Abstract
We report an approach to the core of the manzamine alkaloid keramaphidin B that relies on the strain-promoted cycloaddition of an azacyclic allene with a pyrone trapping partner. The cycloaddition is tolerant of nitrile and primary amide functional groups and can be complemented with a subsequent retro-Diels–Alder step. These efforts demonstrate that strained cyclic allenes can be used to build significant structural complexity and should encourage further studies of these fleeting intermediates.
Graphical Abstract
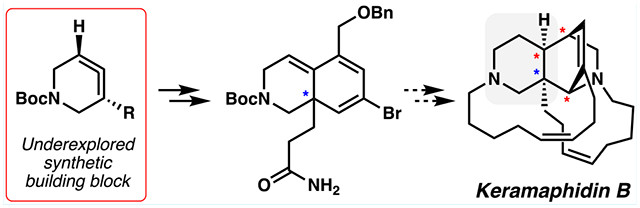
Manzamine alkaloids serve as attractive synthetic targets due to their impressive structural complexity. Some members of this class of natural products display important biological activities, including but not limited to anticancer, antibacterial, and anti-inflammatory properties.1–3 The present study pertains to the manzamine alkaloid keramaphidin B (1, Figure 1), which was first isolated in 1994 as a racemate and features a pentacyclic framework with an azadecalin core.4,5 The eastern portion of the azadecalin core bears a [2.2.2]-bridged bicycle containing four stereogenic centers, one of which is quaternary (C8a). Additionally, the natural product possesses 11- and 13-membered macrocyclic rings. Two prior syntheses of keramaphidin B (1) have been achieved: Baldwin’s biomimetic synthesis of (±)-1 in 19986–8 and Fürstner’s synthesis of (+)-1 via an intermolecular Michael/Michael addition cascade to generate the central core in 2021.9
Figure 1.

(−)-Keramaphidin B (1), a complex manzamine alkaloid, and azacyclic allene 2, an underutilized synthetic building block.
We viewed keramaphidin B (1) as a suitable target to probe the scope and limitations of the strained cyclic allene methodology in a complex setting. Specifically, we questioned whether azacyclic allenes (2) could be used to assemble the azadecalin core and generate the C8a quaternary stereocenter of keramaphidin B (1, Figure 1). The use of strained cyclic allenes in the context of total synthesis has remained underexplored, especially in comparison to related aryne chemistry, which has been used in >100 total syntheses to date.10–12 Encouraged by our group’s recent success in using an azacyclic allene (2) in the total synthesis of (−)-lissodendoric acid A,13 we sought to further expand cyclic allene chemistry by utilizing these fleeting intermediates to build the highly complex bridged azadecalin core of keramaphidin B (1).
Strained cyclic allenes provide new tools for the rapid generation of highly complex sp3-rich scaffolds under mild reaction conditions. Although they were experimentally discovered over 50 years ago,14–17 they have recently emerged as valuable synthetic building blocks.13,18–41 Most relevant to the studies described herein, it has been demonstrated that azacyclic allenes (2) serve as competent dienophiles in strain-promoted Diels–Alder cycloadditions due to their appreciable strain energy of ~27 kcal/mol.37 Generally, studies have focused on the use of electron-rich dienes, but in the context of our total synthesis studies,13 we found that regioselectivity of the cycloaddition with respect to the olefins of the cyclic allene can be modulated by using relatively electron-deficient pyrones. This approach has also proven useful in assembling the azadecalin core of keramaphidin B (1) in the presence of potentially reactive functional groups, as we describe in this report.
Figure 2 shows our retrosynthetic analysis of keramaphidin B (1). The natural product 1 was expected to arise from [2.2.2]-azabicycle 3 through late-stage functional group manipulations and the installation of the 13-membered macrocycle. Next, we envisioned the assembly of [2.2.2]-azabicycle 3 via a series of cycloaddition processes. [2.2.2]-Azabicycle 3 would be accessed from intermediate 4, which would be generated in situ from precursor 5, via an intramolecular iminium or N-acyl iminium Diels–Alder (ImDA) cycloaddition.42–44 In turn, intermediate 5 would arise from cycloadduct 6 via a retro-Diels–Alder reaction that proceeds with the loss of carbon dioxide. Lastly, cycloadduct 6 would be accessed from strained cyclic allene 7 and pyrone 8 via the key strain-promoted Diels–Alder cycloaddition. This step would simultaneously introduce a quaternary center and construct the azadecalin core of the natural product. As will be discussed herein, several variations of this overall strategy were considered,45 including the tether length (n) for the ImDA step, the oxidation state for the ImDA precursor (i.e., iminium vs N-acyl iminium),46 and whether cycloadduct 6 was isolated in the Diels–Alder/retro-Diels–Alder sequence.
