Abstract
Bacteria inhibitory to Escherichia coli O157:H7 were isolated from cattle and evaluated for their potential for reducing carriage of E. coli O157:H7 in calves. Eighteen of 1,200 bacterial isolates from cattle feces and intestinal tissue samples were screened and determined to inhibit the growth of E. coli O157:H7 in vitro. Seventeen of the isolates were E. coli and one was Proteus mirabilis. None produced Shiga toxin. Genomic DNA fingerprinting by pulsed-field gel electrophoresis revealed 13 distinguishable profiles among the 18 isolates. Two calves inoculated perorally with a mixture of all 18 isolates (1010 CFU) appeared to be normal and did not develop signs of clinical disease throughout a 25- to 27-day observation period. These bacteria colonized segments of the gastrointestinal tract and were in feces at the termination of the experiment (25 and 27 days postinoculation) at levels of 50 to 200 CFU/g. Fifteen cannulated calves were studied to determine the efficiency of the probiotic bacteria in reducing or eliminating the carriage of E. coli O157:H7. Nine calves served as controls, with each animal receiving perorally 1010 CFU of E. coli O157:H7. E. coli O157:H7 was detected intermittently in the rumen samples from all control animals throughout 3 weeks postinoculation, whereas E. coli O157:H7 was shed at various levels in feces continuously throughout the experiment (mean, 28 days). E. coli O157:H7 was isolated from the rumens and colons of eight of nine and nine of nine calves, respectively, at the termination of the study. Six calves each received perorally 1010 CFU of probiotic bacteria and then 2 days later received 1010 CFU of E. coli O157:H7. E. coli O157:H7 was detected in the rumen for only 9 days postinoculation in two animals, for 16 days in one animal, for 17 days in two animals, and for 29 days in one animal. E. coli O157:H7 was detected in feces for only 11 days postinoculation in one animal, for 15 days in one animal, for 17 days in one animal, for 18 days in one animal, for 19 days in one animal, and for 29 days in one animal. At the end of the experiment (mean, 30 days), E. coli O157:H7 was not recovered from the rumen of any of the six animals treated with probiotic bacteria; however, E. coli O157:H7 was recovered from the feces of one of the animals. This animal was fasted twice postinoculation. These studies indicate that selected probiotic bacteria administered to cattle prior to exposure to E. coli O157:H7 can reduce the level of carriage of E. coli O157:H7 in most animals.
During the past decade, Escherichia coli O157:H7, an important human pathogen causing hemorrhagic colitis and hemolytic-uremic syndrome, has been reported as a cause of human illness with increased frequency (1, 10, 16). Cattle, especially young animals, have been implicated as a principal reservoir of E. coli O157:H7 (4, 7, 19, 21, 22, 24, 26), with undercooked ground beef being a major vehicle of food-borne outbreaks.
A recent national survey performed by the National Animal Health Monitoring System of the U.S. Department of Agriculture revealed that 1.6% of feedlot cattle shed E. coli O157:H7 in their feces and that 0.4% shed E. coli O157:nonmotile (O157:NM) in their feces (6). A major study of calves on dairy farms revealed that 1.5 to 2.9% of animals between 24 h of age and weaning and 4.9 to 5.3% of animals between the age of weaning and 4 months shed E. coli O157:H7 in their feces (26). Experimental infection of calves and adult cattle with E. coli O157:H7 infection revealed that shedding of E. coli O157:H7 varies widely among animals of the same age group (14 to >20 weeks) but persists longer in calves than in adults, and previous infection does not prevent reinfection with the same strain of E. coli O157:H7 (5).
Many concerns have been raised regarding E. coli O157:H7 contamination of foods. Such concerns have been heightened by the tolerance of E. coli O157:H7 to acidic conditions. Proper cooking is an effective method of killing E. coli O157:H7 in foods. Insanitary practices in preparing foods often result in food-borne illness; hence, methods for reducing or eliminating the carriage of E. coli O157:H7 in cattle are needed to reduce the level of exposure to the pathogen in food and the environment (23). The purpose of this study was to isolate potential probiotic bacteria and to evaluate their efficacy at reducing the level of carriage of E. coli O157:H7 by cattle. Probiotic bacteria are those that beneficially affect the host by improving its microbial balance, including eliminating or reducing microorganisms that are carried by the host and that are harmful to humans.
MATERIALS AND METHODS
Source and isolation of potential probiotic bacteria.
Bacteria to be screened for activity bactericidal for or inhibitory to E. coli O157:H7 were isolated from cattle feces or cattle gastrointestinal tissue (intestine and colon). Fecal samples were collected from cattle that were confirmed to be negative for E. coli O157:H7 by fecal testing (26). Fifty-five fecal samples were serially diluted (1:10) in 0.1 M phosphate buffer (phosphate-buffered saline [PBS]; pH 7.2), 0.1 ml of each dilution was plated onto sorbitol MacConkey agar (SMA), and the plates were incubated for 16 h at 37°C. Up to 10 colonies were randomly selected, and each one was transferred to a test tube containing 10 ml of Trypticase soy broth (TSB; BBL, Becton Dickinson Co., Cockeysville, Md.). Cultures were incubated for 16 h at 37°C. Sixty-eight tissue samples (1 g each in 9 ml of PBS) were homogenized individually (Ultra-Turrax T25 homogenizer; Janke & Kunkel IKA-Labortechnik, Staufen, Germany) at 9,500 rpm for 1 min, and 0.1-ml portions were plated onto the surfaces of SMA plates. The plates were incubated for 16 h at 37°C. Up to 10 colonies were each transferred to test tubes containing 10 ml of TSB, and the tubes were incubated for 16 h at 37°C.
