Abstract
Adolescents are particularly attuned to popularity within peer groups, which impacts behaviors such as risk-taking and prosocial behavior. Neurodevelopmental changes orient adolescents toward salient social cues in their environment. We examined whether neural regions that track popularity are associated with longitudinal changes in risk-taking and prosocial behavior. During an fMRI scan, adolescents (n = 109, Mage=13.59, SD=0.59) viewed pictures of their popular and unpopular classmates based on sociometric nominations from their social networks. Neural tracking of high popularity in the dorsomedial prefrontal cortex was associated with increases in risk-taking behavior, whereas tracking of low popularity in the right insula was associated with increases in prosocial behavior. Results suggest that individual differences in neural tracking of popularity relate to longitudinal changes in adolescents’ social behaviors.
Keywords: Peer status, Adolescence, Popularity, Risk-taking, Prosocial, fMRI
Adolescence is a sensitive period for social development wherein peer hierarchies become particularly salient, and enhancing peer status is highly valued (LaFontana and Cillessen, 2010). Such sensitivity to peer status may arise from neural changes in the developing brain that orient adolescents to social cues in their environment. Heightened sensitivity to peer status plays a large role in shaping goals and behavior (Crone and Dahl, 2012), such that adolescents are more likely to prioritize peer status gains over other social and academic goals (LaFontana and Cillessen, 2010). Indeed, adolescents with high peer status (i.e., rated as most popular by their peers) tend to engage in a variety of risky behaviors (Mayeux et al., 2008, Prinstein et al., 2011, van den Broek et al., 2016), and it has been theorized that those who desire to increase their status may engage in greater risk-taking to more closely align themselves with their popular peers (Crone and Dahl, 2012, Op de Macks et al., 2017). On the other hand, adolescents who tend to be more sensitive to peers with low peer status (i.e., rated as least popular; are rejected by their peers) may show increased prosocial behavior that is subserved by an empathic response (Masten et al., 2010, Masten et al., 2011). In the present study, we examined whether adolescents’ neural sensitivity to their high and low status peers from their real-life social networks predicted longitudinal changes in risk-taking and prosocial behavior.
1. Neurobiological Sensitivity to Social Hierarchies
Navigating social hierarchies and detecting valued members is a critical aspect of thriving in social groups. From an evolutionary perspective, these processes are essential for advanced social species and likely played a key role in the evolution of complex neural circuitry in primates and humans (Dunbar, 2012, Silk, 2007). There are numerous studies that probe the cognitive and perceptual processes involved with status detection in adult humans and non-human primates (for a review, see Koski et al., 2015). Yet researchers have only recently investigated the neural mechanisms by which individuals track peer status in real-world networks. These studies have identified that regions associated with social cognition (dorsomedial prefrontal cortex (dmPFC), temporoparietal junction (TPJ), and precuneus) and valuation (ventromedial prefrontal cortex (vmPFC), ventral striatum (VS), and amygdala) spontaneously encode peer status information from real-world peer groups in young adults (Morelli et al., 2018, Parkinson et al., 2017, Zerubavel et al., 2015). However, little is known about these mechanisms and behavioral consequences in adolescence when social hierarchies become especially salient.
Adolescents show an increased orientation towards social cues and peer contexts (Blakemore and Mills, 2014, Nelson et al., 2016, Foulkes and Blakemore, 2016, Perino et al., 2016). For instance, adolescents focus more on social hierarchies within their peer networks compared to children and adults (Prinstein, 2017). Moreover, when viewing images of peers and social rewards, adolescents show greater activation than adults in brain regions associated with valuation (e.g., vmPFC, VS, and amygdala; Perino et al., 2016; Somerville et al., 2011) and social cognition (e.g., dmPFC and TPJ; Blakemore et al., 2007; Pfeifer et al., 2009; Somerville et al., 2013; van den Bos et al., 2011; Wang et al., 2006). Although no research to date has examined how these neural regions associated with valuation and social cognition track peer status in adolescents, it is plausible that they would track status cues within the peer group because high status peers are highly valued and because adolescents wish to understand the thoughts and intentions of these peers.
While many socio-cognitive and perceptual processes preferentially encode information pertaining to high status individuals (Koski et al., 2015), few studies have probed whether any neural systems are particularly involved with attending to information about individuals with low status. However, numerous studies have investigated the neural correlates of witnessing rejection in an experimental setting. These paradigms have consistently elicited activations in regions associated with affective empathy (insula and dorsal anterior cingulate cortex (dACC)), which involves sharing the emotional experiences or pain of others, and cognitive empathy (mPFC and precuneus), which involves taking the perspective of others (Masten et al., 2010, Masten et al., 2011, Meulen et al., 2016). However, these findings are limited to specific incidents of exclusion in an experiment and may not extend to the passive viewing of chronically rejected peers. For example, in a task in which young adults passively viewed peers within their social network, mPFC activation discriminated high status peers from others, but did not differentiate between medium and low status peers (Morelli et al., 2018).
2. The role of peer status in adolescents’ risky and prosocial behavior
Adolescents are more sensitive to feedback related to peer acceptance and rejection (Silk et al., 2012) and feel greater distress following social exclusion compared to children and adults (Sebastian et al., 2010). Thus, it is not surprising that adolescents are more likely to adjust their behaviors to conform with high status individuals within their social networks (Brechwald and Prinstein, 2011, Crone and Dahl, 2012). Adolescents with higher status are more likely to engage in tobacco, marijuana, and alcohol use, as well as risky sexual behavior (Agan et al., 2015, Choukas-Bradley et al., 2015, Mayeux et al., 2008, Prinstein et al., 2011), and their peers can recognize this association (Gibbons et al., 2003, Mayeux et al., 2008). Since adolescents recognize that risky behaviors are associated with higher status, those who value status and are highly sensitive to it may be more likely to engage in risky behaviors as a status-seeking behavior (Crone and Dahl, 2012, Gibbons et al., 2003). Indeed, engagement in risky behaviors has been associated with greater orientation towards high-status peers (Fuligni et al., 2001, Prinstein et al., 2011) and status-seeking behaviors in digital contexts (Nesi and Prinstein, 2019).
While low status peers are less likely to be prosocial themselves, they are more likely to be the beneficiaries of prosocial behavior (van Rijsewijk et al., 2016), indicating that others’ orientation towards rejected peers may subserve increased prosociality. This is supported by neuroimaging findings that witnessing rejection is associated with heightened activation in brain regions involved in cognitive and affective empathy (e.g., insula dACC, mPFC and precuneus), which in turn predicts increased prosociality towards the victim (Masten et al., 2010, Masten et al., 2011, Meulen et al., 2016). Thus, variance in neural sensitivity to unpopular peers may reflect individual differences in empathic responses, which may ultimately drive differences in prosocial behavior.
