Abstract
Mycobacterium celatum is a recently described mycobacterium isolated from patients who have suppressed cell-mediated immunity, such as AIDS. We present here, to our knowledge, the first report of a fatal pulmonary infection caused by M. celatum in a 73-year-old immunocompetent female patient. The mycobacterium was identified by a 16S rRNA sequence analysis.
Mycobacterium celatum is a recently described nontuberculous mycobacterium (3, 18). These nontuberculous, or atypical, mycobacteria are found widely in nature. Only a few species are pathogenic in humans. The epidemiology of these organisms is not well understood, but person-to-person transmission has never been demonstrated. Most infections occur in patients with suppressed cell-mediated immunity, such as AIDS (6, 9, 13, 17). Immunocompetent patients are rarely infected. Single cases of pulmonary infection or lymphadenitis caused by Mycobacterium scrofulaceum, Mycobacterium avium complex, or Mycobacterium kansasii have been observed (1). One child with lymphadenitis caused by M. celatum has been reported (8). To our knowledge, a pulmonary infection by M. celatum is an immunocompetent patient has not been described.
Case report.
A 73-year-old female Caucasian patient (163 cm, 61 kg) developed a nonproductive cough. Her medical and family histories were unremarkable, apart from diabetes mellitus type 2 diagnosed in 1985 and treated with glyburide (glibenclamide) (HbA1, 10.5%). Physical examination revealed no pathological findings except moist rales in the upper left lung.
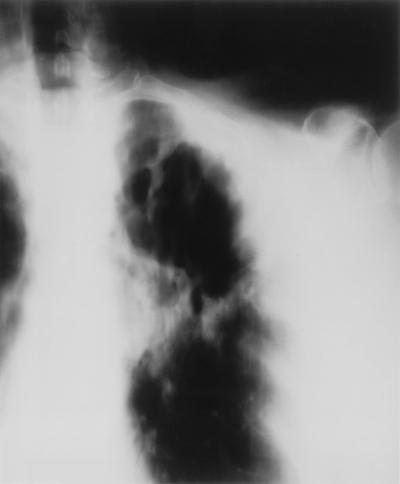
A chest X ray (Fig. 1) and the presence of acid-fast bacteria in the sputum indicated a mycobacterial pulmonary infection. As the strongly positive tine test suggested immunocompetence, infection with Mycobacterium tuberculosis was assumed and treatment was started with isoniazid, pyrazinamide, ethambutol, and rifampin. In the following 4 weeks, the general condition of the patient deteriorated. Her temperature increased to 39°C, and respiratory insufficiency developed. When atypical mycobacteria with resistance to isoniazid and rifampin were isolated, ciprofloxacin and clarithromycin were added to pyrazinamide, ethambutol, theophylline, and inhalation of terbutaline and fluticasone. The patient felt better for 3 weeks. Thereafter, her condition worsened, and a chest X ray showed an extended infiltrate including the left lower pulmonary lobe (Fig. 2). The patient died 10 weeks after admission. M. celatum was identified by a 16S rRNA sequence analysis only after her death.
FIG. 1.
X ray tomogram showing inflammatory infiltrate with cavitation in the upper left lung.
FIG. 2.
X ray taken 10 weeks after that in Fig. 1; the infiltrate had progressed to include the left lower pulmonary lobe.
Laboratory analysis revealed acid-fast bacteria in the gastric juice and the sputum. The erythrocyte sedimentation rate was 120/120 mm/h, hemoglobin was 131 g/liter, thrombocytes were 438/nl, and leukocytes were 8.1/nl (neutrophils, 83%; monocytes, 8%; eosinophils, 1%; lymphocytes, 8%). The lymphocyte subpopulations were T4, 36.5% (237/μl), and T8, 14.7% (95/μl); the T4/T8 ratio was 2.5. Analysis of the blood gases showed a pH of 7.53, a partial CO2 pressure of 5.25 kPa, a partial O2 pressure of 7.5 kPa, and oxygen saturation of 92%. The tine test was strongly positive. Serum protein electrophoresis and clotting tests, serum aminotransferases and electrolytes, and the creatinine clearance rate were within normal limits, and antibodies against human immunodeficiency virus types 1 and 2 were not present.