Figure 2.

Retrosynthetic analysis of (−)-keramaphidin B (1).
We first attempted to prepare a cycloadduct of type 6 with a pendant primary amine (see Figure 2; n = 7), as this could plausibly allow for direct assembly of the core of keramaphidin B (1) and install all the carbons necessary for the 11-membered macrocycle (Figure 3). The model system47 shown is proposed to provide valuable insight into the fundamental reactivity of the strained cyclic allene for constructing the bicyclic core of the natural product. The necessary cyclic allene precursor 11 was derived from silyl ketone 948 using a scalable three-step cross-metathesis, hydrogenation, and triflation sequence. This route, which mimics a general strategy developed by our laboratory for prior studies, gave access to 11 in 26% yield over three steps. To evaluate the key step, 11 was subjected to pyrone 12 in the presence of CsF at 23 °C. Notably, pyrone 12 was chosen as the trapping partner for this cycloaddition, as it has been demonstrated to be a competent diene for the strain-promoted Diels–Alder cycloaddition with azacyclic allenes.13 We were delighted to obtain cycloadduct 14 in 56% yield. Of note, this key step forms two new C–C bonds and sets three new stereocenters, including a quaternary carbon at C8a, which highlights the utility of cyclic allene methodology to generate structural complexity in a single synthetic step.
Figure 3.

Model system for assembly of the azadecalin scaffold (14).
The strain-promoted Diels–Alder cycloaddition of the cyclic allene proceeds with high regio- and diastereoselectivity, presumably via an approach shown in transition structure 13 (Figure 3). With regard to regioselectivity,49 cycloaddition could occur at either olefin of the cyclic allene; however, the more substituted, relatively electron-rich olefin preferentially undergoes the reaction with the relatively electron-deficient pyrone, presumably via an inverse electron demand Diels–Alder cycloaddition.13 Considering the diastereoselectivity, the pyrone is thought to approach the allene in an endo fashion, with favorable orbital overlap between the diene and the nonreactive olefin of the cyclic allene.34 In this case, these stereoelectronic factors led to the formation of 14 with excellent regio- and diastereoselectivity.
With cycloadduct 14 in hand, we aimed to construct the desired [2.2.2]-azabicycle via an ImDA reaction (Figure 4). We first sought to reduce the C4a–C5 alkene in the piperidinyl ring to install the requisite stereochemistry at C4a.50 This was achieved by subjecting cycloadduct 14 to allylic oxidation conditions, followed by a diastereoselective 1,4-reduction using modified Stryker’s reagent.13 Product 15 was obtained in 48% yield over two steps with >20:1 dr. Next, heating 15 to 80 °C in acetonitrile facilitated a retro-Diels–Alder reaction. Treatment of the product with TFA led to the removal of the Boc protecting groups, thus affording the desired substrate 16. Considerable effort was put forth to enable the desired intramolecular ImDA51 and generate the [2.2.2]-azabicyclic core. Although the formation of the iminium intermediate was validated,52 exhaustive attempts to access the desired product 17 were not successful. We hypothesize that there is a significant entropic penalty to achieve the necessary reactive conformation for the ImDA reaction, resulting from the lengthy tether.53 Consequently, we opted to pursue a modified substrate bearing a shorter and more geometrically constrained tether.
Figure 4.

Synthesis of ImDA precursor 16 and evaluation of the intramolecular ImDA reaction.
Our revised approach to synthesize the [2.2.2]-azabicycle is shown in Figure 5. We envisioned accessing cyclic allenes 18, which bear a shorter tether with either pendant nitrile or primary amide functional groups for the introduction of N-substituents. Trapping with pyrone 8 and a subsequent retro-Diels–Alder reaction furnished cycloadducts 19. If the primary amide and nitrile functional groups are tolerated in this transformation, this step would expand upon known azacyclic allene cycloaddition chemistry. Subsequently, the s-cis diene present in 19 could engage in the intramolecular N-acyl ImDA or ImDA cycloaddition,46 depending on the oxidation state at C16, to furnish 20. Cleavage of the C16–N bond54 could enable introduction of the 11-membered macrocycle.
Figure 5.

Revised strategy to construct the [2.2.2]-azabicycle.
