Abstract
Lung cancer is a major public health problem and a leading cause of cancer-related deaths worldwide. Despite advances in treatment options, the five-year survival rate for lung cancer patients remains low, emphasizing the urgent need for innovative diagnostic and therapeutic strategies. MicroRNAs (miRNAs) have emerged as potential biomarkers and therapeutic targets for lung cancer due to their crucial roles in regulating cell proliferation, differentiation, and apoptosis. For example, miR-34a and miR-150, once delivered to lung cancer via liposomes or nanoparticles, can inhibit tumor growth by downregulating critical cancer promoting genes. Conversely, miR-21 and miR-155, frequently overexpressed in lung cancer, are associated with increased cell proliferation, invasion, and chemotherapy resistance. In this review, we summarize the current knowledge of the roles of miRNAs in lung carcinogenesis, especially those induced by exposure to environmental pollutants, namely, arsenic and benzopyrene, which account for up to 1/10 of lung cancer cases. We then discuss the recent advances in miRNA-based cancer therapeutics and diagnostics. Such information will provide new insights into lung cancer pathogenesis and innovative diagnostic and therapeutic modalities based on miRNAs.
Keywords: lung cancer, environmental carcinogens, microRNAs, delivery, therapeutics, diagnosis
1. Introduction
Lung cancer is one of the leading causes of cancer-related deaths worldwide, affecting both developed and developing countries. Despite advancements in early detection and treatment, the prognosis of lung cancer remains poor, with a five-year survival rate of less than 20% [1]. The etiology of lung cancer is complex and multifactorial, contributed to by environmental, genetic, and lifestyle factors. Above all, exposure to environmental pollutants, such as arsenic and benzopyrene (BaP), accounts for up to 1/10 of lung cancer cases [2]. Arsenic is commonly found in groundwater and soil, whereas BaP is present in cigarette smoke, diesel exhaust, and other combustion products [3]. Although the mechanisms by which these carcinogens induce lung cancer are still not fully understood, recent studies have unveiled pivotal contributions by miRNAs. MiRNAs are a class of small non-coding RNAs that play important roles in the post-transcriptional regulation of gene expression in eukaryotic cells. They are typically 18–25 nucleotides long and are involved in gene silencing, translational repression, and mRNA degradation [4]. MiRNAs bind to the 3′ untranslated region (UTR) of target mRNAs and induce their degradation or translational inhibition [5]. This regulates the expression of genes involved in a variety of biological processes, such as cell differentiation, proliferation, and apoptosis [6]. Dysregulation of discrete sets of miRNAs is implicated in numerous diseases, including cancer, cardiovascular disease, and neurological disorders [7]. New studies have indicated that miRNAs have significant functions in the development of lung cancer, specifically cases triggered by arsenic and BaP exposure, and could serve as viable targets for therapeutic intervention [8]. A set of miRNAs that regulate cancer-related signals like cell growth and proliferation have been observed to have varying levels of expression in lung cells following exposure to these carcinogens, demonstrating their involvement in the formation of cancer in the lungs caused by these toxins [9,10]. In this review, we will summarize the roles of miRNAs in lung carcinogenesis, especially in cases induced by exposure to arsenic and BaP, and discuss their diagnostic and therapeutic potentials. This review will help advance our insight into the role of miRNAs in lung cancer and justify their utility in improving patient outcomes.
2. The Genesis and Amplification of Human Lung Cancer
2.1. The Pathogenesis of Lung Cancer
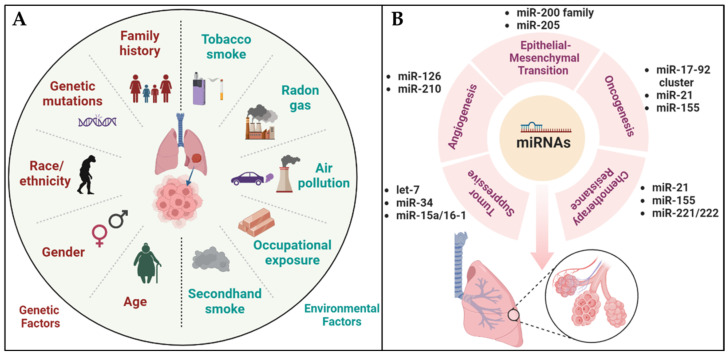
Lung cancer progresses through multiple stages. The first stage, known as initiation, involves the occurrence of genetic mutations in normal cells, rendering them more vulnerable to the progression of cancer. These initiated cells then accumulate further genetic and epigenetic changes and begin to proliferate, forming pre-cancerous lesions termed dysplasia [11]. Over time, these pre-cancerous lesions progress to invasive cancer and eventually spread to other parts of the body. Lung cancer is divided into two major types: non-small cell lung cancer (NSCLC) (85% of cases) and small cell lung cancer (SCLC) (15% of cases), depending on the type of affected cells [12]. Recent advances in genomic and molecular profiling technologies have provided new insights into the genetic and molecular profiles of these two types, contributing to the development of specific targeted therapies [13]. However, lung cancer development is complex and multifactorial under the influences of numerous factors, including exposure to environmental carcinogens and lifestyle factors. Above all, tobacco smoke is the major environmental/lifestyle factor for lung cancer [14]. In addition, prolonged exposure to high concentrations of radon and air pollution, as well as work-related substances and secondhand smoke, increases the risks of lung cancer [15]. Thus, reducing exposure to these carcinogens will undoubtedly help to prevent lung cancer development [16]. Furthermore, genetic factors, including a family history of lung cancer and specific mutations, as well as gender, age, race, and ethnicity, could also influence the predisposition of individuals to the disease (Figure 1A) [17]. For example, a large proportion of lung cancers carry mutations in the EGFR, ALK, and KRAS genes. However, the mutation patterns can change over time and under targeted therapies, allowing them to acquire resistance towards previously effective treatments [18]. Such difficulty could possibly be circumvented by earlier detection and prompt treatment of this formidable disease [19].
Figure 1.
Representative diagrams of the causative factors of lung carcinogenesis and influence of miRNAs on lung cancer development. (A) A visual representation that shows how genetic and environmental factors come together to cause lung cancer. (B) A graphical portrayal of miRNAs with their different modulatory functions in lung cancer.
2.2. Arsenic- and BaP-Induced Human Lung Carcinogenesis
Environmental exposure to arsenic and BaP serves as the major contributor to lung carcinogenesis [20]. Although exposure to each carcinogen increases the risk of lung cancer, co-exposure induces synergistic effects [21].
Arsenic is a naturally occurring metalloid widely distributed in the environment in soil, rocks, and minerals, while also being present in some groundwater sources [22]. Chronic exposure to arsenic is linked to lung cancer, skin cancer, bladder cancer, and other diseases [23]. Arsenic exists in several forms, including a highly toxic inorganic form that causes cancer, skin lesions, and cardiovascular disease [24]. Organic forms of arsenic, on the other hand, are less toxic and are typically found in plants and animals. Arsenic has been used for a variety of purposes throughout history, including pesticides, herbicides, insecticides, and certain industrial processes [25]. However, due to its toxicity, the use of arsenic has been restricted or banned in many countries, including the US. The mechanism of arsenic-induced lung cancer involves the induction of oxidative stress, DNA damage, and genomic instability, leading to gene mutations [26].
BaP is a polycyclic aromatic hydrocarbon (PAH) and a potent environmental carcinogen [27]. BaP is generated from burning organic materials, for example, the combustion of fossil fuels, tobacco smoking, grilling, or charring meat [28]. BaP is one of the most potent carcinogens found in cigarette smoke [29]. Exposure to BaP is linked to lung, skin, and bladder cancer. In addition, BaP elicits other harmful effects on human health, including respiratory problems, such as asthma and chronic bronchitis, as well as cardiovascular diseases [30]. BaP could also exert harmful effects on the ecosystem, particularly reproductive problems, developmental abnormalities, and the reduced growth and survival of aquatic organisms [31]. BaP is particularly harmful because it can easily enter the body through inhalation, ingestion, or skin contact. Once inside the body, BaP is converted into highly reactive metabolites that damage DNA and other cellular components [32]. The mechanism of BaP-induced lung cancer involves the formation of DNA adducts, triggering mutations in critical tumor suppressor genes and oncogenes [33]. One of the genes affected by BaP exposure is p53, a critical tumor suppressor gene regulating cell cycle arrest, DNA repair, and apoptosis. Mutations in p53 are found in many types of cancer and are associated with poor prognoses [34]. BaP exposure also causes epigenetic changes, such as DNA methylation, which could silence the expression of tumor suppressor genes and promote cancer development. BaP exposure may also activate oncogenes such as c-Myc, which is a transcription factor that regulates cell proliferation and apoptosis [35]. BaP additionally promotes the expression of growth factors and their receptors, such as the epidermal growth factor (EGF) and EGF receptor (EGFR), the major contributors to lung cancer development [36].
As described above, arsenic and BaP induce lung cancer through different mechanisms. Arsenic interferes with DNA damage repair, increases oxidative stress, and promotes cell proliferation. BaP, on the other hand, causes mutations in tumor suppressor genes, disrupts cell signaling pathways, and suppresses the immune system [37]. When arsenic and BaP are present together, however, their harmful effects are amplified and synergized, serving as a profound risk factor for lung cancer [38]. For example, their co-existence leads to upregulation of the pro-tumor mitogen-activated protein kinase (MAPK) pathway involved in cell growth and survival and cancer development [39]. In addition, these carcinogens alter the expression of genes involved in DNA damage repair, cell cycle progression, and apoptosis, further contributing to carcinogenesis [40]. Moreover, co-exposure to both carcinogens could impair cellular defense mechanisms that otherwise protect lung cells from environmental toxins. For example, arsenic interferes with glutathione, a critical antioxidant that helps neutralize reactive oxygen species (ROS) and prevents oxidative damage [41]. BaP, on the other hand, antagonizes aryl hydrocarbon receptors (AhRs), which play a key role in detoxifying environmental pollutants. When these defense mechanisms are compromised, lung cells become more vulnerable to the harmful effects of arsenic and BaP, further increasing the risk of lung cancer. Any preemptive measures, such as reducing exposure to these carcinogens, especially in occupational settings and areas with high environmental contamination, are essential for reducing the risk of lung cancer [42].
3. MiRNA-Based Mechanisms of Lung Carcinogenesis
Dysregulation of miRNA expression has been implicated in various diseases, including cancer. MiRNAs have been shown to play key roles in mediating lung carcinogenesis in response to environmental carcinogens (Supplementary Table S1) [43]. Recent research has demonstrated the potential utility of miRNAs as diagnostic and prognostic biomarkers for lung cancer. Moreover, miRNAs have been explored as therapeutic targets for lung cancer treatment, with promising results from preclinical studies. The targeted delivery of miRNA analogs and anti-miRNA oligonucleotides to cancer cells has emerged as a highly promising avenue for therapeutic advancement.
3.1. MiRNA Biogenesis and Regulatory Roles in Human Lung Cancer
MiRNA biogenesis is a crucial mechanism for the post-transcriptional regulation of gene expression in cells. It involves a series of enzymatic steps that result in the processing of primary miRNA transcripts into mature miRNAs, which then bind to target mRNAs and regulate their expression. MiRNA biogenesis is a multi-step process that involves the transcription of DNA into a primary miRNA (pri-miRNA) molecule by RNA polymerase II, followed by the processing of the pri-miRNA in the nucleus by the Drosha enzyme and its cofactor DGCR8 to produce a precursor miRNA (pre-miRNA) molecule [44]. The pre-miRNA is then exported to the cytoplasm, where it is cleaved by the Dicer enzyme to form a miRNA duplex. The duplex is then loaded into the RNA-induced silencing complex (RISC), which includes Argonaute (AGO) proteins [45]. The miRNA strand serves as a guide for the RISC complex to bind with target mRNA molecules that possess matching sequences. The RISC complex binds with the target mRNA, and this interaction can potentially cause degradation of the mRNA or its translational inhibition, which ultimately lead to the silencing of the gene [46]. MiRNA biogenesis is tightly regulated by a complex network of molecular interactions involving multiple protein complexes and regulatory factors. In fact, aberrant expression of key miRNA biogenesis factors, such as Drosha, DGCR8, Dicer, and Exportin-5, is found in various types of lung cancer [47]. In particular, decreased expression of Dicer is often found in lung cancer [48].
Dysregulation of specific sets of miRNAs has indeed been associated with the development of lung cancer. The dysregulation of miRNAs involved in cell proliferation, apoptosis, and metastasis can contribute to the progression of lung cancer [49]. In lung cancer, certain miRNAs that have oncogenic properties, such as miR-21, miR-155, and miR-221/222, are found to be elevated [50]. These miRNAs promote cell proliferation and metastasis, and they can target tumor suppressor genes like PTEN and PDCD4, leading to their downregulation and loss of their tumor-suppressing functions [51]. Furthermore, oncogenic miRNAs like miR-21, miR-155, and miR-221/222 have also been associated with chemotherapy resistance in lung cancer. They can regulate drug transporters, apoptosis, and DNA repair pathways, thereby affecting the efficacy of chemotherapy treatment [52]. On the other hand, tumor suppressor miRNAs, such as miR-34, let-7, and members of the miR-200 family, are downregulated in lung cancer [53]. These miRNAs normally inhibit cell growth and metastasis, but their reduced expression levels in lung cancer can contribute to uncontrolled cell growth and metastatic spread. For example, miR-34a, which targets oncogenes like c-Met and Notch1, is downregulated in lung cancer [54]. Furthermore, several miRNAs, including the miR-200 family and miR-205, have been shown to regulate the epithelial–mesenchymal transition (EMT) of lung cancer [55]. The EMT is a process in which epithelial cells lose their polarity and cell–cell adhesion and gain mesenchymal properties, promoting invasion and metastasis [56]. Other miRNAs, including miR-126 and miR-210, have been implicated in regulating the angiogenesis of lung cancer (Figure 1B) [57]. Angiogenesis is the process of forming new blood vessels, crucial for tumor growth and metastasis [58]. These dysregulated miRNAs also interact with various signaling pathways that are frequently mutated in lung cancer, such as the EGFR and KRAS pathways, further highlighting their roles in the disease [59]. Identification of dysregulated miRNAs in lung cancer may provide new opportunities for the development of miRNA-based therapeutic strategies.
3.2. MiRNAs Mediate Lung Carcinogenesis by Arsenic and BaP Co-Exposure
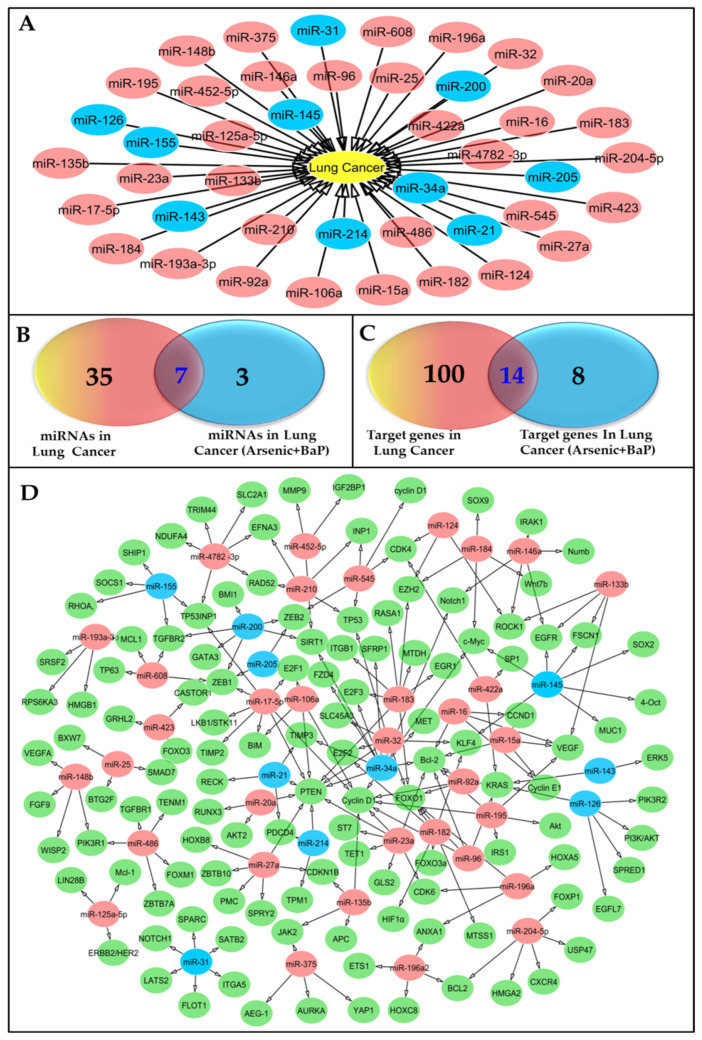
Exposure of lung cells to carcinogens, like arsenic and BaP, could lead not only to mutations in oncogenes and tumor suppressor genes, but also to changes in miRNA expression that facilitate cancer development (Figure 2A and Supplementary Table S2). For instance, pro-tumor miR-21, which targets tumor suppressor genes such as PTEN, PDCD4, and RECKS, is elevated in arsenic-exposed lung cells [60]. Conversely, antitumor miR-200c is downregulated in arsenic-exposed lung cells to promote the EMT [61]. Furthermore, antitumor miR-31, targeting the JAK/STAT pathway involved in cell proliferation and survival, is downregulated in BaP-exposed lung cells [62,63]. We will describe more details of molecular pathways regulated by miRNAs below. Given the potential roles of miRNAs in carcinogen-induced lung cancer, miRNA-based therapies may hold promise as a novel approach to treating this disease [64,65]. For example, a group of miRNAs, miR-21, miR-155, miR-200c, miR-145, miR-34a, miR-31, and miR-126, are linked to lung carcinogenesis whether or not it is induced by arsenic and BaP exposure (Figure 2A,B). They all regulate the expression of lung cancer-associated genes KRAS, c-Myc, SOCS1, SATB2, PTEN, PDCD4, Bcl-2, TGFBR2, ZEB1, Cyclin D1, ZEB2, RECK, EGFL7, and KLF4 (Figure 2C,D). Thus, modulation of the miRNA–cancer driver gene axis may represent a potential therapeutic approach for lung cancer. In fact, miRNA mimics have been utilized to restore the expression of tumor suppressor miRNAs, while miRNA inhibitors have been used to target oncogenic miRNAs [66]. Additionally, miRNA-targeting nanoparticles or exosomes have been developed as more targeted and efficient miRNA delivery systems [67].
Figure 2.
Representative diagrams of the miRNAs that have been associated with human lung cancer and their corresponding targets. (A) The implicated miRNAs in human lung cancer progression; those labeled in blue are involved in lung cancer induced by arsenic and BaP exposure. (B) The shared miRNAs that play a role in lung cancer and are also involved in the development of lung cancer caused by exposure to arsenic and BaP. (C) The target genes responsible for the progression of lung cancer and those that are also responsible for the development of lung cancer triggered by exposure to arsenic and BaP. (D) Representative diagram for miRNAs and their gene targets in lung cancer tissue. The miRNAs linked to lung cancer are depicted in red, whereas those associated with lung cancer induced by arsenic and BaP are indicated in blue. The genes that are targeted by these miRNAs are visually represented in green.
