Abstract
Influenza is a respiratory disease caused by the influenza virus, which is highly transmissible in humans. This paper presents a systematic review and meta-analysis of randomized controlled trials (RCTs) and test-negative designs (TNDs) to assess the vaccine effectiveness (VE) of seasonal influenza vaccines (SIVs) in humans aged 15 to 64 years. An electronic search to identify all relevant studies was performed. The outcome measure of interest was VE on laboratory-confirmed influenza (any strain). Quality assessment was performed using the Cochrane risk-of-bias tool for RCTs and the ROBINS-I tool for TNDs. The search identified a total of 2993 records, but only 123 studies from 73 papers were included in the meta-analysis. Of these studies, 9 were RCTs and 116 were TNDs. The pooled VE was 48% (95% CI: 42–54) for RCTs, 55.4% (95% CI: 43.2–64.9) when there was a match between the vaccine and most prevalent circulating strains and 39.3% (95% CI: 23.5–51.9) otherwise. The TNDs’ adjusted VE was equal to 39.9% (95% CI: 31–48), 45.1 (95% CI: 38.7–50.8) when there was a match and 35.1 (95% CI: 29.0–40.7) otherwise. The match between strains included in the vaccine and strains in circulation is the most important factor in the VE. It increases by more than 25% when there is a match with the most prevalent circulating strains. The laboratorial method for confirmation of influenza is a possible source of bias when estimating VE.
Keywords: influenza, test-negative design, clinical trials, efficacy, effectiveness, strains
1. Introduction
Influenza is a respiratory disease resulting from infection with the influenza virus. It is more prevalent during cold periods, with the peak of infections between November and April in the Northern Hemisphere and between June and October in the Southern Hemisphere. The influenza virus is highly transmissible in humans [1]. The World Health Organization (WHO) estimates that there are 1 billion cases of influenza worldwide each year, of which 3–5 million are severe cases [2]. An estimated 650,000 deaths per year result from influenza infection [3]. The most effective way to prevent influenza infection is through vaccination [4]. Seasonal flu vaccination campaigns represent a major investment for countries and governments. It is therefore important to assess the effectiveness of the vaccine.
The two main types of studies used to assess the seasonal influenza vaccine (SIV) performance are randomized controlled trials (RCTs) and observational studies. Among these, the most used are cohort studies and, mainly, the case-control study, known as test-negative design (TND) [5]. RCTs are always conducted for the marketing authorization of the vaccine regardless of the year in question [6]. Vaccine performance is determined by vaccine efficacy (VER), which is equal to
| (1) |
where RR is the relative risk. These trials are very expensive and time-consuming [7]. Therefore, the application of (1) is not a parsimonious method for real-time monitoring of the efficacy of a SIV.
For monitoring the annual effectiveness of different vaccines, TND is used when laboratory confirmation is required [8]. The TND sample is composed of individuals with influenza-like illness (ILI) who access a hospital or other healthcare facilities for a consultation. These individuals are tested for influenza disease, and positive cases are recognized as cases, while negative cases are identified as the controls. The effectiveness of the vaccine is measured by comparing the odds of infection between those vaccinated and unvaccinated [9,10]. The vaccine effect is measured by its effectiveness (VE), which is equal to
| (2) |
where OR stands for the odds ratio. Several factors can affect the VE and introduce bias in the estimates of the VE. For example, VE can be seriously affected by the mismatch of the virus strains included in the vaccine and those in circulation in each vaccination season. The WHO has developed influenza surveillance and monitoring systems in order to understand which strains are circulating worldwide. Five reference centers located in the US, UK, Australia, Japan and China are responsible for collecting the information issued by each country and pinpointing the strains that are expected to be most prevalent in the following year, and these are the ones that should be in the next SIV [11]. The high rate of viral mutation, which includes fewer marked processes such as antigenic drift and profound changes called antigenic shift, mean that strains are not always as expected, to the notable detriment of the VE.
Previous vaccination may bias VE estimation. Natural infection and vaccination interfere with the individual’s immune system. On a theoretical level, it is expected that there may be some pre-existing immunity, either from previous infection or vaccination. Thus, resistance to the disease may be favored [12]. However, differences are not always significant [13]. The presence of comorbidities can affect VE. One of the groups at high risk of a severe influenza illness comprehends people with associated health problems. The influence of comorbidities can be analyzed in two ways: the ability of the interaction with the vaccine to be sufficiently robust for good protective capacity and the comorbidities as a determining influence on resistance to infection [14]. The type of substrate used for viral replication, using eggs or cultured cells may affect VE [15]. The time of the vaccination uptake is also relevant [16]. Finally, individual characteristics such as age [14,17], sex [14], conditions such as pregnancy [18] and even mood [19] at the time of the vaccine uptake seem to affect the VE.
Thus, RCTs and TNDs are used in different contexts. RCTs are the gold standard for licensing of use and TNDs are the main tool for monitoring the annual effectiveness of the SIVs [20,21]. However, these test designs apply different measures (VER and VE, respectively) and no relationship has been established between the results observed in RCTs and TNDs. This paper describes a systematic review and meta-analysis of RCTs and TNDs conducted to assess the VER and VE of SIVs in humans aged 15 to 64 years. Despite several papers describing performed reviews of the VER of the SIV or its effectiveness are available [22,23,24,25,26,27], information regarding VE is scarce. In this sense, the main objective of this work is to measure the effect of a vaccine assessed in RCTs and TNDs using a common measure: VE. Other information related to the individuals who participated in each study was also collected to identify possible factors that may influence VE.
2. Methods
This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [28]. The proposed methodology for the systematic review was registered in the international prospective register of systematic reviews PROSPERO [CRD42023397974].
2.1. Search Strategy and Eligibility Criteria
An electronic search was conducted to identify all relevant studies. The literature search was performed in MEDLINE (via PubMed) and Cochrane’s library. The search in PubMed was performed using  4.3.0 software [29], package RISmed [30] available at https://www.r-project.org/. The last search date was March 2023. We also screened the reference list of included studies and relevant systematic reviews. The detailed search strategy is available in the register on PROSPERO. Only studies written in English were included to avoid any bias related to a mistranslation.
4.3.0 software [29], package RISmed [30] available at https://www.r-project.org/. The last search date was March 2023. We also screened the reference list of included studies and relevant systematic reviews. The detailed search strategy is available in the register on PROSPERO. Only studies written in English were included to avoid any bias related to a mistranslation.
The included studies verified the following inclusion criteria:
-
–
Inclusion of sufficient information to compute the vaccine efficacy/effectiveness;
-
–
Articles published between 2013 and 2023;
-
–
Influenza was confirmed by a laboratorial method;
-
–
Articles that include the human population aged 15 to 65 years;
-
–
The participants in TNDs must have received a seasonal influenza vaccine at least 14 days before symptoms onset to be regarded as vaccinated;
-
–
Comparator was a placebo (for RCTs) or non-vaccinated (for TND);
-
–
Articles published in English.
TND studies were excluded if they reported only pooled data for more than one season.
2.2. Interventions
The intervention of interest was vaccination with one of the following seasonal influenza vaccines: trivalent inactivated (TIV), tetravalent inactivated (QIV) or live attenuated (LAIV). Monovalent and noncommercial vaccines were not considered.
2.3. Outcome Measure
The outcome of interest was vaccine efficacy or effectiveness on laboratory-confirmed influenza (any strain). PCR (polymerase chain reaction) and rapid virus detection tests were considered as possible methods for the confirmation of infection.
When studies reported both unadjusted and adjusted vaccine effectiveness, the adjusted figure was used in the results as it was considered the less biased estimate of the treatment effect.
Additional outcomes of interest were collected for subgroup analysis.
2.4. Data Collection and Analysis
Two authors of this review independently assessed the study eligibility by inspecting the title and abstract. All articles selected from the title/abstract reading were inspected for inclusion with a full-text review by both authors. The information of all selected papers was independently extracted to a form that included study design, participants, sample size, description of intervention, outcomes and quality assessment indicators. Discrepancies in study selection were resolved through consensus.
After the systematic review, a meta-analysis was performed using  software’s metafor package [31]. When any of the outcome measures were zero, the value of 0.5 was added. The associated confidence intervals are based on the logarithm transformation.
software’s metafor package [31]. When any of the outcome measures were zero, the value of 0.5 was added. The associated confidence intervals are based on the logarithm transformation.
Forest plots were obtained to present a graphical overview (values of VE lower than −200% were omitted).
Between-study heterogeneity was assessed by Cochran’s Q and the statistic. A value higher than 0.75 was regarded as high heterogeneity and the pooled estimate was interpreted as low-certainty evidence. If the statistic was lower than 0.25, a fixed effects model was chosen. Otherwise, if the statistic was higher than 0.25 and lower than 0.75, a random effects model was used. Model estimation was performed using a restricted estimation maximum likelihood methodology with Knapp and Hartung adjustment. The adjusted estimates found in RCTs/TNDs were obtained using logistic regression. Thus, pooling was based on the log RR/OR and standard deviation, with the exponential of the pooled result re-expressed as VER/VE.
Funnel plots provided a visual assessment of possible publication bias. The trim and fill method and Egger’s test were applied to screen for possible publication bias [32].
2.5. Quality Assessment
Two authors independently assessed the included studies for risk of bias using validated critical appraisal tools. Inconsistencies were resolved by a third reviewer.
The Cochrane risk-of-bias tool for randomized trials (RoB 2) was used for RCTs [33]. Data were inputted in the RoB 2 Excel tool to implement them (available on riskofbiasinfo.org website, accessed on 1 March 2023).
TND studies were assessed for risk of bias using the ROBINS-I (Risk of Bias In Non-randomized Studies of Interventions) tool [34]. Results are presented in tabular form, with the agreed consensus of risk of bias for each of the seven included domains and the overall risk of bias for each study denoted by the highest risk of bias score in any singular domain, as per the ROBINS-I methodology. While unadjusted effectiveness was used where adjusted was not reported, there is clearly a risk of bias associated with the unadjusted estimate.
A sensitivity analysis was produced to control the effect of the high risk of bias studies.
3. Results
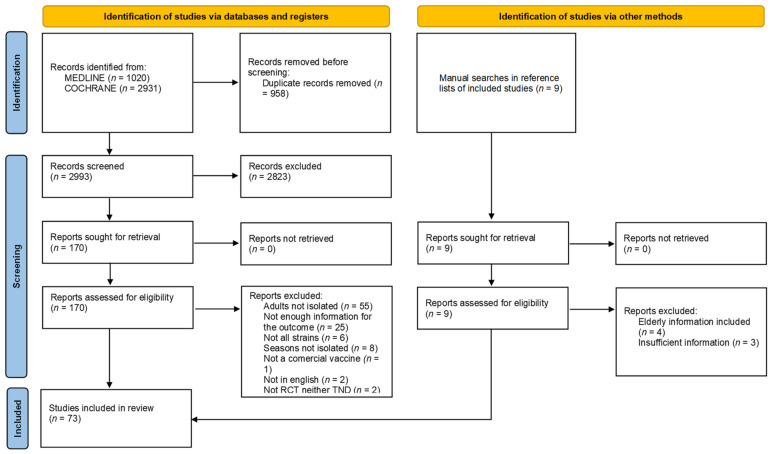
The search identified a total of 2993 records after removing duplicates (see Figure 1). The full text of 172 records was screened for eligibility, 99 of which were excluded. References of excluded studies are reported in Supplementary Table S1. A total of 123 studies from 73 papers were included in the meta-analysis. Of these studies, 9 are RCTs [35,36,37,38,39] and 114 are TNDs [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]. The selection process is detailed in the PRISMA flowchart (see Figure 1).
Figure 1.
PRISMA study search flow diagram.
These articles comprised 86 studies from the Northern Hemisphere and 31 studies from the Southern Hemisphere. Six articles have information from countries in the Northern and in the Southern Hemisphere. In studies performed in more than one European country, the country is referred to as Europe (e.g., I-MOVE studies).
The main characteristics of the RCT studies reported in the articles are summarized in Table 1. Table 2 presents the characteristics of the TND studies.
Table 1.
Summary of the included RCT studies.
| Author | Country | Season | Vaccine | VE 100 (95% CI) | n | Strain Match | Test |
|---|---|---|---|---|---|---|---|
| Madhi 2014 [35] | South Africa | 2011–2012 | TIV | 51 (15–72) | 2049 | Mismatched | PCR |
| Madhi 2014 [35] | South Africa | 2011–2012 | TIV | 66 (13–87) | 188 | Unclear | PCR |
| Petrie 2016 [36] | USA | 2007–2008 | TIV | 70 (51–82) | 1139 | Matched | PCR |
| Petrie 2016 [36] | USA | 2007–2008 | LAIV | 39 (4–61) | 1138 | Matched | PCR |
| Mcbride 2016 [37] | Australia | 2008–2009 | TIV | 42 (22–57) | 7515 | Matched | PCR |
| Mcbride 2016 [37] | Australia | 2008–2009 | TIV | 44 (27–57) | 7334 | Unclear | PCR |
| Steinhoff 2017 [38] | Nepal | 2011–2012 | TIV | 48 (15–68) | 3693 | Unclear | PCR |
| Steinhoff 2017 [38] | Nepal | 2012–2013 | TIV | 0 (−78–43) | 3693 | Matched | PCR |
| Liebowitiz 2020 [39] | USA | 2012–2013 | QIV | 42 (−42–76) | 2049 | Matched | PCR |
Table 2.