Screening of cultures for anti-E. coli O157:H7 properties.
A five-strain mixture of E. coli O157:H7 from our culture collection, including strains 932 (human isolate), C7927 (human isolate), E009 (meat isolate), E0018 (cattle isolate), and E0122 (cattle isolate), was used to screen culture supernatants for anti-E. coli O157:H7 activity. Approximately 107 cells of E. coli O157:H7 of approximately equal populations of each strain in 0.1 ml were plated onto the surfaces of duplicate SMA and Trypticase soy agar (TSA) plates. Cultures were sedimented by centrifugation (4,000 × g for 20 min), and the supernatant from each culture was filter sterilized (0.2-μm-pore-size cellulose acetate membrane; Nalgene Co., Rochester, N.Y.) for determination of anti-E. coli O157:H7 properties. A disc (diameter, 12 mm; Dispens-O-Disc; Difco Laboratories, Detroit, Mich.) was placed on the surface of each SMA and TSA plate, and 0.1 ml of filter-sterilized supernatant from a single culture was applied to the surface of the disc. In addition, a disc with Vibax LA-5 (Triclosan) (1:2,000 in PBS; Benchmark Co., Salt Lake City, Utah) and another disc with 70% ethanol, which were used as positive controls, and a disc with PBS, which was used as a negative control, were applied to each plate. The cultures were incubated for 18 h at 37°C and were observed for zones of growth inhibition. Probiotic bacteria were selected as those which produced a clear zone of 1 mm or greater surrounding each disc.
Preparation of E. coli O157:H7 cultures for inoculation into calves.
The same five-strain mixture of E. coli O157:H7 described above was used to inoculate calves. To facilitate enumeration of these bacterial isolates, the strains were selected for resistance to nalidixic acid (50 μg/ml) by exposure to serially increased (1:2; i.e., 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8, 25, and 50 μg/ml) concentrations of nalidixic acid in MacConkey agar every 24 h. Each strain of nalidixic acid-resistant E. coli O157:H7 was transferred into 10 ml of TSB containing nalidixic acid (50 μg/ml) and was incubated for 16 to 18 h at 37°C with agitation (150 rpm). A 2-ml suspension of each isolate was transferred to 300 ml of TSB. After incubating at 37°C for 16 to 18 h with agitation (150 rpm), the bacteria were sedimented by centrifugation (4,000 × g for 20 min) and were washed three times in PBS. PBS was added to sedimented bacteria in an amount needed to obtain an optical density (OD) at 630 nm of 0.5 (ca. 108 CFU/ml). The five isolates (2 × 109 CFU of each strain) of E. coli O157:H7 were mixed in 250 ml of 2% sterilized skim milk just prior to oral inoculation of the calves. Enumeration of the bacteria was confirmed by plate counts on TSA and SMA plates containing nalidixic acid at 50 μg/ml (SMA-NA plates).
Preparation of probiotic bacteria for inoculation into calves.
All 18 probiotic bacterial isolates were selected for resistance to nalidixic acid (50 μg/ml) by the procedure described above for ease of enumeration of the organisms in feces. The bacteria were grown individually in 10 ml of TSB containing nalidixic acid (50 μg/ml). A 1-ml portion of each isolate was transferred to 100 ml of TSB. After incubation at 37°C for 16 to 18 h, the bacteria were sedimented by centrifugation (4,000 × g for 20 min), washed, and adjusted to an OD at 630 nm of 0.5 by the method described above. The 18 strains of probiotic bacteria (1010 CFU), with approximately equal populations of each strain, were mixed into 250 ml of 2% sterilized skim milk immediately before oral inoculation of the calves. The bacterial population was confirmed by enumeration of serial dilutions on TSA and SMA-NA plates, in duplicate.
Preparation of calves before receipt of bacterial inoculation.
Fifteen single-source male dairy calves were reared on milk replacer and were weaned at 6 weeks of age prior to transfer to the University of Georgia. The calves were housed individually in climate-controlled biolevel-2 concrete rooms. Each room had an individual floor drain and was cleaned once daily. Calves were fed a mixture of alfalfa pellets and sweet feed twice daily and had free access to water. During a 2-week conditioning period, feces from all calves were sampled by fluorescent-antibody staining for bovine diarrhea virus, coronavirus, rotavirus, E. coli pilus antigens, and cryptosporidia, and all samples tested negative. Fecal flotation for intestinal parasites and bacterial culture for Salmonella and E. coli O157:H7 were also negative prior to inoculation. After a 2-week preconditioning period, calves were surgically fitted with rumen cannulas (flexible rumen cannulas).