4. Deciphering the Molecular Signaling Pathways of MiRNAs in Lung Cancer
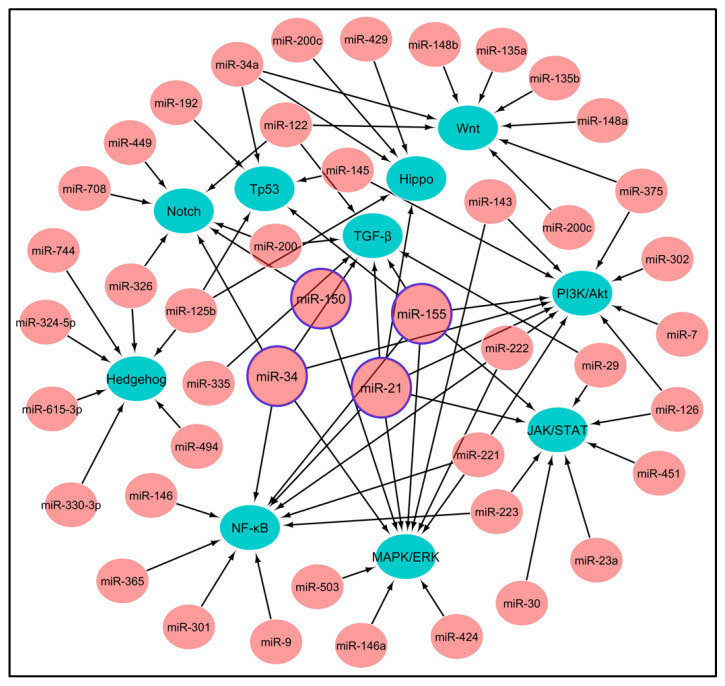
To develop miRNA-targeted therapeutics, it is essential to know which signaling pathways regulate miRNAs. In lung cancer, a group of oncogenes or tumor suppressor genes are dysregulated, leading to aberrant expression of the downstream miRNAs. The dysregulated genes and pathways upstream of miRNAs include the epidermal growth factor receptor (EGFR), KRAS, PI3K-Akt-mTOR, Wnt, Notch, Hedgehog, TGF-β, JAK/STAT, NF-κB, and Hippo pathways. These genes are commonly upregulated in lung cancer and promote cell proliferation, invasion, survival, and therapeutic resistance (Figure 3 and Supplementary Table S3). The genes/pathways are regulated by miRNAs including miR-21, miR-31, miR-34a, miR-155, and miR-221/222 [68,69], and they are involved in the apoptosis, cell proliferation, angiogenesis, and metastasis of cancer cells.
Figure 3.
Illustrating network of the signaling mechanisms in lung cancer through miRNA-mediated regulation. The image shows the signaling pathways that are involved in lung cancer, including Wnt, TGF-β, Notch, Hedgehog, PI3K/Akt, MAPK/ERK, JAK/STAT, NF-κB, Hippo, and Tp53. Each of these signaling pathways is regulated by many miRNAs. Four specific miRNAs, namely miR-21, miR-150, miR-155, and miR-34, are known to have a significant impact on the regulation and progression of lung cancer, and they will be focused on by miRNA therapeutics.
5. Different Types and Mechanisms of MiRNA-Based Therapies for Lung Cancer
MiRNA-based therapies utilize different approaches, including inhibiting oncogenic miRNAs, restoring tumor suppressor miRNAs, modulating the immune response, and sensitizing cancer cells to chemotherapy and radiation therapy. These strategies demonstrate the versatility of miRNA-based therapies in targeting cancer and hold promise for improving treatment outcomes.
5.1. Inhibition of Oncogenic MiRNAs
Oncogenic miRNAs have been implicated in the progression of tumorigenesis by suppressing the expression of tumor suppressor genes. Consequently, targeting oncogenic miRNAs has emerged as a promising therapeutic strategy for the treatment of lung cancer. MiRNA-based therapeutics, such as anti-miRNA oligonucleotides (AMOs), locked nucleic acids (LNA), and antisense oligonucleotides (ASOs), have been developed to inhibit the function of these oncogenic miRNAs. Another approach, known as miRNA sponges, has also been explored for sequestering miRNAs and inhibiting their activity. Notably, studies focusing on AMOs specifically designed to target miR-21, an oncogenic miRNA, have demonstrated their effectiveness in restraining lung cancer cell proliferation and inducing apoptosis [70].
5.2. Restoration of Tumor Suppressor MiRNAs
Tumor suppressor miRNAs play a pivotal role in impeding tumor growth and metastasis. In the context of lung cancer, a notable observation is the downregulation of tumor suppressor miRNAs. Hence, an enticing strategy for treating lung cancer involves the restoration of these miRNAs. By reinstating tumor suppressor miRNAs, it is anticipated that the inhibition of tumor growth and suppression of metastasis can be achieved [71]. MiRNA therapeutics, such as miRNA mimics, can restore the function of tumor suppressor miRNAs by binding to their target genes and inhibiting their expression. For example, a miR-34a mimic has been shown to inhibit lung cancer cell growth and induce apoptosis by targeting multiple oncogenic genes [71].
5.3. Modulation of Immune Response
MiRNAs have been implicated in the regulation of immune cell functions, suggesting their potential involvement in the modulation of antitumor immune responses. Dysregulation of these miRNAs can lead to impaired immune responses against tumors. MiRNA-based therapeutics, including miRNA antagonists and mimics, offer a promising approach to modulate the expression of immune-related miRNAs. For instance, studies have demonstrated that the use of a miR-155 antagonist can enhance the antitumor immune response in lung cancer by increasing the expression of key immune mediators, such as interferon-γ and interleukin-2. This highlights the potential of targeting specific miRNAs to manipulate immune-related pathways and improve antitumor immunity [72].
5.4. Sensitization to Chemotherapy and Radiation Therapy
MiRNAs could also play roles in the regulation of resistance to chemotherapy and radiation therapy, and dysregulation of these miRNAs could confer therapeutic resistance. MiRNA therapeutics, such as miRNA inhibitors, can modulate the expression of drug-resistance-related miRNAs and sensitize lung cancer cells to cancer treatments. For example, a miR-221 inhibitor has been shown to target multiple drug-resistance-related genes and sensitize lung cancer cells to chemotherapy [73].
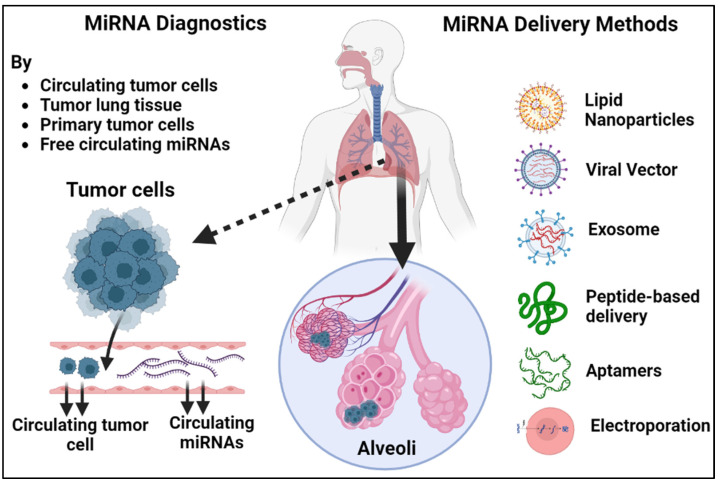
6. MiRNA Therapeutics and Delivery Methods
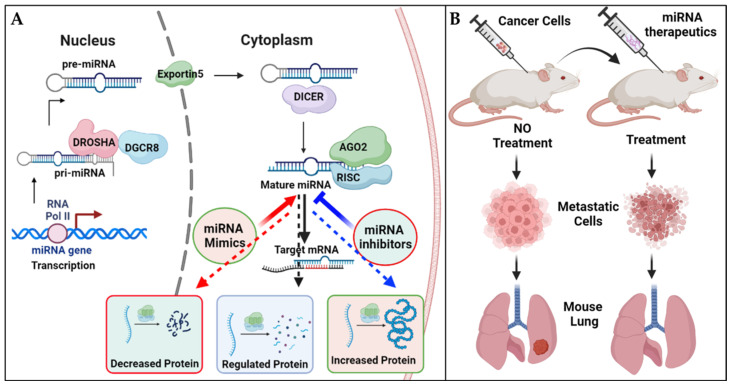
Over the past decade, an extensive array of therapeutics based on miRNAs has been meticulously crafted and extensively explored in preclinical settings. MiRNA-based treatments have demonstrated compelling efficacy in animal models, effectively restricting metastasis and offering promising prospects for combating cancer spread (Figure 4A,B). The development of effective delivery systems is a critical aspect of miRNA therapeutics. Various delivery methods have been developed and tested, including lipid-based delivery, viral vectors, exosomes, aptamers, peptide-based delivery, and electroporation (Figure 5). Lipid-based nanoparticles have been shown to be effective in delivering miRNA therapeutics to target cells and can be designed to selectively target specific tissues and organs. The use of nanocarriers for delivering miRNA therapeutics offers a potential solution to address off-target effects and toxicity concerns. By encapsulating miRNAs in nanocarriers, targeted delivery to lung cancer cells can be achieved. Through the incorporation of targeting ligands on the nanocarrier surface, specific binding to lung cancer cell receptors can be achieved, minimizing exposure to normal tissues and reducing off-target effects. Additionally, nanocarriers provide protection for miRNAs, improving their stability and bioavailability. Controlled release mechanisms ensure sustained and localized delivery to the tumor site. Nanocarriers can also be designed to possess other advantageous properties, such as enhanced cellular uptake and triggered release, further optimizing the therapeutic potential of miRNA-based treatments for lung cancer [74].
Figure 4.
A detailed model illustrating the biogenesis of miRNAs and demonstrating the effectiveness of miRNA-based therapies for managing lung metastases. (A) The biogenesis of miRNAs involves transcription by RNA polymerase II, processing by Drosha and Dicer enzymes, and incorporation into the RNA-induced silencing complex (RISC) to regulate gene expression at both the cellular and animal levels through oncology-directed miRNA replacement therapy. (B) Experimental animals have been used to test the efficacy of miRNA-based treatments in restricting metastasis, with studies conducted to assess the ability of these therapies to prevent the spread of cancer to other parts of the body.
Figure 5.
An illustrated guide to the diagnostic and therapeutic potential of miRNAs and methods for delivering miRNA therapeutics. MiRNAs can be extracted from circulating miRNAs, circulating tumor cells, primary tumor cells, and tumor lung tissue and analyzed for their expression patterns. These miRNA profiles can then be used to develop non-invasive diagnostic tools for cancer detection and monitoring and to guide personalized treatment strategies. The delivery methods include lipid-based nanoparticles, viral vectors, exosomes, aptamers, peptide-based delivery, and electroporation. Each method has its own advantages and limitations, and the choice of delivery method depends on factors such as the type of miRNA therapeutic and the target tissue.
Viral vectors, such as adenoviruses and lentiviruses, can also be used as delivery vehicles for miRNA therapeutics [75]. Exosomes, small vesicles that are naturally produced by cells, have shown promise as delivery vehicles for miRNA therapeutics due to their ability to target specific cells and tissues [76]. Aptamers, small molecules that can specifically bind to target cells, have also been investigated for their potential as delivery vehicles for miRNA therapeutics [77]. Peptide-based delivery methods have been developed to target specific cell types or tissues and have been shown to be effective in delivering miRNA therapeutics to these targets [78]. Electroporation, which involves the use of an electric field to introduce miRNA molecules into cells, has also been explored as a method for delivering miRNA therapeutics to specific tissues [79]. While each delivery method has its own advantages and limitations, continued research in this area is necessary to optimize delivery systems and maximize the therapeutic potential of miRNA-based therapies. MiRNA therapeutics encompasses two distinct categories aimed at manipulating the expression of specific miRNAs: miRNA mimics and miRNA inhibitors. MiRNA mimics serve to enhance the expression of a particular miRNA, while miRNA inhibitors work to decrease its expression (Figure 4A).
6.1. MiRNA Mimics
MiRNA mimics are synthetic RNA molecules that mimic the function of endogenous miRNAs. They are designed to increase the expression of a specific miRNA that is downregulated in cancer cells, thereby restoring its tumor-suppressive function [80]. MiRNA mimics are typically chemically modified to enhance their stability and reduce off-target effects. One of the most widely used miRNA mimics is miR-34a, which is downregulated in lung cancer and functions as a tumor suppressor by regulating multiple oncogenic pathways [81]. Several preclinical studies have shown that systemic delivery of miR-34a mimics can inhibit lung tumor growth and metastasis in mouse models [82]. Another example of miRNA mimics is miR-16, which is downregulated in lung cancer and targets multiple oncogenes [83]. Delivery of miR-16 mimics has been shown to induce apoptosis and inhibit lung cancer cell proliferation [84].
6.2. MiRNA Inhibitors
MiRNA inhibitors, also known as antagomirs or anti-miRNAs, are synthetic RNA molecules that inhibit the function of endogenous miRNAs. They are designed to target and bind to the mature miRNA, thereby preventing its interaction with target mRNAs [85]. MiRNA inhibitors are also chemically modified to enhance their stability and reduce off-target effects. One of the most studied miRNA inhibitors is the miR-21 inhibitor, which targets a miRNA that is overexpressed in lung cancer and promotes tumor growth and metastasis [86]. In preclinical studies, systemic delivery of miR-21 inhibitors has been shown to inhibit lung tumor growth and sensitize cancer cells to chemotherapy [87,88]. Another example of miRNA inhibitors is the miR-155 inhibitor; miR-155 is upregulated in lung cancer and promotes tumor growth and immune evasion [89]. Delivery of miR-155 inhibitors has been shown to suppress lung tumor growth and enhance antitumor immune responses [90].
6.3. Delivery Methods for MiRNA Therapeutics
The success of miRNA therapeutics depends on their efficient delivery to the target tissues and cells. The delivery methods for miRNA therapeutics can be broadly classified into viral and non-viral vectors [91].
6.3.1. Viral Vectors
Viral vectors are the most used delivery vehicles for miRNA therapeutics. They include retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses (AAVs). These vectors are engineered to express the desired miRNA mimic or inhibitor and are capable of efficient transduction of both dividing and non-dividing cells (Figure 5). Several preclinical and clinical studies have shown the efficacy of viral-vector-based delivery of miRNA therapeutics for lung cancer treatment [92,93]. For example, a phase I clinical trial tested the safety and efficacy of intravenous delivery of a lentiviral vector expressing miR-16 in patients with advanced NSCLC. The results showed that the treatment was well-tolerated and resulted in stable disease in some patients [94].
6.3.2. Non-Viral Vectors
Non-viral vectors for miRNA delivery are an attractive alternative to viral vectors because they are generally safer, less immunogenic, and more easily customizable. They include lipid-based nanoparticles, polymers, and inorganic nanoparticles. Non-viral vectors can be designed to encapsulate miRNA mimics or inhibitors and deliver them to the target cells through various mechanisms, such as endocytosis and membrane fusion [95,96]. Lipid-based nanoparticles are the most extensively studied non-viral vectors for miRNA delivery. They consist of a cationic lipid core and a polyethylene glycol (PEG) shell, which enhance their stability and reduce their immunogenicity [97]. Several preclinical studies have shown the efficacy of lipid-based nanoparticles in delivering miRNA therapeutics to lung cancer cells [98,99]. For example, a recent study demonstrated that the intravenous delivery of lipid-based nanoparticles containing miR-34a mimics can inhibit lung tumor growth and metastasis in a mouse model of NSCLC [100].
Polymers are another type of non-viral vector for miRNA delivery. They can be designed to have chemical and physical properties suitable for optimal stability, biocompatibility, and release kinetics. Polyethyleneimine (PEI) is one of the most used polymers for miRNA delivery because of its high cationic charge and ability to condense miRNAs into nanoparticles [101,102]. Several preclinical studies have shown the efficacy of PEI-based nanoparticles in delivering miRNA therapeutics to lung cancer cells [103,104]. Inorganic nanoparticles, such as gold nanoparticles and magnetic nanoparticles, are also being explored as non-viral vectors for miRNA delivery. They have unique physicochemical properties, such as high surface areas and magnetic responsiveness, making them applicable for magnetic-resonance-guided miRNA delivery to lung cancer cells [105,106].
7. Clinical Trials of MiRNA Therapeutics in Lung Cancer Treatment
There has been a surge in new lung cancer therapies utilizing miRNAs to alter the activity of lung cancer cells. Such interest stems from the effectiveness, reduced toxicity, and improved specificity of miRNA-based therapies compared to traditional cancer treatments. The safety and efficacy of each miRNA-based cancer therapy has been tested through clinical trials. Here, we summarize some of the recent clinical trials investigating the use of miRNA therapeutics in lung cancer treatment.
A phase I clinical trial evaluating the miR-34a mimic, MRX34, in patients with advanced solid tumors, including lung cancer, yielded compelling results. This groundbreaking study demonstrated the safety and efficacy of MRX34, as it was well tolerated by the patients. Encouragingly, a significant number of patients experienced positive outcomes, with two achieving a partial response and five stabilizing their diseases [107]. A Phase I/II clinical trial assessing MRX34’s safety and efficacy in patients with unresectable primary liver cancer or liver metastases, including cases originating from lung cancer, yielded promising results. The trial confirmed MRX34′s safety profile and provided evidence of its antitumor activity. Notably, three out of twenty-four patients achieved a partial response, while eight patients experienced disease stabilization. Another Phase I/IIa clinical trial evaluated the safety and efficacy of MRX34 in combination with the immune checkpoint inhibitor pembrolizumab in patients with advanced solid tumors, including NSCLC. The trial demonstrated that the combination therapy was safe and well-tolerated, with evidence of antitumor activity in some patients, including those who had previously progressed on immunotherapy. Specifically, out of seventeen patients, two patients achieved a partial response and six patients showed disease stabilization [108].
MiR-16 mimic TargomiRs was also tested in a phase I clinical trial for safety and pharmacokinetics in patients with advanced solid tumors, including lung cancer. The trial showed that TargomiRs was safe and well-tolerated, with no dose-limiting toxicities observed. In addition, TargomiRs demonstrated evidence of antitumor activity in some patients, with one patient achieving a partial response and four patients achieving disease stabilization [109]. Another Phase I/IIa clinical trial also evaluated the safety and efficacy of TargomiRs in combination with the chemotherapy drug docetaxel in patients with advanced NSCLC. The trial demonstrated that the combination therapy was well-tolerated and showed evidence of antitumor activity, with seven out of twelve patients achieving partial response or stable disease conditions [110]. These findings suggest that MRX34 and TargomiRs may hold promise as potential therapeutic options for lung cancer patients [111]. Combining miRNA therapeutics with immunotherapy or chemotherapy may enhance their antitumor effects.
8. Potential Benefits and Limitations of MiRNA Therapeutics in Lung Cancer Treatment
MiRNA therapeutics offers a potential new treatment modality for lung cancer, with several potential benefits over traditional therapies. However, there are also several limitations and challenges that must be overcome before these therapies can be widely adopted in clinics.
