Abstract
Background:
Aging is not a disease; rather, it is a process. As people age, visual impairment (VI) becomes more common. In 2010, the overall prevalence rate of vision impairment in all races was 25.66% in individuals aged ≥ 80 years, according to the estimate of the National Eye Institute at the National Institutes of Health. This review aimed to address the common causes of VI in the elderly.
Methods:
In this narrative review, an electronic search of the PubMed/MEDLINE database was conducted using “visual impairment” and “elderly” for the period between January 2010 and April 2021, to include randomized clinical trials and observational studies concerning VI in the elderly. The selected time period was chosen to provide an updated review.
Results:
The search yielded 2955 articles published over the period of more than 11 years. The relevant randomized clinical trials or observational studies were included and reviewed. Cataracts, refractive errors, open-angle glaucoma, age-related macular degeneration, and diabetic retinopathy were the most common age-related ocular disorders leading to VI if untreated in the elderly. The loss of visual acuity can adversely affect quality of life in the elderly. Difficulty with activities of daily living related to VI can lead to social isolation, depression, and anxiety. Loss of vision in the elderly is linked to an increased risk of falls, hip fracture, depression, and poor quality of life.
Conclusions:
The most common causes of VI in the elderly are cataracts and refractive errors. VI in most ocular diseases is more prevalent in women than in men due to longer lifespan. The overall prevalence of the main causes of VI in the elderly is expected to increase; therefore, health policymakers should consider this when planning for the health-enhancement program of the population.
Key Words: visual impairment, elderly, cataracts, refractive error, age-related macular degeneration, glaucoma, diabetic retinopathy, policymaker, health-enhancement program
INTRODUCTION
Aging is a process that is not considered as a disease. Individuals aged 65 or more years are considered elderly [1]. Presbyopia, reduced contrast sensitivity, reduced dark/light adaptation, and glare delay recovery are common visual changes that are considered part of normal aging physiology. However, with age, visual impairment (VI) becomes more common [1-5]. VI is classified according to visual acuity of the best-seeing eye into mild (< 20/30 to ≥ 20/60), moderate (< 20/60 to ≥ 20/200), and severe (< 20/200 to ≥ 20/400). Blindness is defined as a visual acuity of < 20/400 in the best-seeing eye [6]. In 2010, among individuals aged ≥ 80 years, the overall prevalence rates of VI in all races, white Americans, Hispanics, and African Americans (AAs) were 25.66%, 27.17%, 19.11%, and 16.68%, respectively [7]. Additionally, in individuals aged ≥ 80 years, the overall blindness rates among AAs, white Americans, other races, and Hispanics were 9.21%, 8.41%, 6.32%, 3.46%, and 2.15%, respectively [7]. In a systematic review and meta-analysis, the global number of blind individuals in 2020 was estimated at 43.3 million, of whom 23.9 million were female; the number with moderate and severe VI was estimated at 295 million, of whom 163 million were female; and the number with mild VI was estimated at 258 million, of whom 142 million were female. By 2050, the global numbers of individuals with blindness, moderate and severe VI, mild VI, and uncorrected presbyopia are predicted to reach 61.0 million, 474 million, 360 million, and 866 million, respectively [8]. The prevalence of VI differs based on the place of study; for example, the prevalence in individuals aged ≥ 80 years in Yugan County, China, was reported as 56.6% [9].
This review aimed to address common causes of VI in the elderly, including cataracts, refractive errors, open-angle glaucoma, age-related macular degeneration (ARMD), and diabetic retinopathy (DR).
METHODS
In this narrative review, an electronic search of the PubMed/MEDLINE database was conducted using the keywords “visual impairment” and “elderly”, including relevant randomized clinical trials and observational studies published between January 2010 and April 2021. The search syntax (“visual impairment” AND elderly) was used to provide an updated comprehensive review with inclusion of the most relevant articles.
RESULTS
The search yielded 2955 articles published over the period of more than 11 years. The relevant randomized clinical trials or observational studies were included and reviewed. Cataracts [6, 9-18], refractive error [6, 10, 14, 16, 18], glaucoma [13], ARMD [6, 12, 13, 17], DR [13], and myopic degeneration [12, 14, 16] were the main causes of age-related ocular disorders in the elderly. Articles concerning the main causes of VI in the elderly are reviewed in the Discussion section below. Table 1 summarizes the most common causes of ocular disorders in the elderly outlined in some included studies.