3. The current study
In the current study, we examined how individual differences in neural sensitivity to peers with high and low status predicts longitudinal changes in risk-taking and prosocial behavior in early adolescence. It is important to note that, while research has differentiated between two forms of status in adolescence – popularity (whether one is viewed by their peers as being popular, regardless of whether they are well liked) and social preference (whether one is well-liked by their peers) – the current study solely focuses on popularity, since enhancing popularity is particularly valued by adolescents and has the greatest potential to influence behavior (Gommans et al., 2017). Social preference data (i.e., liking) was collected, and the associated analyses can be found in the supplement.
Participants completed a novel fMRI task, the “Classmates Task”, wherein they viewed yearbook photos of their classmates from their school and grade. We used peer-nominated sociometric ratings from 873 students across 3 middle-schools to sort these photos by popularity, such that participants saw sequences of highly popular and unpopular peers from their school grade. Studying sensitivity to popularity is particularly appropriate for this age range given that prioritization of status peaks in early adolescence (Duell et al., 2021). By utilizing sociometric data and ecologically valid stimuli of real-world peers, this study allows us to further our understanding of how adolescents perceive popularity in their social networks. Moreover, by leveraging longitudinal data, this study helps elucidate how sensitivity to social hierarchies contributes to positive and negative behavioral trajectories.
We assessed whether brain regions that track the status of peers from their real-life social network predict changes in adolescents’ risk-taking and prosocial behavior over the course of a year. Based on research in adults (Morelli et al., 2018, Parkinson et al., 2017, Zerubavel et al., 2015), we expected to find neural tracking of high popularity in regions associated with social cognition (dmPFC, TPJ and precuneus) and valuation (vmPFC, ventral striatum, and amygdala), and that heightened neural tracking to popularity in these regions would predict longitudinal increases in risk-taking behavior over the course of a year. Given the lack of findings regarding neural sensitivity to low popularity, these analyses are exploratory. However, previous work on neural sensitivity to witnessing rejection suggests that neural tracking of low popularity may occur in regions associated with affective (insula and dACC) and cognitive (mPFC) empathy, and that individual differences in such activation may predict subsequent prosocial behavior (Masten et al., 2010, Masten et al., 2011, Meulen et al., 2016).
4. Methods
4.1. Participants
This study consists of data collection across 3 waves when participants were in the 6th and 7th grade (wave 1, 2016–2017 school year), 7th and 8th grade (wave 2, 2017–2018 school year), and 8th and 9th grade (wave 3, 2018–2019 school year). For the purposes of this study, sociometric nominations were obtained during school-based testing at wave 1 and 2, fMRI data were collected at wave 2, and self-report measures were obtained in waves 2 and 3.
Participants were recruited from three rural public middle schools in the southeast United States (N = 1385) as part of a five-year longitudinal study. Letters of consent were mailed to all caregivers of students, with an option to grant or deny consent for their child to participate in the study. Approximately 77 % of families (n = 1059 families) returned signed forms; 88 % (n = 935) of these gave consent for their child to participate, yielding a sample that represented 67.5 % of the population in this community. A total of 873 consented students participated in school-based assessments at wave 1 (see supplement for demographics of the full school sample). School-based assessments were collected in the schools annually. Assent and data were obtained using computer-assisted self-interviews administered by trained research staff in school. At each wave, peer nominations and self-report measures were collected, including the prosocial tendencies measure. Participants were compensated with a monetary remuneration of $5 at each wave.
Participants from the larger study who provided interest in being contacted for a future study were called and screened for eligibility (i.e., MRI contraindications) and recruited for the fMRI study within the same academic year as the larger study. Participants completed annual fMRI scans, each one year apart. For the first wave of data collection, 284 families were screened, of whom 91 were ineligible due to learning disabilities, braces, head trauma, or other MRI contraindications, and 45 were eligible but did not participate due to scheduling difficulties or no longer interested in participating, resulting in a final sample of 148 adolescents. To account for attrition, an additional 30 participants were recruited at wave 2 to the neuroimaging subsample. Adolescents and parents gave written assent/consent in accordance with the university’s Institutional Review Board. At wave 2, participants completed an fMRI scan that lasted approximately 1.5 h, during which they completed the Classmates task, as well as four other tasks that are not the focus of this manuscript. Following the scan, adolescents completed several self-report measures using computer-assisted software in a private room, including risk-taking behaviors, as well as other measures which are not the focus of this manuscript. Adolescents were compensated with a monetary remuneration of $90, a $20 gift card for performing well in the scanner (e.g., minimum motion), an extra $25 as a bonus for returning to our study for wave 2, snacks during the visit, and a meal. Parents were compensated with a monetary remuneration of $50, as well as a meal, compensation for gas, and parking.
Of the 141 participants who completed an fMRI scan at wave 2, 135 participants completed the Classmates fMRI task. Of these, 3 were excluded from analyses for excessive head motion (> 2 mm in any direction), 15 were excluded due to an error in calculating the sociometric ratings of target images in the Classmates task (see the “fMRI task” subsection for more details) and 8 were excluded for missing sociometric data, resulting in a final sample of 109 adolescents (57 female) ages 12–15 (Mage = 13.59, SD = 0.59). Of these 109 participants, 103 had the risk-taking measure and 100 had the prosocial tendencies measure across waves 2 and 3. Participants were from diverse racial/ethnic backgrounds (39 Hispanic/Latino, 33 White, 23 Black/African American, 12 multiracial, 2 Native American). Overall, the sample was from low to middle socioeconomic status in terms of parental reported household income (32 % <$30,000, 40% $30–$60,000, 25% over $60,000, 3 % did not respond), and parental education of the primary caregiver (24% less than high school, 11 % high school diploma, 38 % some college, 27 % associate degree or higher).
4.2. Measures
Data used in the current study include sociometric ratings of peer status collected at wave 1 and 2, the Classmates fMRI task collected at wave 2, and self-report measures of risk-taking and prosocial behaviors collected at wave 2 and 3.
4.2.1. Sociometric ratings of peer status
Sociometric procedures were used to measure peer status as part of the larger study. During the school based assessment, participants were given a full list of their peers within their school and grade level and were asked to identify (1) who they like the most, (2) who they like the least, 3) who is the most popular, and (4) who is the least popular. There was no limit to the number of peers they could nominate. Based on the peer nominations from the school based assessment, we calculated social preference (z-score of “liked the most” minus z-score of “liked the least”) and popularity (z-score of “most popular” minus z-score of “least popular”) scores for each participant in the school. This z-score for each sociometric index is based on their relative rating compared to other students within their school and grade. These peer nominations collected during wave 1 were used to create the stimuli in the fMRI task, which was administered at wave 2. Peer nominations were also collected during wave 2, which were used to control for participants’ own popularity in analyses.