Summary of the included TND studies.
| Author | Country | Season | Vaccine | VE 100 (95% CI) | n | Strain Match | Test |
|---|---|---|---|---|---|---|---|
| Kissling 2023 [40] | Europe | 2021–2022 | QIV and LAIV | 41 (25–64) | 6876 | Mismatched | PCR |
| Tenforde 2023 [41] | USA | 2021–2022 | QIV | 29 (24–33) | 59,150 | Mismatched | PCR |
| Kim 2022 [42] | Canada | 2021–2022 | QIV | 53 (−35–84) | 176 | Mismatched | PCR |
| Price 2022 [43] | USA | 2021–2022 | QIV | 44 (22–59) | 1850 | Mismatched | PCR |
| Richard 2022 [44] | USA | 2012–2013 | LAIV | 14 (0–27) | 2580 | Mismatched | PCR, RT, culture |
| Richard 2022 [44] | USA | 2013–2014 | LAIV | –6 (−24–10) | 2613 | Mismatched | PCR, RT |
| Richard 2022 [44] | USA | 2014–2015 | LAIV | 6 (−5–16) | 4715 | Mismatched | PCR, RT |
| Richard 2022 [44] | USA | 2012–2013 | TIV | 23 (9–36) | 2311 | Mismatched | PCR, RT |
| Richard 2022 [44] | USA | 2013–2014 | TIV | 33 (21–44) | 2517 | Mismatched | PCR, RT |
| Richard 2022 [44] | USA | 2014–2015 | TIV | 13 (3–22) | 5043 | Mismatched | PCR, RT |
| Sominina 2021 [45] | Russia | 2018–2019 | TIV | 62 (16–83) | 925 | Matched | PCR |
| Hyder 2021 [46] | India | 2017–2018 | QIV | 24 (−68–66) | 547 | Mismatched | PCR |
| Hyder 2021 [46] | India | 2018–2019 | QIV | 49 (−76–85) | 306 | Mismatched | PCR |
| Stuurmann 2021 [47] | Europe | 2019–2020 | TIV and QIV | 29 (−5–52) | 1055 | Mismatched | PCR |
| Stuurman 2021 [47] | Europe | 2019–2020 | TIV and QIV | 30 (−35–64) | 2041 | Mismatched | PCR |
| Grijalva 2021 [48] | USA | 2019–2020 | TIV and QIV | 38 (−22–68) | 638 | Mismatched | PCR |
| Grijalva 2021 [48] | USA | 2019–2020 | TIV and QIV | 80 (63–89) | Unclear | Mismatched | PCR |
| Hu 2021 [49] | USA | 2019–2020 | Unclear | 46 (36–55) | 5817 | Mismatched | PCR |
| Martin 2020 [50] | USA | 2016–2017 | QIV | 31 (12–45) | 2605 | Matched | PCR |
| Martin 2020 [50] | USA | 2017–2018 | QIV | 34 (22–45) | 3524 | Matched | PCR |
| Stuurman 2020 [51] | Europe | 2018–2019 | QIV | 40 (2–63) | 1095 | Matched | PCR, RT |
| Stuurman 2020 [51] | Europe | 2018–2019 | QIV | 45 (18–63) | 2036 | Matched | PCR, RT |
| Rizzo 2020 [52] | Italy | 2018–2019 | QIV | 40 (19–56) | 290 | Mismatched | PCR |
| Qahtami 2020 [53] | Saudi Arabia | 2018–2019 | TIV | 42 (14–64) | 556 | Unclear | PCR |
| Redlberger–Fritz 2020 [54] | Austria | 2016–2017 | QIV, TIV, aTIV, LAIV | −7 (−132–51) | 492 | Mismatched | PCR |
| Redlberger–Fritz 2020 [54] | Austria | 2017–2018 | QIV, TIV, aTIV, LAIV | 19 (−64–60) | 668 | Mismatched | PCR |
| Redlberger–Fritz 2020 [54] | Austria | 2018–2019 | QIV, TIV, aTIV, LAIV | 51 (−1–76) | 614 | Matched | PCR |
| Rose 2020 [55] | Europe | 2019–2020 | QIV, TIV, LAIV | 36 (2–58) | 13,630 | Mismatched | PCR |
| Ando 2019 [56] | Japan | 2018–2019 | QIV | 43 (17–61) | 555 | Unclear | RT |
| Segaloff 2019 [57] | USA | 2014–2015 | TIV | 41 (2–65) | 624 | Mismatched | PCR |
| Segaloff 2019 [57] | USA | 2015–2016 | TIV | 69 (44–82) | 441 | Matched | PCR |
| Flannery 2019 [58] | USA | 2018–2019 | TIV and QIV | 16 (4–26) | 5022 | Mismatched | PCR |
| Kissling 2019 [59] | Europe | 2016–2017 | TIV | 34 (19–46) | 5840 | Mismatched | PCR |
| Blanchette 2019 [60] | Canada | 2010–2011 | TIV | 34 (20–40) | 9288 | Unclear | PCR |
| Constantino 2019 [61] | Italia | 2018–2019 | TIV and QIV | 60 (1–83) | 308 | Probable | PCR |
| Pebody 2019 [62] | United Kingdom | 2017–2018 | TIV and QIV | 12 (−17–34) | 1896 | Unclear | PCR |
| Kissling 2019 [63] | Denmark | 2018–2019 | TIV and QIV | 55 (44–64) | 5807 | Mismatched | PCR |
| Kissling 2019 [63] | European Union | 2018–2019 | TIV and QIV | 32 (−32–65) | 1142 | Mismatched | PCR |
| Kissling 2019 [63] | United Kingdom | 2018–2019 | TIV and QIV | 37 (−20–67) | 575 | Mismatched | PCR |
| Kissling 2019 [63] | Denmark | 2018–2019 | TIV and QIV | 39 (2–62) | 727 | Mismatched | PCR |
| Chon 2019 [64] | Japan | 2015–2016 | QIV | −9 (−200–68) | 99 | Unclear | PCR, RT |
| Molgaard–Nielsen 2019 [65] | Denmark | 2010–2011 | TIV | 64 (29–82) | 626 | Unclear | Unclear |
| Regan 2019 [66] | Australia | 2016 | QIV | 31 (3–51) | 713 | Mismatched | PCR |
| Showronski 2019 [67] | Canada | 2017–2018 | Unclear | 63 (46–75) | 946 | Matched | PCR |
| Regan 2019 [68] | Australia | 2012 | TIV | 46 (22–63) | 825 | Unclear | PCR |
| Regan 2019 [68] | Australia | 2013 | TIV | 57 (26–75) | 577 | Unclear | PCR |
| Regan 2019 [68] | Australia | 2014 | TIV | 60 (41–73) | 1112 | Unclear | PCR |
| Regan 2019 [68] | Australia | 2015 | TIV | 50 (32–64) | 1491 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2010–2011 | Unclear | 72 (−5–93) | 167 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2011–2012 | Unclear | 47 (−98–86) | 84 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2012–2013 | Unclear | 23 (−85–68) | 202 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2013–2014 | Unclear | 51 (−30–82) | 200 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2014–2015 | Unclear | 24 (−189–47) | 171 | Unclear | PCR |
| Thompson 2018 [69] | USA | 2015–2016 | Unclear | 40 (−33–72) | 216 | Unclear | PCR |
| Flannery 2018 [70] | USA | 2017–2018 | TIV and QIV | 19 (0–34) | 20,165 | Matched | PCR |
| Flannery 2018 [70] | USA | 2017–2018 | TIV and QIV | 40 (24–53) | 1362 | Matched | PCR |
| Chan 2018 [71] | China | 2017–2018 | TIV and QIV | 71 (42–86) | 383 | Unclear | PCR |
| Seki 2018 [72] | Japan | 2016–2017 | QIV | 36 (−7–62) | 299 | Matched | RT |
| Wu 2018 [73] | China | 2016–2017 | TIV | 4 (−200–76) | 6009 | Mismatched | PCR |
| Yaron–Yakobi 2018 [74] | Israel | 2014–2015 | QIV, TIV, LAIV | −54 (−200–72) | 417 | Mismatched | PCR |
| Yaron–Yakobi 2018 [74] | Israel | 2015–2016 | QIV, TIV, LAIV | 39 (8–60) | 783 | Mismatched | PCR |
| Skowronski 2018 [75] | Canada | 2017–2018 | TIV and QIV | 31 (6–49) | 895 | Unclear | PCR |
| Stein 2017 [76] | Israel | 2016–2017 | TIV and QIV | 12 (−110–64) | 151 | Matched | PCR |
| Stein 2017 [76] | Israel | 2016–2017 | TIV and QIV | 59 (1–83) | 165 | Matched | PCR |
| Pelody 2017 [77] | United Kingdom | 2017–2018 | TIV and QIV | 14 (−7–32) | 1896 | Mismatched | PCR |
| Skowronski 2017 [78] | Canada | 2015–2016 | TIV | 42 (20–59) | 1076 | Matched | PCR |
| Skowronski 2017 [78] | Canada | 2015–2016 | TIV | 32 (−1–54) | 520 | Matched | PCR |
| Kuliese 2017 [79] | Lithuania | 2015–2016 | TIV | 61 (−345–97) | 72 | Unclear | PCR |
| Ma 2017 [80] | China | 2014–2015 | TIV | −60 (−200–50) | 4990 | Mismatched | PCR |
| Seki 2017 [81] | Japan | 2013–2014 | QIV | 56 (21–76) | 262 | Unclear | RT |
| Seki 2017 [81] | Japan | 2014–2015 | QIV | 8 (−64–48) | 235 | Unclear | RT |
| Seki 2017 [81] | Japan | 2015–2016 | QIV | 53 (20–72) | 427 | Matched | RT |
| McAnerney 2016 [82] | South Africa | 2015 | TIV | 54 (−14–82) | 599 | Unclear | PCR |
| Fielding 2016 [83] | Australia | 2015 | TIV | 52 (37–63) | 1492 | Mismatched | PCR |