The requisite cyclic allene precursors were synthesized, as shown in Figure 6. Precursor 21, accessible in one step from ketone 9 (see SI for details), was elaborated to nitrile-containing cyclic allene precursor 22a through a hydroboration/oxidation, oxidation, and nitrile formation sequence.55 To access the other desired cyclic allene precursor, nitrile 22a was treated with the Ghaffar–Parkins catalyst to furnish primary amide 22b in 84% yield.56 It is notable that the silyl group and triflate motif withstand the various reaction conditions shown in Figure 6, thus demonstrating the utility of these cyclic allene precursors in multistep synthesis.
Figure 6.

Synthesis of cyclic allene precursors 22a and 22b.
With cyclic allene precursors 22a and 22b in hand, we evaluated each compound in a strain-promoted Diels–Alder cycloaddition/retro-Diels–Alder cascade (Figure 7). Each substrate was independently subjected to pyrone 12 and CsF in acetonitrile. At ambient temperatures, as described previously, cyclic allene generation and Diels–Alder trapping occurred, leading to the formation of two new C–C bonds and installation of the C8a quaternary carbon. By directly heating the reactions to 80 °C, the subsequent CO2 extrusion was achieved in one pot to generate trienes 23a and 23b in 51 and 27% yield,57 respectively. The presumed mechanism is depicted for the reaction of amide 22b (22b → 25 → 26 →23b), with regio- and diastereoselectivity in the key cyclic allene trapping step, paralleling observations described earlier (see Figure 3). Despite the low yield of 23b, it is notable that the primary amide functional group is tolerated in this complexity-generating step given the known electrophilicity of strained cyclic allenes.58 Although extensive efforts to access 24 using ImDA and N-acyl ImDA approaches were ultimately curtailed due to the exploration of an alternative approach, our current results underscore the synthetic utility of strained cyclic allene intermediates for the generation of complex scaffolds in the context of a total synthesis study.
Figure 7.

One-pot cycloaddition and CO2 extrusion to generate trienes 23a and 23b.
Strained cyclic allenes have seen sparse use in total synthesis, especially in comparison to their close relatives, arynes, and cyclic alkynes. In this study, we have validated that these relatively understudied fleeting intermediates can be used to generate significant structural complexity in the context of a synthetic approach to the manzamine alkaloid keramaphidin B (1). Our key step involves the regio- and diastereoselective trapping of a strained azacyclic allene with a pyrone, leading to the formation of two new C–C bonds and three stereocenters, including a quaternary carbon center. These efforts should inform future synthetic design plans, including our ongoing efforts to assemble the bridged azadecalin core of keramaphidin B (1). Furthermore, we expect that these studies will help fuel the exploration of strained azacyclic allenes for the assembly of complex structural architectures.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to the University of California, Los Angeles, NIH-NIGMS (R35-GM139593, T32-GM136614 for J.A.M.G. and F32-GM136171 for J.L.B.), the Trueblood Family (N.K.G.), and the National Science Foundation GRFP (DGE-1650604 for M.M.M.). These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH NCRR (S10RR025631).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c01489.
Experimental details, compound characterization data, and NMR spectra (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.3c01489
The authors declare no competing financial interest.