4.2.2. Prosocial Tendencies
During the school based assessment, participants completed the Prosocial Tendencies Measure (Carlo and Randall, 2002). Adolescents reported on how much they feel 19 different behavioral tendencies apply to them on a 5-point scale (1 = Does not describe me at all, 2 = Describes me a little, 3 = Somewhat describes me, 4 = Describes me well, 5 = Describes me greatly). The scale includes 5 subscales that assess different motivations for prosocial behavior. In the current study, we focused on the 4 subscales that are most closely associated with positive, other-oriented motivations for prosocial behavior, including anonymous (4 questions; e.g., “Most of the time, I help others who do not know who helped them”), emotional (5 questions; e.g., “I tend to help others when they are very emotional”), dire (3 questions; e.g., “I tend to help people who are in a real crisis or need”), and altruism (4 questions, reverse-scored; e.g., “I feel that if I help someone, they should help me in the future”). We took the average of all items across these subscales to create one index of prosocial behavior (Cronbach's αWave2 = 0.84, αWave3 = 0.87).
4.2.3. Risk-Taking Behaviors
Participants completed a modified version of the Adolescent Risk-taking Scale (Alexander et al., 1990). Adolescents reported on their frequency of engaging in 14 risky behaviors on a 4-point scale (0 = never, 1 = once or twice, 2 = several times, 3 = many times). The scale included questions about rule breaking (e.g., “I have snuck out of my house without my parents knowing”), sexual activity (e.g., “I have had sex with someone I just met”), substance use (e.g., “I have gotten drunk or high at a party”), and dangerous behavior (e.g., “I did something risky or dangerous on a dare”). A total mean score for all items was calculated (Cronbach's αWave2 = 0.72, αWave3 = 0.75).
4.3. fMRI Task
During the Classmates task, which was adapted from Parkinson et al. (2017), participants viewed yearbook photos of their peers from school. The yearbook photos (i.e., targets) used in the task were selected based on the sociometric data from the previous year (wave 1), because of the time required to process the sociometric data and create the scan task. However, we ran correlations between wave 1 and wave 2 for all four sociometric categories to establish that peer status was highly stable across years (i.e. High Popularity: r = 0.67, p < .001; Low Popularity: r = 0.76, p < .001; High Social Preference: r = 0.80, p < .001; Low Social Preference: r = 0.81, p < .001) in line with prior research (Dijkstra et al., 2013). To be selected as a target for the task, the peer needed to have a sociometric z-score between 1 and 5 (representing 1–5 SD above the mean on popularity/social preference in their school and grade) or between − 1 and − 5 (representing 1–5 SD below the mean on popularity/social preference in their school and grade). One version of the task was created for each grade level within each school (three middle schools, each with 2 grades, resulting in six versions total). The task had four conditions: High social preference (i.e., z-score between 1 and 5 on social preference), low social preference (i.e., z-score between −1 and −5 on social preference), high popularity (e.g., z-score between 1 and 5 on popularity), and low popularity (i.e., z-score between −1 and −5 on popularity). Within each condition, there were 10 targets, and we aimed for an equal number of boys and girls within each condition. Due to a data management error, z-scores for the targets from one of the schools (two of the six task versions) were incorrect and did not necessarily fall within the criteria (i.e., z-score between ± 1 and 5). Popularity and social preference scores were recalculated for the target images in these task versions. For one group of participants (n = 20), there were a sufficient number of target images that still fit the criteria for each condition (High Popularity = 9, Low Popularity = 8, High Social Preference = 8, Low Social Preference = 10), so these participants were included. For the other group, there were an insufficient number of target images fitting the criteria (e.g., as few as 5) in the conditions, so these participants (n = 15) were excluded. Across all versions of the task that were retained, the average z-score within each condition was approximately 2 (see supplement for details). There was no overlap in the targets across conditions, such that each target belonged in only one sociometric category and appeared in only one condition. No participants were included as targets so that no participant would see their own image. Target photos were obtained from school yearbooks from the previous school year, the same year the sociometric ratings were collected. Yearbook photos were digitized into JPEG images.
The task was programmed in E-Prime and presented across two runs. Each run consisted of eight blocks, two blocks per condition, each with 10 faces. Within each run, participants were presented with the eight blocks in a randomized order. The order in which their faces were shown was fixed within the block with the order pre-selected based on a randomization algorithm. We used an n-back task design (Parkinson et al., 2017) to ensure that the participants were paying attention, such that each block contained one target that appeared twice in a row. Participants were instructed to press with their right pointer finger when a target repeated. Due to the limited number of peers who satisfied our criteria for high and low status (greater or less than one standard deviation from the average) and in order to achieve the power for neuroimaging analysis on visual stimuli, we repeated each peer face across the conditions, in line with previous studies (e.g., Zerubavel et al., 2015; Parkinson et al., 2017). Having participants passively view faces was also in line with these previous studies in young adults, allowing us to probe the spontaneous neural processes adolescents use to encode social status information. The repeated targets were fixed in the task and balanced so that no target was shown more than another (i.e., when a target was shown twice in one block, that target was absent from the next block). Thus, participants saw each face 4 times total (2 in each run), with each condition having 40 total trials each. Each target face was presented for 1750 ms, separated by a jittered inter-trial interval (M=2300 ms, range: 565.8 – 4936.8 ms). Each block was approximately 40.5 s, and the total time of each run was 336 s
4.4. fMRI data acquisition and preprocessing
Imaging data were collected using a 3 Tesla Siemens Prisma MRI scanner. The Classmates Task was presented on a computer screen and projected through a mirror. A high-resolution structural T2 * -weighted echo-planar imaging (EPI) volume (TR = 2000 ms; TE = 25 ms; matrix = 92 ×92; FOV = 230 mm; 37 slices; slice thickness = 3 mm; voxel size 2.5 × 2.5 × 3 mm3) was acquired coplanar with a T2 * -weighted structural matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 5700 ms; TE = 65 ms; matrix = 192 ×192; FOV = 230 mm; 38 slices; slice thickness = 3 mm). In addition, a T1 * magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 2400 ms; TE = 2.22 ms; matrix = 256 × 256; FOV = 256 mm; sagittal plane; slice thickness = 0.8 mm; 208 slices) was acquired. The orientation for the EPI and MBW scans was oblique axial to maximize brain coverage and to reduce noise. Preprocessing was conducted using FSL (FMRIB’s Software Library, version 6.0; www.fmrib.ox.ac.uk/fsl) and included the following steps: Skull stripping using BET (Smith, 2002); motion correction with MCFLIRT (Jenkinson et al., 2002); spatial smoothing with Gaussian kernel of full width at half maximum (FWHM) 6 mm; high-pass temporal filtering with a filter width of 128 s (Gaussian-weighted least-squares straight line fitting, with sigma=64.0 s); grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; and individual level ICA denoising for motion and physiological noise using MELODIC (version 3.15; Beckmann and Smith, 2004), combined with an automated signal classifier (Tohka et al., 2008; Neyman-Pearson threshold =0.3). For the spatial normalization, the EPI data were registered to the T1 image with a linear transformation, followed by a white-matter boundary based transformation (BBR; Greve and Fischl, 2009) using FLIRT, linear and non-linear transformations to standard Montreal Neurological Institute (MNI) 2-mm brain were performed using Advanced Neuroimaging Tools (ANTs; Avants et al., 2011), and then spatial normalization of the EPI image to the MNI. Quality check during preprocessing and analyses ensured adequate signal coverage.
