Abstract
Rabbits immunised with virulent Treponema paraluis-cuniculi were challenged intradermally with graded doses of Treponema pallidum at three, five, seven, 12, and 30 months to ascertain the level of protection to T pallidum at various intervals after immunisation.
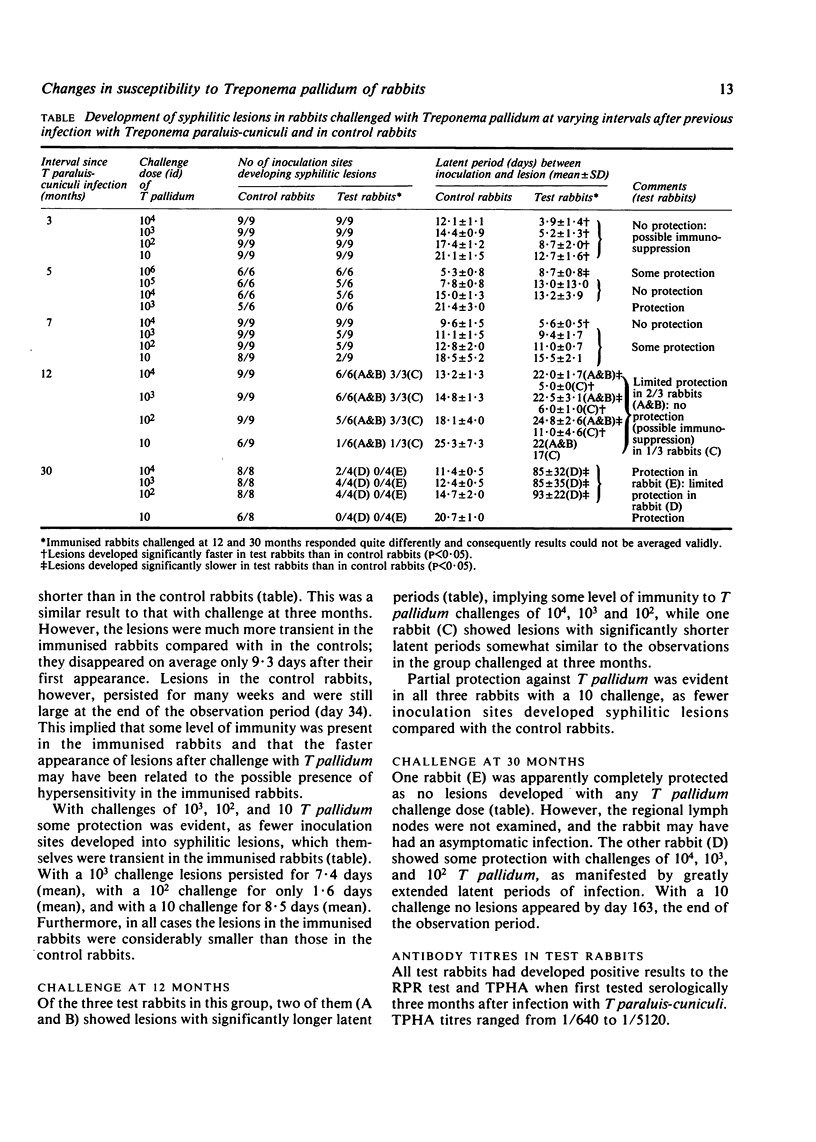
Rabbits challenged at three months after immunisation showed no protection against T pallidum and developed syphilitic lesions significantly faster than the control rabbits, which suggests that the former rabbits were immunosuppressed. Some protection was evident at five and seven months after immunisation, as fewer inoculation sites developed syphilitic lesions with challenges of 103, 102, and 10 T pallidum and lesions developed significantly slower with 106 challenge. Two rabbits showed significant protection at 12 months after immunisation but a third, presumably still immunosuppressed, developed lesions significantly faster than the control rabbits after challenge. At 30 months after immunisation one rabbit was completely protected and developed no lesions after challenge; the other rabbit showed only partial protection against challenge with 104, 103, and 102 but complete protection against challenge with 10 T pallidum.
T paraluis-cuniculi appeared to induce a state of immunosuppression by three months after infection; in one rabbit this may have been 12 months. In most immunised rabbits, however, limited cross-protection to low challenge doses of T pallidum developed by five months and was also detectable at seven and 12 months. Only one rabbit was completely resistant to challenge with 104T pallidum after 30 months and another was only partly immune. Thus, T paraluis-cuniculi infection does not produce a rapid pronounced cross-immunity to T pallidum in rabbits, which may thus limit its usefulness as a vaccine against syphilis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E., Musher D. M. Aberrant secondary antibody responses to sheep erythrocytes in rabbits with experimental syphilis. Infect Immun. 1979 Jul;25(1):133–138. doi: 10.1128/iai.25.1.133-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- From E., Thestrup-Pedersen K., Thulin H. Reactivity of lymphocytes from patients with syphilis towards T. pallidum antigen in the leucocyte migration and lymphocyte transformation tests. Br J Vener Dis. 1976 Aug;52(4):224–229. doi: 10.1136/sti.52.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford K. W., Brostoff J. Leucocyte migration and cell-mediated immunity in syphilis. Br J Vener Dis. 1972 Dec;48(6):483–488. doi: 10.1136/sti.48.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Downes J. Experimental infection of man with rabbit-virulent Treponema paraluis-cuniculi. Br J Vener Dis. 1981 Feb;57(1):7–10. doi: 10.1136/sti.57.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. Susceptibility of rabbits venereally infected with Treponema paraluis-cuniculi to superinfections with Treponema pallidum. Br J Vener Dis. 1980 Dec;56(6):387–389. doi: 10.1136/sti.56.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M., Zeigler J. A., Jones R. H. Experimental syphilis vaccines in rabbits. I. Differential protection with an adjuvant spectrum. Br J Vener Dis. 1976 Feb;52(1):9–17. doi: 10.1136/sti.52.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. The immunology of syphilis. Int J Dermatol. 1976 Oct;15(8):566–576. doi: 10.1111/j.1365-4362.1976.tb04891.x. [DOI] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARING G. W., Jr, FLEMING W. L. Further attempts to immunize rabbits with killed Treponema pallidum. Am J Syph Gonorrhea Vener Dis. 1951 Nov;35(6):568–572. [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. III. Impairment in production of lymphocyte mitogenic factor. Clin Exp Immunol. 1977 Sep;29(3):496–500. [PMC free article] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