Rumen cannulation and surgical aftercare.
Feed was withheld from the calves for 12 h. The left paralumbar fossa was clipped and scrubbed for standard surgical preparation. The fossa was anesthetized with lidocaine by using a paravertebral nerve block. A circular incision slightly smaller than the diameter of the cannula was made, and the circular piece of skin and underlying cuticular and external abdominal oblique muscles were removed. Vessels were ligated as necessary, and internal abdominal oblique muscles, transverse muscles, and the peritoneum were bluntly separated and retracted to create an opening to expose the rumen wall. The rumen wall was grasped with two large towel clamps for traction to exteriorize the rumen. The rumen wall was then sutured to the skin with no. 3 catgut, incorporating the muscle layers with a continuous suture pattern. The rumen wall was incised, and a circular piece of rumen was removed and the cannula was inserted.
The calves were treated for 5 days after surgery with procaine penicillin G (3,000 U/lb) intramuscularly. The area between the cannula and the rumen wall was gently cleansed daily with 10% povidone-iodine (Betadine) solution. At least 10 days were allowed for surgical recovery and aftercare prior to experimental inoculation.
Inoculation of calves with probiotic and challenge bacteria.
Following a 12-h fast, six calves were inoculated via a rumen cannula with 250 ml of skim milk containing probiotic bacteria. After 48 h, the five-strain mixture of E. coli O157:H7 was inoculated via the same route. Nine control calves were challenged with the five-strain mixture of E. coli O157:H7 only. Following challenge, the calves were examined daily for clinical signs including depression, pyrexia, diarrhea, and anorexia. Rectal feces or rumen samples collected from the fistula were assayed for pH (rumen) and counts of E. coli O157:H7 and probiotic bacteria (rumen contents and feces). Six of the nine control calves (calves 2, 3, 4, 7, 8, and 9) were twice fasted for 48 h each at days 15 and 16 or days 16 and 17 and at days 22 and 23 or days 23 and 24 to provide conditions that can exhance the fecal excretion of E. coli O157:H7 (3). Similarly, four of six probiotic bacteria-treated calves (calves 1, 3, 4, and 6) were twice fasted at days 15 and 16 or days 16 and 17 and at days 22 and 23 or days 23 and 24.
Recovery of bacteria inoculated into calves.
A sample of 10 g of feces or rumen content was collected by retrieval from the rectum or cannulation of the rumen daily up to the end of the study following inoculation with E. coli O157:H7. Samples were placed in a tube containing 15 ml of Cary-Blair transport medium, kept at 5°C, and transported to the Center for Food Safety and Quality Enhancement for analysis. A volume containing 1 g of feces or rumen fluid was serially (1:10) diluted in 0.85% NaCl to 10−6, and 0.1 ml of each dilution was plated in duplicate onto SMA-NA plates. Tissue samples from sites throughout the entire gastrointestinal tract collected at necropsy (see below) were held at 5°C until analysis. Luminal contents from each segment were separated and weighed, and the tissue was rinsed with 100 ml of PBS. The rinsed tissue was added to 9 ml of PBS and was homogenized for 1 min at 9,500 rpm with an Ultra-Turrax tissue homogenizer. A 0.1-ml sample of tissue or tissue content suspension was inoculated onto SMA-NA plates in quadruplicate, and the plates were incubated at 37°C for 24 h for enumeration of E. coli O157:H7 or probiotic bacteria. If these bacteria were not detected by the direct plating method, a selective enrichment method (17) (modified TSB containing 50 μg of nalidixic acid/ml) was performed. The samples were each placed in 100 ml of selective enrichment medium and were incubated at 37°C for 24 h with agitation at 150 rpm. Dilutions of cultures were plated onto SMA-NA plates, and isolates were selected and further tested. Colonies typical of E. coli O157:H7 (sorbitol negative) were replated onto SMA-NA plates and were confirmed to be E. coli by biochemical methods and as O157 (26) and H7 (8) by serological methods. Colonies typical of probiotic bacteria (sorbitol positive) were randomly selected from plates with the highest dilution having colonies and were confirmed to be the inoculated probiotic bacteria by genomic DNA fingerprinting by a pulsed-field gel electrophoresis (PFGE) procedure (14).
Genomic fingerprinting of bacterial isolates.
PFGE procedures similar to those described previously were used (14). Bacteria were grown in 10 ml of TSB at 37°C for 24 h with agitation at 200 rpm. The bacteria were sedimented by centrifugation (4,000 × g for 20 min), washed three times in 75 mM NaCl containing 25 mM EDTA at pH 7.4 (SE), and resuspended in 0.5 ml of SE. The bacterial suspension was mixed with 0.5 ml of 2% (wt/vol) low-melting-point agarose in buffer consisting of 10 mM Tris, 10 mM MgCl2, and 0.1 mM EDTA (pH 7.5). This mixture was dispensed into sample molds, and the agarose plugs were digested with proteinase K (2 mg of proteinase K, 50 mM Tris, 50 mM EDTA, 1% N-lauroylsarcosine/ml [pH 8.0]) at 56°C overnight. The samples were washed in 10 mM Tris–5 mM EDTA (pH 7.5) and digested with 50 U of XbaI. After incubation at 37°C overnight, the reaction was stopped by the addition of 20 μl of 0.5 M EDTA. The DNA samples were electrophoresed on a 1.2% agarose gel in 0.5× TBE buffer (10× TBE buffer is 0.89 M Tris, 0.025 M EDTA, and 0.89 M boric acid) with a contour-clamped homogeneous electric field device (CHEF MAPPER; Bio-Rad). After electrophoresis for 24 h at 200 V with pulse times of 5 to 50 s and linear ramping and an electrical field angle of 120° at 14°C, the gels were stained with ethidium bromide and the bands were visualized and photographed with UV transillumination.
