Abstract
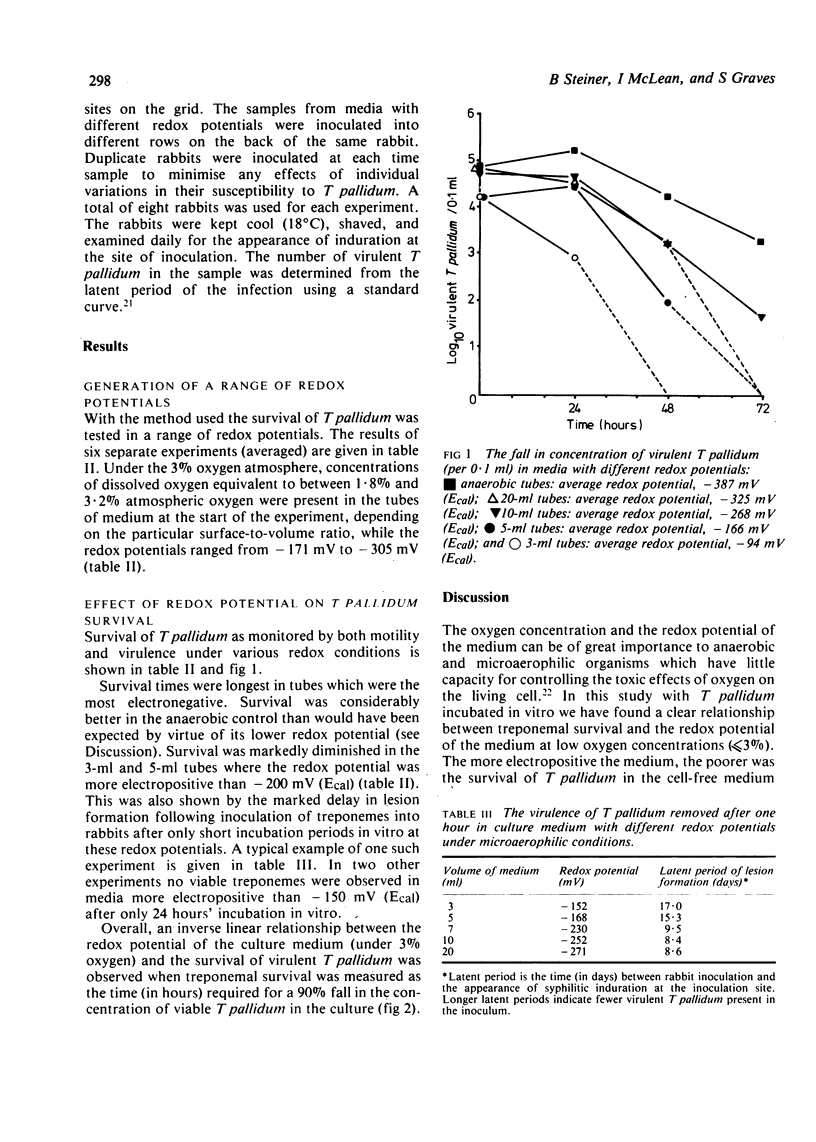
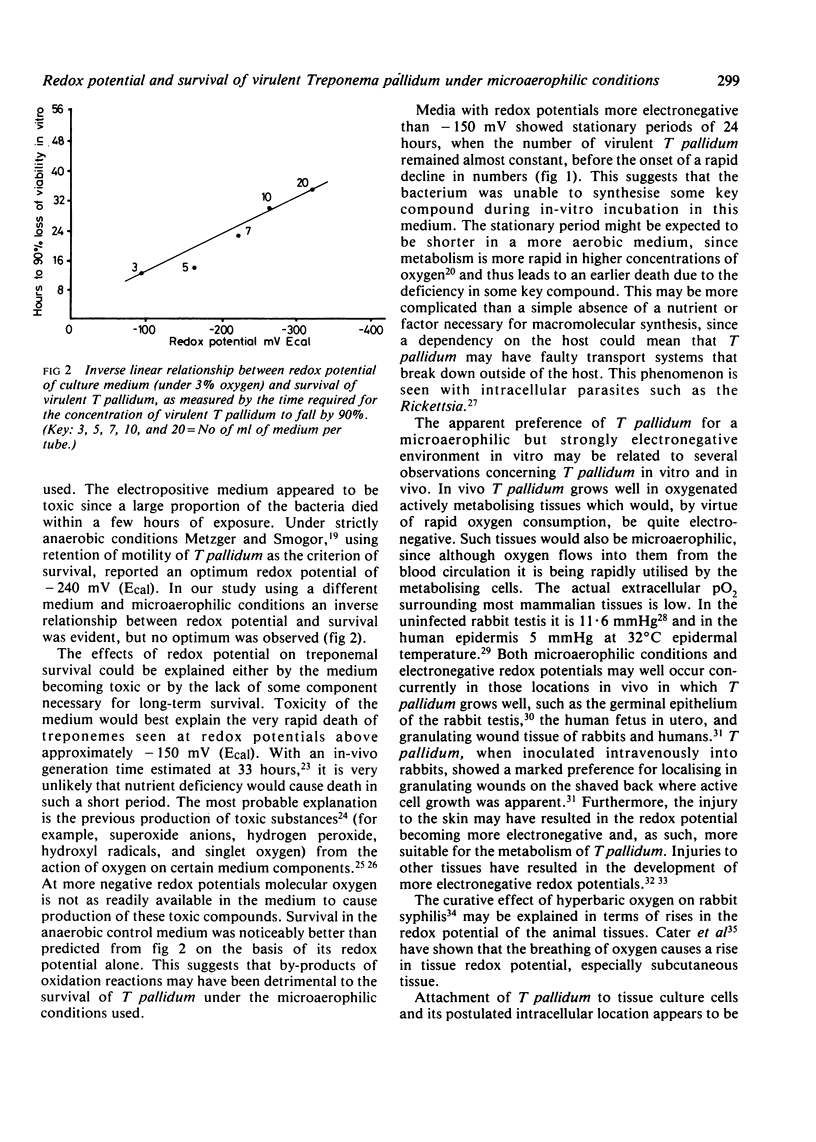
A strongly reduced culture medium, capable of maintaining the virulence of Treponema pallidum (Nichols) for several days, was exposed to an atmosphere of 3% oxygen in nitrogen for 2-3 days before inoculation with T pallidum. By using various volumes of medium in uniform tubes a range of redox potentials (Ecal) from -94 mV to -325 mV was produced depending on the surface area-to-volume ratios of the medium. The anaerobic medium had an Ecal value of -387 mV. The medium was inoculated with T pallidum and incubated in an atmosphere of 3% oxygen. The survival of treponemes at different redox potentials was monitored by observing the retention of motility and by measuring the latent period of infection after inoculation of the cultures into the shaved backs of rabbits. Under these conditions T pallidum survived longest at low (electronegative) redox potential. An inverse linear relationship was observed between the redox potential of the culture medium and the survival of T pallidum, as measured by the time required for a 90% reduction of virulent organisms. No optimum redox potential was detected, the most electronegative medium (-325 mV, Ecal) giving the best survival.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbieri J. T., Cox C. D. Glucose incorporation by Treponema pallidum. Infect Immun. 1979 Apr;24(1):291–293. doi: 10.1128/iai.24.1.291-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J. T., Cox C. D. Pyruvate oxidation by Treponema pallidum. Infect Immun. 1979 Jul;25(1):157–163. doi: 10.1128/iai.25.1.157-163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Anabolic potential of virulent Treponema pallidum. Infect Immun. 1977 Dec;18(3):857–859. doi: 10.1128/iai.18.3.857-859.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Hayes N. C. Virulent Treponema pallidum: aerobe or anaerobe. Infect Immun. 1976 Mar;13(3):704–711. doi: 10.1128/iai.13.3.704-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Mogerley S. Capacity of virulent Treponema pallidum (Nichols) for deoxyribonucleic acid synthesis. Infect Immun. 1979 Feb;23(2):392–397. doi: 10.1128/iai.23.2.392-397.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATER D. B., PHILLIPS A. F., SILVER I. A. The measurement of oxidation-reduction potentials, pH, and oxygen tension in tumours. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):382–399. doi: 10.1098/rspb.1957.0019. [DOI] [PubMed] [Google Scholar]