| Petrie 2016 [84] | USA | 2014–2015 | QIV | 67 (11–88) | 165 | Mismatched | PCR |
| Petrie 2016 [84] | USA | 2014–2015 | QIV | 10 (−127–64) | 239 | Mismatched | PCR |
| Rizzo 2016 [85] | Italy | 2014–2015 | TIV | −6 (−134–52) | 1183 | Mismatched | PCR |
| Lytras 2016 [86] | Greece | 2014–2015 | TIV | 46 (17–65) | 363 | Mismatched | PCR |
| Rondy 2016 [87] | France, Italy e Spain | 2013–2014 | TIV | 43 (−5–69) | 305 | Matched | PCR |
| Gherasim 2016 [88] | Spain | 2014–2015 | TIV | 36 (16–51) | 2957 | Mismatched | PCR |
| Redlberger–Fritz 2016 [89] | Austria | 2014–2015 | TIV | 54 (−15–82) | 532 | Mismatched | PCR |
| Kelly 2016 [90] | Australia | 2011 | TIV | −5 (−99–44) | 227 | Matched | PCR |
| Kelly 2016 [90] | Australia | 2011 | TIV | 77 (52–89) | 409 | Matched | PCR |
| Kelly 2016 [90] | Australia | 2012 | TIV | 22 (−20–49) | 415 | Mismatched | PCR |
| Kelly 2016 [90] | Australia | 2012 | TIV | 43 (9–64) | 460 | Mismatched | PCR |
| Kelly 2016 [90] | Australia | 2013 | TIV | 54 (16–75) | 190 | Mismatched | PCR |
| Kelly 2016 [90] | Australia | 2013 | TIV | 58 (12–80) | 258 | Mismatched | PCR |
| Bissielo 2016 [91] | New Zealand | 2015 | TIV | 27 (−8–51) | 618 | Mismatched | PCR |
| Bissielo 2016 [91] | New Zealand | 2015 | TIV | 46 (2–70) | 246 | Mismatched | PCR |
| Cheng 2015 [92] | Australia | 2014 | TIV | 50 (35–61) | 1234 | Matched | PCR |
| Levy 2015 [93] | Thailand | 2009–2010 | TIV | 73 (26–90) | 240 | Mismatched | PCR |
| Levy 2015 [93] | Thailand | 2010–2011 | TIV | 52 (−102–88) | 62 | Matched | PCR |
| Levy 2015 [93] | Thailand | 2011–2012 | TIV | 30 (−200–84) | 129 | Matched | PCR |
| Levy 2015 [93] | Thailand | 2012–2013 | TIV | 59 (16–80) | 411 | Mismatched | PCR |
| McAnerney 2015 [94] | South Africa | 2010 | TIV | 48 (−22–78) | 354 | Matched | PCR |
| McAnerney 2015 [94] | South Africa | 2011 | TIV | 59 (10–81) | 548 | Matched | PCR |
| McAnerney 2015 [94] | South Africa | 2012 | TIV | 67 (−15–91) | 749 | Mismatched | PCR |
| McAnerney 2015 [94] | South Africa | 2013 | TIV | 91 (38–99) | 460 | Matched | PCR |
| McAnerney 2015 [95] | South Africa | 2014 | TIV | 43 (−107–84) | 812 | Mismatched | PCR |
| Rondy 2015 [96] | Europe | 2012–2013 | TIV | 86 (77–92) | 564 | Mismatched | PCR |
| McLean 2015 [97] | USA | 2012–2013 | TIV | 467 | 3307 | Matched | PCR |
| Filipovic 2014 [98] | Croatia | 2010–2011 | TIV | −19 (−200–58) | 240 | Matched | PCR |
| Turner 2014 [99] | New Zealand | 2014 | TIV | 59 (23–79) | 190 | Unclear | PCR |
| Turner 2014 [99] | New Zealand | 2014 | TIV | 57 (28–74) | 498 | Unclear | PCR |
| Levy 2014 [100] | Australia | 2010 | TIV | 60 (0–84) | 355 | Matched | PCR |
| Levy 2014 [100] | Australia | 2011 | TIV | 40 (−21–70) | 348 | Matched | PCR |
| Levy 2014 [100] | Australia | 2012 | TIV | 47 (19–65) | 804 | Matched | PCR |
| Yang 2014 [101] | China | 2012–2013 | TIV | 70 (−159–96) | 1246 | Matched | Virus isolation |
| Sullivan 2014 [102] | Australia | 2012 | TIV | 12 (−21–36) | 926 | Mismatched | PCR |
| Skowronski 2014 [103] | Canada | 2011–2012 | TIV | 56 (26–74) | 975 | Mismatched | PCR |
| Skowronski 2014 [104] | Canada | 2013–2014 | TIV | 70 (53–82) | 562 | Matched | PCR |
| Skowronski 2014 [105] | Canada | 2012–2013 | TIV | 36 (11–54) | 979 | Mismatched | PCR |
| Kavanagh 2013 [106] | Scotland | 2010–2011 | TIV | 100 (−349–100) | 457 | Matched | PCR |
| Castilla 2013 [107] | Spain | 2011–2012 | TIV | 44 (−11–72) | 650 | Matched | PCR |
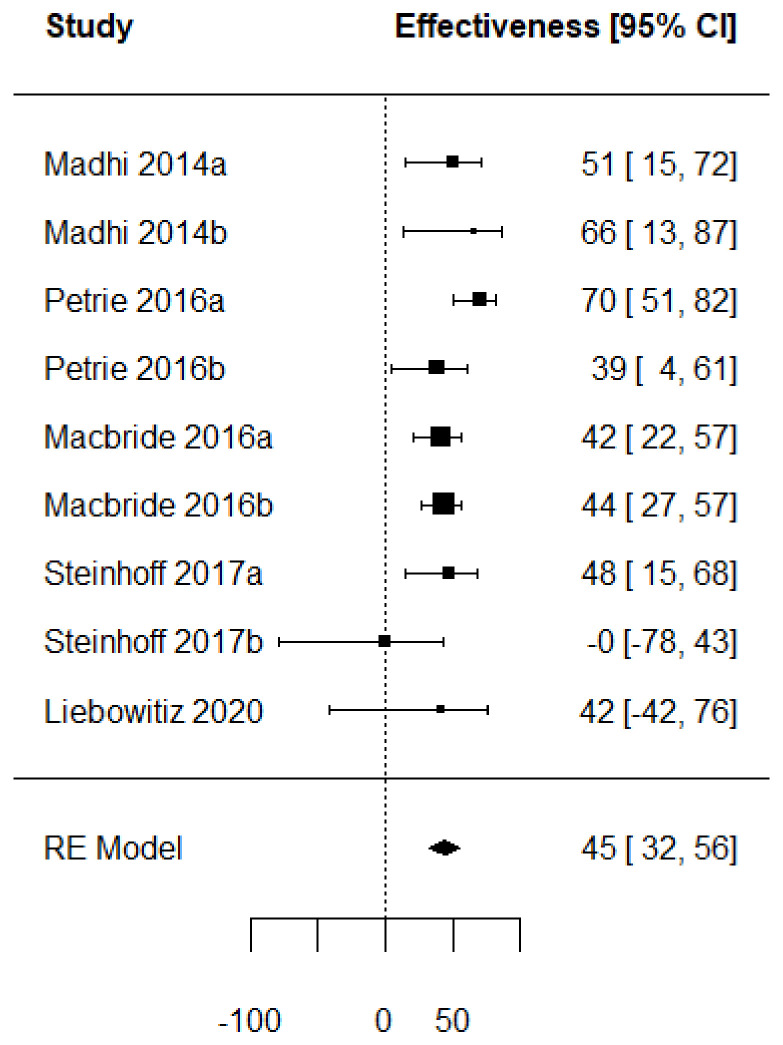
The RCT sample size ranged from 85 to 7515 in the 9 studies. The reported VER ranged from to . The heterogeneity between studies is low: and Cochran’s (p-value 0.18). The TIV was the most-used vaccine (seven studies). QIV and LAIV were used in only one study each. All individuals from one of the studies reported in [35] were HIV positive. Contact with the influenza virus in the study extracted from [39] was deliberately provoked. We find a match between the vaccine strains and the virus in circulation in six studies [36,37,38,39]. In two of the three studies where we verified a mismatch [35,37], it was possible to extract data for the matched strains. The studies extracted from [38] include only pregnant women.
The study by Liebowitz, D. et al. reported positive results for influenza for both ILI and non-ILI patients [39]. As our goal was to compare RCTs to TNDs, the efficacy concerning the influenza-positive illness was reported. The studies extracted from Mcbride, W.J. et al. [37] and Steinhoff, M.C. et al. [38] included two consecutive seasons.
The TND sample size ranged from 62 to 59,150. The TIV was the most-used vaccine (59 studies) followed by QIV (19 studies) in studies that used only 1 type of vaccine. Eighteen studies used both the TIV and QIV vaccines. LAIV was the only choice in three studies. Six studies used all three types of vaccines. The type of vaccine used in eight studies was unclear.
For the RCTs studies, the pooled adjusted VER is equal to ( CI: 30.5–52.9), which is similar to the pooled non-adjusted VER: ( CI: 30.1–53.0). In both models, (forest plots not shown).
Figure 2 presents a forest plot with the VE computed from the extracted crude values. This allows us to have a common measure to assess a vaccine performance regardless of the type of study (RCT or TND). The VE is represented by individual squares proportional to the precision of the estimates, and the horizontal lines represent the 95% CIs for each included study. The diamond indicates the pooled VE, which is equal to ( CI: 32.1–55.8).
Figure 2.
Forest plot of the vaccine effectiveness (RCT studies [35,36,37,38,39]).
For the TND studies, the reported VE ranged from to . The heterogeneity between studies is very high: and Cochran’s (p-value < 0.001).
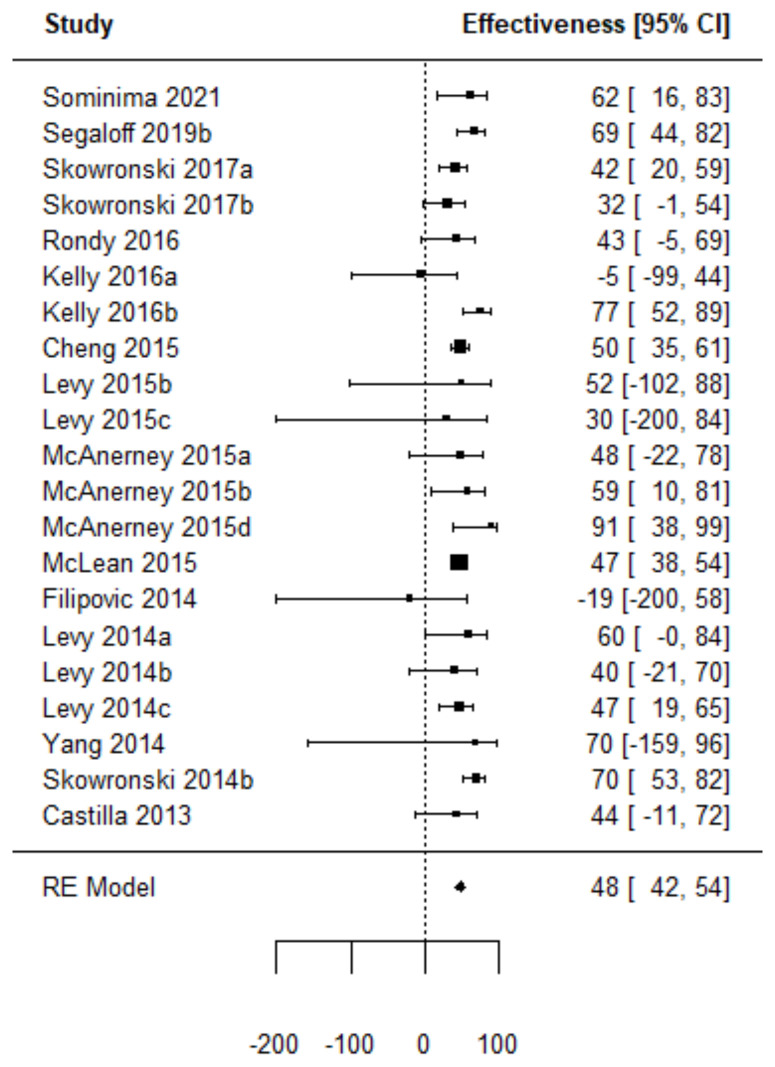
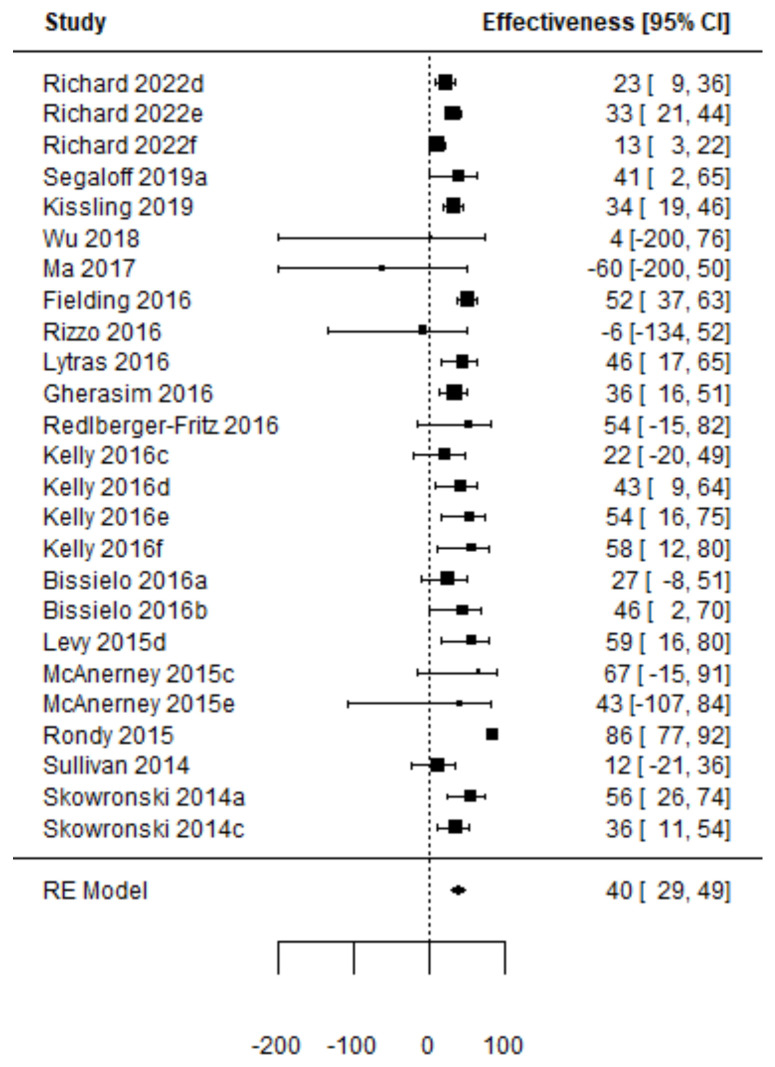
The pooled VE of the TIV is (95% CI: 41.7–54.2) when there is a match between the strains included in the vaccine and the most prevalent in circulation, while it decreases to (95% CI: 29.1–49.4) when there is a mismatch.
As for the QIV-type vaccine, studies show an overall effectiveness of 34.3% (95% CI: 29.6–38.7). When both the TIV and QIV vaccines are used in the same study, the effectiveness rate is 37.3% (95% CI: 24.5–47.8). In LAIV studies, the overall effectiveness is only 5.4% (95% CI: −20.7–25.9). Finally, when all three types of vaccines are used within the same study, the effectiveness is equal to 32.8% (95%CI: 12.3–48.5).
Forest plots of the adjusted VE of the TIV when vaccine strains match or mismatch are shown in Figure 3 and Figure 4. Figure 5, Figure 6, Figure 7 and Figure 8 show the results for the other type of vaccines (except for the only study that uses both QIV and LAIV). Further details about RCT and TND studies are given in Supplementary Tables S2 and S3.
Figure 3.