Necropsy of calves.
The calves were killed with intravenous sodium pentobarbital. The gastrointestinal tract was clamped at the esophagus and rectum and was removed in toto. Lengths (4 to 6 cm) of duodenum, proximal, middle, and distal jejunum, proximal and distal ileum, proximal and distal cecum, proximal loop of the ascending colon, centripetal turn and centrifugal turn of the spiral colon, transverse colon, and descending colon were double tied to allow sampling of all sections for the enumeration of E. coli O157:H7 and probiotic bacteria in both the tissues and their contents with minimal cross-contamination. Sections and contents of the rumen, reticulum, omasum, and abomasum and sections of the kidney, spleen, liver, gall bladder, jejunal lymph node, ileal lymph node, cecal lymph node, and tonsil also were collected for culture and enumeration of E. coli O157:H7 and/or probiotic bacteria. Sections from all of these sites, as well as sections of prescapular lymph node, skeletal muscle, skin, tonsil, thyroid, thymus, esophagus, heart, pancreas, umbilicus, adrenal, urinary bladder, and testes, also were placed in 10% buffered formalin for histologic examination.
Histopathology of tissues.
Fixed tissues were embedded in paraffin by standard methods, sectioned at 5 μm, and stained with hematoxylin and eosin by the protocol described by Brown et al. (3). Selected sections were Gram stained (3).
Statistical analysis.
Significant differences between treatments were determined by using the Statistical Analysis System’s t test procedure (20).
RESULTS
In vitro screening of potential probiotic bacteria that secrete a metabolite(s) inhibitory to E. coli O157:H7.
A total of 1,200 bacterial colonies were isolated from the gastrointestinal tissues and feces of cattle determined not to excrete E. coli O157:H7 in their feces. These bacteria were screened for their ability to inhibit the growth of or kill E. coli O157:H7 in vitro, and 18 were determined to secrete antimicrobial metabolites. Among them, five colonies were isolated from feces, five were isolated from the small intestine, and eight were isolated from the colon. Seventeen of the 18 colonies were identified as E. coli and the other was identified as Proteus mirabilis (Table 1). All colonies were assayed for Shiga toxin production (26), and none produced Shiga toxin. Genomic DNA fingerprinting by PFGE revealed 13 different profiles among the 18 isolates.
TABLE 1.
Selected characteristics of potential probiotic bacteria with antimicrobial activity against E. coli O157:H7 and isolated from cattle
| Isolate no. | Source | DNA fingerprintinga | Dominant strainb |
|---|---|---|---|
| E. coli 34 | Feces | Unique | No |
| E. coli 71 | Feces | Unique | No |
| P. mirabilis 208 | Feces | Unique | No |
| E. coli 271 | Intestine | Unique | Yes |
| E. coli 276 | Intestine | Same as 271 | |
| E. coli 277 | Intestine | Same as 271 | |
| E. coli 278 | Intestine | Same as 271 | |
| E. coli 282 | Intestine | Unique | No |
| E. coli 368 | Colon | Unique | No |
| E. coli 457 | Colon | Same as 271 | |
| E. coli 460 | Colon | Unique | No |
| E. coli 769 | Colon | Unique | No |
| E. coli 770 | Colon | Same as 769 | No |
| E. coli 779 | Colon | Unique | No |
| E. coli 786 | Colon | Unique | Yes |
| E. coli 797 | Colon | Unique | Yes |
| E. coli 1018 | Feces | Unique | No |
| E. coli 1019 | Feces | Unique | Yes |
DNA fingerprinting as determined by PFGE; unique indicates that the PFGE profile is different from those of the other strains in this study.
Dominant strain indicates that the strain was recovered at 26 days postinoculation (termination of study) from the contents of the gastrointestinal tracts of two calves experimentally administered all 18 potential probiotic bacterial isolates.
Colonization of calves by probiotic bacteria.
One calf was initially fed one strain of probiotic bacteria (E. coli 271 at 1010 CFU). The calf appeared to be clinically normal, and this E. coli strain was recovered by the enrichment procedure only from the contents of the ileum and the cecum at the termination of the experiment (12 days). Two calves were then fed all 18 strains (at approximately equal concentrations of each strain; 5 × 108 CFU each) of probiotic bacteria (1 × 1010 CFU per calf) as a mixture. The calves’ feces were of normal consistency, and the bacteria were isolated from the gastrointestinal tract (both tissue and the contents of the rumen and colon) for up to 27 days (at the termination of the study, the counts were 50 to 200 CFU/g of feces).