- CROSS B. A., SILVER I. A. Neurovascular control of oxygen tension in the testis and epididymis. J Reprod Fertil. 1962 Jun;3:377–395. doi: 10.1530/jrf.0.0030377. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers W. S., Taylor-Robinson D. The effect of reducing and other agents on the motility of Treponema pallidum in an acellular culture medium. J Gen Microbiol. 1979 Oct;114(2):443–447. doi: 10.1099/00221287-114-2-443. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Ploegmakers F., Gondrie P., Abboud R. Myocardial myoglobin oxygen tension. Am J Physiol. 1973 Apr;224(4):870–876. doi: 10.1152/ajplegacy.1973.224.4.870. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Barber M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 1974 Jul;10(1):123–127. doi: 10.1128/iai.10.1.123-127.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Becker F. A., Stout J. G. Prolonged survival of virulent Treponema pallidum (Nichols strain) in cell-free and tissue culture systems. Infect Immun. 1977 Oct;18(1):173–182. doi: 10.1128/iai.18.1.173-182.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Stout J. G., Becker F. A. Comparative behavior of virulent strains of Treponema pallidum and Treponema pertenue in gradient cultures of various mammalian cells. Infect Immun. 1979 May;24(2):337–345. doi: 10.1128/iai.24.2.337-345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Billington T. Optimum concentration of dissolved oxygen for the survival of virulent Treponema pallidum under conditions of low oxidation-reduction potential. Br J Vener Dis. 1979 Dec;55(6):387–393. doi: 10.1136/sti.55.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grin E. I., Nadazdin M., Svob M. Effect of hyperbaric oxygen on experimental syphilis in the rabbit. Br J Vener Dis. 1973 Oct;49(5):405–412. doi: 10.1136/sti.49.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M. D., Smith R. N., Couch N. P. Surface pH and redox potential of skeletal muscle in graded hemorrhage. Surgery. 1969 Mar;65(3):457–461. [PubMed] [Google Scholar]
