting and Prevention. URL https://www.nccmerp.org/statement-medication-error-rates (accessed 5.1.21).
- Papanicolas I., Mossialos E., Gundersen A., Woskie L., Jha A.K. Performance of UK National Health Service compared with other high income countries: observational study. BMJ. 2019;l6326 doi: 10.1136/bmj.l6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathi A., Puvvada R., Patel H., Bhandari P., Nagpal S. Evaluation of medication errors in a tertiary care hospital of a low- to middle-income country. Cureus. 2021 doi: 10.7759/cureus.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins Carolyn, Wolf Marti. COMMUNITY HEALTH FORUM Summer/Fall; 2016. Collaborative benchmarking to improve patient medication safety. [Google Scholar]
- Smith J. NHS; 2004. Building a safer NHS for patients: Improving medication safety. [Google Scholar]
- Taylor N., Fisher S., Butler C. Benchmarking in-patient medication errors in specialist palliative care. Palliat. Med. 2010;24:350–351. doi: 10.1177/0269216309354397. [DOI] [PubMed] [Google Scholar]
- Tchijevitch O., Hallas J., Bogh S.B., Birkeland S.F. Medication incidents and medication errors in Danish healthcare: A descriptive study based on medication incident reports from the Danish Patient Safety Database, 2014–2018. Basic Clin. Paharmacol. Toxicol. 2023;132:416–424. doi: 10.1111/bcpt.13846. [DOI] [PubMed] [Google Scholar]
- Thomas B., Pallivalapila A., El Kassem W., Al Hail M., Paudyal V., McLay J., MacLure K., Stewart D. Investigating the incidence, nature, severity and potential causality of medication errors in hospital settings in Qatar. Int. J. Clin. Pharm. 2021;43:77–84. doi: 10.1007/s11096-020-01108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D., Yazdi F., Kanji S., Burry L., Beck A., Butler C., Esmaeilisaraji L., Hamel C., Hersi M., Skidmore B., Moher D., Hutton B. Incidence, causes, and consequences of preventable adverse drug reactions occurring in inpatients: A systematic review of systematic reviews. PLoS One. 2018;13:e0205426. doi: 10.1371/journal.pone.0205426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.