Contributor Information
Milauni M. Mehta, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, United States;
Jordan A. M. Gonzalez, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, United States;
James L. Bachman, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, United States
Neil K. Garg, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, United States;
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- (1).Magnier E; Langlois Y Manzamine Alkaloids, Syntheses and Synthetic Approaches. Tetrahedron 1998, 54, 6201–6258. [Google Scholar]
- (2).Radwan M; Hanora A; Khalifa S; Abou-El-Ela SH Manzamines. Cell Cycle 2012, 11, 1765–1772. [DOI] [PubMed] [Google Scholar]
- (3).Ashok P; Ganguly S; Murugesan S Manzamine Alkaloids: Isolation, Cytotoxicity, Antimalarial Activity and SAR Studies. Drug Discovery Today 2014, 19, 1781–1791. [DOI] [PubMed] [Google Scholar]
- (4).Kobayashi J. i.; Tsuda M; Kawasaki N; Matsumoto K; Adachi T Keramaphidin B, A Novel Pentacyclic Alkaloid from a Marine Sponge Amphimedon sp.: A Plausible Biogenetic Precursor of Manzamine Alkaloids. Tetrahedron Lett. 1994, 35, 4383–4386. [Google Scholar]
- (5).Tsuda M; Inaba K; Kawasaki N; Honma K; Kobayashi J Chiral Resolution of (±)–Keramaphidin B and Isolation of Manzamine L, a New β-Carboline Alkaloid from a Sponge Amphimedon sp. Tetrahedron 1996, 52, 2319–2324. [Google Scholar]
- (6).Baldwin JE; Claridge TDW; Culshaw AJ; Heupel FA; Lee V; Spring DR; Whitehead RC Studies on the Biomimetic Synthesis of the Manzamine Alkaloids. Chem. Eur. J 1999, 5, 3154–3161. [Google Scholar]
- (7).Baldwin JE; Claridge TDW; Culshaw AJ; Heupel FA; Lee V; Spring DR; Whitehead RC; Boughtflower RJ; Mutton IM; Upton RJ Investigations into the Manzamine Alkaloid Biosynthetic Hypothesis. Angew. Chem., Int. Ed 1998, 37, 2661–2663. [DOI] [PubMed] [Google Scholar]
- (8).Baldwin JE; Whitehead RC On the Biosynthesis of Manzamines. Tetrahedron Lett. 1992, 33, 2059–2062. [Google Scholar]
- (9).Meng Z; Spohr SM; Tobegen S; Farès C; Fürstner A A Unified Approach to Polycyclic Alkaloids of the Ingenamine Estate: Total Syntheses of Keramaphidin B, Ingenamine, and Nominal Njaoamine I. J. Am. Chem. Soc 2021, 143, 14402–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Tadross PM; Stoltz BM A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev 2012, 112, 3550–3577. [DOI] [PubMed] [Google Scholar]
- (11).Gampe CM; Carreira EM Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem., Int. Ed 2012, 51, 3766–3778. [DOI] [PubMed] [Google Scholar]
- (12).Takikawa H; Nishii A; Sakai T; Suzuki K Aryne-Based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds. Chem. Soc. Rev 2018, 47, 8030–8056. [DOI] [PubMed] [Google Scholar]
- (13).Ippoliti FM; Adamson NJ; Wonilowicz LG; Nasrallah DJ; Darzi ER; Donaldson JS; Garg NK Total Synthesis of Lissodendoric Acid A via Stereospecific Trapping of a Strained Cyclic Allene. Science 2023, 379, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Moser WR Reactions of gem-Dihalocyclopropanes with Organometallic Reagents. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, 1964. [Google Scholar]
- (15).Wittig G; Fritze P On the Intermediate Occurrence of 1,2-Cyclohexadiene. Angew. Chem., Int. Ed 1966, 5, 846. [Google Scholar]
- (16).Uyegaki M; Ito S; Sugihara Y; Murata I 1-Benzoxepin and Its Valence Isomers, 4,5-benz-3-oxatricyclo[4.1.0.02,7]heptane and 3,4-benz-2-oxabicyclo-[3.2.1]-hepta-3,6-diene. Tetrahedron Lett. 1976, 17, 4473–4476. [Google Scholar]
- (17).Christl M; Braun M; Wolz E; Wagner W Cycloallene, 9. 1-Phenyl-1-aza-3,4- Cyclohexadien, Das Erste Isodihydropyridin: Ertzeugung und Abfangreaktionen. Chem. Ber 1994, 127, 1137–1142. [Google Scholar]
- (18).Quintana I; Peña D; Pérez D; Guitián E Generation and Reactivity of 1,2-Cyclohexadiene Under Mild Reaction Conditions. Eur. J. Org. Chem 2009, 2009, 5519–5524. [Google Scholar]
- (19).Peña D; Iglesias B; Quintana I; Pérez D; Guitián E; Castedo L Synthesis and Reactivity of New Strained Cyclic Allene and Alkyne Precursors. Pure Appl. Chem 2006, 78, 451–455. [Google Scholar]
- (20).Jankovic CL; West FG 2 + 2 Trapping of Acyloxy-1,3-cyclohexadienes with Styrenes and Electron-Deficient Olefins. Org. Lett 2022, 24, 9497–9501. [DOI] [PubMed] [Google Scholar]
- (21).Lofstrand VA; West FG Efficient Trapping of 1,2-Cyclohexadienes with 1,3-Dipoles. Chem. Eur. J 2016, 22, 10763–10767. [DOI] [PubMed] [Google Scholar]
- (22).Lofstrand VA; McIntosh KC; Almehmadi YA; West FG Strain-activated Diels–Alder Trapping of 1,2-Cyclohexadienes: Intramolecular Capture by Pendent Furans. Org. Lett 2019, 21, 6231–6234. [DOI] [PubMed] [Google Scholar]