Table 1.
The most common causes of visual impairment in the elderly according to the some included studies
| Author (year) | Region | Type of Study | Age and number of participants | First Major Cause | Second Major Cause |
|---|---|---|---|---|---|
| Thapa et al., 2018 [ 15 ] | Nepal | Cross sectional survey | Age ≥ 60 y; n = 1860 | Cataract | Retinal disorders |
| Gan et al., 2018 [ 19 ] | China | Cross sectional survey | Age ≥ 60 y; n = 3789 | Cataract | Refractive error |
| Baldev et al., 2017 [ 10 ] | Northern India | A population based cross-sectional | Age > 60 y; n = 450 | Cataract | Refractive error |
| Cypel et al., 2017 [ 6 ] | Brazil | Cross-sectional | Age > 80 y; n = 150 | Cataract | Refractive error |
| Pan et al., 2016 [ 18 ] | Eastern China | Community-based survey | Age > 60 y; n = 4579 | Refractive error | Cataract |
| Varma et al., 2016 [ 14 ] | Chinese-American | Cross-sectional | Age ≥ 50 y; 4582 | Refractive error | Cataract |
| Nowak et al., 2015 [ 13 ] | Poland | Cross-sectional | Age ≥ 60 y; n = 1107 | ARMD | Cataract |
| Zhou et al., 2012 [ 20 ] | China | Cross-sectional | Age ≥ 60 y; n = 1305 | Cataract | Fundus diseases |
| Chen et al., 2012 [ 17 ] | Taiwan | Cross-sectional | Age ≥ 65 y; n = 2316 | Cataract | ARMD |
| Tong et al., 2011 [ 4 ] | China | Cross-sectional | Age ≥ 60 y; n = 5199 | Cataract | Refractive error |
Abbreviations: y, years; n, number of participants; ARMD, age-related macular degeneration.
DISCUSSION
The loss of visual acuity in the elderly can adversely affect their quality of life [21]. VI may cause visual difficulties in activities of daily living, leading to social isolation, depression, and anxiety [22]. Loss of vision in the elderly is associated with an increased risk of falls [23], hip fracture [24], depression [25], and poor quality of life [26].
Cataracts
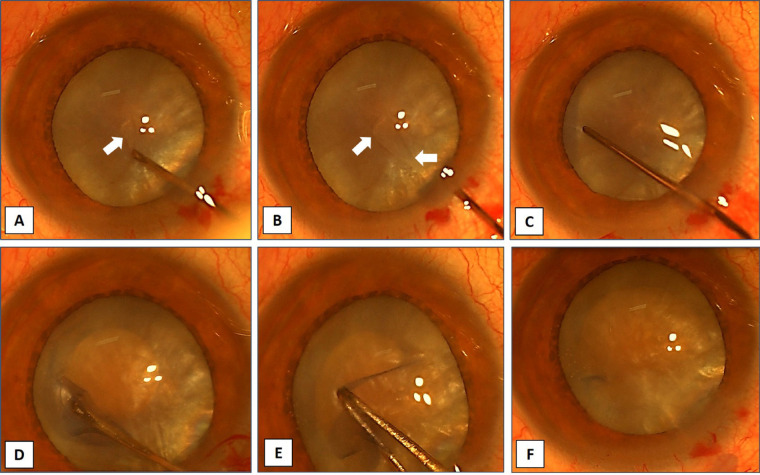
The main causes of VI in the elderly are reported as cataracts [6, 9-18] and refractive error [6, 10, 14, 16, 18] in most of the included studies (Table 1). According to a meta-analysis of 288 studies involving 3,983,541 participants from 98 countries spanning more than three decades, among adults aged ≥ 50 years, most cases of moderate or severe VI and blindness are due to cataracts and refractive error, which are both reversible [27]. Population growth and aging have contributed to an increase in vision loss [27]. Cataracts (Figure 1) are the primary worldwide cause of visual loss in the elderly [6, 9-16]. Cataracts account for almost half of the world’s 37 million cases of blindness [28]. Delaying cataract development by ten years might reduce the need for surgery by 50% [29].
Figure 1.