The organism was initially cultured from sputum with liquid medium (BACTEC; Becton Dickinson) over 3 weeks. Primary culture on solid medium (Löwenstein-Jensen) was unsuccessful. The acid-fast organism from the gastric juice was not cultured. The isolated mycobacterium grew at 31 to 45°C; it was Tween 80 hydrolysis negative, nitrate reductase negative, arylsulfatase negative, and nicotinic acid and pyrazinamidase positive. DNA probes (Accuprobe; Gen-Probe Inc., San Diego, Calif.) specific for M. tuberculosis complex were positive after 5 min but negative after 10 min of hybridization. The gene fragment of the 16S rRNA was sequenced as described previously (3) and identified as belonging to M. celatum. Susceptibility testing by the proportion method with Löwenstein-Jensen medium revealed resistance to isoniazid, rifampin, and pyrazinamide but sensitivity to ethambutol. These results were confirmed by a radiometric method (BACTEC 460TB).
Discussion.
To our knowledge, this is the first report of a pulmonary infection by M. celatum in a patient with apparently normal cellular immunity. This species was initially recognized by biochemical reactions similar to those of M. avium but presented a mycolic acid pattern that was like that of Mycobacterium xenopi (4, 15). Also, the DNA probe used for culture confirmation may give misleading results, because the M. tuberculosis DNA probe shows cross-reactivity in cases of M. celatum (type I) infection, causing false-positive hybridization signals (5 min hybridization time) (2, 16). Identification of M. celatum has been made possible by restriction fragment length polymorphism analysis of the amplified sequence of the Hsp65 gene, multilocus enzyme electrophoresis, and 16S rRNA sequence analysis (3).
M. celatum cannot be identified by biochemical characteristics. At present the most practical way to distinguish M. celatum from other mycobacteria seems to be a positive DNA hybridization signal for M. tuberculosis complex at 5 min but negative hybridization at 10 min with the Accuprobe test.
Our patient did not have AIDS, and the strongly positive intracutaneous reaction to tuberculin indicated cellular immunocompetence. The total T4 helper cell number was low due to lymphopenia. The T4/T8 ratio was in the normal range. Lymphocytopenia in the circulating blood is a characteristic feature of active tuberculosis (10, 12); it may be caused by local recruitment of CD4 T lymphocytes to the sites of infection, such as granulomas and pleural and ascitic exudates, where lymphocytes are abundant (11, 14). A normal peripheral lymphocyte count is rapidly restored when treatment is successful (12). In AIDS, in contrast, total CD4 T lymphocyte numbers are depleted due to human immunodeficiency virus-induced lymphocyte destruction and secondary to impaired lymphocyte production due to loss of the normal thymic-lymphoid architecture (7).
The patient’s mild diabetes mellitus is unlikely to have contributed much to her susceptibility to infection. This means that pulmonary infection with M. celatum occurred in an apparently immunocompetent host. The delay of 4 weeks until the correct diagnosis of a nontuberculous mycobacteriosis was made and treatment with antimicrobials to which the offending organism was resistant may have contributed to the fatal outcome.
This report of a pulmonary infection with M. celatum indicates that not only the known nontuberculous mycobacteria, M. kansasii, M. avium, and M. scrofulaceum, can cause infections in immunocompetent humans (1). An exact and rapid diagnosis with direct amplified tests, as described for M. tuberculosis (5), and advances in diagnostic technology may be crucial for successful treatment of a nontuberculous mycobacterial infection.