- (23).Almehmadi YA; West FG A Mild Method for the Generation and Interception of 1,2-Cycloheptadienes with 1,3-dipoles. Org. Lett 2020, 22, 6091–6095. [DOI] [PubMed] [Google Scholar]
- (24).Wang B; Constantin MG; Singh S; Zhou Y; Davis RL; West FG Generation and Trapping of Electron-Deficient 1,2-Cyclohexadienes. Unexpected Hetero-Diels–Alder Reactivity. Org. Biomol. Chem 2021, 19, 399–405. [DOI] [PubMed] [Google Scholar]
- (25).Hioki Y; Mori A; Okano K Steric Effects on Deprotonative Generation of Cyclohexynes and 1,2-Cyclohexadienes from Cyclohexenyl Triflates by Magnesium Amides. Tetrahedron 2020, 76, 131103. [Google Scholar]
- (26).Inoue K; Nakura R; Okano K; Mori A One-pot Synthesis of Silylated Enol Triflates from Silyl Enol Ethers for Cyclohexynes and 1,2-Cyclohexadienes. Eur. J. Org. Chem 2018, 2018, 3343–3347. [Google Scholar]
- (27).Westphal MV; Hudson L; Mason JW; Pradeilles JA; Zecri FJ; Briner K; Schreiber SL Water-Compatible Cycloadditions of Oligonucleotide-Conjugated Strained Allenes for DNA-Encoded Library Synthesis. J. Am. Chem. Soc 2020, 142, 7776–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Anthony SM; Wonilowicz LG; McVeigh MS; Garg NK Leveraging Fleeting Strained Intermediates to Access Complex Scaffolds. JACS Au 2021, 1, 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kelleghan AV; Witkowski DC; McVeigh MS; Garg NK Palladium-Catalyzed Annulations of Strained Cyclic Allenes. J. Am. Chem. Soc 2021, 143, 9338–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yamano MM; Kelleghan AV; Shao Q; Giroud M; Simmons BJ; Li B; Chen S; Houk KN; Garg NK Intercepting Fleeting Cyclic Allenes with Asymmetric Nickel Catalysis. Nature 2020, 586, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McVeigh MS; Kelleghan AV; Yamano MM; Knapp RR; Garg NK Silyl Tosylate Precursors to Cyclohexyne, 1,2-Cyclohexadiene, and 1,2-Cycloheptadiene. Org. Lett 2020, 22, 4500–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yamano MM; Knapp RR; Ngamnithiporn A; Ramirez M; Houk KN; Stoltz BM; Garg NK Cycloadditions of Oxacyclic Allenes and a Catalytic Asymmetric Entryway to Enantioenriched Cyclic Allenes. Angew. Chem., Int. Ed 2019, 58, 5653–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Barber JS; Styduhar ED; Pham HV; McMahon TC; Houk KN; Garg NK Nitrone Cycloadditions of 1,2-Cyclohexadiene. J. Am. Chem. Soc 2016, 138, 2512–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ramirez M; Svatunek D; Liu F; Garg NK; Houk KN Origins of Endo Selectivity in Diels–Alder Reactions of Cyclic Allene Dienophiles. Angew. Chem., Int. Ed 2021, 60, 14989–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).McVeigh MS; Garg NK Interception of 1,2-Cyclohexadiene with TEMPO Radical. Tetrahedron Lett. 2021, 87, 153539–153543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Spence KA; Tena Meza A; Garg NK Merging Metals and Strained Intermediates. Chem. Catalysis 2022, 2, 1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Barber JS; Yamano MM; Ramirez M; Darzi ER; Knapp RR; Liu F; Houk KN; Garg NK Diels–Alder Cycloadditions of Strained Azacyclic Allenes. Nat. Chem 2018, 10, 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Nendel M; Tolbert LM; Herring LE; Islam MN; Houk KN Strained Allenes as Dienophiles in the Diels–Alder Reaction: An Experimental and Computational Study. J. Org. Chem 1999, 64, 976–983. [DOI] [PubMed] [Google Scholar]
- (39).For a comprehensive review of strained cyclic allene chemistry through 2003, see: Christl M Cyclic Allenes Up to Seven-Membered Rings. Modern Allene Chemistry 2004, 243–357. [Google Scholar]
- (40).Xu Q; Hoye TR A Distinct Mode of Strain-Driven Cyclic Allene Reactivity: Group Migration to the Central Allene Carbon Atom. J. Am. Chem. Soc 2023, 145, 9867–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Witkowski DC; McVeigh MS; Scherer GM; Anthony SM; Garg NK Catalyst-Controlled Annulations of Strained Cyclic Allenes with π-Allylpalladium Complexes. J. Am. Chem. Soc 2023, 145, 10491–10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wu P; Nielsen TE Scaffold Diversity from N-Acyliminium Ions. Chem. Rev 2017, 117, 7811–7856. [DOI] [PubMed] [Google Scholar]
- (43).Memeo MG; Quadrelli P Iminium Ions as Dienophiles in Aza-Diels–Alder Reactions: A Closer Look. Chem. Eur. J 2012, 18, 12554–12582. [DOI] [PubMed] [Google Scholar]
- (44).Erkkilä A; Majander I; Pihko PM Iminium Catalysis. Chem. Rev 2007, 107, 5416–5470. [DOI] [PubMed] [Google Scholar]
- (45).Although our exploratory studies were performed without attempts to control absolute stereochemistry, our prior studies have demonstrated the feasibility of asymmetric synthesis of an allene precursor to provide enantioenriched cycloadducts; see reference 13.