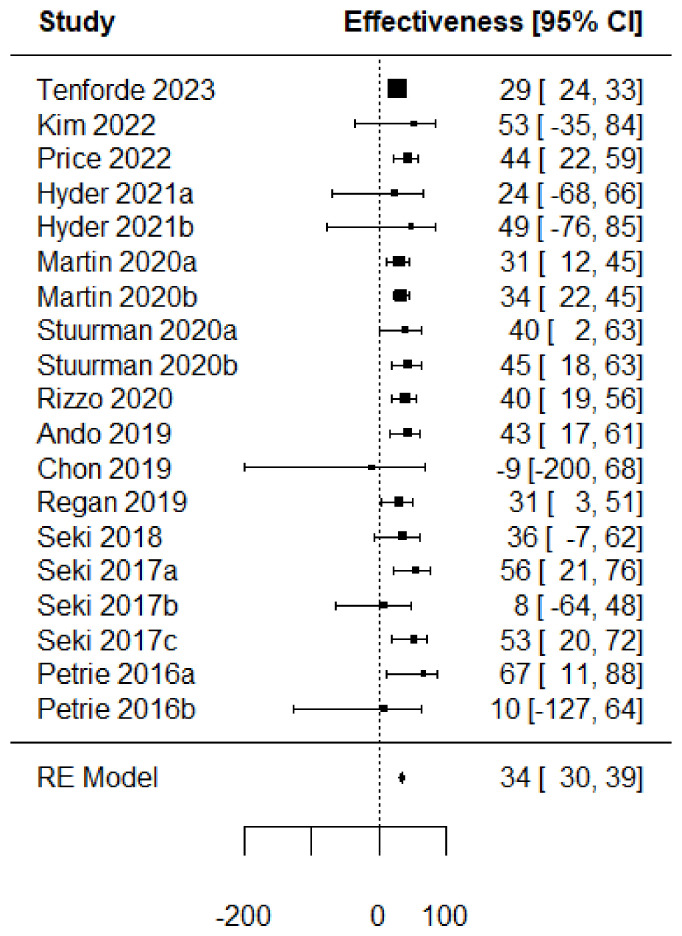
Forest plot of the vaccine effectiveness. (TND studies, TIV vaccine only, vaccine strains match circulating strains [45,57,78,87,90,92,93,94,97,98,100,101,104,107]).
Figure 4.
Forest plot of the vaccine effectiveness (TND studies, TIV vaccine only, mismatch between vaccine and circulating strains [44,57,59,73,80,83,85,86,88,89,90,91,93,94,95,96,102,103,105]).
Figure 5.
Forest plot of the vaccine effectiveness. (QIV vaccine only [41,42,43,46,50,51,52,56,64,72,81,84]).
Figure 6.
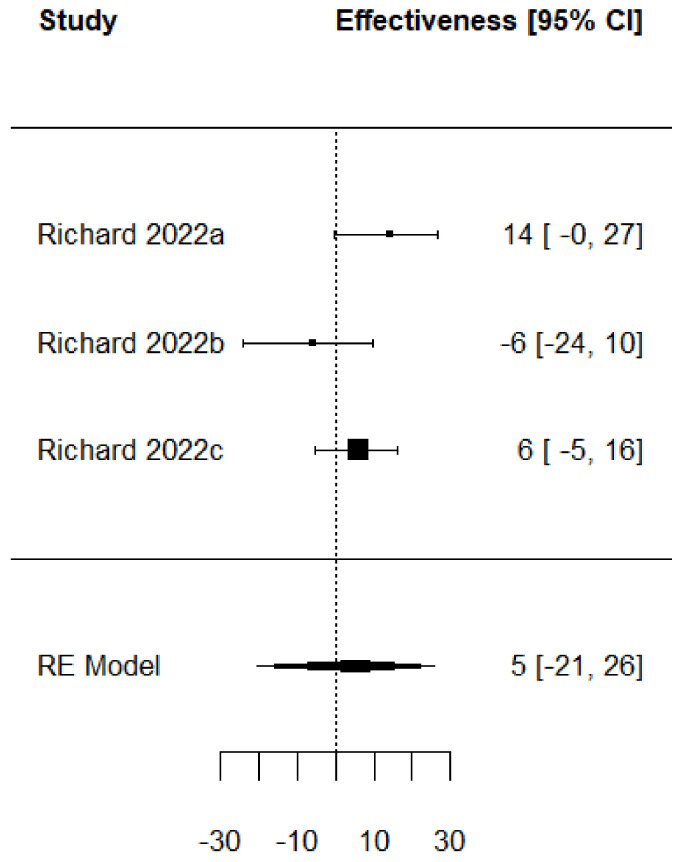
Forest plot of the vaccine effectiveness (LAIV vaccine only [44]).
Figure 7.
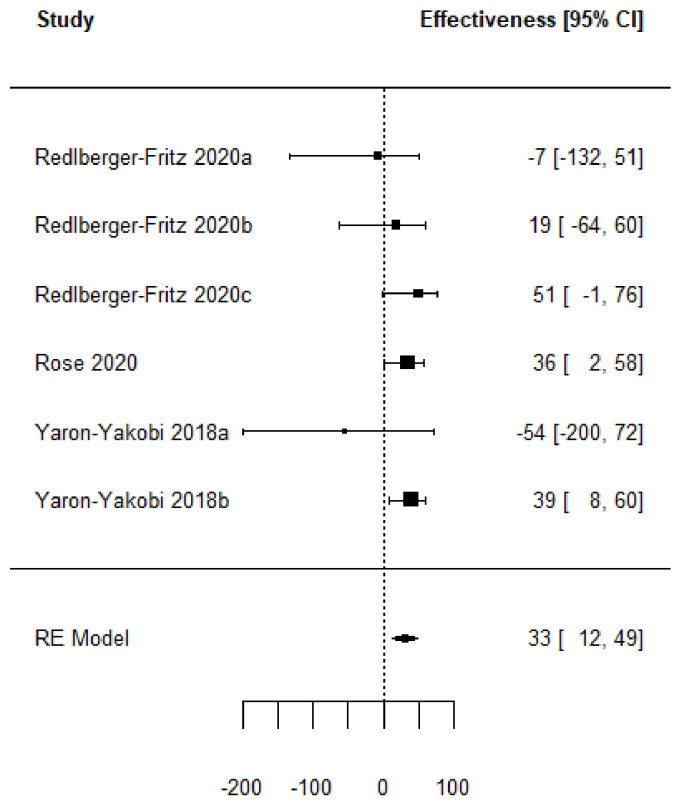
Forest plot of the vaccine effectiveness (TIV, QIV and LAIV simultaneously used [54,55,74]).
Figure 8.
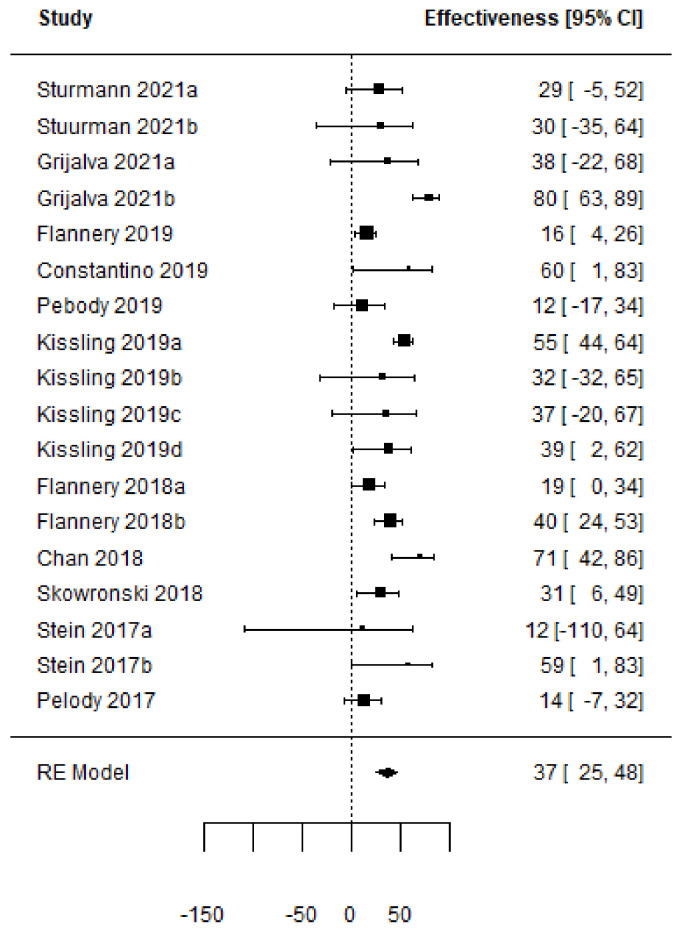
Forest plot of the vaccine effectiveness (TIV and QIV simultaneously used [47,48,58,61,62,63,70,71,75,76,77]).
3.1. Risk of Bias Assessment
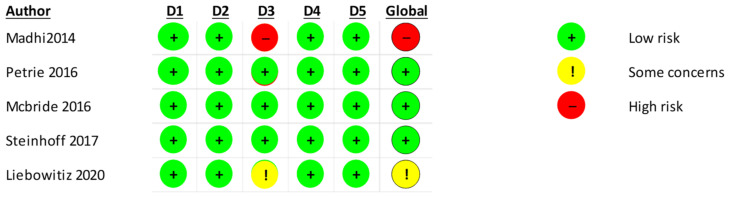
The RoB2.0 assessment indicates that one study [33] has a high risk of bias. A summary of the results is presented in Figure 9.
Figure 9.
Rob 2.0 assessment of the included RCT studies [35,36,37,38,39] of the 5 domains. (D1: randomization process, D2: deviations from the intended interventions, D3: missing outcome data; D4: measurement of the outcome, D5: selection of the reported result).
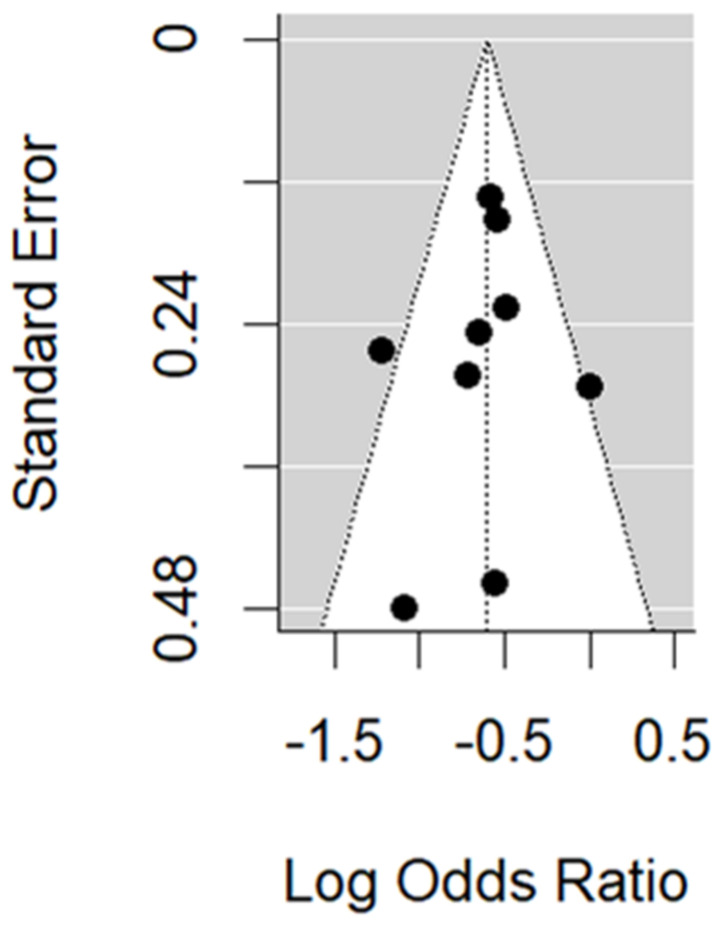
In the RCT studies, no evidence of publication bias is found through the funnel plot (Figure 10). Trim and fill methods and Egger’s test (p-value 0.19) do not identify any missing RCT study.
Figure 10.
Funnel plot of the RCT studies.
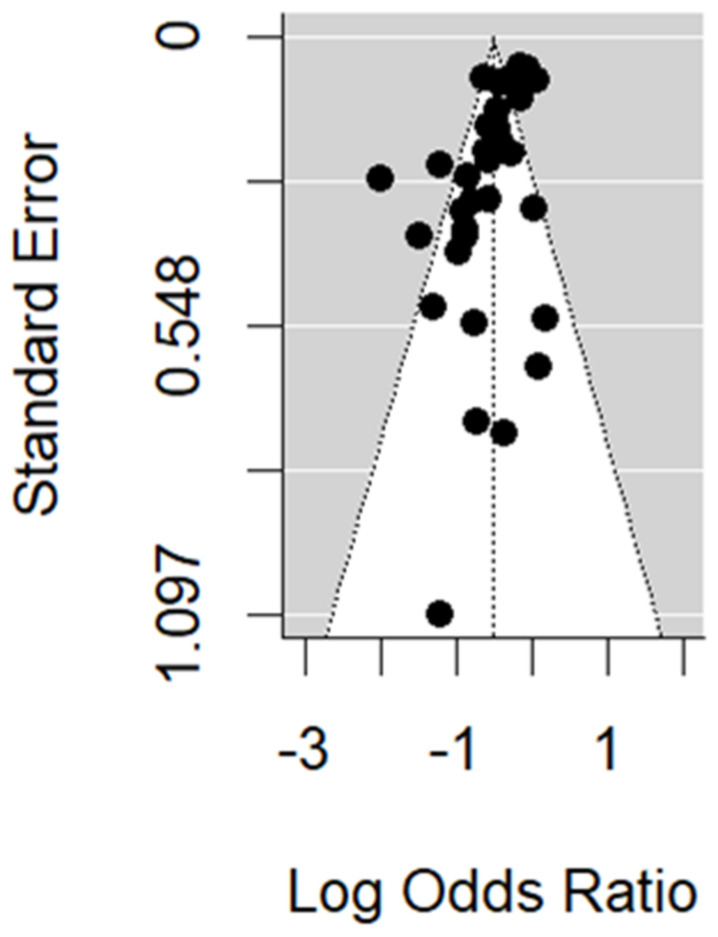
Figure 11 is the funnel plot for the TND studies. We found some evidence of asymmetry, which is confirmed by the trim and fill method (10 missing studies) and Egger’s test (p-value < 0.001). However, when restricting to studies where vaccine and circulating strains match, no missing studies were identified by the trim and fill method, and Egger’s test p-value increased to 0.04. When restricting to studies where vaccine and circulating strains mismatch, results were similar to the general case.