4.5. fMRI Data analysis
Individual level, fixed-effects analyses were estimated using the general linear model convolved with a canonical hemodynamic response function in SPM12. We modeled the task as an event-related design with four separate conditions wherein sociometric ratings were used as parametric modulators: high popularity, low popularity, high social preference, and low social preference. Inter-trial jitters were not explicitly modeled and thus served as an implicit baseline. The absolute value of the sociometric rating for the target (i.e., social preference score for the low and high social preference conditions; popularity score for the low and high popular conditions) was included as a parametric modulator (PM) at the trial level. Importantly, the sociometric ratings ranged from relatively lower to high scores within each of the four conditions, which allowed us to examine whether adolescents linearly track peer status at the neural level within each condition (e.g., linear tracking of peers with sociometric popularity between 1 and 5 SD above average for the high popularity condition, see supplement for details). Within each block, one target was repeated as an attention check. The repeated target was treated as a separate condition and was modeled as a contrast of no interest. TRs with motion greater than.5 framewise displacement were modeled as a nuisance regressor. Since our primary aim was to examine adolescents’ sensitivity to popularity, we focus our analyses on this condition. Moreover, we decided to analyze popularity without controlling for social preference ratings. While popularity and social preference are different constructs, they share contributing factors (e.g. attractiveness, social competency, extraversion, etc.) (Sandstrom and Cillessen, 2006), and are often correlated with one another (in our sample, r = 0.45, p < .001). Since these constructs are correlated with one another, controlling for social preference among target images may remove important variance. Analyses solely focusing on social preference can be found in the supplement.
The individual-level contrast images were submitted to random effects group-level analyses using GlmFlex. We examined if neural sensitivity to high and low popularity (using popularity scores as parametric modulators in each condition) differentially predicted longitudinal changes in risk-taking and prosocial behavior. To this end, we ran whole brain regressions in which we regressed neural tracking of popularity at wave 2 onto risk-taking at wave 3 controlling for risk-taking at wave 2. We ran the same model with prosocial behaviors. In addition, we controlled for participants’ own sociometric popularity from wave 2. Results from these whole brain analyses reveal whether brain regions which show increasing sensitivity to popularity, measured via the parametric modulators, predict longitudinal changes in risk-taking and prosocial behavior.
To correct for multiple comparisons, the spatial autocorrelation function (acf) option was used in AFNI’s 3dFWHMx to estimate intrinsic smoothness. The individual-level residuals were entered into 3dFWHMx along with the brain mask. Because real fMRI data does not have a true Gaussian-shaped ACF, 3dFWHMx estimates smoothness by fitting the ACF to a mixed model that is Gaussian plus mono-exponential. 3dClustSim was used to estimate the probability of false positives using 2-sided thresholding and the NN1 option using the corrected approach recommended by Eklund et al. (2016). Cluster size corrections for multiple comparisons at a < 0.05 over the whole brain were achieved at a voxel-wise threshold of p < .005. Results of the simulation yielded a voxel-wise threshold of p < .005 combined with a minimum cluster size of 208 voxels for the main effects, 197 voxels for the regression of risk-taking, and 204 voxels for the regression of prosocial behavior (given the different sample sizes), corresponding to p < .05, family-wise error corrected. The group level acf values were as follows: 0.546944793, 4.685530941, 12.73257414 (main effects); 0.549268783, 4.667489394, 12.68986948 (risk-taking); 0.547707491, 4.68737725, 12.67997578 (prosocial behavior).
5. Results
5.1. Descriptive analyses
First, we examined correlations among adolescents’ own popularity and their self-reported risk-taking and prosocial behaviors (Table 1). Adolescents’ own popularity at wave 2 was correlated with their risk-taking behavior at wave 2 but was not associated with their prosocial behavior in either wave.
Table 1.
Summary statistics and correlations of all study variables.
| Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| 1. Risk Taking Wave 2 | 0.27 | 0.23 | |||||
| 2. Risk Taking Wave 3 | 0.35 | 0.29 | 0.52** | ||||
| 3. Prosocial Behavior Wave 2 | 3.44 | 0.58 | 0.17 | 0.12 | |||
| 4. Prosocial Behavior Wave 3 | 3.55 | 0.60 | -0.12 | -0.09 | 0.59** | ||
| 5. Popularity Wave 2 | -0.21 | 1.28 | 0.20* | 0.19 | -0.04 | -0.02 | |
| 6. Age Wave 1 | 13.59 | 0.59 | -0.01 | 0.13 | -0.01 | 0.03 | 0.17 |
Note. Computed correlation used Pearson-method, * p < .05, ** p < .01.
5.2. Main effects of neural tracking of high popularity
To examine the main effect of neural tracking of high popularity, we first conducted a random-effects, whole-brain analysis at the group level for the High Popularity condition (i.e., when viewing peers whose popularity was > 1 SD above mean), using popularity as a parametric modulator. We did not find main effects for any regions that linearly track increases in popularity. Instead, the precuneus, among other regions (Table 2A), negatively tracked popularity, such that activation decreased as the popularity of target images increased.
Table 2.