REFERENCES
- 1.Brodt H R. Aktuelle Therapie atypischer Mycobacteriosen. Immun Infekt. 1992;20:39–45. [PubMed] [Google Scholar]
- 2.Butler W R, O’Connor S P, Yakrus M A, Gross W M. Cross-reactivity of genetic probe for detection of Mycobacterium tuberculosis with newly described species Mycobacterium celatum. J Clin Microbiol. 1994;32:536–538. doi: 10.1128/jcm.32.2.536-538.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler W R, O’Connor S P, Yakrus M A, Smithwick R W, Plikaytis B B, Moss C W, Floyd M M, Woodley C L, Kilburn J O, Vadney F S, Gross W M. Mycobacterium celatum sp. nov. Int J Syst Bacteriol. 1993;43:539–548. doi: 10.1099/00207713-43-3-539. [DOI] [PubMed] [Google Scholar]
- 4.Butler W R, Thibert L, Kilburn J O. Identification of Mycobacterium avium complex strains and some similar species by high-performance liquid chromatography. J Clin Microbiol. 1992;30:2698–2704. doi: 10.1128/jcm.30.10.2698-2704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canzaro A. Value of direct amplified test for diagnosis of tuberculosis. Lancet. 1996;347:1500–1501. doi: 10.1016/s0140-6736(96)90666-2. [DOI] [PubMed] [Google Scholar]
- 6.Emler S, Praplan P, Robner P, Auckenthaler R, Hirschel B. Disseminierte Infektion mit Mycobacterium celatum. Schweiz Med Wochenschr. 1996;126:1062–1065. [PubMed] [Google Scholar]
- 7.Finkel T H, Banda N K. Indirect mechanism of HIV pathogenesis: how does HIV kill T cells? Curr Opin Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 8.Haase G, Skopnik H, Bätge S, Böttger E C. Cervical lymphadenitis caused by Mycobacterium celatum. Lancet. 1994;344:1020–1021. doi: 10.1016/s0140-6736(94)91680-2. [DOI] [PubMed] [Google Scholar]
- 9.Horsburg C R. Mycobacterium avium complex in the acquired immune deficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 10.Maartens G, Willcox P A, Benatar S R. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;89:291–296. doi: 10.1016/0002-9343(90)90340-j. [DOI] [PubMed] [Google Scholar]
- 11.Montes Santiago J, Gambon Deza F, Pacheco Carracedo M, Cerda Mota T. Cellular immune response in tuberculosis: analysis of T-lymphocytes and their subsets, B-lymphocytes and natural cytotoxic cells in different tuberculosis states and body fluids. Rev Clin Esp. 1996;196:223–227. [PubMed] [Google Scholar]
- 12.Onwubalili J K. Untreated tuberculosis may be associated with lymphopenia, not lymphocytosis. Afr J Med Med Sci. 1990;19:181–183. [PubMed] [Google Scholar]
- 13.Piersimoni C, Tortoli E, De Sio G. Disseminated infection due to Mycobacterium celatum in patient with AIDS. Lancet. 1994;344:332. doi: 10.1016/s0140-6736(94)91369-2. [DOI] [PubMed] [Google Scholar]
- 14.Rook G A W, Carswell J W, Stanford J L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of ‘anergic’ tuberculosis. Clin Exp Immunol. 1976;26:129–132. [PMC free article] [PubMed] [Google Scholar]
- 15.Saubolle M A, Roberts G D, Goodman N L, Davis T, Jonas V. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. 1993. Isolates of nontuberculous mycobacteria (NTM) identified as members of tuberculosis (MTB) complex by commercial genetic probes, abstr. U93; p. 185. [Google Scholar]
- 16.Stockman L, Springer B, Böttger E C, Roberts G D. Mycobacterium tuberculosis nucleic acid probes for rapid diagnosis. Lancet. 1993;341:1486. doi: 10.1016/0140-6736(93)90935-a. [DOI] [PubMed] [Google Scholar]
- 17.Tortoli E, Piersimoni C, Bacosi D, Bartoloni A, Betti F, Bono L, Burrini C, De Sio G, Lacchini C, Mantella A, Orsi P G, Penati V, Simonetti M T, Böttger E C. Isolation of the newly described species Mycobacterium celatum from AIDS Patients. J Clin Microbiol. 1995;33:137–140. doi: 10.1128/jcm.33.1.137-140.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakrus M, Butler W, Kilburn J, Plikaytis B, Moss C, Silcox V, Floyd M, Vadney F, Gross W. Abstracts of the 92nd General Meeting of the American Society for Microbiology 1992. 1992. Characterization of a new drug-resistant mycobacterium species associated with pulmonary disease in AIDS patients, abstr. U-24; p. 169. [Google Scholar]