Abstract
Recently, Fang et al. in Cancer Cell report that methionine restriction increases antitumor immunity by enhancing cyclic GMP-AMP synthase (cGAS) activity and promoting its dissociation from chromatin. This finding identifies a potential strategy to target cGAS demethylation in cancer therapy by altering methionine metabolism.
Keywords: methionine restriction, cGAS methylation, antitumor immunity
The metabolism of tumors as a target for cancer therapy has been a difficult topic to address. Understanding the regulatory mechanisms of the tumor immune microenvironment holds potential clinical value as it helps assess patient response to immunotherapy and identifies potential combinations with metabolic interventions. Dietary amino acids can reprogram the tumor microenvironment, which is a promising approach in cancer therapy [1–3]. However, our understanding of the impact of diet on cancer metabolism is in the very early stages. The cGAS-STING-type I interferon (IFN) signaling pathway is a crucial player in the innate immune response as well as tumor immunity [4–6]. Its activation has shown promise as an effective antitumor strategy when combined with immune checkpoint inhibitors [7–8]. cGAS is a major sensor for cytoplasmic double-stranded DNA (dsDNA) [4] and is also abundantly enriched in the nucleus [3], but the interaction between cGAS and chromatin is poorly understood. Likewise, it remains unclear whether the metabolism of amino acids affects cGAS-STING signaling and whether it can be effectively combined with ionizing radiation–another major therapy for consideration-along with immune checkpoint inhibitors. To address such questions Fang et al. assessed the regulation of cGAS-STING signaling by nutrient stress and highlighted the potential of targeting cGAS demethylation in cancer therapy [9].
Notably, they identified methylation sites at K362 (K350 in mice) that were responsive to methionine (Met) and S-adenosylmethionine (SAM) availability, suggesting that methionine plays a role in limiting activation of the cGAS-STING pathway by inhibiting cGAS activity. Further, they found that cGAS methylation requires the regulation of the methyltransferase SUV39H1, which can inhibit cGAS activity by blocking cGAS binding to DNA and dimerization. K362 methylation promotes nuclear aggregation and chromatin tethering of cGAS. UHRF1, ubiquitin-like with PHD and ring finger domains 1, a methylation recognition protein [10], recruits methylated cGAS to promote its binding to chromatin. The authors showed that short-term methionine restriction or targeted intervention of the SUV39H1-UHRF1 axis reduces cGAS methylation.
Fang et al. next investigated the pathological role of targeting the SUV39H1-cGAS-UHRF1 axis in tumor growth. The authors demonstrated that tumor cells and host methylation loss or a methylation mimic modification can inhibit endogenous cGAS activity and promote tumor immune escape. The combination of methionine restriction and the SUV39H1 inhibitor reshapes the tumor immune microenvironment, improves the efficacy of radiotherapy, and inhibits tumor growth. The authors further investigated the methylation of cGAS in colorectal cancer samples and found it to have a negative correlation with the prognosis of individuals.
In summary, Fang et al. revealed that methionine-SUV39H1-UHRF1 regulates cGAS chromatin tethering and activity through methylation, which provides an important potential targeted intervention strategy for activating the cGAS-STING pathway to promote anti-tumor immunity (Fig. 1). Many mechanisms remain to be fully understood, such as the long-term methionine deprivation that may cause complex consequences, and the high metabolic demand of cancer cells that results in their competition with immune cells for amino acid resources. Nevertheless, in view of cGAS in antiviral infection, autoimmune diseases, and tumor immunity, the inhibition of cGAS activity by methylation modification may be a key switch for immune response regulation, which is worthy of further exploration and adds a new dimension to understanding nutrient function in cancer therapy.
Figure 1.