Twenty-one colonies were isolated by direct plating methods from SMA-NA and TSA-nalidixic acid plates with the highest dilutions of tonsil, omasum, reticulum, rumen, proximal ileum, distal cecum, proximal loop of ascending colon, transverse colon, and feces of two calves at 26 days postinoculation with the probiotic bacteria. These calves were not challenged with E. coli O157:H7. The 21 colonies were analyzed by PFGE and were determined to have only four distinguishable DNA profiles. These four dominant isolates were all E. coli. Among the 21 colonies, 9 were strain 797, 7 were strain 786, 3 were strain 271, and 2 were strain 1019. Strains 786 and 797 were isolated from both calves, whereas strains 271 and 1019 were isolated from only one of the two calves.
Although some strains of the inoculated bacteria were recovered at necropsy from tissue specimens from different parts of the gastrointestinal tract, there were no pathological changes in any of the tissue samples assayed.
Efficiency of probiotic bacteria in reducing carriage of E. coli O157:H7 in calves.
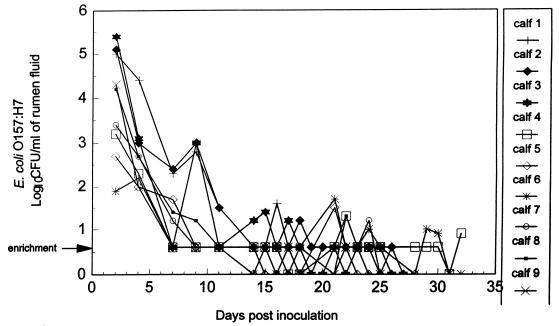
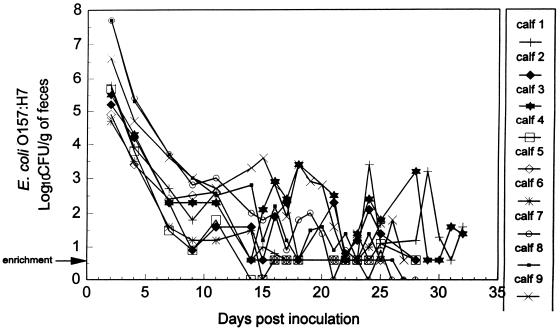
Of the nine control calves administered only E. coli O157:H7, all remained clinically healthy, with no evidence of fever or diarrhea. E. coli O157:H7 was isolated intermittently from the rumen fluid of all animals during the first 3 weeks postinoculation (Fig. 1). Shedding of E. coli O157:H7 in the feces at various levels was generally detected continuously for the duration of the experiment (mean, 28 days) (Fig. 2). At necropsy, E. coli O157:H7 was isolated from all nine calves, as follows: rumen contents of five of nine calves, reticulum contents of three of nine calves, omasum contents of one of nine calves, and the colons of nine of nine calves (Table 2). No pathological changes were observed in any of the tissue samples examined microscopically.
FIG. 1.
Detection of E. coli O157:H7 in rumen fluid of nine control calves administered only E. coli O157:H7. Bacterial enumeration was performed by surface plating on SMA-NA plates, in duplicate. The arrow indicates that detection of E. coli O157:H7 was by an enrichment procedure in which 9 ml of rumen fluid was positive for E. coli O157:H7.
FIG. 2.
Detection of E. coli O157:H7 in feces of nine control calves administered only E. coli O157:H7. Bacterial enumeration was performed by surface plating on SMA-NA plates, in duplicate. The arrow indicates that detection of E. coli O157:H7 was by an enrichment procedure in which 9 g of feces was positive for E. coli O157:H7.
TABLE 2.
Recovery at necropsy of E. coli O157:H7 from the contents of tissue specimens from nine control calves administered only E. coli O157:H7
| Site of tissue sample | No. positive calves | CFU/g of tissue content
|
|
|---|---|---|---|
| Rangea | Meanb | ||
| Rumen | 5 | <0.5 × 101–4.0 × 101 | 0.7 × 101 |
| Reticulum | 3 | <0.5 × 101–4.0 × 101 | 0.6 × 101 |
| Omasum | 1 | <0.5 × 101–0.5 × 101 | <0.5 × 101 |
| Ileum | 2 | <0.5 × 101–0.5 × 101 | <0.5 × 101 |
| Cecum | 2 | <0.5 × 101–0.6 × 101 | <0.5 × 101 |
| Spiral colon | 6 | <0.5 × 101–1.0 × 101 | <0.5 × 101 |
| Descending colon | 3 | <0.5 × 101–1.2 × 101 | <0.5 × 101 |
Range of recovery. Bacterial enumeration was performed by surface plating samples onto SMA-NA plates in quadruplicate. Enrichment culture positive is expressed as <0.5 × 101 CFU/g.
Geometric mean.