Figure 11.
Funnel plot of the TND studies.
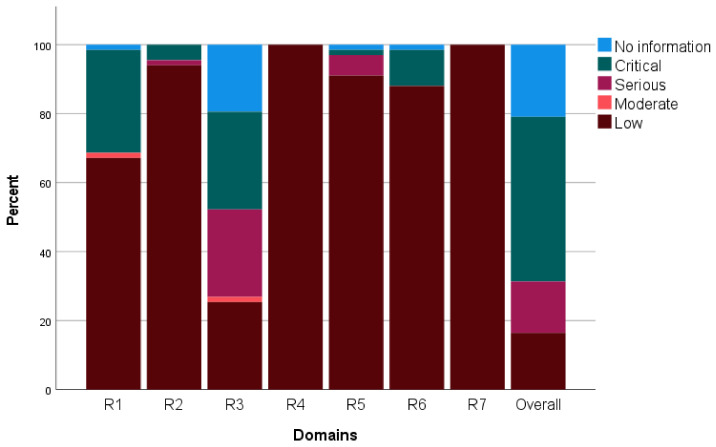
The ROBINS-I assessment tool for intervention was applied to papers involving TND studies. Most of the studies were at a serious/critical risk of bias as the vaccination status was not always based on the individual’s records (classification bias). Eleven papers were determined to have a low risk of bias. Fourteen studies failed to provide sufficient information to be classified in at least one of the seven domains analyzed by the tool. The overall results are summarized in Figure 12, and Supplementary Table S4 presents the results by assessment domain.
Figure 12.
ROBINS-I overall assessment of the included TND studies and by domain. (R1: confounding, R2: selection, R3: classification, R4: deviation from intended interventions; R5: missing data; R6: outcome measurement; R7: reported results).
3.2. Subgroup Analysis
A sensitivity analysis was performed by removing the high-bias RCT study [38]. The relative adjusted vaccine efficacy increased by 1.4% and the vaccine effectiveness by less than 0.5% As for the TND studies, those at a critical risk of bias were removed for the sensitivity analysis. The pooled VE showed non-significant variations. However, for the TIV vaccines when there was a mismatch between the circulating and vaccine strains, it decreased by nearly 10%.
Table 3 presents the vaccine effectiveness for the studies where the vaccine and circulating strains match and mismatch separately. A 15% increase in vaccine effectiveness was observed when the strains match. When our analysis is restricted to the TIV, the variation is even greater with an increase of more than 20%.
Table 3.
Comparison of vaccine effectiveness according to the match between circulating and vaccine strains verified in the RCT studies (95% confidence intervals in brackets; a p-value lower than 0.05 is identified with *).
| Match | Mismatch | p-Value | (%) | |
|---|---|---|---|---|
| All vaccines | 55.4 (43.2,64.9) | 39.3 (23.5, 51.9) | 0.068 | 0.01 |
| TIV | 59.4 (46.4,69.2) | 39.3 (23.5, 51.9) | 0.035 * | 0 |
Table 4 presents a subgroup analysis for the TND studies. The TIV showed a better performance compared to studies that did not use a TIV (p-value 0.010). A match between the vaccine and circulating strains improves the VE by more than 10% (p-value 0.017). Confirming influenza by PCR results in a higher VE estimate (p-value 0.012).
Table 4.
Comparison of vaccine effectiveness according to several factors observed in TND studies. (95% confidence intervals are presented in brackets; a p-value lower than 0.05 or 0.01 is identified with * or **; a comparison between studies that only use the TIV vaccine and the other; b comparison between studies where vaccine and circulating strains match and mismatch, only for TIV studies; c comparison between studies that only use the QIV vaccine and the other; d comparison between studies that only use the LAIV vaccine and the other, e comparison between studies where vaccine and circulating strains match and mismatch, all studies; f comparison between studies that include individuals with severe symptoms and those that do not; g comparison between studies that include inpatient individuals and those that do not; h comparison between studies that confirm the presence of the influenza virus using only PCR and those that do not).
| Yes | No | p-Value | (%) | |
|---|---|---|---|---|
| Adjusted estimate? | 39.9 (30.5–47.9) | 41.0 (36.8–44.9) | 0.419 | 77.9 |
| TIV? a | 44.9 (39.1–50.1) | 30.3 (22.0–37.7) | 0.010 * | 76.9 |
| Match circulating strains, TIV? b | 48.3 (41.7–54.2) | 40.1 (29.1–49.4) | 0.080 | 61.4 |
| QIV? c | 34.3 (29.6–38.7) | 42.7 (36.4–48.3) | 0.454 | 80.8 |
| LAIV? d | 5.4 (−20.7–25.9) | 41.4 (37.1–45.4) | 0.001 ** | 78.5 |
| Match circulating strains? e | 45.1 (38.7–50.8) | 35.1 (29.0–40.7) | 0.017 * | 69.8 |
| Not only ILI? f | 38.7 (33.5–43.5) | 43.9 (37.7–49.5) | 0.176 | 73.9 |
| Not only outpatients? g | 43.1 (33.2–51.6) | 39.6 (35.1–43.8) | 0.497 | 80.3 |
| Only PCR? h | 42.7 (38.3–46.8) | 29.7 (20.4–37.9) | 0.012 * | 76.3 |
| Northern Hemisphere? | 39.1 (34.4–43.4) | 44.7 (36.1–52.2) | 0.196 | 80.2 |
No significant differences were found between pooled adjusted and non-adjusted VE estimates, studies that include individuals with severe symptoms and studies that included only ILI individuals, studies that included or not only inpatients, and studies performed in different hemispheres.
TIV pooled effectiveness observed in RCT studies is higher than the pooled value obtained from the TND studies when vaccine and circulating strains match, although the difference is not significant (p-value = 0.27, I2 = 17.4%). When there is a mismatch, the values are similar (around 40% in both cases).
4. Discussion
RCTs and TNDs are the most-used study designs to assess the performance of the SIV. Comparing RCT and TND estimates through a common measure (VE) is a relevant subject as the usefulness of TNDs is still discussed in the literature [10]. This explains the focus on individuals aged 15–64 years as they are not, in general, a high-risk group for severe influenza illness. Elderly people were excluded as it would increase the risk of dealing with results extracted from individuals with comorbidities. In addition, RCTs in the elderly population have another vaccine as a comparator [108,109]. Placebo is not used, as expected, because vaccination is recommended [110].
The number of RCT studies found was small. This fact limits the possibility of comparing RCT and TND studies except for TIV vaccines. The VE estimated by RCT studies is 10% higher than the VE estimated through the TNDs, although the difference is not significant. When there is a mismatch, similar values were obtained for both designs. It seems that TND studies are a reliable alternative for the assessment of a vaccine’s performance, as it is referred to in [111].
One of the main purposes of a meta-analysis is to compute pooled estimates. However, the pooled VE for the TND is not shown as it would be pointless. The VE of the individual studies varies over a wide range and the measure of the between-studies heterogeneity is close to . However, it is possible to identify some reasons that explain this high heterogeneity.
From this review, the match between the vaccine and circulating strains arises as the most important factor. In TND studies, a difference of close to 10% was observed. This is in line with what was found in a systematic review of 2016 [112]. In elderly people, even greater differences were reported, between 20 and 30% [113,114]. When comparing TIV and QIV vaccines, we found higher effectiveness values for TIV vaccines. This is true for both RCT and TND studies. This was a surprising result as VE should increase with the number of strains included in the vaccine, although this was already observed in previous work on children [115]. Our understanding is that a match between the strains included in the vaccine and those that are predominantly circulating is the most influential factor. Hence, it is not relevant to have a high number of strains in a vaccine if they do not match the strains the vaccine aims to prevent. This also explains why the RCT study with a sample in which all individuals were HIV positive did not have a low VE, as there was a match. The relevance of the vaccine strains emerges as a key factor in effectiveness. This conclusion is supported by other meta-analyses whose results also point in this direction [116,117,118]. It is also interesting to observe that pooled VE obtained from adjusted and non-adjusted estimates are not significantly different. This leads one to believe that the impact of some of the confounding variables identified in the literature as influencing VE (e.g., prior vaccination) is limited, as some authors have already referred to [119].
PCR tests were used in most studies, although in some cases they were not the exclusive method for the detection of the influenza virus. It was not possible to compare the use of PCR tests with their non-use. Thus, it was only possible to compare the exclusive use of PCR tests with the combined use of more than one type of laboratory test. One of the alternative tests used was the rapid test, which has a lower sensitivity [120]. This lack of sensitivity might be the explanation for a significantly higher VE in the studies that used only PCR tests.
Limitations of the Study
Despite the interesting results found herein, some limitations were evident. LAIV vaccines have very low effectiveness values. The number of studies (3) in which these vaccines are involved is low, so it is not possible to generalize the results. For instance, a VE equal to 44% was found in a systematic review reported in [115].
The TND is validated to assess vaccine effectiveness in outpatients but not in inpatients [57], which limited the possibility to compare disease severity with vaccine effectiveness. This may explain why only in a few studies individuals with acute respiratory infections were found. Thus, it was not possible to assess the impact of symptom severity.
As the number of studies reporting VE by age and strain was very low, it was not possible to assess the effect of the different strains in the VE estimation. Other limitations arise from the high number of TND studies at critical risk of bias and the lack of control over some variables, which could impact the reliability of the results. However, the majority of studies presented VE estimates adjusted for several confounding variables, although these variables were not always the same across the different studies.
5. Conclusions
This meta-analysis provides important insights into the effectiveness of influenza vaccines, highlighting the crucial role of the match between vaccine strains and those circulating in the population. The findings observed herein provide a basis for future research on the effectiveness of influenza vaccines and suggest that efforts should focus on improving the match between vaccine strains and those circulating in the population.
Abbreviations
aTIV, adjuvanted trivalent inactivated vaccine; CI, confidence interval; LAIV, live attenuated vaccine; n, sample size; PCR, polymerase chain reaction; QIV, tetravalent inactivated vaccine; RE, random effects; RT, rapid test; TIV, trivalent inactivated vaccine; VE, vaccine effectiveness.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines11081322/s1, Table S1: references of excluded studies; Table S2: characteristics of the included RCT studies; Table S3: characteristics of the included TND studies; Table S4: ROBINS-I overall assessment of the included TND studies by domain.
Author Contributions
J.P.M., M.S. and A.M. completed the study design, study identification and data extraction; J.P.M., A.M., M.F. and R.S. performed the statistical analysis. J.P.M., M.S. and A.M. wrote the manuscript. M.F. and R.S. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work is partially financed by national funds through FCT-Fundação para a Ciência e a Tecnologia under the project UIDB/00006/2020.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.European Centre for Disease Prevention and Control . Systematic Review of the Efficacy, Effectiveness and Safety of Newer and Enhanced Seasonal Influenza Vaccines for the Prevention of Laboratory-Confirmed Influenza in Individuals Aged 18 Years and Over. Publications Office; Luxembourg: 2020. [Google Scholar]
- 2.World Health Organization (WHO) The Global Influenza Surveillance and Response System. [(accessed on 1 January 2023)]. Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system.