Anterior segment images show surgical steps for capsulorhexis in a dense mature fluid-filled cataract (A-F). This figure has been reused with the permission of Med Hypothesis Discov Innov Ophthalmol [30].
Cataracts reduce performance-based visual function compared with that of unaffected individuals. Ni et al. found that uneventful cataract surgery could result in visual improvement, enhancing both performance-based and self-assessed functional vision [31]. The prevalence of cataract-related visual loss increases with age [6, 31]. Increasing age and impaired visual function are independently linked with loss of independence, including driving difficulties and placement in a nursing facility [6]. There are several modifiable risk factors for cataract development. Smoking is linked to nuclear sclerosis and posterior subcapsular cataracts [32], diabetes to cortical and posterior subcapsular cataracts [33, 34], ultraviolet light exposure to cortical and posterior subcapsular cataracts [35, 36], and myopia to all forms of cataracts. Oral corticosteroids, and to a lesser extent, inhaled corticosteroids, have been associated with posterior subcapsular cataracts [33, 34].
Cataract-associated vision loss is also related to sex and age [13, 33, 37]. Women are more likely than age-matched men to lose vision due to cataracts [13]. The risk of cataracts increases with age; by age ≥ 80 years, 70.38%, 53.48%, 60.66%, 60.86%, and 68.30% of white Americans, AAs, Hispanics, other races, and the overall population in the United States have cataracts, respectively [38].
The prevalence of blindness in men and women is 4.17% and 5.68%, respectively. Women have a 35% higher likelihood of blindness and a 69% greater chance of blindness due to cataracts [39]. Female sex accounts for 35% of the blindness prevalence and 33% of the cataract prevalence [39]. The influences of postmenopausal hormonal changes, genetics, and serum inflammatory markers in cataract development are being studied [32, 40, 41].
Considering that patients aged > 65 years with impaired vision are more likely to fall, fracture bones, and require walking aids [42], elimination of the causes of VI, including cataracts, should be emphasized in the elderly. Since the first Rapid Evaluation of Avoidable Blindness (RAAB) Study in 2009 in Bhutan, a country in south-central Asia, blindness has decreased by 33%, from 1.5% in 2009 to 1.0% in 2018 [43].
The prevalence of cataracts in Chinese individuals aged 45–49 years and 85–89 years ranged from 6.71% to 73.0% in men and 8.39% to 77.51% in women, respectively [44]. The number of any cataract cases in individuals aged 45–89 years increased from 50.75 million in 1990 to 111.74 million in 2015 [44]. Between 2000 and 2010, South Central China had the most cataract cases, while Northwest China had the fewest. Because of China’s aging population, cataract and cataract blindness prevalence will remain a major public health concern [44].
The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial showed that late ARMD, bilateral cataract surgery, and visual acuity < 20/40 were all associated with a lower chance of survival [45]. In contrast, oral supplementation with omega-3 fatty acids, lutein plus zeaxanthin, zinc, or beta-carotene had no statistically significant effect on mortality [45].
Refractive Error
The major cause of VI in the United States is uncorrected refractive error [46]. In the Canadian Longitudinal Study on Aging, the most prevalent cause of vision loss was refractive error [47]. Blindness due to uncorrected refractive error rose from 6.2 million cases in 1990 to 7.4 million cases in 2015; the figures for overall VI were 84.8 million and 116.3 million cases, respectively. By 2020, the estimated number of global cases of moderate or severe VI caused by uncorrected refractive error was 127.7 million (51.0–225.3 million) [27]. It is estimated that by 2050, 866 million people will have uncorrected presbyopia [8]. Geriatric visual acuity screening is still recommended; however, the United States Preventive Services Task Force (USPSTF) found insufficient evidence to recommend screening for impaired visual acuity in adults aged ≥ 65 years. Despite convincing evidence that visual acuity tests can identify individuals with a refractive error, the USPSTF found that screening using only visual acuity testing does not accurately detect early ARMD or cataracts [48, 49]. In addition, Clarke et al. in a recent systematic review and meta-analysis found a comparable risk of poor vision in communities with and without vision screening [50]. More research is required to identify any benefits of vision screening in the elderly. Screening is intended to determine if older individuals require glasses to function normally and to avoid depression or falls that may result from impaired vision.