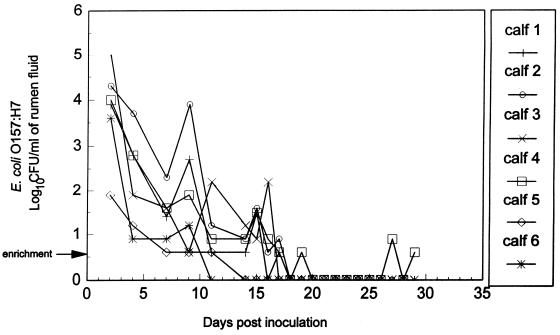
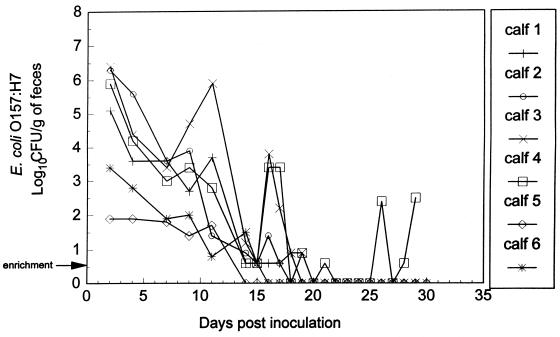
All six calves that were administered probiotic bacteria 2 days before treatment with E. coli O157:H7 remained healthy, with no evidence of fever or diarrhea during the entire experiment. E. coli O157:H7 was detected in rumen samples, collected through a rumen cannula, for up to 9 days after challenge in two animals, 16 days in one animal, 17 days in two animals, and 29 days in one animal (Fig. 3). The number of E. coli O157:H7 in the rumens of calves treated with probiotic bacteria was significantly less (P < 0.05) at 18 days posttreatment and thereafter than in the control group treated only with E. coli O157:H7. E. coli O157:H7 was detected in the feces of probiotic-treated calves for up to 11, 15, 17, 18, 19, and 29 days (at the termination of the experiment) in one animal each (Fig. 4). The number of E. coli O157:H7 in the feces of calves treated with probiotic bacteria was significantly less (P < 0.05) at 15 and 18 days posttreatment and thereafter than in the control group treated only with E. coli O157:H7. At necropsy (mean, 30 days), E. coli O157:H7 was not recovered from rumen samples from any of these six animals (Table 3); however, E. coli O157:H7 was recovered from the colon of one of the six animals (Table 3). Probiotic bacteria were not detected in any segments of the gastrointestinal tract of the E. coli O157:H7-positive animal which was twice fasted for 2-day periods (days 16 and 17 and days 23 and 24) postinoculation during the study.
FIG. 3.
Detection of E. coli O157:H7 in rumen fluid of six calves administered probiotic bacteria and administered E. coli O157:H7 2 days later. Bacterial enumeration was performed by surface plating on SMA-NA plates, in duplicate. The arrow indicates that detection of E. coli O157:H7 was by an enrichment procedure in which 9 ml of rumen fluid was positive for E. coli O157:H7.
FIG. 4.
Detection of E. coli O157:H7 in feces of six calves administered probiotic bacteria and administered E. coli O157:H7 2 days later. Bacterial enumeration was performed by surface plating on SMA-NA plates, in duplicate. The arrow indicates that detection of E. coli O157:H7 was by an enrichment procedure in which 9 g of feces was positive for E. coli O157:H7.
TABLE 3.
Recovery of E. coli O157:H7 at necropsy from the contents of tissue specimens from six calves treated with probiotic bacteria
| Site of tissue sample | No. of positive tissue samples/no. of tissue samples tested | CFU/g of tissue content |
|---|---|---|
| Rumen | 0/6 | |
| Reticulum | 0/6 | |
| Omasum | 0/6 | |
| Ileum | 0/6 | |
| Cecum | 1/6 | 3.9 × 103 |
| Spiral colon | 1/6 | 5.0 × 103 |
| Decending colon | 1/6 | 2.5 × 103 |
Sites of localization of probiotic bacteria.
All calves treated with probiotics were necropsied between 29 and 30 days after the administration of E. coli O157:H7. Of the six calves administered probiotic bacteria 2 days before treatment with E. coli O157:H7, probiotic bacteria were recovered at necropsy from the contents of the rumens, reticulums, omasa, and colons of five of six calves. However, probiotic bacteria were not isolated at necropsy from the one animal from which E. coli O157:H7 was isolated from cecal and colonic contents at populations of 2.5 × 103 to 5.0 × 103 CFU/g (Tables 3 and 4).
TABLE 4.
Recovery at necropsy of probiotic bacteria from tissue specimens from six calves treated with probiotic bacteria
| Site of tissue sample | No. of tissue samples positive/ total no. tested | CFU/g of tissue content
|
|
|---|---|---|---|
| Rangea | Meanb | ||
| Rumen | 5/6 | <0.5 × 101–1.0 × 103 | 1.3 × 102 |
| Reticulum | 5/6 | <0.5 × 101–4.0 × 102 | 6.9 × 101 |
| Omasum | 5/6 | <0.5 × 101–3.2 × 102 | 6.0 × 101 |
| Ileum | 2/6 | 2.5 × 101–6.3 × 103 | 1.1 × 103 |
| Cecum | 5/6 | <0.5 × 101–2.5 × 103 | 8.6 × 102 |
| Spiral colon | 5/6 | <0.5 × 101–5.0 × 103 | 1.4 × 103 |
| Decending colon | 5/6 | <0.5 × 101–3.2 × 103 | 6.0 × 102 |
Range of recovery. Bacterial enumeration was performed by surface plating samples onto SMA-NA plates in quadruplicate. Enrichment culture positive is expressed as <0.5 × 101 CFU/g.