- (46).We opted to pursue both variants of the ImDA reaction simultaneously to maximize the likelihood of success and provide options for late-stage manipulations. In general, N-acyl ImDA reactions tend to utilize harsher reaction conditions. For pertinent reviews, see references 42–44.
- (47).This approach would omit the macrocyclic alkene but was pursued as a model system due to relative ease of substrate synthesis.
- (48).Silyl ketone 9 can be accessed in two synthetic steps from a known compound (reference 13). See the SI for further details.
- (49).The regioisomeric ratios reported in this study are reflective of the selectivity with respect to the two olefins of the cyclic allene. A single regioisomer is consistently observed where bonds are formed between C8a of the allene with C1 of the pyrone and C4a of the allene with C4 of the pyrone (see Figure 2 for atom numbering).
- (50).We opted to introduce the required stereochemistry at C4a early in the synthesis for the model system route to investigate the intramolecular ImDA with a diene trapping partner (16). However, if this olefin is not reduced, retro-Diels–Alder with loss of CO2 leads to a triene motif, which we also explored as a trapping partner for the iminium and N-acyl-iminium Diels–Alder reaction (see Figure 7).
- (51).Grieco PA; Parker DT Quinolizidine Synthesis via Intramolecular Immonium Ion Based Diels–Alder Reactions. Total Synthesis of (+)-Lupine, (+)-Epilupine, (+)-Cryptopleurine, and (+)-Julandine. J. Org. Chem 1988, 53, 3325–3330. [Google Scholar]
- (52).Formation of the iminium intermediate was validated via reductive amination control experiments following Fukuyama’s approach; see the following reference for conditions employed: Ueda H; Satoh H; Matsumoto K; Sugimoto K; Fukuyama T; Tokuyama H Total Synthesis of (+)-Haplophytine. Angew. Chem., Int. Ed 2009, 48, 7600–7603. [DOI] [PubMed] [Google Scholar]
- (53).Such challenges are inherent to macrocyclization processes. For a pertinent review, see: Marti-Centelles V; Pandey MD; Burguete MI; Luis SV Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem. Rev 2015, 115, 8736–8834. [DOI] [PubMed] [Google Scholar]
- (54).One attractive approach involves cleavage of a twisted amide; for a pertinent review, see: Liu C; Szostak M Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J 2017, 23, 7157–7173. [DOI] [PubMed] [Google Scholar]
- (55).Haydl AM; Xu K; Breit B Regio- and Enantioselective Synthesis of N-Substituted Pyrazoles by Rhodium-Catalyzed Asymmetric Addition to Allenes. Angew. Chem., Int. Ed 2015, 54, 7149–7153. [DOI] [PubMed] [Google Scholar]
- (56).Cadierno V Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review. Appl. Sci 2015, 5, 380–401. [Google Scholar]
- (57).The poor mass balance for the cycloaddition with cyclic allene precursor 22b is attributed to the general decomposition observed in the 1H NMR spectrum of the crude reaction mixture.
- (58).Dillon PW; Underwood GR Cyclic Allenes. I. The Electronic Structure and Probable Deformation of the Allene Linkage When Included in a Ring. An INDO-MO Study. J. Am. Chem. Soc 1974, 96, 779–787. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