Although uncorrected refractive error can be as disabling as VI caused by non-correctable causes, it is easily addressed using glasses. The aging process affects several aspects of vision. The elder’s vision may decrease considerably as a result of glare. Color vision and contrast sensitivity are known to decrease with age [51, 52].
Increasing time outdoors has been associated with myopia in the elderly [53]. Myopia is more prevalent in individuals aged > 70 years than in other age groups, indicating a nuclear cataract-induced myopic shift in refraction [54]. In 2010, the estimated prevalence rates of myopia in those aged ≥ 80 years among white Americans, Hispanics, other races, and AAs were 18.37%, 17.07%, 15.13%, and 10.25%, respectively [55]. Between ages 55 and 59 years, the estimated prevalence rates in white Americans, other races, Hispanics, and AAs were 22.99%, 16.76%, 14.53%, and 12.21%, respectively [55]. A meta-analysis highlighted the link between myopia and increased risks of myopic macular degeneration, retinal detachment, posterior subcapsular cataract, nuclear cataract, and open-angle glaucoma. Longer axial length, higher degree of myopia, and age ≥ 60 years were all associated with a higher risk of VI. Therefore, the priority of myopia prevention and treatment has been highlighted in the literature [56, 57].
The prevalence of hyperopia increases with age in all races. In 2010, the estimated prevalence rates of hyperopia in individuals aged ≥ 80 years among white Americans, Hispanics, AAs, and other races were 25.12%, 17.50%, 9.66%, and 1.07%, respectively, and were higher in women than in men among all age groups [58].
Glaucoma
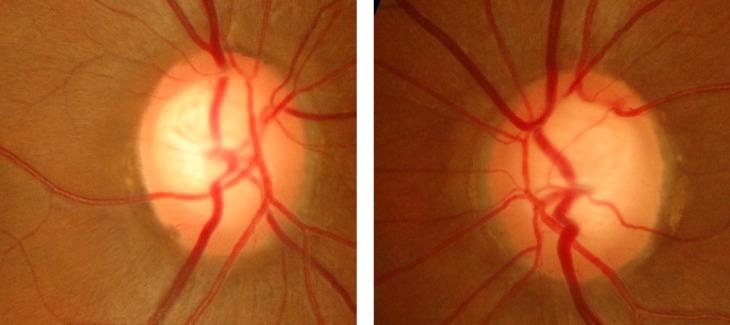
Glaucoma increases in prevalence with age [59] (Figure 2). In 2010, 1.9% of people aged > 40 years and 7.89% of people aged > 80 years in the United States were affected by glaucoma [60]. Flaxman et al. reported that in 2015, glaucoma caused moderate or severe VI in 4 million people, and 2.9 million were blind. They predicted that glaucoma would lead to moderate or severe VI in 4.5 million people in 2020, and 3.2 million would be blind [27].
Figure 2.
Fundus photographs of the right and left eyes of a patient with glaucoma. Note the large cup-to-disc ratio, bayoneting sign, vessel barring, regional pallor, and subtle retinal nerve fiber layer defect.
Race, sex, and age can affect the likelihood of glaucoma. Glaucoma is more common among women due to their longer lifespan [60]. AAs have the highest prevalence, and glaucoma is the leading cause of blindness in AAs [60, 61]. Undiagnosed visual disorders affect 10% of the elderly population. One of the most frequently used methods of glaucoma screening is tonometry. A dilated fundus exam is recommended by the United States Centers for Disease Control and Prevention every 1–2 years [18, 62, 63]. Goldmann applanation tonometry remained the gold standard to which the other devices may underestimate or overestimate the intraocular pressure measurement. However, the central corneal thickness and other factors such as ethnicity could affect the accuracy of intraocular pressure measurement [64].
The prevalence of glaucoma has been reported to increase with age. Glaucoma is diagnosed in approximately 6% of AAs by the age of 69 years, and the proportion increases to 12% after the age of 80 years. Sixty-one percent of glaucoma cases in the United States are in women. It has been predicted that in 2050, 6.3 million people will have a diagnosis of glaucoma [60]. Clinical trials on pharmaceutical treatment of primary open-angle glaucoma have found that medical treatment is effective, safe, and positively affects quality of life [65, 66]. Glaucoma interventions have been summarized in a recently published review article. The novel Rho-kinase inhibitors showed significant intraocular pressure reduction [66].