- 3.WHO Regional Office for Europe . WHO Regional Office for Europe Recommendations on Influenza Vaccination for the 2020/2021 Season during the Ongoing COVID-19 Pandemic. WHO; Copenhagen, Denmark: 2020. [Google Scholar]
- 4.Buchy P., Badur S. Who and When to Vaccinate against Influenza. Int. J. Infect. Dis. 2020;93:375–387. doi: 10.1016/j.ijid.2020.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Sullender W.M., Fowler K.B., Gupta V., Krishnan A., Ram Purakayastha D., Srungaram VLN R., Lafond K.E., Saha S., Palomeque F.S., Gargiullo P., et al. Efficacy of Inactivated Trivalent Influenza Vaccine in Rural India: A 3-Year Cluster-Randomised Controlled Trial. Lancet Glob. Health. 2019;7:e940–e950. doi: 10.1016/S2214-109X(19)30079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean H.Q., Belongia E.A. Influenza Vaccine Effectiveness: New Insights and Challenges. Cold Spring Harb. Perspect. Med. 2021;11:a038315. doi: 10.1101/cshperspect.a038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan S.G., Feng S., Cowling B.J. Potential of the Test-Negative Design for Measuring Influenza Vaccine Effectiveness: A Systematic Review. Expert Rev. Vaccines. 2014;13:1571–1591. doi: 10.1586/14760584.2014.966695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Jin P.F., Li J.X., Zhu F.C. Application of test-negative design in vaccine efficacy evaluation. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:280–283. doi: 10.3760/cma.j.issn.0254-6450.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Balasubramani G.K., Zimmerman R.K., Eng H., Lyons J., Clarke L., Nowalk M.P. Comparison of Local Influenza Vaccine Effectiveness Using Two Methods. Vaccine. 2021;39:1283–1289. doi: 10.1016/j.vaccine.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Wei M., Jin P., Li J., Zhu F. An Evaluation of a Test-Negative Design for EV-71 Vaccine from a Randomized Controlled Trial. Hum. Vaccines Immunother. 2021;17:2101–2106. doi: 10.1080/21645515.2020.1859900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., et al. Influenza. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foppa I.M., Ferdinands J.M., Chung J., Flannery B., Fry A.M. Vaccination History as a Confounder of Studies of Influenza Vaccine Effectiveness. Vaccine X. 2019;1:100008. doi: 10.1016/j.jvacx.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenciano M., Kissling E., Larrauri A., Nunes B., Pitigoi D., O’Donnell J., Reuss A., Horváth J.K., Paradowska-Stankiewicz I., Rizzo C., et al. Exploring the Effect of Previous Inactivated Influenza Vaccination on Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza: Results of the European I-MOVE Multicentre Test-Negative Case-Control Study, 2011/2012-2016/2017. Influenza Other Resp. Viruses. 2018;12:567–581. doi: 10.1111/irv.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott A.N., Buchan S.A., Kwong J.C., Drews S.J., Simmonds K.A., Svenson L.W. Using Population-Wide Administrative and Laboratory Data to Estimate Type- and Subtype-Specific Influenza Vaccine Effectiveness: A Surveillance Protocol. BMJ Open. 2019;9:e029708. doi: 10.1136/bmjopen-2019-029708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Álvarez Aldeán J., Salamanca I., Ocaña D., Barranco J.L., Walter S. Effectiveness of Cell Culture-Based Influenza Vaccines Compared with Egg-Based Vaccines: What Does the Literature Say? Rev. Esp. Quim. 2022;35:241–248. doi: 10.37201/req/117.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahni L.C., Naioti E.A., Olson S.M., Campbell A.P., Michaels M.G., Williams J.V., Staat M.A., Schlaudecker E.P., McNeal M.M., Halasa N.B., et al. Sustained Within-Season Vaccine Effectiveness Against Influenza-Associated Hospitalization in Children: Evidence from the New Vaccine Surveillance Network, 2015–2016 Through 2019–2020. Clin. Infect. Dis. 2023;76:e1031–e1039. doi: 10.1093/cid/ciac577. [DOI] [PubMed] [Google Scholar]
- 17.Feng S., Sullivan S.G., Tchetgen Tchetgen E.J., Cowling B.J. The Causal Interpretation of “Overall Vaccine Effectiveness” in Test-Negative Studies. Am. J. Epidemiol. 2021;190:1993–1999. doi: 10.1093/aje/kwab101. [DOI] [PubMed] [Google Scholar]
- 18.Donzelli A. Influenza Vaccination in Pregnancy: Careful Assessment Confirms Safety Concerns for the Offspring. Hum. Vaccines Immunother. 2019;15:2168–2170. doi: 10.1080/21645515.2019.1605818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayling K., Fairclough L., Buchanan H., Wetherell M.A., Vedhara K. Mood and Influenza Vaccination in Older Adults: A Randomized Controlled Trial. Health Psychol. 2019;38:984–996. doi: 10.1037/hea0000786. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker A.C., Gallagher S., Drayson M. Time of Day of Vaccination Does Not Relate to Antibody Response to Thymus-Independent Vaccinations. Vaccine X. 2022;11:100178. doi: 10.1016/j.jvacx.2022.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagovkina N.V., Zheleznov L.M., Subbotina K.A., Tsaan A.A., Kozlovskaya L.I., Gordeychuk I.V., Korduban A.K., Ivin Y.Y., Kovpak A.A., Piniaeva A.N., et al. Vaccination with Oral Polio Vaccine Reduces COVID-19 Incidence. Front. Immunol. 2022;13:907341. doi: 10.3389/fimmu.2022.907341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comber L., Murchu E.O., Jordan K., Hawkshaw S., Marshall L., O’Neill M., Teljeur C., Ryan M., Carnahan A., Pérez Martín J.J., et al. Systematic Review of the Efficacy, Effectiveness and Safety of High-dose Seasonal Influenza Vaccines for the Prevention of Laboratory-confirmed Influenza in Individuals ≥18 Years of Age. Rev. Med. Virol. 2022;33:e2330. doi: 10.1002/rmv.2330. [DOI] [PubMed] [Google Scholar]
- 23.Murchu E.O., Comber L., Jordan K., Hawkshaw S., Marshall L., O’Neill M., Ryan M., Teljeur C., Carnahan A., Pérez J.J., et al. Systematic Review of the Efficacy, Effectiveness and Safety of Recombinant Haemagglutinin Seasonal Influenza Vaccines for the Prevention of Laboratory-confirmed Influenza in Individuals ≥18 Years of Age. Rev. Med. Virol. 2022;33:e2331. doi: 10.1002/rmv.2331. [DOI] [PubMed] [Google Scholar]
- 24.Díez-Domingo J., Torcel-Pagnon L., Carmona A., Launay O., Dos Santos G., Rizzo C., Haag M., Stuurman A., Nauta J., Vannacci A., et al. The Value of Public-Private Collaborative Real-World Evidence Platforms to Monitor Vaccine Performance Post Authorization: DRIVE—A European Initiative. Expert Rev. Vaccines. 2022;21:1701–1710. doi: 10.1080/14760584.2022.2137144. [DOI] [PubMed] [Google Scholar]
- 25.Boddington N.L., Pearson I., Whitaker H., Mangtani P., Pebody R.G. Effectiveness of Influenza Vaccination in Preventing Hospitalization Due to Influenza in Children: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2021;73:1722–1732. doi: 10.1093/cid/ciab270. [DOI] [PubMed] [Google Scholar]
- 26.Okoli G.N., Racovitan F., Abdulwahid T., Righolt C.H., Mahmud S.M. Variable Seasonal Influenza Vaccine Effectiveness across Geographical Regions, Age Groups and Levels of Vaccine Antigenic Similarity with Circulating Virus Strains: A Systematic Review and Meta-Analysis of the Evidence from Test-Negative Design Studies after the 2009/10 Influenza Pandemic. Vaccine. 2021;39:1225–1240. doi: 10.1016/j.vaccine.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 27.Mallory R.M., Bandell A., Ambrose C.S., Yu J. A Systematic Review and Meta-Analysis of the Effectiveness of LAIV4 and IIV in Children Aged 6 Months to 17 Years during the 2016–2017 Season. Vaccine. 2020;38:3405–3410. doi: 10.1016/j.vaccine.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2023. [Google Scholar]
- 30.Kovalchik S. Package ‘RISmed. [(accessed on 1 January 2023)]. Available online: https://cran.r-project.org/web/packages/RISmed/RISmed.pdf.
- 31.Viechtbauer W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Soft. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 32.Hartung J., Knapp G., Sinha B.K. Statistical Meta-Analysis with Applications. Wiley; Hoboken, NJ, USA: 2008. (Wiley series in probability and statistics). [Google Scholar]
- 33.Shi L., Lin L. The Trim-and-Fill Method for Publication Bias: Practical Guidelines and Recommendations Based on a Large Database of Meta-Analyses. Medicine. 2019;98:e15987. doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S., Adrian P.V., van Niekerk N., Treurnicht F., Ortiz J.R., et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N. Engl. J. Med. 2014;371:918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 36.Petrie J.G., Ohmit S.E., Truscon R., Johnson E., Braun T.M., Levine M.Z., Eichelberger M.C., Monto A.S. Modest Waning of Influenza Vaccine Efficacy and Antibody Titers During the 2007–2008 Influenza Season. J. Infect. Dis. 2016;214:1142–1149. doi: 10.1093/infdis/jiw105. [DOI] [PubMed] [Google Scholar]
- 37.Mcbride W.J.H., Abhayaratna W.P., Barr I., Booy R., Carapetis J., Carson S., De Looze F., Ellis-Pegler R., Heron L., Karrasch J., et al. Efficacy of a Trivalent Influenza Vaccine against Seasonal Strains and against 2009 Pandemic H1N1: A Randomized, Placebo-Controlled Trial. Vaccine. 2016;34:4991–4997. doi: 10.1016/j.vaccine.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 38.Steinhoff M.C., Katz J., Englund J.A., Khatry S.K., Shrestha L., Kuypers J., Stewart L., Mullany L.C., Chu H.Y., LeClerq S.C., et al. Year-Round Influenza Immunisation during Pregnancy in Nepal: A Phase 4, Randomised, Placebo-Controlled Trial. Lancet Infect. Dis. 2017;17:981–989. doi: 10.1016/S1473-3099(17)30252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebowitz D., Gottlieb K., Kolhatkar N.S., Garg S.J., Asher J.M., Nazareno J., Kim K., McIlwain D.R., Tucker S.N. Efficacy, Immunogenicity, and Safety of an Oral Influenza Vaccine: A Placebo-Controlled and Active-Controlled Phase 2 Human Challenge Study. Lancet Infect. Dis. 2020;20:435–444. doi: 10.1016/S1473-3099(19)30584-5. [DOI] [PubMed] [Google Scholar]
- 40.Kissling E., Pozo F., Martínez-Baz I., Buda S., Vilcu A., Domegan L., Mazagatos C., Dijkstra F., Latorre-Margalef N., Kurečić Filipović S., et al. Influenza Vaccine Effectiveness against Influenza A Subtypes in Europe: Results from the 2021–2022 I-MOVE Primary Care Multicentre Study. Influenza Resp. Viruses. 2023;17:e13069. doi: 10.1111/irv.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenforde M.W., Weber Z.A., DeSilva M.B., Stenehjem E., Yang D.-H., Fireman B., Gaglani M., Kojima N., Irving S.A., Rao S., et al. Vaccine Effectiveness Against Influenza-Associated Urgent Care, Emergency Department, and Hospital Encounters During the 2021–2022 Season, VISION Network. J. Infect. Dis. 2023;228:jiad015. doi: 10.1093/infdis/jiad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S., Chuang E.S., Sabaiduc S., Olsha R., Kaweski S.E., Zelyas N., Gubbay J.B., Jassem A.N., Charest H., De Serres G., et al. Influenza Vaccine Effectiveness against A(H3N2) during the Delayed 2021/22 Epidemic in Canada. Eurosurveillance. 2022;27:2200720. doi: 10.2807/1560-7917.ES.2022.27.38.2200720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price A.M., Flannery B., Talbot H.K., Grijalva C.G., Wernli K.J., Phillips C.H., Monto A.S., Martin E.T., Belongia E.A., McLean H.Q., et al. Influenza Vaccine Effectiveness Against Influenza A(H3N2)-Related Illness in the United States During the 2021–2022 Influenza Season. Clin. Infect. Dis. 2023;76:1358–1363. doi: 10.1093/cid/ciac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard S.A., Fairchok M., Coles C., Burgess T.H., Colombo R.E. Influenza Vaccine Effectiveness: Analysis of the Impact of Repeated Vaccinations in Military Health System Beneficiaries. Open Forum Infect. Dis. 2022;9:ofac497. doi: 10.1093/ofid/ofac497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sominina A., Danilenko D., Komissarov A., Pisareva M., Musaeva T., Bakaev M., Afanasieva O., Stolyarov K., Smorodintseva E., Rozhkova E., et al. Age-Specific Etiology of Severe Acute Respiratory Infections and Influenza Vaccine Effectivity in Prevention of Hospitalization in Russia, 2018–2019 Season. J. Epidemiol. Glob. Health. 2021;11:413–425. doi: 10.1007/s44197-021-00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mir H., Haq I., Koul P.A. Poor Vaccine Effectiveness against Influenza B-Related Severe Acute Respiratory Infection in a Temperate North Indian State (2019–2020): A Call for Further Data for Possible Vaccines with Closer Match. Vaccines. 2021;9:1094. doi: 10.3390/vaccines9101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuurman A.L., Biccler J., Carmona A., Descamps A., Díez-Domingo J., Muñoz Quiles C., Nohynek H., Rizzo C., Riera-Montes M. Brand-Specific Influenza Vaccine Effectiveness Estimates during 2019/20 Season in Europe—Results from the DRIVE EU Study Platform. Vaccine. 2021;39:3964–3973. doi: 10.1016/j.vaccine.2021.05.059. [DOI] [PubMed] [Google Scholar]