Main effects for neural tracking of high and low popularity.
| Anatomical Region | x | y | z | t | k |
|---|---|---|---|---|---|
| A. High Popularity: | |||||
| R Fusiform Gyrus | 26 | -40 | -42 | -4.54 | 285 |
| L Cerebellum/Precuneus | 4 | -40 | 6 | -4.48 | 437 |
| L Cerebellum | -30 | -72 | -18 | -4.29 | 492 |
| L Precentral Gyrus | -42 | -10 | 32 | -4.11 | 351 |
| B. Low Popularity: | |||||
| Dorsal Anterior Cingulate Cortex | 8 | 12 | 38 | -5.58 | 2572 |
| R Insula | 44 | 10 | 2 | -5.29 | 4125 |
| L Insula | -36 | 12 | 8 | -4.78 | 4937 |
| Thalamus | 8 | -24 | 16 | -4.37 | 1047 |
| Posterior Cingulate Cortex | 2 | -22 | 34 | -3.89 | 338 |
| L Middle Temporal Gyrus | -58 | -64 | 4 | -3.78 | 250 |
Note. L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to the MNI coordinates. Corrected cluster size: 207 contiguous voxels. All results are p < .005, corrected. All test statistics for the main effect were negative, such that these regions decreased in activation as the popularity increased within the high popularity condition, whereas they decreased in activation as popularity decreased within the low popularity condition.
5.3. Main effects of neural tracking of low popularity
We also conducted a random-effects, whole-brain analysis at the group level for the Low Popularity condition (i.e., when viewing peers whose popularity was < 1 SD below mean), using the absolute value of popularity as a parametric modulator. The insula and cingulate cortex, among other regions (Table 2B) negatively tracked low popularity, such that these regions exhibited less activation for the least popular target images.
5.4. Neural tracking of high popularity and longitudinal links to risk-taking and prosocial behavior
Our primary analyses examined how individual differences in neural tracking of highly popular peers were associated with changes in adolescents’ self-reported risk-taking and prosocial behavior over one year. In whole-brain analyses, we regressed self-reported risk-taking at wave 3 onto neural tracking of popularity, controlling for risk-taking at wave 2 as well as participants’ own popularity at wave 2.
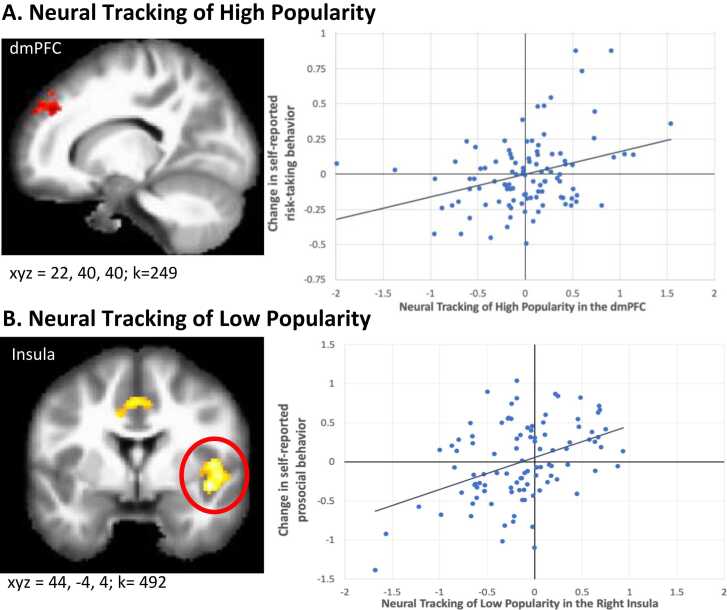
Neural tracking of high popularity in the right dorsomedial prefrontal cortex (dmPFC) was associated with changes in risk-taking behavior (Table 3A). For descriptive purposes, we extracted parameter estimates of signal intensity from the dmPFC, where estimates reflect neural tracking of popularity (i.e., linear increases in the dmPFC as popularity increased). We plotted neural tracking of popularity in the dmPFC against risk-taking at wave 3 controlling for risk-taking at wave 2. As shown in Fig. 1A, positive tracking (i.e., linear increases in activation) of high popularity in the dmPFC was associated with increases in risk-taking behaviors, whereas negative tracking (i.e., linear decreases in activation) of high popularity in the dmPFC was associated with decreases in risk-taking behaviors. We found no significant clusters for the regression of prosocial behavior onto neural tracking of high popularity.
Table 3.
Neural regions that linearly tracked high (A) and low (B) popularity that correlated with changes in (A) Risk-Taking and (B) Prosocial Behavior.
| Anatomical region | x | y | z | t | k |
|---|---|---|---|---|---|
| A. High Popularity: Changes in Risk-Taking Behavior | |||||
| R dmPFC | 22 | 40 | 40 | 3.40 | 249 |
| B. Low Popularity: Changes in Prosocial Behavior | |||||
| R Insula | 44 | -4 | 4 | 4.27 | 492 |
| MCC | -8 | -12 | 40 | 3.60 | 234 |
| Sensorimotor Cortex | -8 | -14 | 64 | 4.14 | 456 |
Note. L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to the MNI coordinates. Corrected cluster size: 197 (for Risk-taking analysis), 204 (for prosocial behavior analysis) contiguous voxels. All results are p < .005, corrected.
Fig. 1.
(A) Neural tracking of high popularity in the right dmPFC correlates with increases in risk taking and (B) Neural tracking of low popularity in the right insula correlates with increases in prosocial behaviors. The x-axes represent neural tracking of popularity; values reflect the linear slopes of voxel activation with respect to 1-unit increases (A) or decreases (B) in popularity. The y-axes represent changes in behavior such that (A) indicates risk-taking at wave 3 controlling for risk-taking at wave 2, and (B) represents prosocial behavior at wave 3 controlling for prosocial behavior at wave 2.
5.5. Neural tracking of low popularity and longitudinal links to risk-taking and prosocial behavior
Neural tracking of low popularity in the right insula, midcingulate cortex (MCC), and sensorimotor cortex were associated with changes in prosocial behavior (Table 3B).1 For descriptive purposes, we extracted parameter estimates of signal intensity from the insula, where estimates reflect neural tracking of low popularity (i.e., linear increases in the insula as low popularity increased). We plotted neural tracking of low popularity in this region against prosocial behavior at wave 3 controlling for prosocial behavior at wave 2. As shown in Fig. 1B, positive tracking (i.e., linear increases in activation) of low popularity in this region was associated with increases in prosocial behavior, whereas negative tracking (i.e., linear decreases in activation) of low popularity in this region was associated with decreases in risk-taking behaviors.2 We found no significant clusters for the regression of risk-taking onto neural tracking of low popularity.
6. Discussion
Adolescence is characterized by an increased sensitivity to peer status and social evaluation, as well as changes in behaviors that may increase an individual’s peer status. This study evaluated how neural sensitivity to popular and unpopular peers in adolescents’ school-based social networks was associated with changes in risk-taking and prosocial behavior. We identified regions wherein individual differences in neural tracking of popularity related to longitudinal changes in risk-taking and prosocial behavior. In particular, we found that adolescents who showed increased neural tracking to popularity in the dmPFC showed longitudinal increases in risk-taking behavior over the course of one year. Additionally, heightened sensitivity in the insula to participants’ least popular peers was associated with increases in prosocial behavior. These results suggest that heightened neural sensitivity to popular and unpopular peers may influence the trajectories of risk-taking and prosocial behavior, respectively.