Geometric mean.
DISCUSSION
Ruminants including cattle (3, 5), deer (12), and sheep (13) have been identified as carriers of E. coli O157:H7. The primary sites of E. coli O157:H7 localization in calves are the rumen and colon (3). The rumen appears to be the most important site for long-term carriage of E. coli O157:H7 because it may serve as the source of the bacteria found in the colon (3). Histologic examination of colonic tissue revealed no evidence of attachment of E. coli O157:H7 to colonic tissue. Hence, the presence of E. coli O157:H7 in the colon may be a transient state whereby the bacteria are passing through rather than colonizing the colon. In addition, changes that affect the conditions of the rumen, such as fasting, may influence the presence of E. coli O157:H7. Studies by Rasmussen et al. (18) revealed that E. coli O157:H7 could grow unrestricted in rumen fluid collected from fasted animals. Factors influencing the conditions in the rumen include nutrition, feeding regimens, and animal handling at the farm (9).
The presence of bacteria that produce metabolites inhibitory to E. coli O157:H7 at sites where O157:H7 strains localize is another factor that may influence the localization of O157:H7 in the gastrointestinal tract. Some strains of E. coli can produce colicins that are inhibitory in vitro to diarrheagenic E. coli strains, including strains of serotype O157:H7 (2, 15). Murinda et al. (15) assayed 24 E. coli colicin-producing strains and determined that all E. coli O157:H7 strains evaluated were sensitive to ColE1 to ColE8, K, and N on mitomycin C-containing agar and to ColG, ColH, and Mcc B17 on Luria agar. Colicins could be one of many metabolites produced by the probiotic bacteria in the rumen and other sections of the gastrointestinal tract. The findings from this study indicate that the probiotic bacteria localize at the same sites of calves as E. coli O157:H7 (Tables 2 and 4); hence, the anti-E. coli O157:H7 metabolites that the probiotic bacteria produce would be in the same proximity as the target bacteria.
The administration of probiotic bacteria, used in this study to treat calves prior to exposure to E. coli O157:H7, decreases the duration of ruminal carriage of E. coli O157:H7. Our studies with probiotic bacteria revealed that E. coli O157:H7 was detectable in rumen fluid an average of 14 days (range, 9 to 17 days) postinoculation in five of six animals given probiotic bacteria, whereas O157:H7 was detected in rumen fluid for an average of 26 days (range, 22 to 32 days) in control calves not receiving probiotic bacteria. At necropsy, E. coli O157:H7 was no longer detected in the rumens of six of six calves receiving probiotic bacteria. In contrast, E. coli O157:H7 was detected at necropsy in the contents of the rumen, reticulum, or omasum of seven of nine control animals.
Cray and Moon (5) reported that experimentally treated calves shed E. coli O157:H7 in their feces at least 7 weeks postinoculation. In our study, fecal shedding of E. coli O157:H7 was reduced from 25 to 32 days, when the calves were necropsied (control group), to 14 to 19 days in five of the six calves treated with probiotic bacteria. At necropsy, E. coli O157:H7 was recovered from the feces of only one of the six probiotic-treated animals, whereas it was recovered from all nine of the control group from which E. coli O157:H7 was recovered at 25 to 32 days postinoculation. The persistence of E. coli O157:H7 in one animal in the group treated with probiotic bacteria may be due to the apparent failure of probiotic bacteria to colonize this animal. It is possible that greater protection or clearance can be conferred by multiple treatments with probiotic bacteria.
Although the mechanism(s) by which our selected probiotic bacteria reduced the level of carriage of E. coli O157:H7 remains to be elucidated, these studies indicate that microbial interactions could be an important factor that contributes to the homeostasis of the bacterial flora in the gastrointestinal tract (11, 25). Hence, treatment of cattle with probiotic bacteria can reduce the level of carriage and fecal shedding of E. coli O157:H7 and may thereby reduce environmental contamination with this pathogen. Reducing E. coli O157:H7 carriage in cattle should decrease the likelihood of meat, vegetable, fruit, and water contamination, thereby decreasing the potential for food-, water-, and environment-associated E. coli O157:H7 illness in humans.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the American Meat Institute Foundation.
We thank Ping Zhao, Darnisha L. Grant, Christy R. Neeley, and Mary K. Flowe for invaluable technical assistance and M. R. S. Clavero for statistical analysis.
