Abstract
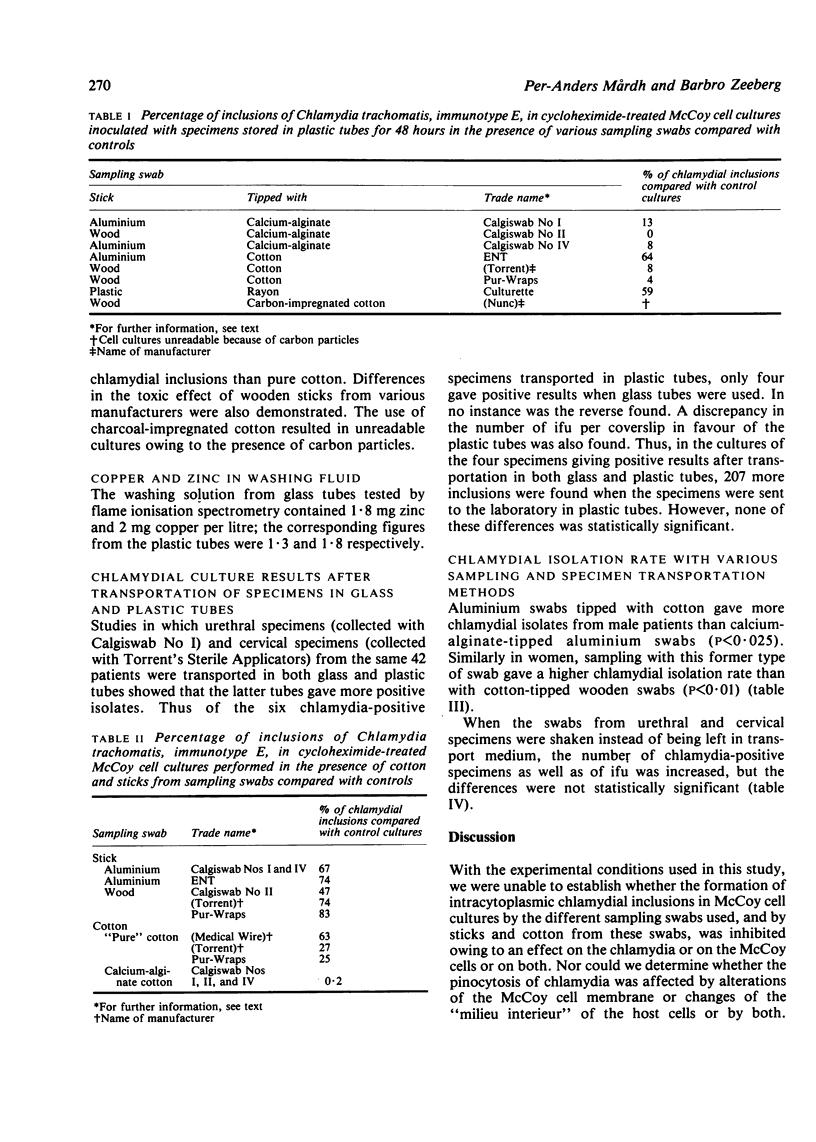
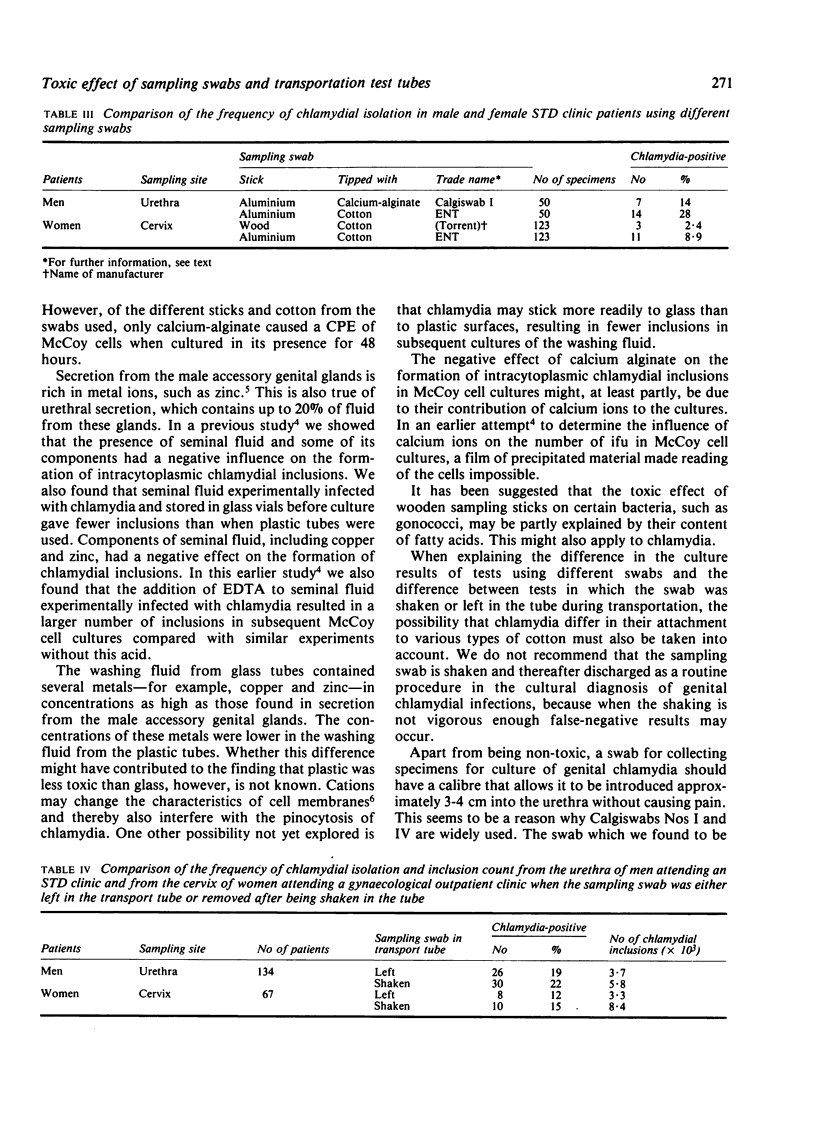
The ability of Chlamydia trachomatis, immunotype E, to produce intracytoplasmic inclusions in cycloheximide-treated McCoy cells after being exposed to different types of sampling swabs in experimentally infected transport medium was studied. A larger number of inclusions was obtained with cotton-tipped aluminium and rayon-tipped plastic swabs than with calcium-alginate-tipped aluminium and cotton-tipped wooden swabs (P less than 0.0001). Transport medium stored in glass tubes caused a cytopathic effect when inoculated on to McCoy cell cultures; no such effect occurred with plastic tubes. When cotton-tipped aluminium instead of calcium-alginate-tipped aluminium swabs were used to collect 50 make urethral specimens significantly more were chlamydia-positive (P less than 0.025). This was also true when cotton-tipped aluminium swabs were used instead of alginate-tipped swabs in a study of 123 cervical specimens (P less than 0.01). When the calcium-alginate-tipped aluminium and cotton-tipped wooden swabs were shaken in the transport medium after sampling from the male urethra and the cervix, instead of being left in the medium during transport to the laboratory, more specimens were chlamydia-positive and a greater number of chlamydial inclusions were found per culture-positive sample; these results were, however, not statistically significant (P greater than 0.05).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colleen S., Mårdh P. A., Schytz A. Magnesium and zinc in seminal fluid of healthy males and patients with non-acute prostatitis with and without gonorrhoea. Scand J Urol Nephrol. 1975;9(3):192–197. doi: 10.3109/00365597509134210. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Comparison of various McCoy cell treatment procedures used for detection of Chlamydia trachomatis. J Clin Microbiol. 1979 Aug;10(2):198–201. doi: 10.1128/jcm.10.2.198-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