6.1. Neural sensitivity to high popularity is associated with risk-taking
Given the clear link between popularity and risk-taking in adolescence (Agan et al., 2015, Choukas-Bradley et al., 2015, Mayeux et al., 2008, Prinstein et al., 2011), we hypothesized that greater neural sensitivity to popular peers would be associated with longitudinal increases in risky behaviors. Indeed, adolescents who showed increased neural tracking of popularity in the dmPFC at wave 2 were more likely to increase their risk-taking over the course of one year. Interestingly, those who showed lower neural tracking of popularity in the dmPFC (i.e., those who had higher neural activation in response to viewing their less popular peers) were more likely to decrease risk-taking behaviors over the same time period. The dmPFC is often associated with the process of mentalizing, wherein individuals consider the thoughts, beliefs and intentions of others, (Blakemore and Mills, 2014). Additionally, in adolescence the dmPFC plays a large role in processing information about the self, and has also been activated when incorporating information about distant or dissimilar others (Crone and Fuligni, 2020, van Buuren et al., 2022). Thus, stronger neural tracking of popularity in the dmPFC could reflect a heightened tendency to consider the thoughts and beliefs of more popular (relative to less popular) peers, and potentially a higher tendency to incorporate these ideas into evaluations of the self. This could, in turn, lead to increases in behaviors that are typically characteristic of popularity in the peer group, such as risk-taking. Moreover, lower neural tracking of popularity was generally associated with decreases in risk-taking, which might suggest that lower mental state reasoning and consideration of the thoughts of popular peers is associated with less engagement in their characteristic behaviors. However, given that our fMRI task only involved the passive viewing of peers’ faces, we cannot say for certain that our participants were engaged in the process of mentalizing or self-reference, and there may be other explanations for dmPFC activation. Importantly, we controlled for participants’ own popularity given that individual’s own peer status is associated with longitudinal increases in risk-taking (Mayeux et al., 2008). Thus, our results suggest that adolescents’ neural sensitivity to popularity is predictive of changes in their risky behavior beyond those explained by their own social standing.
6.2. Neural sensitivity to low popularity is associated with prosocial behavior
Although less is known about sensitivity to low popularity, previous studies have linked heightened neural sensitivity when witnessing rejection to prosocial behavior (Masten et al., 2010, Masten et al., 2011, Meulen et al., 2016). We found that increased tracking of low popularity in the insula predicted increased prosocial behavior over the course of one year. One potential explanation for this finding is that increased activation of these regions in response to the least popular peers reflects heightened affective empathy, and that this subserves increased tendencies for prosocial behavior in various contexts. Notably, we did not find similar effects for regions associated with the cognitive component of empathy, such as the mPFC or precuneus. Given that previous studies found activation in these regions when actually performing a prosocial action toward a rejected individual (Meulen et al., 2016), it may be the case that affective empathy subserves the increased tendency for prosocial behavior overall, whereas cognitive empathy is employed during specific instances of prosociality, particularly toward rejected or unpopular peers.
6.3. Addressing null results
Although caution must be exercised when interpreting null results, it is notable that, contrary to our hypotheses, no main effects were found for the high popularity condition in regions we hypothesized based off of findings in adults (e.g., Morelli et al., 2018; Parkinson et al., 2017; Zerubavel et al., 2015). This could potentially be the result of methodological differences (e.g., only evaluating tracking within high popularity, as opposed to across the full continuum) or developmental differences (e.g., adolescents may exhibit greater variance in neural tracking of popular peers than adults, such that some negatively track high popularity and others positively track it, resulting in a null main effect). In addition, no effects were found for regions of the brain associated with valuation, such as the vmPFC, VS, and amygdala. One potential explanation is that these regions may be engaged, but do not linearly track popularity, as measured by the parametric modulator. Alternatively, it may be the case that simply observing popular peers does not induce valuation, but rather that such activation is contingent on actions or feedback from these peers. Additionally, these regions are relatively small, and our task may not yield large enough activations to surpass our whole-brain threshold.
6.4. Contributions, limitations, and future directions
This study has several strengths. While prior work has identified neural systems that track popularity in adults (Morelli et al., 2018, Parkinson et al., 2017, Zerubavel et al., 2015), this study provides a novel contribution by assessing how individual differences in sensitivity to peer status in adolescents are associated with longitudinal changes in social behaviors. Moreover, this study is the first to gauge neural sensitivity to peer status and its behavioral correlates in early adolescence, when popularity is the most valued (LaFontana and Cillessen, 2010) and most likely to influence behavior (Crone and Dahl, 2012). Additionally, we utilized a large longitudinal sample and robust sociometric data, allowing us to capture individual differences across multiple school social networks. Lastly, by using stimuli of peers from adolescent’s real-world social networks, the Classmates task has high ecological validity and contributes to our knowledge of the social processes that underlie adolescents’ status-related behaviors.
Nonetheless, the study has some key limitations. Primarily, while the relations between neural tracking of popularity and behavioral outcomes may lay groundwork for future theories, this study cannot determine mechanisms by which the two may be related. For example, to explain changes in risk-taking, it may be the case that greater sensitivity to popularity results in greater orientation toward the beliefs and behaviors of popular peers, which in turn leads to greater conformity to their ideals (Cialdini and Goldstein, 2004). Alternatively, those who track popularity in the brain may be more likely to seek out friendships with popular peers, and in turn have increased social opportunities to engage in risk-taking. Individuals often strive to befriend peers who are more popular in order to enhance their own peer status (Dijkstra et al., 2010, Dijkstra et al., 2013, Shin, 2017), so it is also possible that behavioral changes over the course of the year are the result of both peer status goals and changes in friendship networks. Additionally, risk-taking behavior was fairly low in our sample (means of 0.27 and 0.35 across two years on a 0–4 scale). While it is important to capture variance in risk-taking behavior in early adolescence (Collado et al., 2014, Crone et al., 2016), it is important to recognize these low rates and understand that our findings may not translate to populations whose risk taking is higher (such as older adolescents or those at greater risk for externalizing behavior and psychopathology). Lastly, it is important to recognize that risk-taking is not only affected by popularity, but also by other factors such as mood, interpersonal conflict, and social exclusion (Arnett, 1999, Peake et al., 2013). Nonetheless, future studies should evaluate potential mediators, such as peer conformity or friendship network dynamics, that may explain the relations between neural sensitivity to peer status and risk-taking behavior. For understanding the link between sensitivity to low popularity and prosocial behavior, future work could probe whether increased prosociality is primarily directed toward rejected peers, or whether greater sensitivity to low popularity is indicative of stronger empathy and prosociality overall. Additionally, our task only involves the passive viewing of faces, so our ability to make inferences about broader social cognition and empathy processes is limited. To better understand the processes that underlie neural sensitivity to popularity, future work should investigate whether peer status sensitivity is associated with broader perspective taking and empathic abilities. Moreover, while our results held when controlling for participants’ sex, it is well established that sex and gender play a large role in social status and its behavioral correlates, including risk-taking and prosocial behavior (Prinstein et al., 2011, Shin, 2017), so future studies may benefit from examining the moderating role of sex or gender. Lastly, our task does not include peers with average levels of popularity, so future studies should include this group for a full understanding of popularity tracking across the entire peer group.