- 48.Grijalva C.G., Feldstein L.R., Talbot H.K., Aboodi M., Baughman A.H., Brown S.M., Casey J.D., Erickson H.L., Exline M.C., Files D.C., et al. Influenza Vaccine Effectiveness for Prevention of Severe Influenza-Associated Illness Among Adults in the United States, 2019–2020: A Test-Negative Study. Clin. Infect. Dis. 2021;73:1459–1468. doi: 10.1093/cid/ciab462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu W., Gruner W.E., DeMarcus L.S., Thervil J.W., Kwaah B., Fries A.C., Sjoberg P.A., Robbins A.S. Influenza Surveillance Trends and Influenza Vaccine Effectiveness Among Department of Defense Beneficiaries During the 2019-2020 Influenza Season. MSMR. 2021;28:2–8. [PubMed] [Google Scholar]
- 50.Martin E.T., Cheng C., Petrie J.G., Alyanak E., Gaglani M., Middleton D.B., Ghamande S., Silveira F.P., Murthy K., Zimmerman R.K., et al. Low Influenza Vaccine Effectiveness Against A(H3N2)-Associated Hospitalizations in 2016–2017 and 2017–2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) J. Infect. Dis. 2021;223:2062–2071. doi: 10.1093/infdis/jiaa685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuurman A.L., Bollaerts K., Alexandridou M., Biccler J., Díez Domingo J., Nohynek H., Rizzo C., Turunen T., Riera-Montes M. Vaccine Effectiveness against Laboratory-Confirmed Influenza in Europe—Results from the DRIVE Network during Season 2018/19. Vaccine. 2020;38:6455–6463. doi: 10.1016/j.vaccine.2020.07.063. [DOI] [PubMed] [Google Scholar]
- 52.Rizzo C., Gesualdo F., Loconsole D., Pandolfi E., Bella A., Orsi A., Guarona G., Panatto D., Icardi G., Napoli C., et al. Moderate Vaccine Effectiveness against Severe Acute Respiratory Infection Caused by A(H1N1)Pdm09 Influenza Virus and No Effectiveness against A(H3N2) Influenza Virus in the 2018/2019 Season in Italy. Vaccines. 2020;8:427. doi: 10.3390/vaccines8030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al Qahtani A.A., Selim M., Hamouda N.H., Al Delamy A.L., Macadangdang C., Al Shammari K.H., Al Shamary S.F. Seasonal Influenza Vaccine Effectiveness among Health-Care Workers in Prince Sultan Military Medical City, Riyadh, KSA, 2018–2019. Hum. Vaccines Immunother. 2021;17:119–123. doi: 10.1080/21645515.2020.1764827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redlberger-Fritz M., Kundi M., Popow-Kraupp T. Heterogeneity of Circulating Influenza Viruses and Their Impact on Influenza Virus Vaccine Effectiveness During the Influenza Seasons 2016/17 to 2018/19 in Austria. Front. Immunol. 2020;11:434. doi: 10.3389/fimmu.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rose A., Kissling E., Emborg H.-D., Larrauri A., McMenamin J., Pozo F., Trebbien R., Mazagatos C., Whitaker H., Valenciano M., et al. Interim 2019/20 Influenza Vaccine Effectiveness: Six European Studies, September 2019 to January 2020. Eurosurveillance. 2020;25:2000153. doi: 10.2807/1560-7917.ES.2020.25.10.2000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ando S. Estimation of the Effectiveness of Quadrivalent Influenza Vaccines by Distinguishing Between Influenza A (H1N1) Pdm09 and Influenza A (H3N2) Using Rapid Influenza Diagnostic Tests During the 2018-2019 Season. Intern. Med. 2020;59:933–940. doi: 10.2169/internalmedicine.3616-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segaloff H.E., Cheng B., Miller A.V., Petrie J.G., Malosh R.E., Cheng C., Lauring A.S., Lamerato L.E., Ferdinands J.M., Monto A.S., et al. Influenza Vaccine Effectiveness in the Inpatient Setting: Evaluation of Potential Bias in the Test-Negative Design by Use of Alternate Control Groups. Am. J. Epidemiol. 2020;189:250–260. doi: 10.1093/aje/kwz248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flannery B., Kondor R.J.G., Chung J.R., Gaglani M., Reis M., Zimmerman R.K., Nowalk M.P., Jackson M.L., Jackson L.A., Monto A.S., et al. Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018–2019 Season. J. Infect. Dis. 2020;221:8–15. doi: 10.1093/infdis/jiz543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kissling E., Rose A., Emborg H.-D., Gherasim A., Pebody R., Pozo F., Trebbien R., Mazagatos C., Whitaker H., Valenciano M., et al. Interim 2018/19 Influenza Vaccine Effectiveness: Six European Studies, October 2018 to January 2019. Eurosurveillance. 2019;24:1900121. doi: 10.2807/1560-7917.ES.2019.24.1900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanchette P.S., Chung H., Pritchard K.I., Earle C.C., Campitelli M.A., Buchan S.A., Schwartz K.L., Crowcroft N.S., Gubbay J.B., Karnauchow T., et al. Influenza Vaccine Effectiveness Among Patients with Cancer: A Population-Based Study Using Health Administrative and Laboratory Testing Data From Ontario, Canada. JCO. 2019;37:2795–2804. doi: 10.1200/JCO.19.00354. [DOI] [PubMed] [Google Scholar]
- 61.Costantino C., Restivo V., Amodio E., Colomba G.M.E., Vitale F., Tramuto F. A Mid-Term Estimate of 2018/2019 Vaccine Effectiveness to Prevent Laboratory Confirmed A(H1N1)Pdm09 and A(H3N2) Influenza Cases in Sicily (Italy) Vaccine. 2019;37:5812–5816. doi: 10.1016/j.vaccine.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Pebody R., Djennad A., Ellis J., Andrews N., Marques D.F.P., Cottrell S., Reynolds A.J., Gunson R., Galiano M., Hoschler K., et al. End of Season Influenza Vaccine Effectiveness in Adults and Children in the United Kingdom in 2017/18. Eurosurveillance. 2019;24:1800488. doi: 10.2807/1560-7917.ES.2019.24.31.1800488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kissling E., Pozo F., Buda S., Vilcu A.-M., Rizzo C., Gherasim A., Horváth J.K., Brytting M., Domegan L., Meijer A., et al. Effectiveness of Influenza Vaccine against Influenza A in Europe in Seasons of Different A(H1N1)Pdm09 and the Same A(H3N2) Vaccine Components (2016–17 and 2017–18) Vaccine X. 2019;3:100042. doi: 10.1016/j.jvacx.2019.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chon I., Saito R., Hibino A., Yagami R., Dapat C., Odagiri T., Kondo H., Sato I., Kimura S., Kawashima T., et al. Effectiveness of the Quadrivalent Inactivated Influenza Vaccine in Japan during the 2015–2016 Season: A Test-Negative Case-Control Study Comparing the Results by Real Time PCR, Virus Isolation. Vaccine X. 2019;1:100011. doi: 10.1016/j.jvacx.2019.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mølgaard-Nielsen D., Fischer T.K., Krause T.G., Hviid A. Effectiveness of Maternal Immunization with Trivalent Inactivated Influenza Vaccine in Pregnant Women and Their Infants. J. Intern. Med. 2019;286:469–480. doi: 10.1111/joim.12947. [DOI] [PubMed] [Google Scholar]
- 66.Regan A.K., Fielding J.E., Chilver M.B., Carville K.S., Minney-Smith C.A., Grant K.A., Thomson C., Hahesy T., Deng Y.-M., Stocks N., et al. Intraseason Decline in Influenza Vaccine Effectiveness during the 2016 Southern Hemisphere Influenza Season: A Test-Negative Design Study and Phylogenetic Assessment. Vaccine. 2019;37:2634–2641. doi: 10.1016/j.vaccine.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Skowronski D.M., Leir S., Sabaiduc S., Murti M., Dickinson J.A., Olsha R., Gubbay J.B., Croxen M.A., Charest H., Chan T., et al. Interim Estimates of 2018/19 Vaccine Effectiveness against Influenza A(H1N1)Pdm09, Canada, January 2019. Eurosurveillance. 2019;24:1900055. doi: 10.2807/1560-7917.ES.2019.24.4.1900055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Regan A.K., Gibbs R., Bloomfield L., Effler P.V. Estimating Influenza Vaccine Effectiveness Using Data Routinely Available in Electronic Primary Care Records. Vaccine. 2019;37:755–762. doi: 10.1016/j.vaccine.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Thompson M.G., Kwong J.C., Regan A.K., Katz M.A., Drews S.J., Azziz-Baumgartner E., Klein N.P., Chung H., Effler P.V., Feldman B.S., et al. Influenza Vaccine Effectiveness in Preventing Influenza-Associated Hospitalizations During Pregnancy: A Multi-Country Retrospective Test Negative Design Study, 2010–2016. Clin. Infect. Dis. 2019;68:1444–1453. doi: 10.1093/cid/ciy737. [DOI] [PubMed] [Google Scholar]
- 70.Flannery B., Chung J.R., Monto A.S., Martin E.T., Belongia E.A., McLean H.Q., Gaglani M., Murthy K., Zimmerman R.K., Nowalk M.P., et al. Influenza Vaccine Effectiveness in the United States During the 2016–2017 Season. Clin. Infect. Dis. 2019;68:1798–1806. doi: 10.1093/cid/ciy775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan Y.D., Wong M., Au K., Chuang S. Seasonal Influenza Vaccine Effectiveness at Primary Care Level, Hong Kong SAR, 2017/2018 Winter. Hum. Vaccines Immunother. 2019;15:97–101. doi: 10.1080/21645515.2018.1514222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seki Y., Onose A., Murayama T., Koide C., Sugaya N. Influenza Vaccine Showed a Good Preventive Effect against Influenza-Associated Hospitalization among Elderly Patients, during the 2016/17 Season in Japan. J. Infect. Chemother. 2018;24:873–880. doi: 10.1016/j.jiac.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Wu S., Pan Y., Zhang X., Zhang L., Duan W., Ma C., Zhang Y., Zhang M., Sun Y., Yang P., et al. Influenza Vaccine Effectiveness in Preventing Laboratory-Confirmed Influenza in Outpatient Settings: A Test-Negative Case-Control Study in Beijing, China, 2016/17 Season. Vaccine. 2018;36:5774–5780. doi: 10.1016/j.vaccine.2018.07.077. [DOI] [PubMed] [Google Scholar]
- 74.Yaron-Yakoby H., Sefty H., Pando R., Dichtiar R., Katz M.A., Stein Y., Mandelboim M., Mendelson E., Shohat T., Glatman-Freedman A., et al. Effectiveness of Influenza Vaccine in Preventing Medically-Attended Influenza Virus Infection in Primary Care, Israel, Influenza Seasons 2014/15 and 2015/16. Eurosurveillance. 2018;23:17–00026. doi: 10.2807/1560-7917.ES.2018.23.7.17-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skowronski D.M., Chambers C., De Serres G., Dickinson J.A., Winter A.-L., Hickman R., Chan T., Jassem A.N., Drews S.J., Charest H., et al. Early Season Co-Circulation of Influenza A(H3N2) and B(Yamagata): Interim Estimates of 2017/18 Vaccine Effectiveness, Canada, January 2018. Eurosurveillance. 2018;23:18–00035. doi: 10.2807/1560-7917.ES.2018.23.5.18-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein Y., Mandelboim M., Sefty H., Pando R., Mendelson E., Shohat T., Glatman-Freedman A., Muhamed A., Arkadi A., Israeli Influenza Surveillance Network (IISN) et al. Seasonal Influenza Vaccine Effectiveness in Preventing Laboratory-Confirmed Influenza in Primary Care in Israel, 2016–2017 Season: Insights into Novel Age-Specific Analysis. Clin. Infect. Dis. 2018;66:1383–1391. doi: 10.1093/cid/cix1013. [DOI] [PubMed] [Google Scholar]
- 77.Pebody R., Warburton F., Ellis J., Andrews N., Potts A., Cottrell S., Reynolds A., Gunson R., Thompson C., Galiano M., et al. End-of-Season Influenza Vaccine Effectiveness in Adults and Children, United Kingdom, 2016/17. Eurosurveillance. 2017;22:17–00306. doi: 10.2807/1560-7917.ES.2017.22.44.17-00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skowronski D.M., Chambers C., Sabaiduc S., De Serres G., Winter A.-L., Dickinson J.A., Gubbay J.B., Drews S.J., Martineau C., Charest H., et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-Epidemiological Influences on Influenza Vaccine Effectiveness During the 2015–2016 Season in Canada. J. Infect. Dis. 2017;216:1487–1500. doi: 10.1093/infdis/jix526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuliese M., Jancoriene L., Grimalauskaite R., Zablockiene B., Damuleviciene G., Velyvyte D., Lesauskaite V., Ambrozaitis A., Mickiene A., Gefenaite G. Seasonal Influenza Vaccine Effectiveness against Laboratory-Confirmed Influenza in 2015–2016: A Hospital-Based Test-Negative Case—Control Study in Lithuania. BMJ Open. 2017;7:e017835. doi: 10.1136/bmjopen-2017-017835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma C., Pan Y., Zhang L., Zhang Y., Wu S., Sun Y., Duan W., Zhang M., Wang Q., Yang P. Influenza Vaccine Effectiveness against Medically Attended Influenza Illness in Beijing, China, 2014/15 Season. Hum. Vaccines Immunother. 2017;13:2379–2384. doi: 10.1080/21645515.2017.1359364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seki Y., Onose A., Sugaya N. Influenza Vaccine Effectiveness in Adults Based on the Rapid Influenza Diagnostic Test Results, during the 2015/16 Season. J. Infect. Chemother. 2017;23:615–620. doi: 10.1016/j.jiac.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 82.McAnerney J.M., Walaza S., Tempia S., Blumberg L., Treurnicht F.K., Madhi S.A., Valley-Omar Z., Cohen C. Estimating Vaccine Effectiveness in Preventing Laboratory-Confirmed Influenza in Outpatient Settings in South Africa, 2015. Influenza Other Resp. Viruses. 2017;11:177–181. doi: 10.1111/irv.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fielding J.E., Levy A., Chilver M.B., Deng Y.-M., Regan A.K., Grant K.A., Stocks N.P., Sullivan S.G. Effectiveness of Seasonal Influenza Vaccine in Australia, 2015: An Epidemiological, Antigenic and Phylogenetic Assessment. Vaccine. 2016;34:4905–4912. doi: 10.1016/j.vaccine.2016.08.067. [DOI] [PubMed] [Google Scholar]