8.1. Potential Benefits of MiRNA Therapeutics in Lung Cancer Treatment
(I) Targeted Carriers: In miRNA therapeutics, lung cancer treatment involves the utilization of specific carriers to deliver miRNA molecules directly to cancer cells. These carriers, such as liposomes, nanoparticles, or viral vectors, are engineered to protect and transport therapeutic miRNAs to their intended targets within the cancerous tissue. By incorporating miRNAs into these carriers, their stability and bioavailability are enhanced, allowing for efficient delivery and cellular uptake. This targeted approach enables the miRNAs to selectively modulate the expression of cancer-associated genes, thereby exerting precise and potent antitumor effects while minimizing damage to healthy cells [112]. (II) Reduced Toxicity: The utilization of miRNA therapeutics presents a promising approach to mitigate toxicity and minimize side effects through a reduction in off-target effects. By specifically targeting the intended miRNAs, these therapeutic interventions can significantly minimize the likelihood of unintended impacts on other genes, thereby enhancing the safety profile of the treatment [113]. (III) Personalized Therapy: Personalized therapy holds great promise in the realm of miRNA-based treatments. The expression profiles of miRNAs exhibit significant variation among individuals, thereby highlighting the potential for targeted therapies aimed at specific miRNAs. By tailoring treatment strategies to address the unique miRNA landscape of each patient’s tumor, personalized therapies can be developed, offering more effective and precise interventions for improved patient outcomes. [114]. (IV) Combination Therapy: MiRNA therapeutics can be combined with other therapies, such as chemotherapy, radiation therapy, or immunotherapy, to enhance their antitumor effects. Such combinatorial therapy could potentially lead to improved outcomes in lung cancer patients [115]. (V) Overcoming Drug Resistance: MiRNA therapeutics hold significant potential in overcoming drug resistance, a major challenge in the treatment of lung cancer. By targeting dysregulated miRNAs, these interventions can modulate multiple genes and signaling pathways involved in resistance mechanisms. They can restore sensitivity to chemotherapy or targeted therapies by reversing the epithelial–mesenchymal transition, modifying drug efflux, and sensitizing resistant cells through the regulation of key genes and pathways. Additionally, miRNA-based therapies can be combined with existing treatments to enhance efficacy and counteract drug resistance by targeting cancer cells through multiple pathways [116].
8.2. Limitations and Challenges of MiRNA Therapeutics in Lung Cancer Treatment
(I) Delivery challenges: One of the major challenges for miRNA therapeutics is the difficulty of delivering miRNAs to tumor cells. MiRNA therapeutics are often delivered via nanoparticles or other delivery systems, which could be a little complex due to several reasons. Firstly, miRNAs are fragile molecules that can easily degrade in the harsh environment of the body. To protect them, specialized delivery systems such as nanoparticles are employed, which require careful design and optimization. Additionally, the delivery systems must be able to efficiently navigate through various biological barriers, such as the extracellular matrix, blood vessels, and cellular membranes, to reach the tumor cells [117]. (II) Off-Target Effects: Despite the specific design of miRNA therapeutics to target miRNAs, there remains a potential for these miRNAs to affect a diverse range of genes. This introduces the risk of off-target effects and toxicity in normal tissues. Off-target effects pose a concern in miRNA therapeutics as they have the potential to affect a broad range of genes, which may result in unintended consequences and toxicity in healthy tissues. However, the use of nanoparticles offers a promising strategy to mitigate these off-target effects. By encapsulating and delivering miRNA therapeutics within nanoparticles, their release can be tightly controlled, allowing for targeted delivery to specific cells or tissues of interest. This localized delivery approach reduces the likelihood of off-target effects in normal tissues, as the nanoparticles help to enhance the specificity and precision of miRNA therapeutics, maximizing their therapeutic potential while minimizing unintended impacts [118]. (III) Destruction by Immune Cells: Exogenous miRNAs face the risk of immune-cell-mediated elimination, as they have the potential to trigger an immune response, resulting in their destruction. This immune response can limit the efficacy and stability of exogenous miRNAs. Therefore, it is important to consider the immune response as a potential obstacle when utilizing exogenous miRNAs for therapeutic purposes [119]. (IV) Regulatory Challenges: MiRNA therapeutics is a relatively new class of therapeutics, and there remain regulatory hurdles to be overcome before they can be widely utilized in clinics. These challenges include issues related to manufacturing, quality control, and regulatory approval [120]. (V) Limited Clinical Data: Despite recent clinical trials of miRNA-based therapeutics for lung cancer, there is not enough evidence for their safety and effectiveness. To date, most clinical trials have focused on evaluating the safety and tolerability of miRNA-based therapies, and only a few have assessed their therapeutic efficacy. Thus, further clinical studies are awaited to validate the utility of miRNA-based therapies and their potential in lung cancer treatment [121].
9. Diagnostic Potential of MiRNA Signatures in Lung Carcinogenesis
In recent years, the convergence of artificial intelligence (AI) and miRNA therapeutics has shown promising potential in the field of lung cancer diagnosis and classification. AI algorithms have been developed to analyze miRNA expression patterns obtained from patient samples, enabling the identification of specific miRNA signatures associated with different subtypes or stages of lung cancer. By leveraging machine learning techniques, these algorithms can effectively classify lung cancer cases based on their miRNA profiles, providing valuable insights into disease prognosis and personalized treatment strategies [122]. The integration of AI with miRNA therapeutics offers a powerful approach for the precise targeting of dysregulated miRNAs, potentially leading to more effective and tailored treatments for lung cancer patients. Such advancements hold great promise for improving both the accuracy of diagnosis and the development of innovative therapeutic interventions [123].
Several miRNAs have been identified as displaying aberrant expression patterns that actively contribute to the advancement of lung cancer, thereby fostering malignancy [124]. Notably, miR-21, miR-155, and miR-34a frequently exhibit upregulated levels in lung cancer, while miR-126 and miR-145 manifest downregulation [125]. The dysregulation of these miRNAs in the context of lung cancer holds great potential for their utilization as biomarkers, imparting invaluable benefits in the realms of diagnosis, prognosis, and therapeutic monitoring [126]. MiRNA signatures may be used to differentiate lung cancer from non-cancerous lung lesions, such as chronic obstructive pulmonary disease (COPD) and pneumonia. For example, certain miRNA signatures (miR-17-5p, miR-21, miR-27a, and miR-222) could distinguish lung adenocarcinomas from healthy lung tissues with high sensitivity and specificity [127]. MiRNA signatures could also be used to predict the prognosis and therapeutic response of lung cancer patients. For example, a miRNA signature consisting of miR-221, miR-222, and miR-146a has been associated with poor survival in NSCLC patients [128]. Another study has identified a miRNA signature (miR-210, miR-192, and miR-21) that could predict the chemotherapy response of NSCLC patients [129].
9.1. Analysis of MiRNAs in Bodily Fluid for the Better Staging of Lung Cancer Progression
Timely detection and diagnosis of lung cancer play a vital role in enhancing survival rates and enabling optimal treatment outcomes. The assessment of miRNAs in bodily fluids like plasma, serum, and bronchoalveolar lavage fluid (BALF) is an emerging and promising approach for improved staging of lung cancer progression [130]. Aberrant expression of certain miRNAs has been identified in lung cancer, playing a role in its progression. Specifically, miR-21, miR-155, and miR-34a are often found to be upregulated, while miR-126 and miR-145 are frequently downregulated in lung cancer cases. Analyzing these miRNAs in bodily fluids could offer valuable insights into the staging of lung cancer and its progression, providing important information for clinical assessment [131].
Elevated levels of specific miRNAs, namely miR-210, miR-21, and miR-155, were found to be significantly higher in the serum of patients diagnosed with advanced lung cancer when compared to individuals with early-stage disease or healthy controls [132]. Similarly, increased levels of miR-155, miR-210, and miR-21 were observed in the plasma of patients with advanced-stage NSCLC compared to those with early-stage disease or healthy controls [133]. In the BALF of patients with metastatic lung cancer, the levels of miR-148a and miR-152 were found to be notably reduced compared to individuals with localized disease or healthy controls, indicating their potential as biomarkers for distinguishing between different stages of lung cancer [134]. Another study focused on identifying a miRNA signature associated with lymph node metastasis in NSCLC patients. The inclusion of miR-210, miR-21, miR-486-5p, and miR-375 in the signature holds promise as a predictive marker for lymph node involvement in NSCLC patients with lymph node metastasis [135]. These findings highlight the significance of miRNA levels in aiding clinicians with accurate diagnosis and effective management strategies for this specific patient population [136].
9.2. Potential of MiRNA Analyses of Bodily Fluid for Early Detection
Analysis of miRNAs in bodily fluid, such as serum, plasma, and BALF has emerged as a promising approach for the early detection of lung cancer. The levels of miR-205 and miR-21 were significantly higher in the serum of patients with early-stage lung cancer than in patients with benign lung nodules or healthy controls [137]. Similarly, the miRNA signature (miR-21, miR-210, and miR-155) in plasma distinguished between lung cancer patients and healthy controls with high accuracy, even at the earliest stages of the disease [138].
Analysis of miRNAs in bodily fluids has significant potential for the identification of subtype-specific biomarkers in lung cancer. Specifically, a unique miRNA signature comprising miR-29a, miR-let-7f, miR-23a, and miR-27a has been observed in BALF, demonstrating high accuracy in distinguishing between different subtypes of non-small cell lung cancer (NSCLC) [139]. Likewise, the presence of specific miRNAs in serum, such as miR-19a, miR-92a, and miR-29c, has been found to correlate with the EGFR mutation status in NSCLC patients [140]. Furthermore, miRNAs present in bodily fluids offer valuable insights into disease progression and treatment response. Notably, elevated levels of a miRNA signature consisting of miR-1225-5p, miR-328, and miR-548 in serum have been associated with tumor progression and survival in NSCLC patients [141]. Conversely, a decrease in plasma levels of miR-126 is linked to chemotherapy resistance in NSCLC patients [142].
10. Conclusions and Future Perspectives
In conclusion, miRNAs play crucial roles in the development and progression of lung cancer by regulating dysregulated signaling pathways and responding to environmental carcinogens such as arsenic and BaP. MiR-21 has been found to promote cell proliferation and angiogenesis in response to arsenic exposure, while miR-34a inhibits cell growth and induces apoptosis. Similarly, miR-21 and miR-31 are upregulated in response to BaP exposure, promoting cell proliferation, invasion, and the EMT. Certain miRNAs have demonstrated potential as targets for lung cancer treatment, such as miR-34a and miR-150, which have been delivered to lung cancer cells through liposomes and nanoparticles and have effectively suppressed tumor growth by reducing the activity of specific genes involved in cancer advancement. However, miR-21 and miR-155 are often overproduced in lung cancer and have been linked to heightened cell proliferation, invasion, and chemotherapy resistance. Further research is needed to better understand the roles of miRNAs in lung cancer and to develop more effective miRNA-based therapies. Additionally, improving the delivery and efficacy of miRNA-based therapies is crucial, while new strategies such as nanoparticle-based delivery systems and combination therapies are being explored. Furthermore, the use of extracellular miRNAs as biomarkers for lung cancer diagnosis and prognosis is a promising area of research and could be utilized to improve early detection and personalized treatment.
Acknowledgments
We would like to thank Ibrahim Gad for his assistance with network construction, which helped to enrich the content of this review. We are grateful to all authors of the studies we reviewed and their helpful feedback and suggestions during the preparation of this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15082061/s1, Table S1. MiRNAs implicated in lung carcinogenesis with their target genes. Table S2. miRNAs involved in lung carcinogenesis upon exposure to arsenic and BaP. Table S3. The signaling pathways in lung carcinogenesis are subject to regulation by miRNAs. References [143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371] are cited in Supplementary Materials.
Authors Contributions: O.S. and S.F. contributed to the conception and design of the review study, conducted the literature search and review, and drafted the original manuscript. E.Z., M.H., A.A., I.G. and M.A. made significant contributions to the reviewing and editing of the manuscript through their dedicated and strenuous efforts. O.S., A.B., E.T. and S.F. provided critical feedback on the manuscript and revised the manuscript. All authors have read and approved the final version of the manuscript for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supplementary tables.
Conflicts of Interest
The authors declare that they have no conflict of interest regarding this review. The authors declare that they have taken all necessary measures to ensure that the research was conducted without bias or undue influence.
Funding Statement
This work was supported by the startup fund from the Metro Health Medical Center to S.F., an American Cancer Society Research Scholar Grant (RSG-18-238-01-CSM) to S.F., and a National Cancer Institute Research Grant (R01CA248304) to S.F.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sweef O., Yang C., Wang Z. The Oncogenic and Tumor Suppressive Long Non-Coding RNA–microRNA–Messenger RNA Regulatory Axes Identified by Analyzing Multiple Platform Omics Data from Cr(VI)-Transformed Cells and Their Implications in Lung Cancer. Biomedicines. 2022;10:2334. doi: 10.3390/biomedicines10102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang H.-C., Tsou T.-C. Arsenite enhances the benzo[a]pyrene diol epoxide (BPDE)-induced mutagenesis with no marked effect on repair of BPDE-DNA adducts in human lung cells. Toxicol. Vitr. 2009;23:897–905. doi: 10.1016/j.tiv.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Bukowska B., Mokra K., Michałowicz J. Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022;23:6348. doi: 10.3390/ijms23116348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu M., Wang G., Tian W., Deng Y., Xu Y. MiRNA-based Therapeutics for Lung Cancer. Curr. Pharm. Des. 2018;23:5989–5996. doi: 10.2174/1381612823666170714151715. [DOI] [PubMed] [Google Scholar]
- 5.Erdoğan İ., Sweef O., Akgül B. Long Noncoding RNAs in Human Cancer and Apoptosis. Curr. Pharm. Biotechnol. 2023;24:872–888. doi: 10.2174/1389201023666220624094950. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal V., Bell G.W., Nam J.-W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/elife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso A.P.F., Al-Eryani L., States J.C. Arsenic-Induced Carcinogenesis: The Impact of miRNA Dysregulation. Toxicol. Sci. 2018;165:284–290. doi: 10.1093/toxsci/kfy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vorvis C., Koutsioumpa M., Iliopoulos D. Developments in miRNA gene signaling pathways in pancreatic cancer. Futur. Oncol. 2016;12:1135–1150. doi: 10.2217/fon-2015-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso A.P.F., Udoh K.T., States J.C. Arsenic-induced changes in miRNA expression in cancer and other diseases. Toxicol. Appl. Pharmacol. 2020;409:115306. doi: 10.1016/j.taap.2020.115306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceccaroli C., Pulliero A., Geretto M., Izzotti A. Molecular Fingerprints of Environmental Carcinogens in Human Cancer. J. Environ. Sci. Health Part C. 2015;33:188–228. doi: 10.1080/10590501.2015.1030491. [DOI] [PubMed] [Google Scholar]
- 12.Nagano T., Tachihara M., Nishimura Y. Molecular Mechanisms and Targeted Therapies Including Immunotherapy for Non-Small Cell Lung Cancer. Curr. Cancer Drug Targets. 2019;19:595–630. doi: 10.2174/1568009619666181210114559. [DOI] [PubMed] [Google Scholar]
- 13.Yu F., Yu C., Li F., Zuo Y., Wang Y., Yao L., Wu C., Wang C., Ye L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021;6:307. doi: 10.1038/s41392-021-00701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewandowska A., Rudzki M., Rudzki S., Lewandowski T., Laskowska B. Environmental risk factors for cancer—Review paper. Ann. Agric. Environ. Med. 2019;26:1–7. doi: 10.26444/aaem/94299. [DOI] [PubMed] [Google Scholar]