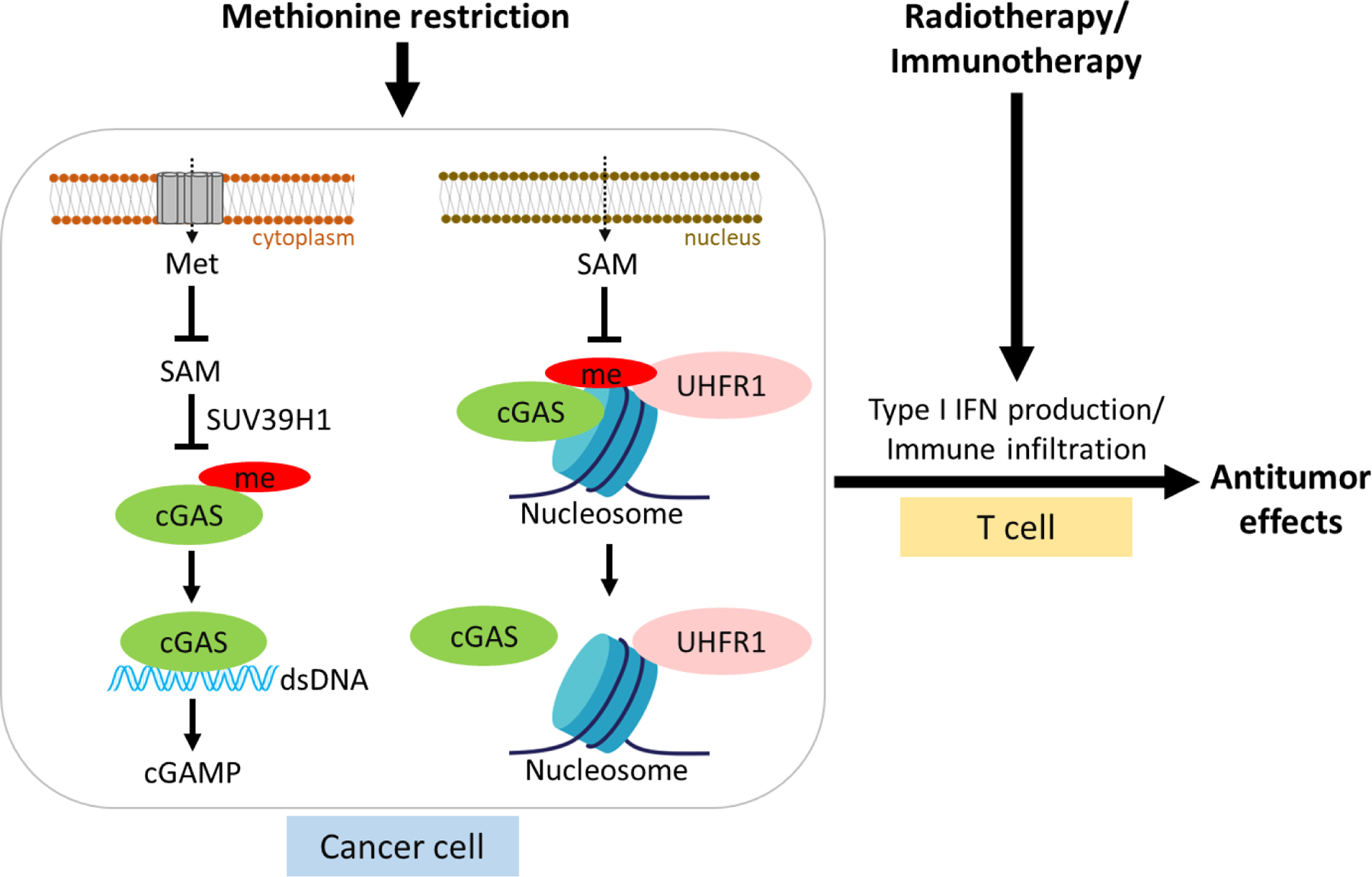
Methionine-mediated methylation recruits UHRF1 to regulate cGAS methylation and its dissociation from chromatin and remodel the tumor immune microenvironment.
Acknowledgments
Support from the National Institutes of Health grant (R01CA193256) (J.W.L.) is gratefully acknowledged. We regret that page limitations have prevented us from including all relevant studies in this spotlight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
JWL advises Restoration Foodworks, Nanocare Technologies, and Cornerstone Pharmaceuticals.
References
- 1.Maddocks ODK et al. (2017). Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376. [DOI] [PubMed] [Google Scholar]
- 2.Gao X et al. (2019). Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan H et al. (2023). Lysine catabolism reprograms tumour immunity through histone crotonylation. Nature 617, 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L et al. (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa H and Barber GN (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature 455, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen T et al. (2019). Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov 9, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin M et al. (2020). Inflammatory microenvironment remodeling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q et al. (2019). Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer. Res 79, 3940–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L et al. (2023). Methionine restriction promotes cGAS activation and chromatin untethering through demethylation to enhance antitumor immunity. Cancer Cell 41, 1118–1133. [DOI] [PubMed] [Google Scholar]
- 10.Bostick M et al. (2007). UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 317, 1760–1764. [DOI] [PubMed] [Google Scholar]