REFERENCES
- 1.Bell P B, Goldoft M, Griffin P M, Davis M A, Gordon D C, Tarr P I, Bartleson C A, Lewis J H, Barrett T J, Wells J G, Baron R, Kobayashi J. A multistate outbreak of Escherichia coli O157:H7-associated blood diarrhea and hemolytic uremic syndrome from hamburgers. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 2.Bradley D E, Howard S P, Lior H. Colicinogeny of O157:H7 enterohemorrhagic Escherichia coli and the shielding of colicin and phage receptors by their O-antigenic side chains. Can J Microbiol. 1991;37:97–104. doi: 10.1139/m91-014. [DOI] [PubMed] [Google Scholar]
- 3.Brown C A, Harmon B G, Zhao T, Doyle M P. Experimental Escherichia coli O157:H7 carriage in calves. Appl Environ Microbiol. 1997;63:27–32. doi: 10.1128/aem.63.1.27-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman P A, Wright D J, Norman P, Fox J, Crick E. Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol Infect. 1993;111:439–447. doi: 10.1017/s0950268800057162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray C W, Moon H W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:1585–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dargatz, D. (Centers for Epidemiology and Animal Health, Animal and Plant Health Inspection Service, Veterinary Service, U.S. Department of Agriculture, Fort Collins, Colo). 1995. Personal communication. Escherichia coli O157:H7 shedding by feedlot cattle.
- 7.Faith N G, Shere J A, Brosch R, Arnold K W, Ansay S E, Lee M S, Luchansky J B, Kaspar C W. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62:1519–1525. doi: 10.1128/aem.62.5.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer J J, III, Davis B R. H7 antiserum-sorbitol fermentation medium: a single-tube screening medium for detecting Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1985;22:620–625. doi: 10.1128/jcm.22.4.620-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber L P, Wells S J, Hancock D D, Doyle M P, Tuttle J, Shere J A, Zhao T. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J Am Vet Med Assoc. 1995;207:46–49. [PubMed] [Google Scholar]
- 10.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 11.Hugdahl M B, Beery J T, Doyle M P. Chemotactic behavior of Campylobacter jejuni. Infect Immun. 1988;51:1560–1566. doi: 10.1128/iai.56.6.1560-1566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene W E, Sazie E, Kok J, Rice D H, Hancock D D, Balan V K, Zhao T, Doyle M P. Outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. JAMA. 1997;277:1229–1231. doi: 10.1001/jama.1997.03540390059036. [DOI] [PubMed] [Google Scholar]
- 13.Kudva I T, Hatfield P G, Hovde C J. Escherichia coli O157:H7 in microbial flora of sheep. J Clin Microbiol. 1996;34:431–433. doi: 10.1128/jcm.34.2.431-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng J, Zhao S, Zhao T, Doyle M P. Molecular characterization of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis and plasmid DNA analysis. J Med Microbiol. 1995;42:258–263. doi: 10.1099/00222615-42-4-258. [DOI] [PubMed] [Google Scholar]
- 15.Murinda S E, Roberts R F, Wilson R A. Evaluation of colicins for inhibitory activity against diarrheagenic Escherichia coli strains, including serotype O157:H7. Appl Environ Microbiol. 1996;62:3196–3202. doi: 10.1128/aem.62.9.3196-3202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padhye N V, Doyle M P. Escherichia coli O157:H7: epidemiology, pathogenesis, and methods for detection in food. J Food Prot. 1992;55:555–565. doi: 10.4315/0362-028X-55.7.555. [DOI] [PubMed] [Google Scholar]
- 17.Padhye N V, Doyle M P. Rapid procedure for detecting enterohemorrhagic Escherichia coli O157:H7 in food. Appl Environ Microbiol. 1991;57:2693–2698. doi: 10.1128/aem.57.9.2693-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen M A, Cray W C, Jr, Casey T A, Whipp S C. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol Lett. 1993;114:79–84. doi: 10.1016/0378-1097(93)90145-r. [DOI] [PubMed] [Google Scholar]
- 19.Renwick S A, Wilson J B, Clarke R C, Lior H, Borczyk A A, Spika J, Rahn K, McFadden K, Brouwer A, Copps A, Anderson N G, Alvens D, Karmali M A. Evidence of direct transmission of Escherichia coli O157:H7 infection between calves and a human. J Infect Dis. 1993;168:792–793. doi: 10.1093/infdis/168.3.792. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute, Inc. Statistical analysis system. Cary, N.C: SAS Institute, Inc.; 1982. [Google Scholar]
- 21.Synge B A, Hopkins G F. Verotoxigenic Escherichia coli O157 in Scottish calves. Vet Rec. 1992;130:583. doi: 10.1136/vr.130.26.583-a. [DOI] [PubMed] [Google Scholar]
- 22.Trevena W B, Hooper R S, Wray C, Willshaw G A, Cheasty T, Domingue G. Vero cytotoxin-producing Escherichia coli O157:H7 associated with companion animals. Vet Rec. 1996;138:400. [PubMed] [Google Scholar]
- 23.Wang G, Zhao T, Doyle M P. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl Environ Microbiol. 1996;62:2567–2570. doi: 10.1128/aem.62.7.2567-2570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whipp S C, Rasmussen M A, Cray W C., Jr Animals as a source of Escherichia coli pathogenic for human beings. J Am Vet Med Assoc. 1994;204:1168–1175. [PubMed] [Google Scholar]
- 25.Zhao S, Meng J, Zhao T, Doyle M P. Use of vaccine and biological control techniques to control pathogens in animals used for food. J Food Safety. 1995;15:193–199. [Google Scholar]
- 26.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]