- 15.Kim J., Park C., Kim K.H., Kim E.H., Kim H., Woo J.K., Seong J.K., Nam K.T., Lee Y.C., Cho S.Y. Single-cell analysis of gastric pre-cancerous and cancer lesions reveals cell lineage diversity and intratumoral heterogeneity. npj Precis. Oncol. 2022;6:9. doi: 10.1038/s41698-022-00251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramírez-Labrada A.G., Isla D., Artal A., Arias M., Rezusta A., Pardo J., Gálvez E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer. 2020;6:86–97. doi: 10.1016/j.trecan.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Duffy M.J., O’Byrne K. Tissue and Blood Biomarkers in Lung Cancer: A Review. Adv. Clin. Chem. 2018;86:1–21. doi: 10.1016/bs.acc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Miller M., Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ. 2021;375:n2363. doi: 10.1136/bmj.n2363. [DOI] [PubMed] [Google Scholar]
- 19.Gurer D.C., Erdogan I., Ahmadov U., Basol M., Sweef O., Cakan-Akdogan G., Akgül B. Transcriptomics Profiling Identifies Cisplatin-Inducible Death Receptor 5 Antisense Long Non-coding RNA as a Modulator of Proliferation and Metastasis in HeLa Cells. Front. Cell Dev. Biol. 2021;9:688855. doi: 10.3389/fcell.2021.688855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H., Lee L.-S., Li G., Tsao S.-W., Chiu J.-F. Upregulation of glycolysis and oxidative phosphorylation in benzo[β]pyrene and arsenic-induced rat lung epithelial transformed cells. Oncotarget. 2016;7:40674–40689. doi: 10.18632/oncotarget.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Yang P., Xie J., Lin H.-P., Kumagai K., Harkema J., Yang C. Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression. Environ. Int. 2020;137:105560. doi: 10.1016/j.envint.2020.105560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya P., Welch A.H., Stollenwerk K.G., McLaughlin M.J., Bundschuh J., Panaullah G. Arsenic in the environment: Biology and Chemistry. Sci. Total. Environ. 2007;379:109–120. doi: 10.1016/j.scitotenv.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 23.Hall A.H. Chronic arsenic poisoning. Toxicol. Lett. 2002;128:69–72. doi: 10.1016/s0378-4274(01)00534-3. [DOI] [PubMed] [Google Scholar]
- 24.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 25.Gadd G.M. Arsenic Toxicity: An Arsenic-Hyperaccumulating Fern Uses a Bacterial-like Tolerance Mechanism. Curr. Biol. 2019;29:R580–R582. doi: 10.1016/j.cub.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Wang W., Zheng F., Zhang A. Arsenic-induced lung inflammation and fibrosis in a rat model: Contribution of the HMGB1/RAGE, PI3K/AKT, and TGF-β1/SMAD pathways. Toxicol. Appl. Pharmacol. 2021;432:115757. doi: 10.1016/j.taap.2021.115757. [DOI] [PubMed] [Google Scholar]
- 27.Tarasco M., Gavaia P.J., Bensimon-Brito A., Cardeira-Da-Silva J., Ramkumar S., Cordelières F.P., Günther S., Bebianno M.J., Stainier D.Y., Cancela M.L., et al. New insights into benzo[⍺]pyrene osteotoxicity in zebrafish. Ecotoxicol. Environ. Saf. 2021;226:112838. doi: 10.1016/j.ecoenv.2021.112838. [DOI] [PubMed] [Google Scholar]
- 28.Levin W., Conney A.H., Alvares A.P., Merkatz I., Kappas A. Induction of Benzo[α]pyrene Hydroxylase in Human Skin. Science. 1972;176:419–420. doi: 10.1126/science.176.4033.419. [DOI] [PubMed] [Google Scholar]
- 29.Chang J., Wang Q., Dong X., Luo T., Liu Z., Xu D. The influencing factors of health hazards of benzo[a]pyrene in cigarette mainstream smoke: The example of one brand in Beijing. Tob. Induc. Dis. 2022;20:80. doi: 10.18332/tid/152419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W., Qi Y., Gao Y., Quan H., Li Q., Zhou H., Huang J. Benzo(a)pyrene exposure in utero exacerbates Parkinson’s Disease (PD)-like α-synucleinopathy in A53T human alpha-synuclein transgenic mice. Toxicol. Appl. Pharmacol. 2021;427:115658. doi: 10.1016/j.taap.2021.115658. [DOI] [PubMed] [Google Scholar]
- 31.Kim J., Park S.-H., Yang S., Oh S.W., Kwon K., Park S.J., Yu E., Kim H., Park J.Y., Choi S., et al. Protective Effects of Maclurin against Benzo[a]pyrene via Aryl Hydrocarbon Receptor and Nuclear Factor Erythroid 2-Related Factor 2 Targeting. Antioxidants. 2021;10:1189. doi: 10.3390/antiox10081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khattab S.A., Hussien W.F., Raafat N., El-Din E.A.A. Modulatory effects of catechin hydrate on benzo[a]pyrene-induced nephrotoxicity in adult male albino rats. Toxicol. Res. 2021;10:542–550. doi: 10.1093/toxres/tfab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G., Yu T., Zhang Q., Zhang H., Xiao M., Cui S., Zhao Y., Lu X. Malignant transformation of human bronchial epithelial cells induced by benzo [a] pyrene suggests a negative feedback of TP53 to PPP1R13L via binding a possible enhancer element. Chem. Biol. Interact. 2021;349:109683. doi: 10.1016/j.cbi.2021.109683. [DOI] [PubMed] [Google Scholar]
- 34.Paget V., Lechevrel M., André V., Le Goff J., Pottier D., Billet S., Garçon G., Shirali P., Sichel F. Benzo[a]pyrene, Aflatoxine B1 and Acetaldehyde Mutational Patterns in TP53 Gene Using a Functional Assay: Relevance to Human Cancer Aetiology. PLoS ONE. 2012;7:e30921. doi: 10.1371/journal.pone.0030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin G., Meng Z. Sulfur dioxide and benzo(a)pyrene modulates CYP1A and tumor-related gene expression in rat liver. Environ. Toxicol. 2010;25:169–179. doi: 10.1002/tox.20484. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Xie J., Li Y., Lin H.-P., Fenske W., Clementino M., Jiang Y., Yang C., Wang Z. Deubiquitinase USP7-mediated MCL-1 up-regulation enhances Arsenic and Benzo(a)pyrene co-exposure-induced Cancer Stem Cell-like property and Tumorigenesis. Theranostics. 2020;10:9050–9065. doi: 10.7150/thno.47897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier A., Schumann B.L., Chang X., Talaska G., Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutat. Res. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 38.Silva R.A., Muñoz S.E., Perez C.A., Eynard A.R. Effects of dietary fat on benz-a-pyrene-induced forestomach tumorigenesis in mice chronically exposed to arsenic. Exp. Toxicol. Pathol. 2000;52:11–16. doi: 10.1016/s0940-2993(00)80007-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen C., Jiang X., Ren Y., Zhang Z. Arsenic Trioxide Co-exposure Potentiates Benzo(a)pyrene Genotoxicity by Enhancing the Oxidative Stress in Human Lung Adenocarcinoma Cell. Biol. Trace Elem. Res. 2013;156:338–349. doi: 10.1007/s12011-013-9819-0. [DOI] [PubMed] [Google Scholar]
- 40.Ho P.C., Ong P.-S., Chan S.-Y., Ho P.C. Differential augmentative effects of buthionine sulfoximine and ascorbic acid in As2O3-induced ovarian cancer cell death: Oxidative stress-independent and -dependent cytotoxic potentiation. Int. J. Oncol. 2011;38:1731–1739. doi: 10.3892/ijo.2011.986. [DOI] [PubMed] [Google Scholar]
- 41.Xie J., Yang P., Lin H.-P., Li Y., Clementino M., Fenske W., Yang C., Wang C., Wang Z. Integrin α4 up-regulation activates the hedgehog pathway to promote arsenic and benzo[α]pyrene co-exposure-induced cancer stem cell-like property and tumorigenesis. Cancer Lett. 2020;493:143–155. doi: 10.1016/j.canlet.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui Q., Chen F.-Y., Chen H.-Y., Peng H., Wang K.-J. Benzo[a]pyrene (BaP) exposure generates persistent reactive oxygen species (ROS) to inhibit the NF-κB pathway in medaka (Oryzias melastigma) Environ. Pollut. 2019;251:502–509. doi: 10.1016/j.envpol.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 43.Zhong S., Golpon H., Zardo P., Borlak J. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl. Res. 2021;230:164–196. doi: 10.1016/j.trsl.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 44.de Sousa M.C., Gjorgjieva M., Dolicka D., Sobolewski C., Foti M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019;20:6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vishnoi A., Rani S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1. [DOI] [PubMed] [Google Scholar]
- 46.Ali Syeda Z., Langden S.S.S., Munkhzul C., Lee M., Song S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020;21:1723. doi: 10.3390/ijms21051723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michlewski G., Cáceres J.F. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherman E.J., Mitchell D.C., Garner A.L. The RNA-binding protein SART3 promotes miR-34a biogenesis and G1 cell cycle arrest in lung cancer cells. J. Biol. Chem. 2019;294:17188–17196. doi: 10.1074/jbc.ac119.010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 50.Soni D.K., Kumar V.P., Biswas S., Holmes-Hampton G.P., Bhattacharyya S., Thomas L.J., Biswas R., Ghosh S.P. CDX-301 prevents radiation-induced dysregulation of miRNA expression and biogenesis. Mol. Ther. Nucleic Acids. 2022;30:569–584. doi: 10.1016/j.omtn.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao M.-Y., Wang L.-M., Liu J., Huang X., Liu J., Zhang Y.-F. MiR-21 Suppresses Anoikis through Targeting PDCD4 and PTEN in Human Esophageal Adenocarcinoma. Curr. Med. Sci. 2018;38:245–251. doi: 10.1007/s11596-018-1872-7. [DOI] [PubMed] [Google Scholar]
- 52.Bayraktar R., Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018;37:33–44. doi: 10.1007/s10555-017-9724-7. [DOI] [PubMed] [Google Scholar]
- 53.O’Bryan S., Dong S., Mathis J.M., Alahari S.K. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur. J. Cancer. 2017;72:1–11. doi: 10.1016/j.ejca.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Liu H., Deng H., Zhao Y., Li C., Liang Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 2018;37:279. doi: 10.1186/s13046-018-0950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhome R., Emaduddin M., James V., House L.M., Thirdborough S.M., Mellone M., Tulkens J., Primrose J.N., Thomas G.J., De Wever O., et al. Epithelial to mesenchymal transition influences fibroblast phenotype in colorectal cancer by altering miR-200 levels in extracellular vesicles. J. Extracell. Vesicles. 2022;11:e12226. doi: 10.1002/jev2.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong C.H., Lou U.K., Fung F.K.-C., Tong J.H.M., Zhang C.-H., To K.-F., Chan S.L., Chen Y. CircRTN4 promotes pancreatic cancer progression through a novel CircRNA-miRNA-lncRNA pathway and stabilizing epithelial-mesenchymal transition protein. Mol. Cancer. 2022;21:10. doi: 10.1186/s12943-021-01481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaccagnini G., Greco S., Voellenkle C., Gaetano C., Martelli F. miR-210 hypoxamiR in Angiogenesis and Diabetes. Antioxidants Redox Signal. 2022;36:685–706. doi: 10.1089/ars.2021.0200. [DOI] [PubMed] [Google Scholar]
- 58.Wang D., Cui L., Yang Q., Wang J. Circular RNA circZFPM2 promotes epithelial-mesenchymal transition in endometriosis by regulating miR-205-5p/ZEB1 signalling pathway. Cell. Signal. 2021;87:110145. doi: 10.1016/j.cellsig.2021.110145. [DOI] [PubMed] [Google Scholar]
- 59.Tan X., Tong L., Li L., Xu J., Xie S., Ji L., Fu J., Liu Q., Shen S., Liu Y., et al. Loss of Smad4 promotes aggressive lung cancer metastasis by de-repression of PAK3 via miRNA regulation. Nat. Commun. 2021;12:4853. doi: 10.1038/s41467-021-24898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu J., Liu B., Wang Z., Wang D., Ni H., Zhang L., Wang Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavallari I., Ciccarese F., Sharova E., Urso L., Raimondi V., Silic-Benussi M., D’agostino D.M., Ciminale V. The miR-200 Family of microRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers. 2021;13:5874. doi: 10.3390/cancers13235874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuo J., Brewer D.S., Arlt V.M., Cooper C.S., Phillips D.H. Benzo pyrene-induced DNA adducts and gene expression profiles in target and non-target organs for carcinogenesis in mice. BMC Genom. 2014;15:880. doi: 10.1186/1471-2164-15-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W.Z., Xi H.Z., Wang Y.J., Ma H.B., Cheng Z.Q., Yang Y., Wu M.L., Liu T.M., Yang W., Wang Q., et al. Design, synthesis, and biological evaluation of benzo[b]thiophene 1,1-dioxide derivatives as potent STAT3 inhibitors. Chem. Biol. Drug Des. 2021;98:835–849. doi: 10.1111/cbdd.13939. [DOI] [PubMed] [Google Scholar]
- 64.He B., Zhao Z., Cai Q., Zhang Y., Zhang P., Shi S., Xie H., Peng X., Yin W., Tao Y., et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020;16:2628–2647. doi: 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumann V., Winkler J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Futur. Med. Chem. 2014;6:1967–1984. doi: 10.4155/fmc.14.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomson D.W., Bracken C.P., Szubert J.M., Goodall G.J. On Measuring miRNAs after Transient Transfection of Mimics or Antisense Inhibitors. PLoS ONE. 2013;8:e55214. doi: 10.1371/journal.pone.0055214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Z., Lin C.-Y., Cheng K. siRNA- and miRNA-based therapeutics for liver fibrosis. Transl. Res. 2019;214:17–29. doi: 10.1016/j.trsl.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavel A.B., Campbell J.D., Liu G., Elashoff D., Dubinett S., Smith K., Whitney D., Lenburg M.E., Spira A., AEGIS Study Team Alterations in Bronchial Airway miRNA Expression for Lung Cancer Detection. Cancer Prev. Res. 2017;10:651–659. doi: 10.1158/1940-6207.capr-17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J., Zhang Z., Nie X., Liu Y., Qi Y., Wang J. Deregulated RNAs involved in sympathetic regulation of sepsis-induced acute lung injury based on whole transcriptome sequencing. BMC Genom. 2022;23:836. doi: 10.1186/s12864-022-09073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu W., Shan X., Wang T., Shu Y., Liu P. miR-21-induced chemoresistance to cisplatin in lung cancer cells by targeting PTEN. Arch. Med. Res. 2011;42:281–285. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Mollaei H., Safaralizadeh R., Rostami Z. MicroRNA replacement therapy in cancer. J. Cell. Physiol. 2019;234:12369–12384. doi: 10.1002/jcp.28058. [DOI] [PubMed] [Google Scholar]
- 72.Hu J., Huang S., Liu X., Zhang Y., Wei S., Hu X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022;2022:7437281. doi: 10.1155/2022/7437281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Y., Shen J.K., Milane L., Hornicek F.J., Amiji M.M., Duan Z. Targeted cancer therapy; nanotechnology approaches for overcoming drug resistance. Curr. Med. Chem. 2015;22:1335–1347. doi: 10.2174/0929867322666150209151851. [DOI] [PubMed] [Google Scholar]
- 74.Kaczmarek J.C., Kowalski P.S., Anderson D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue W., Dahlman J.E., Tammela T., Khan O.F., Sood S., Dave A., Cai W., Chirino L.M., Yang G.R., Bronson R., et al. Small RNA combination therapy for lung cancer. Proc. Natl. Acad. Sci. USA. 2014;111:E3553–E3561. doi: 10.1073/pnas.1412686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wahlgren J., Karlson T.D.L., Brisslert M., Vaziri Sani F., Telemo E., Sunnerhagen P., Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catuogno S., Esposito C.L., De Franciscis V. Aptamer-Mediated Targeted Delivery of Therapeutics: An Update. Pharmaceuticals. 2016;9:69. doi: 10.3390/ph9040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu D., Gao Y., Qi Y., Chen L., Ma Y., Li Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014;351:13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Mir L.M. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochem Bioenerg. 1995;38:251–255. doi: 10.1016/S0302-4598(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 80.Wang K., Li P.-F. Foxo3a Regulates Apoptosis by Negatively Targeting miR-21. J. Biol. Chem. 2010;285:16958–16966. doi: 10.1074/jbc.m109.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasinski A.L., Slack F.J. miRNA-34 Prevents Cancer Initiation and Progression in a Therapeutically Resistant K-ras and p53-Induced Mouse Model of Lung Adenocarcinoma. Cancer Res. 2012;72:5576–5587. doi: 10.1158/0008-5472.can-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen T., Xiao Q., Wang X., Wang Z., Hu J., Zhang Z., Gong Z., Chen S. miR-16 regulates proliferation and invasion of lung cancer cells via the ERK/MAPK signaling pathway by targeted inhibition of MAPK kinase 1 (MEK1) J. Int. Med. Res. 2019;47:5194–5204. doi: 10.1177/0300060519856505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonci D., Coppola V., Musumeci M., Addario A., Giuffrida R., Memeo L., D’Urso L., Pagliuca A., Biffoni M., Labbaye C., et al. The miR-15a–miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 84.Li Z., Jiang W., Wu G., Ju X., Wang Y., Liu W. miR-16 inhibits hyperoxia-induced cell apoptosis in human alveolar epithelial cells. Mol. Med. Rep. 2018;17:5950–5957. doi: 10.3892/mmr.2018.8636. [DOI] [PubMed] [Google Scholar]
- 85.Krützfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M., Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 86.Esau C., Davis S., Murray S.F., Yu X.X., Pandey S.K., Pear M., Watts L., Booten S.L., Graham M., McKay R., et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Elbashir S.M., Lendeckel W., Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding F., You T., Hou X.D., Yi K., Liu X.G., Zhang P., Wang X.K. MiR-21 Regulates Pulmonary Hypertension in Rats via TGF-β1/Smad2 Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:3984–3992. doi: 10.26355/eurrev_201905_17828. [DOI] [PubMed] [Google Scholar]
- 89.Lin J., Chen Y., Liu L., Shen A., Zheng W. MicroRNA-155-5p suppresses the migration and invasion of lung adenocarcinoma A549 cells by targeting Smad2. Oncol. Lett. 2018;16:2444–2452. doi: 10.3892/ol.2018.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu F., Song D., Wu Y., Liu X., Zhu J., Tang Y. MiR-155 inhibits proliferation and invasion by directly targeting PDCD4 in non-small cell lung cancer. Thorac. Cancer. 2017;8:613–619. doi: 10.1111/1759-7714.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 92.Savenkova D.A., Makarova A.-L.A., Shalik I.K., Yudkin D.V. miRNA Pathway Alteration in Response to Non-Coding RNA Delivery in Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2022;23:14954. doi: 10.3390/ijms232314954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Wu W., Wang Y., Han S., Yuan Y., Huang J., Shuai X., Peng Z. Recent development of gene therapy for pancreatic cancer using non-viral nanovectors. Biomater. Sci. 2021;9:6673–6690. doi: 10.1039/d1bm00748c. [DOI] [PubMed] [Google Scholar]