- 84.Petrie J.G., Ohmit S.E., Cheng C.K., Martin E.T., Malosh R.E., Lauring A.S., Lamerato L.E., Reyes K.C., Flannery B., Ferdinands J.M., et al. Influenza Vaccine Effectiveness Against Antigenically Drifted Influenza Higher Than Expected in Hospitalized Adults: 2014–2015. Clin. Infect. Dis. 2016;63:1017–1025. doi: 10.1093/cid/ciw432. [DOI] [PubMed] [Google Scholar]
- 85.Rizzo C., Bella A., Alfonsi V., Puzelli S., Palmieri A.P., Chironna M., Pariani E., Piatti A., Tiberti D., Ghisetti V., et al. Influenza Vaccine Effectiveness in Italy: Age, Subtype-Specific and Vaccine Type Estimates 2014/15 Season. Vaccine. 2016;34:3102–3108. doi: 10.1016/j.vaccine.2016.04.072. [DOI] [PubMed] [Google Scholar]
- 86.Lytras T., Kossyvakis A., Melidou A., Andreopoulou A., Exindari M., Gioula G., Kalliaropoulos A., Tryfinopoulou K., Pogka V., Spala G., et al. Influenza Vaccine Effectiveness in Preventing Hospitalizations with Laboratory-Confirmed Influenza in Greece during the 2014-2015 Season: A Test-Negative Study: Flu Vaccine Effectiveness Against Hospitalization During 2014–2015. J. Med. Virol. 2016;88:1896–1904. doi: 10.1002/jmv.24551. [DOI] [PubMed] [Google Scholar]
- 87.Rondy M., Castilla J., Launay O., Costanzo S., Ezpeleta C., Galtier F., De Gaetano Donati K., Moren A. Moderate Influenza Vaccine Effectiveness against Hospitalisation with A(H3N2) and A(H1N1) Influenza in 2013–14: Results from the InNHOVE Network. Hum. Vaccines Immunother. 2016;12:1217–1224. doi: 10.1080/21645515.2015.1126013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gherasim A., Pozo F., De Mateo S., Aspiritxaga Gamarra I., García-Cenoz M., Vega T., Martínez E., Giménez J., Castrillejo D., Larrauri A. Waning Protection of Influenza Vaccine against Mild Laboratory Confirmed Influenza A(H3N2) and B in Spain, Season 2014–15. Vaccine. 2016;34:2371–2377. doi: 10.1016/j.vaccine.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 89.Redlberger-Fritz M., Kundi M., Popow-Kraupp T. Detailed Report on 2014/15 Influenza Virus Characteristics, and Estimates on Influenza Virus Vaccine Effectiveness from Austria’s Sentinel Physician Surveillance Network. PLoS ONE. 2016;11:e0149916. doi: 10.1371/journal.pone.0149916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelly H.A., Lane C., Cheng A.C. Influenza Vaccine Effectiveness in General Practice and in Hospital Patients in Victoria, 2011–2013. Med. J. Aust. 2016;204:76. doi: 10.5694/mja15.01017. [DOI] [PubMed] [Google Scholar]
- 91.Bissielo A., Pierse N., Huang Q., Thompson M., Kelly H., Mishin V., Turner N., SHIVERS Effectiveness of Seasonal Influenza Vaccine in Preventing Influenza Primary Care Visits and Hospitalisation in Auckland, New Zealand in 2015: Interim Estimates. Eurosurveillance. 2016;21:30101. doi: 10.2807/1560-7917.ES.2016.21.1.30101. [DOI] [PubMed] [Google Scholar]
- 92.Cheng A.C., Kotsimbos T., Kelly P.M. Influenza Vaccine Effectiveness against Hospitalisation with Influenza in Adults in Australia in 2014. Vaccine. 2015;33:7352–7356. doi: 10.1016/j.vaccine.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 93.Levy J.W., Simasathien S., Watanaveeradej V., Bhoomiboonchoo P., Fernandez S., Jarman R.G., Klungthong C., Gibbons R.V., Kerdpanich P., Piboonbanakit D., et al. Influenza Vaccine Effectiveness in the Tropics: Moderate Protection in a Case Test-Negative Analysis of a Hospital-Based Surveillance Population in Bangkok between August 2009 and January 2013. PLoS ONE. 2015;10:e0134318. doi: 10.1371/journal.pone.0134318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McAnerney J.M., Treurnicht F., Walaza S., Cohen A.L., Tempia S., Mtshali S., Buys A., Blumberg L., Cohen C. Evaluation of Influenza Vaccine Effectiveness and Description of Circulating Strains in Outpatient Settings in South Africa, 2014. Influenza Other Resp. Viruses. 2015;9:209–215. doi: 10.1111/irv.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McAnerney J.M., Walaza S., Cohen A.L., Tempia S., Buys A., Venter M., Blumberg L., Duque J., Cohen C. Effectiveness and Knowledge, Attitudes and Practices of Seasonal Influenza Vaccine in Primary Healthcare Settings in South Africa, 2010–2013. Influenza Other Resp. Viruses. 2015;9:143–150. doi: 10.1111/irv.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rondy M., Launay O., Puig-Barberà J., Gefenaite G., Castilla J., De Gaetano Donati K., Galtier F., Hak E., Guevara M., Costanzo S., et al. 2012/13 Influenza Vaccine Effectiveness against Hospitalised Influenza A(H1N1)Pdm09, A(H3N2) and B: Estimates from a European Network of Hospitals. Eurosurveillance. 2015;20:21011. doi: 10.2807/1560-7917.ES2015.20.2.21011. [DOI] [PubMed] [Google Scholar]
- 97.McLean H.Q., Thompson M.G., Sundaram M.E., Kieke B.A., Gaglani M., Murthy K., Piedra P.A., Zimmerman R.K., Nowalk M.P., Raviotta J.M., et al. Influenza Vaccine Effectiveness in the United States During 2012-2013: Variable Protection by Age and Virus Type. J. Infect. Dis. 2015;211:1529–1540. doi: 10.1093/infdis/jiu647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurečić Filipović S., Gjenero-Margan I., Kissling E., Kaić B., Cvitković A. Influenza Vaccine Effectiveness Estimates in Croatia in 2010–2011: A Season with Predominant Circulation of A(H1N1)Pdm09 Influenza Virus. Epidemiol. Infect. 2015;143:2596–2603. doi: 10.1017/S0950268814003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turner N., Pierse N., Huang Q.S., Radke S., Bissielo A., Thompson M.G., Kelly H., SHIVERS Investigation Team Interim Estimates of the Effectiveness of Seasonal Trivalent Inactivated Influenza Vaccine in Preventing Influenza Hospitalisations and Primary Care Visits in Auckland, New Zealand, in 2014. Eurosurveillance. 2014;19:20934. doi: 10.2807/1560-7917.ES2014.19.42.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levy A., Sullivan S.G., Tempone S.S., Wong K.L.M., Regan A.K., Dowse G.K., Effler P.V., Smith D.W. Influenza Vaccine Effectiveness Estimates for Western Australia during a Period of Vaccine and Virus Strain Stability, 2010 to 2012. Vaccine. 2014;32:6312–6318. doi: 10.1016/j.vaccine.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 101.Yang P., Thompson M.G., Ma C., Shi W., Wu S., Zhang D., Wang Q. Influenza Vaccine Effectiveness against Medically-Attended Influenza Illness during the 2012–2013 Season in Beijing, China. Vaccine. 2014;32:5285–5289. doi: 10.1016/j.vaccine.2014.07.083. [DOI] [PubMed] [Google Scholar]
- 102.Sullivan S.G., Chilver M.B., Higgins G., Cheng A.C., Stocks N.P. Influenza Vaccine Effectiveness in Australia: Results from the Australian Sentinel Practices Research Network. Med. J. Aust. 2014;201:109–111. doi: 10.5694/mja14.00106. [DOI] [PubMed] [Google Scholar]
- 103.Skowronski D.M., Janjua N.Z., Sabaiduc S., De Serres G., Winter A.-L., Gubbay J.B., Dickinson J.A., Fonseca K., Charest H., Bastien N., et al. Influenza A/Subtype and B/Lineage Effectiveness Estimates for the 2011–2012 Trivalent Vaccine: Cross-Season and Cross-Lineage Protection with Unchanged Vaccine. J. Infect. Dis. 2014;210:126–137. doi: 10.1093/infdis/jiu048. [DOI] [PubMed] [Google Scholar]
- 104.Skowronski D.M., Chambers C., Sabaiduc S., De Serres G., Dickinson J.A., Winter A.L., Fonseca K., Gubbay J.B., Charest H., Petric M., et al. Interim Estimates of 2013/14 Vaccine Effectiveness against Influenza A(H1N1)Pdm09 from Canada’s Sentinel Surveillance Network, January 2014. Eurosurveillance. 2014;19:20690. doi: 10.2807/1560-7917.ES2014.19.5.20690. [DOI] [PubMed] [Google Scholar]
- 105.Skowronski D.M., Janjua N.Z., De Serres G., Sabaiduc S., Eshaghi A., Dickinson J.A., Fonseca K., Winter A.-L., Gubbay J.B., Krajden M., et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE. 2014;9:e92153. doi: 10.1371/journal.pone.0092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kavanagh K., Robertson C., McMenamin J. Estimates of Influenza Vaccine Effectiveness in Primary Care in Scotland Vary with Clinical or Laboratory Endpoint and Method—Experience across the 2010/11 Season. Vaccine. 2013;31:4556–4563. doi: 10.1016/j.vaccine.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 107.Castilla J., Martínez-Baz I., Martínez-Artola V., Reina G., Pozo F., García Cenoz M., Guevara M., Moran J., Irisarri F., Arriazu M., et al. Decline in Influenza Vaccine Effectiveness with Time after Vaccination, Navarre, Spain, Season 2011/12. Eurosurveillance. 2013;18:20388. doi: 10.2807/ese.18.05.20388-en. [DOI] [PubMed] [Google Scholar]
- 108.DiazGranados C.A., Dunning A.J., Jordanov E., Landolfi V., Denis M., Talbot H.K. High-Dose Trivalent Influenza Vaccine Compared to Standard Dose Vaccine in Elderly Adults: Safety, Immunogenicity and Relative Efficacy during the 2009–2010 Season. Vaccine. 2013;31:861–866. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 109.DiazGranados C.A., Dunning A.J., Kimmel M., Kirby D., Treanor J., Collins A., Pollak R., Christoff J., Earl J., Landolfi V., et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 110.Forrest B.D., Steele A.D., Hiemstra L., Rappaport R., Ambrose C.S., Gruber W.C. A Prospective, Randomized, Open-Label Trial Comparing the Safety and Efficacy of Trivalent Live Attenuated and Inactivated Influenza Vaccines in Adults 60 Years of Age and Older. Vaccine. 2011;29:3633–3639. doi: 10.1016/j.vaccine.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 111.Foppa I.M., Ferdinands J.M., Chaves S.S., Haber M.J., Reynolds S.B., Flannery B., Fry A.M. The Case Test-Negative Design for Studies of the Effectiveness of Influenza Vaccine in Inpatient Settings. Int. J. Epidemiol. 2016;45:dyw022. doi: 10.1093/ije/dyw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belongia E.A., Simpson M.D., King J.P., Sundaram M.E., Kelley N.S., Osterholm M.T., McLean H.Q. Variable Influenza Vaccine Effectiveness by Subtype: A Systematic Review and Meta-Analysis of Test-Negative Design Studies. Lancet Infect. Dis. 2016;16:942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 113.Darvishian M., Van Den Heuvel E.R., Bissielo A., Castilla J., Cohen C., Englund H., Gefenaite G., Huang W.-T., La Bastide-van Gemert S., Martinez-Baz I., et al. Effectiveness of Seasonal Influenza Vaccination in Community-Dwelling Elderly People: An Individual Participant Data Meta-Analysis of Test-Negative Design Case-Control Studies. Lancet Respir. Med. 2017;5:200–211. doi: 10.1016/S2213-2600(17)30043-7. [DOI] [PubMed] [Google Scholar]
- 114.Rondy M., El Omeiri N., Thompson M.G., Levêque A., Moren A., Sullivan S.G. Effectiveness of Influenza Vaccines in Preventing Severe Influenza Illness among Adults: A Systematic Review and Meta-Analysis of Test-Negative Design Case-Control Studies. J. Infect. 2017;75:381–394. doi: 10.1016/j.jinf.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tao Y.Y., Jin P.F., Zhu F.C. [Meta-analysis on effectiveness of live attenuated influenza vaccine against seasonal influenza in children] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:103–110. doi: 10.3760/cma.j.issn.0254-6450.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 116.Lee J.K.H., Lam G.K.L., Shin T., Samson S.I., Greenberg D.P., Chit A. Efficacy and Effectiveness of High-Dose Influenza Vaccine in Older Adults by Circulating Strain and Antigenic Match: An Updated Systematic Review and Meta-Analysis. Vaccine. 2021;39:A24–A35. doi: 10.1016/j.vaccine.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Sekiya T., Ohno M., Nomura N., Handabile C., Shingai M., Jackson D.C., Brown L.E., Kida H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses. 2021;13:971. doi: 10.3390/v13060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smetana J., Chlibek R., Shaw J., Splino M., Prymula R. Influenza Vaccination in the Elderly. Hum. Vaccines Immunother. 2018;14:540–549. doi: 10.1080/21645515.2017.1343226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jackson M.L., Phillips C.H., Benoit J., Kiniry E., Madziwa L., Nelson J.C., Jackson L.A. The Impact of Selection Bias on Vaccine Effectiveness Estimates from Test-Negative Studies. Vaccine. 2018;36:751–757. doi: 10.1016/j.vaccine.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 120.Stripeli F., Sakkou Z., Papadopoulos N., Georgiou V., Gratsia P., Christodoulou I., Tsolia M. Performance of Rapid Influenza Testing in Hospitalized Children. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:683–688. doi: 10.1007/s10096-010-0914-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.