- Lysko P. G., Cox C. D. Respiration and oxidative phosphorylation in Treponema pallidum. Infect Immun. 1978 Aug;21(2):462–473. doi: 10.1128/iai.21.2.462-473.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko P. G., Cox C. D. Terminal electron transport in Treponema pallidum. Infect Immun. 1977 Jun;16(3):885–890. doi: 10.1128/iai.16.3.885-890.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Smogór W. Study of the effect of pH and Eh values of the Nelson-Diesendruck medium on the survival of virulent Treponema pallidum. Arch Immunol Ther Exp (Warsz) 1966;14(4):445–453. [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Ribosomal ribonucleic acid synthesis by virulent Treponema pallidum. Infect Immun. 1978 Mar;19(3):854–860. doi: 10.1128/iai.19.3.854-860.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Miller J. N., Sykes J. A., Fitzgerald T. J. Influence of oxygen tension, sulfhydryl compounds, and serum on the motility and virulence of Treponema pallidum (Nichols strain) in a cell-free system. Infect Immun. 1978 Dec;22(3):689–697. doi: 10.1128/iai.22.3.689-697.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M., Matthews H. M., Roberts M. S. Unsustained multiplication of treponema pallidum (nichols virulent strain) in vitro in the presence of oxygen. Infect Immun. 1978 Feb;19(2):421–429. doi: 10.1128/iai.19.2.421-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M. Radiolabeling of Treponema pallidum (Nichols virulent strain) in vitro with precursors for protein and RNA biosynthesis. Infect Immun. 1978 Oct;22(1):22–28. doi: 10.1128/iai.22.1.22-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Cox C. D. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect Immun. 1977 Apr;16(1):60–68. doi: 10.1128/iai.16.1.60-68.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. M. Redox balance in the body: an approach to quantitation. J Surg Res. 1972 Sep;13(3):138–152. doi: 10.1016/0022-4804(72)90057-1. [DOI] [PubMed] [Google Scholar]
- Stevens K. M. Cultivation requirements for Treponema pallidum, Mycobacterium leprae and other microbial and mammalian microaerophilic cells. Med Hypotheses. 1979 Oct;5(10):1091–1103. doi: 10.1016/0306-9877(79)90024-0. [DOI] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