- 94.Feng Q.Q., Dong Z.Q., Zhou Y., Zhang H., Long C. miR-16-1-3p targets TWIST1 to inhibit cell proliferation and invasion in NSCLC. Bratisl. Lek. Listy. 2018;119:60–65. doi: 10.4149/bll_2018_012. [DOI] [PubMed] [Google Scholar]
- 95.Yan Y., Liu X.-Y., Lu A., Wang X.-Y., Jiang L.-X., Wang J.-C. Non-viral vectors for RNA delivery. J. Control. Release. 2022;342:241–279. doi: 10.1016/j.jconrel.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 97.Yonezawa S., Koide H., Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020;154–155:64–78. doi: 10.1016/j.addr.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsakiri M., Zivko C., Demetzos C., Mahairaki V. Lipid-based nanoparticles and RNA as innovative neuro-therapeutics. Front. Pharmacol. 2022;13:900610. doi: 10.3389/fphar.2022.900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ickenstein L.M., Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019;16:1205–1226. doi: 10.1080/17425247.2019.1669558. [DOI] [PubMed] [Google Scholar]
- 100.Yang Z., Cappello T., Wang L. Emerging role of microRNAs in lipid metabolism. Acta Pharm. Sin. B. 2015;5:145–150. doi: 10.1016/j.apsb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hong S., Leroueil P.R., Janus E.K., Peters J.L., Kober M.-M., Islam M.T., Orr B.G., Baker J.R., Jr., Holl M.M. Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjugate Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 102.Singha K., Namgung R., Kim W.J. Polymers in Small-Interfering RNA Delivery. Nucleic Acid Ther. 2011;21:133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de la Hoz R., Diban N., Berciano M.T., Emeterio C.S., Urtiaga A., Lafarga M., Rodríguez-Rey J.C., Tapia O. Coaxial Synthesis of PEI-Based Nanocarriers of Encapsulated RNA-Therapeutics to Specifically Target Muscle Cells. Biomolecules. 2022;12:1012. doi: 10.3390/biom12081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ewe A., Höbel S., Heine C., Merz L., Kallendrusch S., Bechmann I., Merz F., Franke H., Aigner A. Optimized polyethylenimine (PEI)-based nanoparticles for siRNA delivery, analyzed in vitro and in an ex vivo tumor tissue slice culture model. Drug Deliv. Transl. Res. 2016;7:206–216. doi: 10.1007/s13346-016-0306-y. [DOI] [PubMed] [Google Scholar]
- 105.Revia R.A., Stephen Z.R., Zhang M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Accounts Chem. Res. 2019;52:1496–1506. doi: 10.1021/acs.accounts.9b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeLong R.K., Curtis C.B. Toward RNA nanoparticle vaccines: Synergizing RNA and inorganic nanoparticles to achieve immunopotentiation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1415. doi: 10.1002/wnan.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong D.S., Kang Y.K., Brenner A.J., Sachdev J.C., Ejadi S., Borad M.J., Kim T.-Y., Lim H.Y., Park K., Becerra C., et al. MRX34, a liposomal miR-34 mimic, in patients with advanced solid tumors: Final dose-escalation results from a first-in-human phase I trial of microRNA therapy. J. Clin. Oncol. 2016;34:2508. doi: 10.1200/JCO.2016.34.15_suppl.2508. [DOI] [Google Scholar]
- 108.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I Study of MRX34, a Liposomal miR-34a Mimic, Administered Twice Weekly in Patients with Advanced Solid Tumors. Investig. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Viteri S., Rosell R. An innovative mesothelioma treatment based on miR-16 mimic loaded EGFR targeted minicells (TargomiRs) Transl. Lung Cancer Res. 2018;7((Suppl. S1)):S1–S4. doi: 10.21037/tlcr.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelnar K., Peltier H.J., Leatherbury N., Stoudemire J., Bader A.G. Quantification of Therapeutic miRNA Mimics in Whole Blood from Nonhuman Primates. Anal. Chem. 2014;86:1534–1542. doi: 10.1021/ac403044t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diener C., Keller A., Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022;38:613–626. doi: 10.1016/j.tig.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Ganju A., Khan S., Hafeez B.B., Behrman S.W., Yallapu M.M., Chauhan S.C., Jaggi M. miRNA nanotherapeutics for cancer. Drug Discov. Today. 2017;22:424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kara G., Calin G.A., Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv. Rev. 2022;182:114113. doi: 10.1016/j.addr.2022.114113. [DOI] [PubMed] [Google Scholar]
- 115.Liu S.-H., Hsu K.-W., Lai Y.-L., Lin Y.-F., Chen F.-H., Peng P.-H., Lin L.-J., Wu H.-H., Li C.-Y., Wang S.-C., et al. Systematic identification of clinically relevant miRNAs for potential miRNA-based therapy in lung adenocarcinoma. Mol. Ther. Nucleic Acids. 2021;25:1–10. doi: 10.1016/j.omtn.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan G., Liu Y., Shang L., Zhou F., Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021;41:199–217. doi: 10.1002/cac2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long J., Danesh F.R. Promises and challenges of miRNA therapeutics. Am. J. Physiol. Ren. Physiol. 2022;323:F673–F674. doi: 10.1152/ajprenal.00251.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Titze-De-Almeida S.S., Soto-Sánchez C., Fernandez E., Koprich J.B., Brotchie J.M., Titze-De-Almeida R. The Promise and Challenges of Developing miRNA-Based Therapeutics for Parkinson’s Disease. Cells. 2020;9:841. doi: 10.3390/cells9040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mishra S., Yadav T., Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit. Rev. Oncol. Hematol. 2016;98:12–23. doi: 10.1016/j.critrevonc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 120.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O’Briant K.C., Allen A., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yete S., Saranath D. MicroRNAs in oral cancer: Biomarkers with clinical potential. Oral Oncol. 2020;110:105002. doi: 10.1016/j.oraloncology.2020.105002. [DOI] [PubMed] [Google Scholar]
- 122.Liu M., Wu J., Wang N., Zhang X., Bai Y., Guo J., Zhang L., Liu S., Tao K. The value of artificial intelligence in the diagnosis of lung cancer: A systematic review and meta-analysis. PLoS ONE. 2023;18:e0273445. doi: 10.1371/journal.pone.0273445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bendifallah S., Dabi Y., Suisse S., Jornea L., Bouteiller D., Touboul C., Puchar A., Daraï E. MicroRNome analysis generates a blood-based signature for endometriosis. Sci. Rep. 2022;12:4051. doi: 10.1038/s41598-022-07771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saliminejad K., Khorram Khorshid H.R., Soleymani Fard S., Ghaffari S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 125.Tulinský L., Dzian A., Mataková T., Ihnát P. Overexpression of the miR-143/145 and reduced expression of the let-7 and miR-126 for early lung cancer diagnosis. J. Appl. Biomed. 2022;20:1–6. doi: 10.32725/jab.2022.004. [DOI] [PubMed] [Google Scholar]
- 126.Du X., Zhang J., Wang J., Lin X., Ding F. Role of miRNA in Lung Cancer-Potential Biomarkers and Therapies. Curr. Pharm. Des. 2018;23:5997–6010. doi: 10.2174/1381612823666170714150118. [DOI] [PubMed] [Google Scholar]
- 127.Zhu X., Kudo M., Huang X., Sui H., Tian H., Croce C.M., Cui R. Frontiers of MicroRNA Signature in Non-small Cell Lung Cancer. Front. Cell Dev. Biol. 2021;9:643942. doi: 10.3389/fcell.2021.643942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee S.S., Cheah Y.K. The Interplay between MicroRNAs and Cellular Components of Tumour Microenvironment (TME) on Non-Small-Cell Lung Cancer (NSCLC) Progression. J. Immunol. Res. 2019;2019:3046379. doi: 10.1155/2019/3046379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arab A., Karimipoor M., Irani S., Kiani A., Zeinali S., Tafsiri E., Sheikhy K. Potential circulating miRNA signature for early detection of NSCLC. Cancer Genet. 2017;216–217:150–158. doi: 10.1016/j.cancergen.2017.07.006. Erratum in Cancer Genet. 2018, 228–229, 127. [DOI] [PubMed] [Google Scholar]
- 130.Matthiesen R. MS-based biomarker discovery in bronchoalveolar lavage fluid for lung cancer. Proteom. Clin. Appl. 2020;14:1900077. doi: 10.1002/prca.201900077. [DOI] [PubMed] [Google Scholar]
- 131.Backes C., Meese E., Keller A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016;20:509–518. doi: 10.1007/s40291-016-0221-4. [DOI] [PubMed] [Google Scholar]
- 132.Ge T.-T., Liang Y., Fu R., Wang G.-J., Ruan E.-B., Qu W., Wang X.-M., Liu H., Wu Y.-H., Song J., et al. Expressions of miR-21, miR-155 and miR-210 in plasma of patients with lymphoma and its clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:305–309. (In Chinese) [PubMed] [Google Scholar]
- 133.Gamal-Eldeen A.M., Alrehaili A.A., Alharthi A., Raafat B.M. Effect of Combined Perftoran and Indocyanine Green-Photodynamic Therapy on HypoxamiRs and OncomiRs in Lung Cancer Cells. Front. Pharmacol. 2022;13:844104. doi: 10.3389/fphar.2022.844104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang J.-S., Li B.-J., Lu H.-W., Chen Y., Lu C., Zhu R.-X., Liu S.-H., Yi Q.-T., Li J., Song C.-H. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumor Biol. 2015;36:3035–3042. doi: 10.1007/s13277-014-2938-1. [DOI] [PubMed] [Google Scholar]
- 135.Slaby O., Srovnal J., Radova L., Gregar J., Juracek J., Luzna P., Svoboda M., Hajduch M., Ehrmann J. Dynamic changes in microRNA expression profiles reflect progression of Barrett’s esophagus to esophageal adenocarcinoma. Carcinogenesis. 2015;36:521–527. doi: 10.1093/carcin/bgv023. [DOI] [PubMed] [Google Scholar]
- 136.Ding Y., Wu W., Ma Z., Shao X., Zhang M., Wang Z. Potential value of MicroRNA-21 as a biomarker for predicting the prognosis of patients with breast cancer: A Protocol for Meta-Analysis and Bioinformatics Analysis. Medicine. 2021;100:e25964. doi: 10.1097/md.0000000000025964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X., Hu Z., Wang W., Ba Y., Ma L., Zhang C., Wang C., Ren Z., Zhao Y., Wu S., et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int. J. Cancer. 2012;130:1620–1628. doi: 10.1002/ijc.26177. [DOI] [PubMed] [Google Scholar]
- 138.Bianchi F., Nicassio F., Marzi M., Belloni E., Dall’Olio V., Bernard L., Pelosi G., Maisonneuve P., Veronesi G., Di Fiore P.P. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol. Med. 2011;3:495–503. doi: 10.1002/emmm.201100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu R., Jiang Y., Wu Q., Li Q., Cheng D., Xu L., Zhang C., Zhang M., Ye L. Diagnostic value of microRNA-21 in the diagnosis of lung cancer: Evidence from a meta-analysis involving 11 studies. Tumor Biol. 2014;35:8829–8836. doi: 10.1007/s13277-014-2106-7. [DOI] [PubMed] [Google Scholar]
- 140.Wang H., Wu S., Zhao L., Zhao J., Liu J., Wang Z. Clinical use of microRNAs as potential non-invasive biomarkers for detecting non-small cell lung cancer: A meta-analysis. Respirology. 2015;20:56–65. doi: 10.1111/resp.12444. [DOI] [PubMed] [Google Scholar]
- 141.Canatan D., Sonmez Y., Yılmaz O., Coşkun H., Göksu S.S., Uçar S., Aktekin M.R. The importance microRNAs as a biomarker in lung cancer. Acta Biomed. 2023;94:e2023045. doi: 10.23750/abm.v94i1.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masoumi-Ardakani Y., Najafipour H., Nasri H.R., Aminizadeh S., Jafari S., Moflehi D. Effect of Combined Endurance Training and MitoQ on Cardiac Function and Serum Level of Antioxidants, NO, miR-126, and miR-27a in Hypertensive Individuals. BioMed. Res. Int. 2022;2022:8720661. doi: 10.1155/2022/8720661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Evans C.D., LaDow K., Schumann B.L., Savage R.E., Jr., Caruso J., Vonderheide A., Succop P., Talaska G. Effect of Arsenic on Benzo[a]pyrene DNA Adduct Levels in Mouse Skin and Lung. Carcinogenesis. 2004;25:493–497. doi: 10.1093/carcin/bgg199. [DOI] [PubMed] [Google Scholar]
- 144.Liu X., Xiao J., Zhu H., Wei X., Platt C., Damilano F., Xiao C., Bezzerides V., Bostrom P., Che L., et al. MicroRNA-15a/b Are Up-Regulated in Response to Myocardial Ischemia/Reperfusion Injury. J. Geriatr. Cardiol. 2014;11:53–57. [Google Scholar]
- 145.Bandres E., Cubedo E., Agirre X., Malumbres R., Zárate R., Ramirez N., Abajo A., Navarro A., Moreno I., Monzó M., et al. Identification by Real-Time PCR of 13 Mature MicroRNAs Differentially Expressed in Colorectal Cancer and Non-Tumoral Tissues. Mol. Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kovalchuk O., Filkowski J., Meservy J., Ilnytskyy Y., Tryndyak V.P., Chekhun V.F., Pogribny I.P. Involvement of MicroRNA-451 in Resistance of the MCF-7 Breast Cancer Cells to Chemotherapeutic Drug Doxorubicin. Mol. Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 147.Wu C., Cao Y., He Z., He J., Hu C., Duan H., Jiang J., Xia L. MiR-17-5p Promotes Proliferation by Targeting SOCS6 in Non-Small Cell Lung Cancer Cells. FEBS Lett. 2011;585:903–908. [Google Scholar]
- 148.Wu Q., Luo G., Yang Z., Zhu F., An Y., Shi Y., Fan D. miR-17-5p Promotes Proliferation by Targeting SOCS6 in Gastric Cancer Cells. FEBS Lett. 2014;588:2055–2062. doi: 10.1016/j.febslet.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 149.Liu X.H., Lu K.H., Wang K.M., Sun M., Zhang E.B., Yang J.S., Yin D.D., Liu Z.L., Zhou J., Liu Z.J., et al. MicroRNA-196a Promotes Non-Small Cell Lung Cancer Cell Proliferation and Invasion through Targeting HOXA5. BMC Cancer. 2012;12:348. doi: 10.1186/1471-2407-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiong M., Wang P., Pan B., Nie J., Wang S., He B. The diagnostic and prognostic values of microRNA-196a in cancer. Biosci. Rep. 2021;41:BSR20203559. doi: 10.1042/BSR20203559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shang Y., Wang L.Q., Guo Q.Y., Shi T.L. MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int. J. Clin. Exp. Pathol. 2015;8:2461–2472. [PMC free article] [PubMed] [Google Scholar]
- 152.Larabee S.M., Coia H., Jones S., Cheung E., Gallicano G.I. miRNA-17 members that target Bmpr2 influence signaling mechanisms important for embryonic stem cell differentiation in vitro and gastrulation in embryos. Stem Cells Dev. 2015;24:354–371. doi: 10.1089/scd.2014.0051. [DOI] [PubMed] [Google Scholar]
- 153.Meng F., Henson R., Wehbe-Janek H., Ghoshal K., Jacob S.T., Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu S., Wu H., Wu F., Nie D., Sheng S., Mo Y.Y. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 155.Tsang S.M., Oliemuller E., Howard B.A. Regulatory roles for SOX11 in development, stem cells and cancer. Pt 1Semin Cancer Biol. 2020;67:3–11. doi: 10.1016/j.semcancer.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 156.Gao Z.G., Yang P., Huang J., Ding Y.Q. CircFBXW7 alleviates glioma progression through regulating miR-23a-3p/PTEN axis. Anat. Rec. 2021;304:279–290. doi: 10.1002/ar.24410. [DOI] [PubMed] [Google Scholar]
- 157.Hu X., Wang Y., Liang H., Fan Q., Zhu R., Cui J., Zhang W., Zen K., Zhang C.Y., Hou D., et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017;8:e3059. doi: 10.1038/cddis.2017.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frampton A.E., Castellano L., Colombo T., Giovannetti E., Krell J., Jacob J., Pellegrino L., Roca-Alonso L., Funel N., Gall T.M., et al. Integrated molecular analysis to investigate the role of microRNAs in pancreatic tumour growth and progression. Lancet. 2015;385((Suppl. S1)):S37. doi: 10.1016/S0140-6736(15)60352-X. [DOI] [PubMed] [Google Scholar]
- 159.Rao Y., Fang Y., Tan W., Liu D., Pang Y., Wu X., Zhang C., Li G. Delivery of Long Non-coding RNA NEAT1 by Peripheral Blood Monouclear Cells-Derived Exosomes Promotes the Occurrence of Rheumatoid Arthritis via the MicroRNA-23a/MDM2/SIRT6 Axis. Front. Cell Dev. Biol. 2020;8:551681. doi: 10.3389/fcell.2020.551681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nie M., Yu S., Peng S., Fang Y., Wang H., Yang X. miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD5. Biol. Reprod. 2015;93:98. doi: 10.1095/biolreprod.115.130690. [DOI] [PubMed] [Google Scholar]
- 161.Gallardo E., Navarro A., Viñolas N., Marrades R.M., Diaz T., Gel B., Quera A., Bandres E., Garcia-Foncillas J., Ramirez J., et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30:1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 162.Yan X., Tang B., Chen B., Shan Y., Yang H. Reproducibility Project: Cancer Biology. Replication Study: The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Elife. 2019;8:e43511. doi: 10.7554/eLife.43511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cai X., Hagedorn C.H., Cullen B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.BioMEd Research International Retracted: MicroRNA-124 Regulates the Proliferation of Colorectal Cancer Cells by Targeting iASPP. Biomed. Res. Int. 2023;2023:9847281. doi: 10.1155/2023/9847281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu C.B., Li Q.L., Hu J.F., Zhang Q., Xie J.P., Deng L. miR-124 inhibits growth and invasion of gastric cancer by targeting ROCK1. Asian Pac. J. Cancer Prev. 2014;15:6543–6546. doi: 10.7314/APJCP.2014.15.16.6543. [DOI] [PubMed] [Google Scholar]
- 166.Tang Y., Pan J., Huang S., Peng X., Zou X., Luo Y., Ren D., Zhang X., Li R., He P., et al. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018;37:160. doi: 10.1186/s13046-018-0813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qin Y., Dang X., Li W., Ma Q. miR-133a functions as a tumor suppressor and directly targets FSCN1 in pancreatic cancer. Oncol. Res. 2013;21:353–363. doi: 10.3727/096504014X14024160459122. [DOI] [PubMed] [Google Scholar]
- 168.Li Z., Zhang W., Huang Y. MiRNA-133a is involved in the regulation of postmenopausal osteoporosis through promoting osteoclast differentiation. Acta Biochim. Biophys. Sin. 2018;50:273–280. doi: 10.1093/abbs/gmy006. [DOI] [PubMed] [Google Scholar]