Overall, this research suggests that the neural mechanisms by which adolescents track popularity in real world social networks are related to behavioral changes relevant to peer status hierarchies. Given the importance of promoting positive prosocial behaviors and predicting dangerous risk behaviors in adolescence, this work advances a critical line of research that identifies how aspects of the peer environment interact with intraindividual neural systems to produce social behavior.
Data Statement
Data Statement for: Adolescents’ Neural Sensitivity to High and Low Popularity: Longitudinal Links to Risk-Taking and Prosocial Behavior fMRI results are publicly available at https://neurovault.org/collections/12724/ and other data and materials necessary to reproduce analyses are available upon request to the first author.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was supported by a NIH grant awarded to the final author (R01DA039923). fMRI results are publicly available at https://neurovault.org/collections/12724/ and other data and materials necessary to reproduce analyses are available upon request to the first author. This study was not preregistered.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101290.
While the anonymous, emotional, and dire subscales were all correlated with one another within both waves (correlations ranging between 0.42 and 0.83, p < .001), the altruism subscale was not positively correlated with the other subscales (correlations ranging between −0.20 and 0.05). While these findings are in line with previous studies, and the scale has been validated for adolescents (Carlo et al., 2003; Carlo and Randall, 2002), we ran a sensitivity analysis (which can be found in the supplement) where we excluded the altruism subscale to ensure it was not unduly influencing our results. Our main findings did not change, so the results here reflect the inclusion of all four subscales.
To examine the effect of outliers, we conducted sensitivity analyses on all subjects outside of 3 standard deviations for dmPFC and insula activations (1 participant each). Exclusion of these participants did not change the results of the primary analyses, so these subjects were included in the final analyses. In addition, to ensure that effects were not being affected by differences between schools or by the biological sex or age of participants, we reran our analyses controlling for school, sex, and age. All effects remained, so these controls were not included in the final analyses.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Agan M.L.F., Costin A.S., Deutz M.H.F., Edelsbrunner P.A., Záliš L., Franken A. Associations between risk behaviour and social status in European adolescents. Eur. J. Dev. Psychol. 2015;12(2):189–203. doi: 10.1080/17405629.2014.975790. [DOI] [Google Scholar]
- Alexander C.S., Kim Y.J., Ensminger M., Johnson K.E., Smith B.J., Dolan L.J. A measure of risk taking for young adolescents: reliability and validity assessments. J. Youth Adolesc. 1990;19(6):559–569. doi: 10.1007/BF01537176. [DOI] [PubMed] [Google Scholar]
- Arnett J.J. Adolescent storm and stress, reconsidered. Am. Psychol. 1999;54(5):317–326. doi: 10.1037/0003-066X.54.5.317. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., den Ouden H., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc. Cogn. Affect. Neurosci. 2007;2(2):130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., van Dijk E., Westenberg M., Rombouts S.A.R.B., Crone E.A. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychol. Sci. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Brechwald W.A., Prinstein M.J. Beyond homophily: a decade of advances in understanding peer influence processes. J. Res. Adolesc. 2011;21(1):166–179. doi: 10.1111/j.1532-7795.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek N., Deutz M.H.F., Schoneveld E.A., Burk W.J., Cillessen A.H.N. Behavioral correlates of prioritizing popularity in adolescence. J. Youth Adolesc. 2016;45(12):2444–2454. doi: 10.1007/s10964-015-0352-7. [DOI] [PubMed] [Google Scholar]
- Carlo G., Randall B.A. The development of a measure of prosocial behaviors for late adolescents. J. Youth Adolesc. 2002;31(1):31–44. [Google Scholar]
- Carlo G., Hausmann A., Christiansen S., Randall B.A. Sociocognitive and behavioral correlates of a measure of prosocial tendencies for adolescents. J. Early Adolesc. 2003;23(1):107–134. doi: 10.1177/0272431602239132. [DOI] [Google Scholar]
- Choukas-Bradley S., Giletta M., Neblett E.W., Prinstein M.J. Ethnic differences in associations among popularity, likability, and trajectories of adolescents’ alcohol use and frequency. Child Dev. 2015;86(2):519–535. doi: 10.1111/cdev.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R.B., Goldstein N.J. Social influence: compliance and conformity. Annu. Rev. Psychol. 2004;55(1):591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Collado A., Felton J.W., MacPherson L., Lejuez C.W. Longitudinal trajectories of sensation seeking, risk taking propensity, and impulsivity across early to middle adolescence. Addict. Behav. 2014;39(11):1580–1588. doi: 10.1016/j.addbeh.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Fuligni A.J. Self and others in adolescence. Annu. Rev. Psychol. 2020;71(1):447–469. doi: 10.1146/annurev-psych-010419-050937. [DOI] [PubMed] [Google Scholar]
- Crone E.A., van Duijvenvoorde A.C.K., Peper J.S. Annual research review: Neural contributions to risk-taking in adolescence – developmental changes and individual differences. J. Child Psychol. Psychiatry. 2016;57(3):353–368. doi: 10.1111/jcpp.12502. [DOI] [PubMed] [Google Scholar]
- Dijkstra J.K., Cillessen A.H.N., Lindenberg S., Veenstra R. Basking in reflected glory and its limits: why adolescents hang out with popular peers. J. Res. Adolesc. 2010;20(4):942–958. doi: 10.1111/j.1532-7795.2010.00671.x. [DOI] [Google Scholar]
- Dijkstra J.K., Cillessen A.H.N., Borch C. Popularity and adolescent friendship networks: selection and influence dynamics. Dev. Psychol. 2013;49(7):1242–1252. doi: 10.1037/a0030098. [DOI] [PubMed] [Google Scholar]