- 169.Cho W.C. OncomiRs: The Discovery and Progress of MicroRNAs in Cancers. Mol. Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garzon R., Garofalo M., Martelli M.P., Briesewitz R., Wang L., Fernandez-Cymering C., Volinia S., Liu C.G., Schnittger S., Haferlach T., et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc. Natl. Acad. Sci. USA. 2008;105:3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garzon R., Volinia S., Liu C.G., Fernandez-Cymering C., Palumbo T., Pichiorri F., Fabbri M., Coombes K., Alder H., Nakamura T., et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Campayo M., Navarro A., Benítez J.C., Santasusagna S., Ferrer C., Monzó M., Cirera L. miR-21, miR-99b and miR-375 combination as predictive response signature for preoperative chemoradiotherapy in rectal cancer. PLoS ONE. 2018;13:e0206542. doi: 10.1371/journal.pone.0206542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yanaihara N., Caplen N.J., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R.M., Okamoto A., Yokota J., Tanaka T., et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 174.Hu Z., Chen X., Zhao Y., Tian T., Jin G., Shu Y., Chen Y., Xu L., Zen K., Zhang C., et al. Serum MicroRNA Signatures Identified in a Genome-Wide Serum MicroRNA Expression Profiling Predict Survival of Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010;28:1721–1726. doi: 10.1200/jco.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 175.Cui L., Zhou H., Zhao H., Zhou Y., Xu R., Xu X., Zheng L., Xue Z., Xia W., Zhang B., et al. MicroRNA-99a induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC Cancer. 2012;12:546. doi: 10.1186/1471-2407-12-546. Erratum in: BMC Cancer 2021, 21, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X., Han J., Zhu H., Peng L., Chen Z. miR-181b-5p mediates TGF-β1-induced epithelial-to-mesenchymal transition in non-small cell lung cancer stem-like cells derived from lung adenocarcinoma A549 cells. Int. J. Oncol. 2017;51:158–168. doi: 10.3892/ijo.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang G., Huang Y., Wang L.-L., Zhang Y.-F., Xu J., Zhou Y., Lourenco G.F., Zhang B., Wang Y., Ren R.-J., et al. MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer’s disease. Sci. Rep. 2016;6:26697. doi: 10.1038/srep26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu Z.J., He J.F., Li K.J., Chen J., Xie X.R. Decreased microRNA-146a in CD4+T cells promote ocular inflammation in thy-roid-associated ophthalmopathy by targeting NUMB. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1803–1809. [PubMed] [Google Scholar]
- 179.Huang C., Liu X.-J., Zhou Q., Xie J., Ma T.-T., Meng X.-M., Li J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int. Immunopharmacol. 2016;32:46–54. doi: 10.1016/j.intimp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 180.Palomer X., Capdevila-Busquets E., Botteri G., Davidson M.M., Rodríguez C., Martínez-González J., Vidal F., Barroso E., Chan T.O., Feldman A.M., et al. miR-146a targets c-Fos expression in human cardiac cells. Dis. Model. Mech. 2015;8:1081–1091. doi: 10.1242/dmm.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peng Y., Dong W., Lin T.-X., Zhong G.-Z., Liao B., Wang B., Gu P., Huang L., Xie Y., Lu F.-D., et al. MicroRNA-155 promotes bladder cancer growth by repressing the tumor suppressor DMTF1. Oncotarget. 2015;6:16043–16058. doi: 10.18632/oncotarget.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li X., Liu K., Zhou W., Jiang Z. MiR-155 targeting FoxO3a regulates oral cancer cell proliferation, apoptosis, and DDP resistance through targeting FoxO3a. Cancer Biomarkers. 2019;27:105–111. doi: 10.3233/CBM-190555. [DOI] [PubMed] [Google Scholar]
- 183.Li X., Gong Y., Lin X., Lin Q., Luo J., Yu T., Xu J., Chen L., Xu L., Hu Y. Down-regulation of microRNA-155 suppressed Candida albicans induced acute lung injury by activating SOCS1 and inhibiting inflammation response. J. Microbiol. 2022;60:402–410. doi: 10.1007/s12275-022-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Al-Haidari A.A., Syk I., Thorlacius H. MiR-155-5p positively regulates CCL17-induced colon cancer cell migration by targeting RhoA. Oncotarget. 2017;8:14887–14896. doi: 10.18632/oncotarget.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wu M., Duan Q., Liu X., Zhang P., Fu Y., Zhang Z., Liu L., Cheng J., Jiang H. MiR-155-5p promotes oral cancer progression by targeting chromatin remodeling gene ARID2. Biomed. Pharmacother. 2019;122:109696. doi: 10.1016/j.biopha.2019.109696. [DOI] [PubMed] [Google Scholar]
- 186.Liao W.-W., Zhang C., Liu F.-R., Wang W.-J. Effects of miR-155 on proliferation and apoptosis by regulating FoxO3a/BIM in liver cancer cell line HCCLM3. Eur. Rev. Med. Pharmacol. Sci. 2020;24:7196. doi: 10.26355/eurrev_202007_21845. [DOI] [PubMed] [Google Scholar]
- 187.Suzuki R., Amatya V.J., Kushitani K., Kai Y., Kambara T., Takeshima Y. miR-182 and miR-183 Promote Cell Proliferation and Invasion by Targeting FOXO1 in Mesothelioma. Front. Oncol. 2018;8:446. doi: 10.3389/fonc.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chang H., Liu Y.-H., Wang L.-L., Wang J., Zhao Z.-H., Qu J.-F., Wang S.-F. MiR-182 promotes cell proliferation by suppressing FBXW7 and FBXW11 in non-small cell lung cancer. Am. J. Transl. Res. 2018;10:1131–1142. [PMC free article] [PubMed] [Google Scholar]
- 189.Li X., Zhang X., Zhang Q., Lin R. miR-182 contributes to cell proliferation, invasion and tumor growth in colorectal cancer by targeting DAB2IP. Int. J. Biochem. Cell Biol. 2019;111:27–36. doi: 10.1016/j.biocel.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 190.Wang J., Li J., Shen J., Wang C., Yang L., Zhang X. MicroRNA-182 downregulates metastasis suppressor 1 and contributes to metastasis of hepatocellular carcinoma. BMC Cancer. 2012;12:227. doi: 10.1186/1471-2407-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sarver A.L., Li L., Subramanian S. MicroRNA miR-183 Functions as an Oncogene by Targeting the Transcription Factor EGR1 and Promoting Tumor Cell Migration. Cancer Res. 2010;70:9570–9580. doi: 10.1158/0008-5472.can-10-2074. [DOI] [PubMed] [Google Scholar]
- 192.Fang Z., Tang J., Bai Y., Lin H., You H., Jin H., Lin L., You P., Li J., Dai Z., et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J. Exp. Clin. Cancer Res. 2015;34:1–10. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin X., Zheng L., Song H., Xiao J., Pan B., Chen H., Jin X., Yu H. Effects of microRNA-183 on epithelial-mesenchymal transition, proliferation, migration, invasion and apoptosis in human pancreatic cancer SW1900 cells by targeting MTA1. Exp. Mol. Pathol. 2017;102:522–532. doi: 10.1016/j.yexmp.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 194.Lu Y.-Y., Zheng J.-Y., Liu J., Huang C.-L., Zhang W., Zeng Y. miR-183 induces cell proliferation, migration, and invasion by regulating PDCD4 expression in the SW1990 pancreatic cancer cell line. Biomed. Pharmacother. 2015;70:151–157. doi: 10.1016/j.biopha.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 195.Wang Y.-Y., Duan S.-H., Wang G.-L., Li J.-L. Integrated mRNA and miRNA expression profile analysis of female and male gonads in Hyriopsis cumingii. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-020-80264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang D., Feng W., Zhuang Y., Liu J., Feng Z., Xu T., Wang W., Zhu Y., Wang Z. Long non-coding RNA linc00665 inhibits CDKN1C expression by binding to EZH2 and affects cisplatin sensitivity of NSCLC cells. Mol. Ther. Nucleic Acids. 2021;23:1053–1065. doi: 10.1016/j.omtn.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He J., Ling L., Liu Z., Ren X., Wan L., Tu C., Li Z. Functional interplay between long non-coding RNAs and the Wnt signaling cascade in osteosarcoma. Cancer Cell Int. 2021;21:1–17. doi: 10.1186/s12935-021-02013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nouri N., Shareghi-Oskoue O., Aghebati-Maleki L., Danaii S., Heris J.A., Soltani-Zangbar M.S., Kamrani A., Yousefi M. Role of miRNAs interference on ovarian functions and premature ovarian failure. Cell Commun. Signal. 2022;20:1–12. doi: 10.1186/s12964-022-00992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fisher L. Retraction: Long noncoding RNA ANRIL protects cardiomyocytes against hypoxia/reoxygenation injury by sponging miR-195-5p and upregulating Bcl-2. RSC Adv. 2021;11:6238. doi: 10.1039/d1ra90058g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Patil N., Allgayer H., Leupold J.H. MicroRNAs in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1277:1–31. doi: 10.1007/978-3-030-50224-9_1. [DOI] [PubMed] [Google Scholar]
- 201.Nie H., Mu J., Wang J., Li Y. miR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol. Rep. 2018;40:1370–1378. doi: 10.3892/or.2018.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin X., Wang S., Sun M., Zhang C., Wei C., Yang C., Dou R., Liu Q., Xiong B. RETRACTED ARTICLE: miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J. Hematol. Oncol. 2019;12:1–14. doi: 10.1186/s13045-019-0708-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 203.Yin Y., Hong S., Yu S., Huang Y., Chen S., Liu Y., Zhang Q., Li Y., Xiao H. MiR-195 Inhibits Tumor Growth and Metastasis in Papillary Thyroid Carcinoma Cell Lines by Targeting CCND1 and FGF2. Int. J. Endocrinol. 2017;2017:6180425. doi: 10.1155/2017/6180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wu C.-L., Ho J.-Y., Chou S.-C., Yu D.-S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7:26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu Y., Kanwar S.S., Patel B.B., Oh P.-S., Nautiyal J., Sarkar F.H., Majumdar A.P. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGF R2) in colon cancer cells. Carcinogenesis. 2011;33:68–76. doi: 10.1093/carcin/bgr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tang X., Tu G., Yang G., Wang X., Kang L., Yang L., Zeng H., Wan X., Qiao Y., Cui X., et al. Autocrine TGF-β1/miR-200s/miR-221/DNMT3B regulatory loop maintains CAF status to fuel breast cancer cell proliferation. Cancer Lett. 2019;452:79–89. doi: 10.1016/j.canlet.2019.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yao C.-X., Wei Q.-X., Zhang Y.-Y., Wang W.-P., Xue L.-X., Yang F., Zhang S.-F., Xiong C.-J., Li W.-Y., Wei Z.-R., et al. miR-200b targets GATA-4 during cell growth and differentiation. RNA Biol. 2013;10:465–480. doi: 10.4161/rna.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qiu H., Xie Z., Tang W., Liu C., Wang Y., Gu H., Zheng Q. Association between microRNA-146a, -499a and -196a-2 SNPs and non-small cell lung cancer: A case–control study involving 2249 subjects. Biosci. Rep. 2021;41:BSR20201158. doi: 10.1042/bsr20201158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Belvedere R., Saggese P., Pessolano E., Memoli D., Bizzarro V., Rizzo F., Parente L., Weisz A., Petrella A. miR-196a Is Able to Restore the Aggressive Phenotype of Annexin A1 Knock-Out in Pancreatic Cancer Cells by CRISPR/Cas9 Genome Editing. Int. J. Mol. Sci. 2018;19:1967. doi: 10.3390/ijms19071967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mendiola-Soto D.K., Bárcenas-López D.A., Pérez-Amado C.J., Cruz-Miranda G.M., Mejía-Aranguré J.M., Ramírez-Bello J., Hidalgo-Miranda A., Jiménez-Morales S. MiRNAs in Hematopoiesis and Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2023;24:5436. doi: 10.3390/ijms24065436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Choupani J., Nariman-Saleh-Fam Z., Saadatian Z., Ouladsahebmadarek E., Masotti A., Bastami M. Association of mir-196a-2 rs11614913 and mir-149 rs2292832 Polymorphisms with Risk of Cancer: An Updated Meta-Analysis. Front. Genet. 2019;10:186. doi: 10.3389/fgene.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Szczyrek M., Bitkowska P., Jutrzenka M., Milanowski J. The Role of the Selected miRNAs as Diagnostic, Predictive and Prognostic Markers in Non-Small-Cell Lung Cancer. J. Pers. Med. 2022;12:1227. doi: 10.3390/jpm12081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wu N., Zhang C., Bai C., Han Y.P., Li Q. miR-4782-3p Inhibited Non-Small Cell Lung Cancer growth via USP14. Cell. Physiol. Biochem. 2014;33:457–467. doi: 10.1159/000358626. [DOI] [PubMed] [Google Scholar]
- 214.Xu G., Liu C., Liang T., Qin Z., Yu C.J., Zhang Z., Jiang J., Chen J., Zhan X. Integrated miRNA-mRNA network revealing the key molecular characteristics of ossification of the posterior longitudinal ligament. Medicine. 2020;99:e20268. doi: 10.1097/MD.0000000000020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li J., Li Z., Zhao S., Song Y., Si L., Wang X. Identification key genes, key miRNAs and key transcription factors of lung adenocarcinoma. J. Thorac. Dis. 2020;12:1917–1933. doi: 10.21037/jtd-19-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tian Y., Li X., Bai C., Yang Z., Zhang L., Luo J. MiR-17-5p promotes the endothelialization of endothelial progenitor cells to facilitate the vascular repair of aneurysm by regulating PTEN-mediated PI3K/AKT/VEGFA pathway. Cell Cycle. 2020;19:3608–3621. doi: 10.1080/15384101.2020.1857958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.García-Martínez A., López-Muñoz B., Fajardo C., Cámara R., Lamas C., Silva-Ortega S., Aranda I., Picó A. Increased E2F1 mRNA and miR-17-5p Expression Is Correlated to Invasiveness and Proliferation of Pituitary Neuroendocrine Tumours. Diagnostics. 2020;10:227. doi: 10.3390/diagnostics10040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bao C., Liu T., Qian L., Xiao C., Zhou X., Ai H., Wang J., Fan W., Pan J. Shikonin inhibits migration and invasion of triple-negative breast cancer cells by suppressing epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. J. Cancer. 2021;12:76–88. doi: 10.7150/jca.47553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Khajehdehi M., Khalaj-Kondori M., Ghasemi T., Jahanghiri B., Damaghi M. Long Noncoding RNAs in Gastrointestinal Cancer: Tumor Suppression Versus Tumor Promotion. Dig. Dis. Sci. 2020;66:381–397. doi: 10.1007/s10620-020-06200-x. [DOI] [PubMed] [Google Scholar]
- 220.Zhuang J., Fan J., Zhu L., Zhao L., Huang Y., Pan X., Guo T. miR-452-5p suppressed the metastasis of Non-small cell lung cancer through regulating Moesin. J. Cancer. 2023;14:2015–2022. doi: 10.7150/jca.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ding B., Ma G., Wang Z., Liang W., Gao W. Mechanisms of Kidney Cell Pyroptosis in Chronic Kidney Disease and the Effects of Traditional Chinese Medicine. Evid. Based Complement. Altern. Med. 2021;2021:1173324. doi: 10.1155/2021/1173324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tutarel O., Dangwal S., Bretthauer J., Westhoff-Bleck M., Roentgen P., Anker S.D., Bauersachs J., Thum T. Circulating miR-423_5p fails as a biomarker for systemic ventricular function in adults after atrial repair for transposition of the great arteries. Int. J. Cardiol. 2013;167:63–66. doi: 10.1016/j.ijcard.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 223.Zafari N., Bahramy A., Zolbin M.M., Allahyari S.E., Farazi E., Hassannejad Z., Yekaninejad M.S. microRNAs as novel diagnostic biomarkers in endometriosis patients: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021;22:479–495. doi: 10.1080/14737159.2021.1960508. [DOI] [PubMed] [Google Scholar]
- 224.Liu Z., Zhang L., A Toma M., Li D., Bian X., Pastar I., Tomic-Canic M., Sommar P., Landén N.X. Integrative small and long RNA omics analysis of human healing and nonhealing wounds discovers cooperating microRNAs as therapeutic targets. Elife. 2022;11:e80322. doi: 10.7554/elife.80322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shen X., Li L., Zhang L., Liu W., Wu Y., Ma R. Diagnostic and prognostic value of microRNA-486 in patients with lung cancer: A systematic review and meta-analysis. Int. J. Biol. Markers. 2022;37:377–385. doi: 10.1177/03936155221115750. [DOI] [PubMed] [Google Scholar]
- 226.Tian F., Wang J., Ouyang T., Lu N., Lu J., Shen Y., Bai Y., Xie X., Ge Q. MiR-486-5p Serves as a Good Biomarker in Nonsmall Cell Lung Cancer and Suppresses Cell Growth with the Involvement of a Target PIK3R1. Front. Genet. 2019;10:688. doi: 10.3389/fgene.2019.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang G., Liu Z., Cui G., Wang X., Yang Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumor Biol. 2014;35:11137–11145. doi: 10.1007/s13277-014-2412-0. [DOI] [PubMed] [Google Scholar]
- 228.Hsu T.-K., Asmussen J., Koire A., Choi B.-K., Gadhikar M.A., Huh E., Lin C.-H., Konecki D.M., Kim Y.W., Pickering C.R., et al. A general calculus of fitness landscapes finds genes under selection in cancers. Genome Res. 2022;32:916–929. doi: 10.1101/gr.275811.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Li J., Li P., Shao J., Liang S., Wan Y., Zhang Q., Li C., Li Y., Wang C. Emerging Role of Noncoding RNAs in EGFR TKI-Resistant Lung Cancer. Cancers. 2022;14:4423. doi: 10.3390/cancers14184423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Choi Y.-C., Yoon S., Byun Y., Lee G., Kee H., Jeong Y., Yoon J., Baek K. MicroRNA library screening identifies growth-suppressive microRNAs that regulate genes involved in cell cycle progression and apoptosis. Exp. Cell Res. 2015;339:320–332. doi: 10.1016/j.yexcr.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 231.Wang Y.-F., Ao X., Liu Y., Ding D., Jiao W.-J., Yu Z., Zhai W.-X., Dong S.-H., He Y.-Q., Guo H., et al. MicroRNA-608 Promotes Apoptosis in Non-Small Cell Lung Cancer Cells Treated with Doxorubicin Through the Inhibition of TFAP4. Front Genet. 2019;10:809. doi: 10.3389/fgene.2019.00809. Erratum in: Front Genet. 2021, 12, 649586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang Q., He Y., Kan W., Li F., Ji X., Wu X., Wang X., Zhang Y., Chen J. microRNA-32-5p targets KLF2 to promote gastric cancer by activating PI3K/AKT signaling pathway. Am. J. Transl. Res. 2019;11:4895–4908. [PMC free article] [PubMed] [Google Scholar]
- 233.Al-Marzook F.A., Hassan D.M., Alghazal M.W., Kadheem R.A.A., Jalil A.T., Saleh M.M. MicroRNA-32 Suppression: Its Effects on Prostate Cancer Cells’ Capability to Proliferate and Migrate. Drug Res. 2023;73:170–174. doi: 10.1055/a-1977-8848. [DOI] [PubMed] [Google Scholar]
- 234.Pacholewska A., Kraft M.F., Gerber V., Jagannathan V. Differential Expression of Serum MicroRNAs Supports CD4+ T Cell Differentiation into Th2/Th17 Cells in Severe Equine Asthma. Genes. 2017;8:383. doi: 10.3390/genes8120383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Miao J., Regenstein J.M., Xu D., Zhou D., Li H., Zhang H., Li C., Qiu J., Chen X. The roles of microRNA in human cervical cancer. Arch. Biochem. Biophys. 2020;690:108480. doi: 10.1016/j.abb.2020.108480. [DOI] [PubMed] [Google Scholar]
- 236.Mohamed R.H., Pasha H.F., Gad D.M., Toam M.M. miR-146a and miR-196a-2 genes polymorphisms and its circulating levels in lung cancer patients. J. Biochem. 2019;166:323–329. doi: 10.1093/jb/mvz044. [DOI] [PubMed] [Google Scholar]
- 237.Yan X., Yu H., Liu Y., Hou J., Yang Q., Zhao Y. miR-27a-3p Functions as a Tumor Suppressor and Regulates Non-Small Cell Lung Cancer Cell Proliferation via Targeting HOXB8. Technol. Cancer Res. Treat. 2019;18:1533033819861971. doi: 10.1177/1533033819861971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 238.Li S., Han Y., Liang X., Zhao M. LINC01089 inhibits the progression of cervical cancer via inhibiting miR-27a-3p and increasing BTG2. J. Gene Med. 2021;23:e3280. doi: 10.1002/jgm.3280. [DOI] [PubMed] [Google Scholar]
- 239.Qiao B., He B.-X., Cai J.-H., Tao Q., Lam A.K.-Y. RETRACTED ARTICLE: MicroRNA-27a-3p Modulates the Wnt/β-Catenin Signaling Pathway to Promote Epithelial-Mesenchymal Transition in Oral Squamous Carcinoma Stem Cells by Targeting SFRP1. Sci. Rep. 2017;7:srep44688. doi: 10.1038/srep44688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 240.Zhao X.-R., Zhang Z., Gao M., Li L., Sun P.-Y., Xu L.-N., Qi Y., Yin L.-H., Peng J.-Y. MicroRNA-27a-3p aggravates renal ischemia/reperfusion injury by promoting oxidative stress via targeting growth factor receptor-bound protein 2. Pharmacol. Res. 2020;155:104718. doi: 10.1016/j.phrs.2020.104718. [DOI] [PubMed] [Google Scholar]
- 241.Liu T., Qin W., Hou L., Huang Y. MicroRNA-17 promotes normal ovarian cancer cells to cancer stem cells development via suppression of the LKB1-p53-p21/WAF1 pathway. Tumor Biol. 2014;36:1881–1893. doi: 10.1007/s13277-014-2790-3. [DOI] [PubMed] [Google Scholar]
- 242.Wang X., Han J., Liu Y., Hu J., Li M., Chen X., Xu L. miR-17-5p and miR-4443 Promote Esophageal Squamous Cell Carcinoma Development by Targeting TIMP2. Front. Oncol. 2021;11:605894. doi: 10.3389/fonc.2021.605894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Qu Y., Zhang H., Duan J., Liu R., Deng T., Bai M., Huang D., Li H., Ning T., Zhang L., et al. MiR-17-5p regulates cell proliferation and migration by targeting transforming growth factor-β receptor 2 in gastric cancer. Oncotarget. 2016;7:33286–33296. doi: 10.18632/oncotarget.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Du B., Wang Z., Zhang X., Feng S., Wang G., He J., Zhang B. MicroRNA-545 Suppresses Cell Proliferation by Targeting Cyclin D1 and CDK4 in Lung Cancer Cells. PLoS ONE. 2014;9:e88022. doi: 10.1371/journal.pone.0088022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zaheer U., Faheem M., Qadri I., Begum N., Yassine H.M., Al Thani A.A., Mathew S. Expression profile of MicroRNA: An Emerging Hallmark of Cancer. Curr. Pharm. Des. 2019;25:642–653. doi: 10.2174/1386207322666190325122821. [DOI] [PubMed] [Google Scholar]
- 246.Shi X., Ma W., Li Y., Wang H., Pan S., Pan Y., Xu C., Li L. CircPRKCI relieves lipopolysaccharide-induced HK2 cell injury by upregulating the expression of miR-545 target gene ZEB2. Biofactors. 2020;46:475–486. doi: 10.1002/biof.1620. [DOI] [PubMed] [Google Scholar]
- 247.Deng H., Lv L., Li Y., Zhang C., Meng F., Pu Y., Xiao J., Qian L., Zhao W., Liu Q., et al. miR-193a-3p regulates the multi-drug resistance of bladder cancer by targeting the LOXL4 gene and the Oxidative Stress pathway. Mol. Cancer. 2014;13:234. doi: 10.1186/1476-4598-13-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Khoo C.P., Roubelakis M.G., Schrader J.B., Tsaknakis G., Konietzny R., Kessler B., Harris A.L., Watt S.M. miR-193a-3p interaction with HMGB1 downregulates human endothelial cell proliferation and migration. Sci. Rep. 2017;7:srep44137. doi: 10.1038/srep44137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jiao Y., Hao L., Xia P., Cheng Y., Song J., Chen X., Wang Z., Ma Z., Zheng S., Chen T., et al. Identification of Potential miRNA–mRNA Regulatory Network Associated with Pig Growth Performance in the Pituitaries of Bama Minipigs and Landrace Pigs. Animals. 2022;12:3058. doi: 10.3390/ani12213058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lin C., Zhang S., Wang Y., Wang Y., Nice E., Guo C., Zhang E., Yu L., Li M., Liu C., et al. Functional Role of a Novel Long Noncoding RNA TTN-AS1 in Esophageal Squamous Cell Carcinoma Progression and Metastasis. Clin. Cancer Res. 2018;24:486–498. doi: 10.1158/1078-0432.ccr-17-1851. [DOI] [PubMed] [Google Scholar]
- 251.Xu H., Ma J., Zheng J., Wu J., Qu C., Sun F., Xu S. MiR-31 Functions as a Tumor Suppressor in Lung Adenocarcinoma Mainly by Targeting HuR. Clin. Lab. 2016;62:711–718. doi: 10.7754/clin.lab.2015.150903. [DOI] [PubMed] [Google Scholar]
- 252.Hsu H.-H., Kuo W.-W., Shih H.-N., Cheng S.-F., Yang C.-K., Chen M.-C., Tu C.-C., Viswanadha V.P., Liao P.-H., Huang C.-Y. FOXC1 Regulation of miR-31-5p Confers Oxaliplatin Resistance by Targeting LATS2 in Colorectal Cancer. Cancers. 2019;11:1576. doi: 10.3390/cancers11101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zou G., Ji Q., Geng Z., Du X., Jiang L., Liu T. miR-31-5p from placental and peripheral blood exosomes is a potential biomarker to diagnose preeclampsia. Hereditas. 2022;159:1–12. doi: 10.1186/s41065-022-00250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang M., Meng B., Liu Y., Yu J., Chen Q. MiR-124 Inhibits Growth and Enhances Radiation-Induced Apoptosis in Non-Small Cell Lung Cancer by Inhibiting STAT3. Cell. Physiol. Biochem. 2017;44:2017–2028. doi: 10.1159/000485907. [DOI] [PubMed] [Google Scholar]
- 256.Sun T.-Y., Li Y.-Q., Zhao F.-Q., Sun H.-M., Gao Y., Wu B., Yang S., Ji F.-Q., Zhou D.-S. MiR-1-3p and MiR-124-3p Synergistically Damage the Intestinal Barrier in the Ageing Colon. J. Crohn’s Colitis. 2021;16:656–667. doi: 10.1093/ecco-jcc/jjab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu W., Shen S., Tao M., Wang L. Targeting Notch1 inhibits invasion and growth of ovarian cancer cell through regulation of miR-124/flotillin-1 pathway. Int. J. Clin. Exp. Pathol. 2017;10:1576–1584. [Google Scholar]
- 258.Shirjang S., Mansoori B., Asghari S., Duijf P.H.G., Mohammadi A., Gjerstorff M., Baradaran B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019;139:1–15. doi: 10.1016/j.freeradbiomed.2019.05.017. Correction in Free Radic. Biol. Med. 2019, 146, 402. [DOI] [PubMed] [Google Scholar]
- 259.Ghoshal-Gupta S., Kutiyanawalla A., Lee B.R., Ojha J., Nurani A., Mondal A.K., Kolhe R., Rojiani A.M., Rojiani M.V. TIMP-1 downregulation modulates miR-125a-5p expression and triggers the apoptotic pathway. Oncotarget. 2018;9:8941–8956. doi: 10.18632/oncotarget.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hu H., Du L., Nagabayashi G., Seeger R.C., Gatti R.A. ATM is down-regulated by N-Myc–regulated microRNA-421. Proc. Natl. Acad. Sci. USA. 2010;107:1506–1511. doi: 10.1073/pnas.0907763107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shen Z., Chai T., Luo F., Liu Z., Xu H., Zhang P., Kang M., Chen S. Loss of miR-204-5p Promotes Tumor Proliferation, Migration, and Invasion Through Targeting YWHAZ/PI3K/AKT Pathway in Esophageal Squamous Cell Carcinoma. OncoTargets Ther. 2020;13:4679–4690. doi: 10.2147/ott.s243215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 262.Zhang B., Yin Y., Hu Y., Zhang J., Bian Z., Song M., Hua D., Huang Z. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med. Oncol. 2014;32:331. doi: 10.1007/s12032-014-0331-y. [DOI] [PubMed] [Google Scholar]
- 263.Jia W., Wu Y., Zhang Q., Gao G., Zhang C., Xiang Y. Identification of four serum microRNAs from a genome-wide serum microRNA expression profile as potential non-invasive biomarkers for endometrioid endometrial cancer. Oncol. Lett. 2013;6:261–267. doi: 10.3892/ol.2013.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang H., Li W., Gu W., Yan Y., Yao X., Zheng J. MALAT1 accelerates the development and progression of renal cell carcinoma by decreasing the expression of miR-203 and promoting the expression of BIRC5. Cell Prolif. 2019;52:e12640. doi: 10.1111/cpr.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu L., Wang J., Li X., Ma J., Shi C., Zhu H., Xi Q., Zhang J., Zhao X., Gu M. miR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem. Biophys. Res. Commun. 2015;457:621–626. doi: 10.1016/j.bbrc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 266.Feng Y., Zhu J., Ou C., Deng Z., Chen M., Huang W., Li L. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br. J. Cancer. 2014;110:2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li B., Ding C., Li Y., Peng J., Geng N., Qin W. MicroRNA-145 inhibits migration and induces apoptosis in human non-small cell lung cancer cells through regulation of the EGFR/PI3K/AKT signaling pathway. Oncol. Rep. 2018;40:2944–2954. doi: 10.3892/or.2018.6666. [DOI] [PubMed] [Google Scholar]
- 268.Wu D., Li M., Wang L., Zhou Y., Zhou J., Pan H., Qu P. microRNA-145 inhibits cell proliferation, migration and invasion by targeting matrix metallopeptidase-11 in renal cell carcinoma. Mol. Med. Rep. 2014;10:393–398. doi: 10.3892/mmr.2014.2149. [DOI] [PubMed] [Google Scholar]
- 269.Zhang Y., Yang X., Wu H., Zhou W., Liu Z. MicroRNA-145 inhibits migration and invasion via inhibition of fascin 1 protein expression in non-small-cell lung cancer cells. Mol. Med. Rep. 2015;12:6193–6198. doi: 10.3892/mmr.2015.4163. [DOI] [PubMed] [Google Scholar]
- 270.Chen Y., Wang X., Cheng J., Wang Z., Jiang T., Hou N., Liu N., Song T., Huang C. MicroRNA-20a-5p targets RUNX3 to regulate proliferation and migration of human hepatocellular cancer cells. Oncol. Rep. 2016;36:3379–3386. doi: 10.3892/or.2016.5144. [DOI] [PubMed] [Google Scholar]
- 271.Zhou Q., Dong J., Luo R., Zhou X., Wang J., Chen F. MicroRNA-20a regulates cell proliferation, apoptosis and autophagy by targeting thrombospondin 2 in cervical cancer. Eur. J. Pharmacol. 2018;844:102–109. doi: 10.1016/j.ejphar.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 272.Huang D., Peng Y., Ma K., Deng X., Tang L., Jing D., Shao Z. MiR-20a, a novel promising biomarker to predict prognosis in human cancer: A meta-analysis. BMC Cancer. 2018;18:1189. doi: 10.1186/s12885-018-4907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen H., Pan H., Qian Y., Zhou W., Liu X. MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2. Mol. Cancer. 2018;17:1–11. doi: 10.1186/s12943-017-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xiang J., Hang J.-B., Che J.-M., Li H.-C. MiR-25 is up-regulated in non-small cell lung cancer and promotes cell proliferation and motility by targeting FBXW7. Int. J. Clin. Exp. Pathol. 2015;8:9147–9153. [PMC free article] [PubMed] [Google Scholar]
- 275.Sun Y., Guo F., Bagnoli M., Xue F.-X., Sun B.-C., Shmulevich I., Mezzanzanica D., Chen K.-X., Sood A.K., Yang D., et al. Key nodes of a microRNA network associated with the integrated mesenchymal subtype of high-grade serous ovarian cancer. Chin. J. Cancer. 2015;34:28–40. doi: 10.5732/cjc.014.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jayamohan S., Kannan M., Moorthy R.K., Rajasekaran N., Jung H.S., Shin Y.K., Arockiam A.J.V. Dysregulation of miR-375/AEG-1 Axis by Human Papillomavirus 16/18-E6/E7 Promotes Cellular Proliferation, Migration, and Invasion in Cervical Cancer. Front. Oncol. 2019;9:847. doi: 10.3389/fonc.2019.00847. Erratum in: Front. Oncol. 2021, 11, 694442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Miao L., Liu K., Xie M., Xing Y., Xi T. miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis by blocking JAK2–STAT3 signaling. Cancer Immunol. Immunother. 2014;63:699–711. doi: 10.1007/s00262-014-1550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nishikawa E., Osada H., Okazaki Y., Arima C., Tomida S., Tatematsu Y., Taguchi A., Shimada Y., Yanagisawa K., Yatabe Y., et al. miR-375 Is Activated by ASH1 and Inhibits YAP1 in a Lineage-Dependent Manner in Lung Cancer. Cancer Res. 2011;71:6165–6173. doi: 10.1158/0008-5472.can-11-1020. [DOI] [PubMed] [Google Scholar]
- 279.Kahl I., Mense J., Finke C., Boller A., Lorber C., Győrffy B., Greve B., Götte M., A Espinoza-Sánchez N. The cell cycle-related genes RHAMM, AURKA, TPX2, PLK1, and PLK4 are associated with the poor prognosis of breast cancer patients. J. Cell. Biochem. 2022;123:581–600. doi: 10.1002/jcb.30205. [DOI] [PubMed] [Google Scholar]
- 280.Hassan N., Zhao J.T., Sidhu S.B. The role of microRNAs in the pathophysiology of adrenal tumors. Mol. Cell. Endocrinol. 2017;456:36–43. doi: 10.1016/j.mce.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 281.Katopodis P., Randeva H.S., Spandidos D.A., Saravi S., Kyrou I., Karteris E. Host cell entry mediators implicated in the cellular tropism of SARS-CoV-2, the pathophysiology of COVID-19 and the identification of microRNAs that can modulate the expression of these mediators (Review) Int. J. Mol. Med. 2021;49:1–12. doi: 10.3892/ijmm.2021.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang H., Ye Q., Du Z., Huang M., Zhang M., Tan H. MiR-148b-3p inhibits renal carcinoma cell growth and pro-angiogenic phenotype of endothelial cell potentially by modulating FGF2. Biomed. Pharmacother. 2018;107:359–367. doi: 10.1016/j.biopha.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 283.Kiełbowski K., Ptaszyński K., Wójcik J., Wojtyś M.E. The role of selected non-coding RNAs in the biology of non-small cell lung cancer. Adv. Med. Sci. 2023;68:121–137. doi: 10.1016/j.advms.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 284.Taefehshokr S., Taefehshokr N., Hemmat N., Hajazimian S., Isazadeh A., Dadebighlu P., Baradaran B. The pivotal role of MicroRNAs in glucose metabolism in cancer. Pathol. Res. Pract. 2020;217:153314. doi: 10.1016/j.prp.2020.153314. [DOI] [PubMed] [Google Scholar]
- 285.Li W.-Q., Zhang J.-P., Wang Y.-Y., Li X.-Z., Sun L. RETRACTED ARTICLE: MicroRNA-422a functions as a tumor suppressor in non-small cell lung cancer through SULF2-mediated TGF-β/SMAD signaling pathway. Cell Cycle. 2019;18:1727–1744. doi: 10.1080/15384101.2019.1632135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 286.Liang H., Wang R., Jin Y., Li J., Zhang S. MiR-422a acts as a tumor suppressor in glioblastoma by targeting PIK3CA. Am. J. Cancer Res. 2016;6:1695–1707. [PMC free article] [PubMed] [Google Scholar]
- 287.Gu J., Zhu X., Li Y., Dong D., Yao J., Lin C., Huang K., Hu H., Fei J. miRNA-21 regulates arsenic-induced anti-leukemia activity in myelogenous cell lines. Med. Oncol. 2010;28:211–218. doi: 10.1007/s12032-009-9413-7. [DOI] [PubMed] [Google Scholar]
- 288.Mei Y., Bian C., Li J., Du Z., Zhou H., Yang Z., Zhao R.C. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J. Cell. Biochem. 2012;114:1374–1384. doi: 10.1002/jcb.24479. [DOI] [PubMed] [Google Scholar]
- 289.Liu Z.-L., Wang H., Liu J., Wang Z.-X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2012;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 290.Nakagawa Y., Kuranaga Y., Tahara T., Yamashita H., Shibata T., Nagasaka M., Funasaka K., Ohmiya N., Akao Y. Induced miR-31 by 5-fluorouracil exposure contributes to the resistance in colorectal tumors. Cancer Sci. 2019;110:2540–2548. doi: 10.1111/cas.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Krejbich P., Birringer M. The Self-Administered Use of Complementary and Alternative Medicine (CAM) Supplements and Antioxidants in Cancer Therapy and the Critical Role of Nrf-2—A Systematic Review. Antioxidants. 2022;11:2149. doi: 10.3390/antiox11112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chu K.M., Cho C.H., Shin V.Y. Nicotine and gastrointestinal disorders: Its role in ulceration and cancer development. Curr. Pharm. Des. 2013;19:5–10. doi: 10.2174/13816128130103. [DOI] [PubMed] [Google Scholar]
- 293.Sahni M., Bhandari V. Patho-mechanisms of the origins of bronchopulmonary dysplasia. Mol. Cell. Pediatr. 2021;8:1–10. doi: 10.1186/s40348-021-00129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhang Y., Ren H., Li J., Xue R., Liu H., Zhu Z., Pan C., Lin Y., Hu A., Gou P., et al. Elevated HMGB1 expression induced by hepatitis B virus X protein promotes epithelial-mesenchymal transition and angiogenesis through STAT3/miR-34a/NF-κB in primary liver cancer. Am J Cancer Res. 2021;11:479–494. [PMC free article] [PubMed] [Google Scholar]
- 295.Alvanegh A.G., Ganji S.M., Kamel A., Tavallaie M., Rafati A., Arpanaei A., Dorostkar R., Ghaleh H.E.G. Comparison of oncolytic virotherapy and nanotherapy as two new miRNA delivery approaches in lung cancer. Biomed. Pharmacother. 2021;140:111755. doi: 10.1016/j.biopha.2021.111755. [DOI] [PubMed] [Google Scholar]
- 296.Song L., Li D., Gu Y., Wen Z.-M., Jie J., Zhao D., Peng L.-P. MicroRNA-126 Targeting PIK3R2 Inhibits NSCLC A549 Cell Proliferation, Migration, and Invasion by Regulation of PTEN/PI3K/AKT Pathway. Clin. Lung Cancer. 2016;17:e65–e75. doi: 10.1016/j.cllc.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 297.Chen Q., Chen S., Zhao J., Zhou Y., Xu L. MicroRNA-126: A new and promising player in lung cancer (Review) Oncol. Lett. 2020;21:1. doi: 10.3892/ol.2020.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jalil A.T., Abdulhadi M.A., Al-Ameer L.R., Abbas H.A., Merza M., Zabibah R.S., Fadhil A.A. The emerging role of microRNA-126 as a potential therapeutic target in cancer: A comprehensive review. Pathol. Res. Pr. 2023;248:154631. doi: 10.1016/j.prp.2023.154631. [DOI] [PubMed] [Google Scholar]
- 299.Wang Y., Li H., Shi Y., Wang S., Xu Y., Li H., Liu D. miR-143-3p impacts on pulmonary inflammatory factors and cell apoptosis in mice with mycoplasmal pneumonia by regulating TLR4/MyD88/NF-κB pathway. Biosci. Rep. 2020;40:BSR20193419. doi: 10.1042/bsr20193419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Qian X., Yu J., Yin Y., He J., Wang L., Li Q., Zhang L.-Q., Li C.-Y., Shi Z.-M., Xu Q., et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12:1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gao W., Yu Y., Cao H., Shen H., Li X., Pan S., Shu Y. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed. Pharmacother. 2010;64:399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 302.Mo D., Yang D., Xiao X., Sun R., Huang L., Xu J. MiRNA-145 suppresses lung adenocarcinoma cell invasion and migration by targeting N-cadherin. Biotechnol. Lett. 2017;39:701–710. doi: 10.1007/s10529-017-2290-9. [DOI] [PubMed] [Google Scholar]