- Duell N., van Hoorn J., McCormick E.M., Prinstein M.J., Telzer E.H. Hormonal and neural correlates of prosocial conformity in adolescents. Developmental Cognitive Neuroscience. 2021;48:100936. doi: 10.1016/j.dcn.2021.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R.I.M. The social brain meets neuroimaging. Trends Cogn. Sci. 2012;16(2):101–102. doi: 10.1016/j.tics.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.-J. Is there heightened sensitivity to social reward in adolescence? Curr. Opin. Neurobiol. 2016;40:81–85. doi: 10.1016/j.conb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Fuligni A.J., Eccles J.S., Barber B.L., Clements P. Early adolescent peer orientation and adjustment during high school. Dev. Psychol. 2001;37(1):28–36. doi: 10.1037/0012-1649.37.1.28. [DOI] [PubMed] [Google Scholar]
- Gibbons F.X., Gerrard M., Lane D.J. Social Psychological Foundations of Health and Illness. Blackwell Publishing,; 2003. A social reaction model of adolescent health risk; pp. 107–136. [DOI] [Google Scholar]
- Gommans R., Sandstrom M.J., Stevens G.W.J.M., ter Bogt T.F.M., Cillessen A.H.N. Popularity, likeability, and peer conformity: Four field experiments. J. Exp. Soc. Psychol. 2017;73:279–289. doi: 10.1016/j.jesp.2017.10.001. [DOI] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Koski J.E., Xie H., Olson I.R. Understanding social hierarchies: the neural and psychological foundations of status perception. Soc. Neurosci. 2015;10(5):527–550. doi: 10.1080/17470919.2015.1013223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFontana K.M., Cillessen A.H. Developmental changes in the priority of perceived status in childhood and adolescence. Soc. Dev. 2010;19(1):130–147. [Google Scholar]
- Masten C.L., Eisenberger N.I., Pfeifer J.H., Dapretto M. Witnessing peer rejection during early adolescence: neural correlates of empathy for experiences of social exclusion. Soc. Neurosci. 2010;5(5–6):496–507. doi: 10.1080/17470919.2010.490673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage. 2011;55(1):381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Mayeux L., Sandstrom M.J., Cillessen A.H.N. Is being popular a risky proposition? J. Res. Adolesc. 2008;18(1):49–74. doi: 10.1111/j.1532-7795.2008.00550.x. [DOI] [Google Scholar]
- Meulen M. van der, I Jzendoorn M.H. van, Crone E.A. Neural correlates of prosocial behavior: compensating social exclusion in a four-player cyberball game. PLOS One. 2016;11(7) doi: 10.1371/journal.pone.0159045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Leong Y.C., Carlson R.W., Kullar M., Zaki J. Neural detection of socially valued community members. Proc. Natl. Acad. Sci. USA. 2018;115(32):8149–8154. doi: 10.1073/pnas.1712811115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. Social re-orientation and brain development: an expanded and updated view. Dev. Cogn. Neurosci. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi J., Prinstein M.J. In search of likes: longitudinal associations between adolescents’ digital status seeking and health-risk behaviors. J. Clin. Child Adolesc. Psychol. 2019;48(5):740–748. doi: 10.1080/15374416.2018.1437733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z.A., Bunge S.A., Bell O.N., Kriegsfeld L.J., Kayser A.S., Dahl R.E. The effect of social rank feedback on risk taking and associated reward processes in adolescent girls. Soc. Cogn. Affect. Neurosci. 2017;12(2):240–250. doi: 10.1093/scan/nsw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C., Kleinbaum A.M., Wheatley T. Spontaneous neural encoding of social network position. Nat. Hum. Behav. 2017;1(5):1–7. doi: 10.1038/s41562-017-0072. [DOI] [Google Scholar]
- Peake S.J., Dishion T.J., Stormshak E.A., Moore W.E., Pfeifer J.H. Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. NeuroImage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino M.T., Miernicki M.E., Telzer E.H. Letting the good times roll: adolescence as a period of reduced inhibition to appetitive social cues. Soc. Cogn. Affect. Neurosci. 2016;11(11):1762–1771. doi: 10.1093/scan/nsw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M.J. Penguin,; 2017. Popular: The Power of Likability in a Status-obsessed World. [Google Scholar]
- Prinstein M.J., Choukas-Bradley S.C., Helms S.W., Brechwald W.A., Rancourt D. High peer popularity longitudinally predicts adolescent health risk behavior, or does it?: An examination of linear and quadratic associations. J. Pediatr. Psychol. 2011;36(9):980–990. doi: 10.1093/jpepsy/jsr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M.J., Brechwald W.A., Cohen G.L. Susceptibility to peer influence: using a performance-based measure to identify adolescent males at heightened risk for deviant peer socialization. Dev. Psychol. 2011;47(4):1167. doi: 10.1037/a0023274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom M.J., Cillessen A.H.N. Likeable versus popular: Distinct implications for adolescent adjustment. International Journal of Behavioral Development. 2006;30(4):305–314. doi: 10.1177/0165025406072789. [DOI] [Google Scholar]
- Sebastian C., Viding E., Williams K.D., Blakemore S.-J. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shin H. Friendship dynamics of adolescent aggression, prosocial behavior, and social status: the moderating role of gender. J. Youth Adolesc. 2017;46(11):2305–2320. doi: 10.1007/s10964-017-0702-8. [DOI] [PubMed] [Google Scholar]
- Silk J.B. Social components of fitness in primate groups. Science. 2007 doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Stroud L.R., Siegle G.J., Dahl R.E., Lee K.H., Nelson E.E. Peer acceptance and rejection through the eyes of youth: Pupillary, eyetracking and ecological data from the Chatroom Interact task. Soc. Cogn. Affect. Neurosci. 2012;7(1):93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39(3):1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren M., Sijtsma H., Lute N., van Rijn R., Hollarek M., Walsh R.J., Lee N.C., Krabbendam L. Development of the neural correlates of self- and other-referential processing across adolescence. NeuroImage. 2022;252 doi: 10.1016/j.neuroimage.2022.119032. [DOI] [PubMed] [Google Scholar]
- van Rijsewijk L., Dijkstra J.K., Pattiselanno K., Steglich C., Veenstra R. Who helps whom? Investigating the development of adolescent prosocial relationships. Dev. Psychol. 2016;52:894–908. doi: 10.1037/dev0000106. [DOI] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect. Neurosci. 2006;1(2):107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerubavel N., Bearman P.S., Weber J., Ochsner K.N. Neural mechanisms tracking popularity in real-world social networks. Proc. Natl. Acad. Sci. USA. 2015;112(49):15072–15077. doi: 10.1073/pnas.1511477112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.