- 303.Hu H., Xu Z., Li C., Xu C., Lei Z., Zhang H.-T., Zhao J. MiR-145 and miR-203 represses TGF-β-induced epithelial-mesenchymal transition and invasion by inhibiting SMAD3 in non-small cell lung cancer cells. Lung Cancer. 2016;97:87–94. doi: 10.1016/j.lungcan.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 304.Liu Q., Chen J., Wang B., Zheng Y., Wan Y., Wang Y., Zhou L., Liu S., Li G., Yan Y. Retracted: miR-145 modulates epithelial-mesenchymal transition and invasion by targeting ZEB2 in non–small cell lung cancer cell lines. J. Cell. Biochem. 2018;120:8409–8418. doi: 10.1002/jcb.28126. [DOI] [PubMed] [Google Scholar]
- 305.Pottier N., Maurin T., Chevalier B., Puisségur M.-P., Lebrigand K., Robbe-Sermesant K., Bertero T., Cardenas C.L.L., Courcot E., Rios G., et al. Identification of Keratinocyte Growth Factor as a Target of microRNA-155 in Lung Fibroblasts: Implication in Epithelial-Mesenchymal Interactions. PLoS ONE. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang Y., Wang Z., Lu J., Zhang H. Circular RNA circ-PTEN elevates PTEN inhibiting the proliferation of non-small cell lung cancer cells. Hum. Cell. 2021;34:1174–1184. doi: 10.1007/s13577-021-00526-y. [DOI] [PubMed] [Google Scholar]
- 307.Zhang H., Zhang C., Feng R., Zhang H., Gao M., Ye L. Investigating the microRNA-mRNA regulatory network in acute myeloid leukemia. Oncol. Lett. 2017;14:3981–3988. doi: 10.3892/ol.2017.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhang D., Cao X., Li J., Zhao G. MiR-210 inhibits NF-κB signaling pathway by targeting DR6 in osteoarthritis. Sci. Rep. 2015;5:12775. doi: 10.1038/srep12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gee H.E., Camps C., Buffa F.M., Patiar S., Winter S.C., Betts G., Homer J., Corbridge R., Cox G., West C.M.L., et al. hsa-miR-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer. 2010;116:2148–2158. doi: 10.1002/cncr.25009. [DOI] [PubMed] [Google Scholar]
- 310.Hisakane K., Seike M., Sugano T., Yoshikawa A., Matsuda K., Takano N., Takahashi S., Noro R., Gemma A. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial–mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac. Cancer. 2021;12:1690–1698. doi: 10.1111/1759-7714.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tsuchiya S., Fujiwara T., Sato F., Shimada Y., Tanaka E., Sakai Y., Shimizu K., Tsujimoto G. MicroRNA-210 Regulates Cancer Cell Proliferation through Targeting Fibroblast Growth Factor Receptor-like 1 (FGFRL1) J. Biol. Chem. 2011;286:420–428. doi: 10.1074/jbc.m110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Li Y., Liang C., Ma H., Zhao Q., Lu Y., Xiang Z., Li L., Qin J., Chen Y., Cho W.C., et al. miR-221/222 Promotes S-Phase Entry and Cellular Migration in Control of Basal-Like Breast Cancer. Molecules. 2014;19:7122–7137. doi: 10.3390/molecules19067122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Di Paolo D., Pontis F., Moro M., Centonze G., Bertolini G., Milione M., Mensah M., Segale M., Petraroia I., Borzi C., et al. Cotargeting of miR-126-3p and miR-221-3p inhibits PIK3R2 and PTEN, reducing lung cancer growth and metastasis by blocking AKT and CXCR4 signalling. Mol. Oncol. 2021;15:2969–2988. doi: 10.1002/1878-0261.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lu X., Yin B., Wang X., Wang F., Li Y., Wang N., Yang X., Jiang W. Long non-coding RNA-ZNF281 upregulates PTEN expression via downregulation of microRNA-221 in non-small cell lung cancer. Oncol. Lett. 2020;20:2962–2968. doi: 10.3892/ol.2020.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Li S., Chen H., Ren J., Geng Q., Song J., Lee C., Cao C., Zhang J., Xu N. MicroRNA-223 inhibits tissue factor expression in vascular endothelial cells. Atherosclerosis. 2014;237:514–520. doi: 10.1016/j.atherosclerosis.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 316.Si W., Li Y., Shao H., Hu R., Wang W., Zhang K., Yang Q. MiR-34a Inhibits Breast Cancer Proliferation and Progression by Targeting Wnt1 in Wnt/β-Catenin Signaling Pathway. Am. J. Med. Sci. 2016;352:191–199. doi: 10.1016/j.amjms.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 317.Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nature. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 318.Fathi S., Guessous F., Karkouri M. Diagnostic Value of Potential MicroRNAs in CRC: A Meta-Analysis. Microrna. 2022;11:190–205. doi: 10.2174/2211536611666220523103316. [DOI] [PubMed] [Google Scholar]
- 319.Tang Y., Yang P., Zhu Y., Su Y. LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/β-catenin axis in vitro. Thorac. Cancer. 2019;11:82–94. doi: 10.1111/1759-7714.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hwang W.-L., Jiang J.-K., Yang S.-H., Huang T.-S., Lan H.-Y., Teng H.-W., Yang C.-Y., Tsai Y.-P., Lin C.-H., Wang H.-W., et al. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nature. 2014;16:268–280. doi: 10.1038/ncb2910. [DOI] [PubMed] [Google Scholar]
- 321.Bhatnagar N., Li X., Padi S.K.R., Zhang Q., Tang M.-S., Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Davis B.N., Hilyard A.C., Nguyen P.H., Lagna G., Hata A. Smad Proteins Bind a Conserved RNA Sequence to Promote MicroRNA Maturation by Drosha. Mol. Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gong J., Zhang J.-P., Li B., Zeng C., You K., Chen M.-X., Yuan Y., Zhuang S.-M. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2012;32:3071–3079. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- 324.Yan S., Wang M., Zhao J., Zhang H., Zhou C., Jin L., Zhang Y., Qiu X., Ma B., Fan Q. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 2016;38:201–209. doi: 10.3892/ijmm.2016.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Xiang W., Lin H., Wang Q., Chen W., Liu Z., Chen H., Zhang H., Chen W. miR-34a suppresses proliferation and induces apoptosis of human lens epithelial cells by targeting E2F3. Mol. Med. Rep. 2016;14:5049–5056. doi: 10.3892/mmr.2016.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rusu M.C., Pop F., Hostiuc S., Manta L., Măru N., Grigoriu M. Transdifferentiations and heterogeneity in the stromal niches of uterine leiomyomas. Rom. J. Morphol. Embryol. 2018;59:663–672. [PubMed] [Google Scholar]
- 327.Liu J., Chen W., Zhang H., Liu T., Zhao L. miR-214 targets the PTEN-mediated PI3K/Akt signaling pathway and regulates cell proliferation and apoptosis in ovarian cancer. Oncol. Lett. 2017;14:5711–5718. doi: 10.3892/ol.2017.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Galardi S., Mercatelli N., Giorda E., Massalini S., Frajese G.V., Ciafrè S.A., Farace M.G. miR-221 and miR-222 Expression Affects the Proliferation Potential of Human Prostate Carcinoma Cell Lines by Targeting p27Kip1. J. Biol. Chem. 2007;282:23716–23724. doi: 10.1074/jbc.m701805200. [DOI] [PubMed] [Google Scholar]
- 329.Zhang D.-M., Deng J.-J., Wu Y.-G., Tang T., Xiong L., Zheng Y.-F., Xu X.-M. MicroRNA-223-3p Protect Against Radiation-Induced Cardiac Toxicity by Alleviating Myocardial Oxidative Stress and Programmed Cell Death via Targeting the AMPK Pathway. Front. Cell Dev. Biol. 2022;9:801661. doi: 10.3389/fcell.2021.801661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang X., Zhao X., Shao S., Zuo X., Ning Q., Luo M., Gu S., Zhao X. Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role. Int. J. Oncol. 2014;46:1141–1148. doi: 10.3892/ijo.2014.2809. [DOI] [PubMed] [Google Scholar]
- 331.Guoping M., Ran L., Yanru Q. miR-143 Inhibits Cell Proliferation of Gastric Cancer Cells Through Targeting GATA6. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018;26:1023–1029. doi: 10.3727/096504018x15151515028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jiang Z., Cushing L., Ai X., Lü J. miR-326 Is Downstream of Sonic Hedgehog Signaling and Regulates the Expression of Gli2 and Smoothened. Am. J. Respir. Cell Mol. Biol. 2014;51:273–283. doi: 10.1165/rcmb.2013-0127oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Xie H., Zhao Q., Yu L., Lu J., Peng K., Xie N., Ni J., Li B. Circular RNA circ_0047744 suppresses the metastasis of pancreatic ductal adenocarcinoma by regulating the miR-21/SOCS5 axis. Biochem. Biophys. Res. Commun. 2022;605:154–161. doi: 10.1016/j.bbrc.2022.03.082. [DOI] [PubMed] [Google Scholar]
- 334.Yadav V., Sharma K., Bhattacharya S., Talwar P., Purohit P.K., Saini N. RETRACTED: Hsa-miR-23a∼27a∼24-2 cluster members inhibit aggressiveness of breast cancer cells by commonly targeting NCOA1, NLK and RAP1B. Life Sci. 2022;307:120906. doi: 10.1016/j.lfs.2022.120906. [DOI] [PubMed] [Google Scholar]
- 335.Wang H., Zhu Y., Zhao M., Wu C., Zhang P., Tang L., Zhang H., Chen X., Yang Y., Liu G. miRNA-29c Suppresses Lung Cancer Cell Adhesion to Extracellular Matrix and Metastasis by Targeting Integrin β1 and Matrix Metalloproteinase2 (MMP2) PLoS ONE. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chen C., Tang J., Xu S., Zhang W., Jiang H. miR-30a-5p Inhibits Proliferation and Migration of Lung Squamous Cell Carcinoma Cells by Targeting FOXD1. BioMed. Res. Int. 2020;2020:2547902. doi: 10.1155/2020/2547902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 337.Sun Y., Bai Y., Zhang F., Wang Y., Guo Y., Guo L. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem. Biophys. Res. Commun. 2010;391:1483–1489. doi: 10.1016/j.bbrc.2009.12.098. [DOI] [PubMed] [Google Scholar]
- 338.Wang J., Guo J., Fan H. MiR-155 regulates the proliferation and apoptosis of pancreatic cancer cells through targeting SOCS3. Eur. Rev. Med. Pharmacol. Sci. 2020;24:12625. doi: 10.26355/eurrev_202012_24143. [DOI] [PubMed] [Google Scholar]
- 339.Zhao F.-Y., Han J., Chen X.-W., Wang J., Wang X.-D., Sun J.-G., Chen Z.-T. miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor. Int. J. Mol. Med. 2016;38:183–191. doi: 10.3892/ijmm.2016.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Meng F., Henson R., Lang M., Wehbe H., Maheshwari S., Mendell J.T., Jiang J., Schmittgen T.D., Patel T. Involvement of Human Micro-RNA in Growth and Response to Chemotherapy in Human Cholangiocarcinoma Cell Lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 341.Sorel O., Dewals B.G. MicroRNAs in large herpesvirus DNA genomes: Recent advances. Biomol. Concepts. 2016;7:229–239. doi: 10.1515/bmc-2016-0017. [DOI] [PubMed] [Google Scholar]
- 342.Chen C.-Z. MicroRNAs as Oncogenes and Tumor Suppressors. N. Engl. J. Med. 2005;353:1768–1771. doi: 10.1056/nejmp058190. [DOI] [PubMed] [Google Scholar]
- 343.Tan W., Liao Y., Qiu Y., Liu H., Tan D., Wu T., Tang M., Zhang S., Wang H. miRNA 146a promotes chemotherapy resistance in lung cancer cells by targeting DNA damage inducible transcript 3 (CHOP) Cancer Lett. 2018;428:55–68. doi: 10.1016/j.canlet.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 344.Uddin A., Chakraborty S. Role of miRNAs in lung cancer. J. Cell. Physiol. 2018 doi: 10.1002/jcp.26607. [DOI] [PubMed] [Google Scholar]
- 345.Xue X., Liang X.-J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer. 2012;31:100–109. doi: 10.5732/cjc.011.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gao W., Lu X., Liu L., Xu J., Feng D., Shu Y. MiRNA-21. Cancer Biol. Ther. 2012;13:330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 347.Ma F., Li W., Liu C., Li W., Yu H., Lei B., Ren Y., Li Z., Pang D., Qian C. MiR-23a promotes TGF-β1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/β-catenin signaling. Oncotarget. 2017;8:69538–69550. doi: 10.18632/oncotarget.18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-Cymering C., et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang X., Qiu H., Tang R., Song H., Pan H., Feng Z., Chen L. miR-30a inhibits epithelial-mesenchymal transition and metastasis in triple-negative breast cancer by targeting ROR1. Oncol. Rep. 2018;39:2635–2643. doi: 10.3892/or.2018.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhu X., Li H., Long L., Hui L., Chen H., Wang X., Shen H., Xu W. miR-126 enhances the sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A. Acta Biochim. Biophys. Sin. 2012;44:519–526. doi: 10.1093/abbs/gms026. [DOI] [PubMed] [Google Scholar]
- 351.Sun X., Guan G., Dai Y., Zhao P., Liu L., Wang Q., Li X. microRNA-155-5p initiates childhood acute lymphoblastic leukemia by regulating the IRF4/CDK6/CBL axis. Lab. Investig. 2022;102:411–421. doi: 10.1038/s41374-021-00638-x. [DOI] [PubMed] [Google Scholar]
- 352.Liu Q., Zhang M., Jiang X., Zhang Z., Dai L., Min S., Wu X., He Q., Liu J., Zhang Y., et al. miR-223 suppresses differentiation of tumor-induced CD11b+Gr1+myeloid-derived suppressor cells from bone marrow cells. Int. J. Cancer. 2011;129:2662–2673. doi: 10.1002/ijc.25921. [DOI] [PubMed] [Google Scholar]
- 353.Wang X.-C., Tian L.-L., Jiang X.-Y., Wang Y.-Y., Li D.-G., She Y., Chang J.-H., Meng A.-M. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett. 2011;311:203–209. doi: 10.1016/j.canlet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 354.Jang M.H., Kim H.J., Gwak J.M., Chung Y.R., Park S.Y. Prognostic value of microRNA-9 and microRNA-155 expression in triple-negative breast cancer. Hum. Pathol. 2017;68:69–78. doi: 10.1016/j.humpath.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 355.Coppola V., Musumeci M., Patrizii M., Cannistraci A., Addario A., Maugeri-Saccà M., Biffoni M., Francescangeli F., Cordenonsi M., Piccolo S., et al. BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial–mesenchymal transition. Oncogene. 2012;32:1843–1853. doi: 10.1038/onc.2012.194. [DOI] [PubMed] [Google Scholar]
- 356.Yu G., Li H., Wang J., Gumireddy K., Li A., Yao W., Tang K., Xiao W., Hu J., Xiao H., et al. miRNA-34a Suppresses Cell Proliferation and Metastasis by Targeting CD44 in Human Renal Carcinoma Cells. J. Urol. 2014;192:1229–1237. doi: 10.1016/j.juro.2014.05.094. [DOI] [PubMed] [Google Scholar]
- 357.Ye E.-A., Steinle J.J. miR-146a suppresses STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in primary human retinal microvascular endothelial cells in high glucose conditions. Vis. Res. 2017;139:15–22. doi: 10.1016/j.visres.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zheng L., Xu C.-C., Chen W.-D., Shen W.-L., Ruan C.-C., Zhu L.-M., Zhu D.-L., Gao P.-J. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem. Biophys. Res. Commun. 2010;400:483–488. doi: 10.1016/j.bbrc.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 359.Liang Y.K., Lin H.Y., Dou X.W., Chen M., Wei X.L., Zhang Y.Q., Wu Y., Chen C.F., Bai J.W., Xiao Y.S., et al. MiR-221/222 promote epithelial-mesenchymal transition by targeting Notch3 in breast cancer cell lines. NPJ Breast Cancer. 2018;4:20. doi: 10.1038/s41523-018-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wang R., Wang F.-F., Cao H.-W., Yang J.-Y. MiR-223 regulates proliferation and apoptosis of IL-22-stimulated HaCat human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci. 2019;230:28–34. doi: 10.1016/j.lfs.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 361.Zhang W., Zhang T., Jin R., Zhao H., Hu J., Feng B., Zang L., Zheng M., Wang M. MicroRNA-301a promotes migration and invasion by targeting TGFBR2 in human colorectal cancer. J. Exp. Clin. Cancer Res. 2014;33:1–13. doi: 10.1186/s13046-014-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Li W., Sun Z., Chen C., Wang L., Geng Z., Tao J. Sirtuin7 has an oncogenic potential via promoting the growth of cholangiocarcinoma cells. Biomed. Pharmacother. 2018;100:257–266. doi: 10.1016/j.biopha.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 363.Zhao G.-Z., Niu Y.-Q., Li Z.-M., Kou D., Zhang L. MiR-200c inhibits proliferation and promotes apoptosis of Wilms tumor cells by regulating akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:6623–6631. doi: 10.26355/eurrev_202006_21648. [DOI] [PubMed] [Google Scholar]
- 364.Liu F., Li T., Zhan X. Silencing circular RNAPTPN12 promoted the growth of keloid fibroblasts by activating Wnt signaling pathway via targeting microRNA-21-5p. Bioengineered. 2022;13:3503–3515. doi: 10.1080/21655979.2022.2029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lv L., Wang X., Ma T. microRNA-944 Inhibits the Malignancy of Hepatocellular Carcinoma by Directly Targeting IGF-1R and Deactivating the PI3K/Akt Signaling Pathway [Retraction] Cancer Manag. Res. 2021;13:4765–4766. doi: 10.2147/cmar.s324204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Guan N., Wang R., Feng X., Li C., Guo W. Long non-coding RNA NBAT1 inhibits the progression of glioma through the miR-21/SOX7 axis. Oncol. Lett. 2020;20:3024–3034. doi: 10.3892/ol.2020.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Pan J., Zhou L., Lin C., Xue W., Chen P., Lin J. MicroRNA-34a Promotes Ischemia-Induced Cardiomyocytes Apoptosis through Targeting Notch1. Evid. Based Complement. Altern. Med. 2022;2022:1388415. doi: 10.1155/2022/1388415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zheng Z., Qu J.-Q., Yi H.-M., Ye X., Huang W., Xiao T., Li J.-Y., Wang Y.-Y., Feng J., Zhu J.-F., et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway. Cell Death Dis. 2017;8:e2855. doi: 10.1038/cddis.2017.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Liang J., Cao D., Zhang X., Liu L., Tan Q., Shi S., Chen K., Liang J., Wang Z. miR-192-5p suppresses uterine receptivity formation through impeding epithelial transformation during embryo implantation. Theriogenology. 2020;157:360–371. doi: 10.1016/j.theriogenology.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 370.Pan S., Zhao X., Shao C., Fu B., Huang Y., Zhang N., Dou X., Zhang Z., Qiu Y., Wang R., et al. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021;12:1–15. doi: 10.1038/s41419-020-03304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Li N., Cui T., Guo W., Wang D., Mao L. MiR-155-5p accelerates the metastasis of cervical cancer cell via targeting TP53INP1. OncoTargets Ther. 2019;12:3181–3196. doi: 10.2147/ott.s193097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article and supplementary tables.