Age-Related Macular Degeneration
ARMD is a progressive disease of the macula lutea that results in a gradual decline in central vision and leads to severe VI [67] (Figure 3). Among white adults over 50 years of age, the prevalence rate of ARMD was 2.5% in 2010. The prevalence rates of ARMD among people aged ≥ 80 years are 11.73% overall and 13.59% in white Americans, who have the greatest risk of ARMD compared to other races [68]. The burden of disease is predicted to increase by 2050, affecting 5.44 million people. White Americans will account for most cases, and Hispanics will experience the highest growth rate, with an almost six-fold increase in the number of expected ARMD cases from 2010 to 2050. Dietary supplements, such as vitamins and minerals, are recommended [68]. According to AREDS2, a dietary supplement formulation may lessen the likelihood of intermediate ARMD progressing to the advanced stage [68-70].
Figure 3.
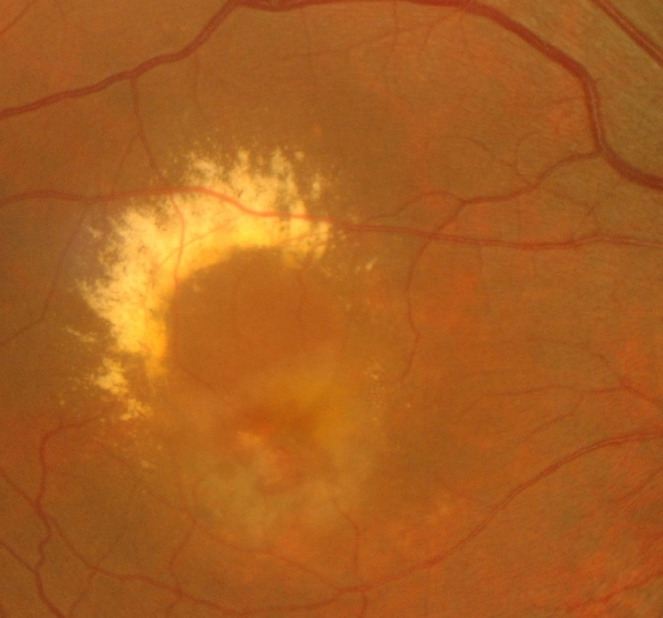
Fundus photograph of the right eye of a patient with age-related macular degeneration. Note the hard exudate and significant macular edema caused by choroidal neovascularization.
Based on a meta-analysis, the global prevalence rate of blindness caused by macular diseases was 6.6% in 2010, accounting for 3.1% of all VI cases worldwide. The prevalence of early ARMD was estimated to be 8.0%, and late ARMD 0.4%, between the ages of 45 and 85 years. The estimated numbers of individuals with ARMD were 196 million in 2020 and 288 million in 2040. Early ARMD was found to be more common in Europeans (11.2%) than in Asians (6.8%) [71, 72]. ARMD was ranked as the fourth most common cause of blindness worldwide in 2015, accounting for an estimated 5.8% of blind people, and as the third most common cause of moderate to severe VI (3.9%) [72]. These data highlight the importance of ARMD as one of the main causes of VI in the elderly. Therefore, policymakers should consider this when planning for health enhancement of the population.
Diabetic Retinopathy
According to the World Health Organization global report on diabetes published in 2016, one in every 12 adults (8.5%) has diabetes [73]. DR is the fifth leading cause of blindness and moderate to severe VI worldwide, and its prevalence is increasing in the United States. DR is a cause of vision loss in individuals aged 20–74 years [74, 75]. DR causes vision loss predominantly through diabetic macular edema and proliferative diabetic retinopathy (Figures 4 and 5) and is mostly mediated by increased retinal vascular injury and subsequent local ischemia [76-79]. From 2008 to 2010, DR accounted for 6.1% to 8.3% of VI cases in the United Kingdom. The prevalence rate of DR in 2010 for people aged ≥ 75 years was 7% in white Americans and AAs, as compared to 19% in Hispanics, who have a higher risk. In 2010, women had a higher prevalence rate than men (51% versus 49%). The prevalence rate of DR is expected to double by the year 2050, affecting 14.6 million individuals [74, 80].
Figure 4.
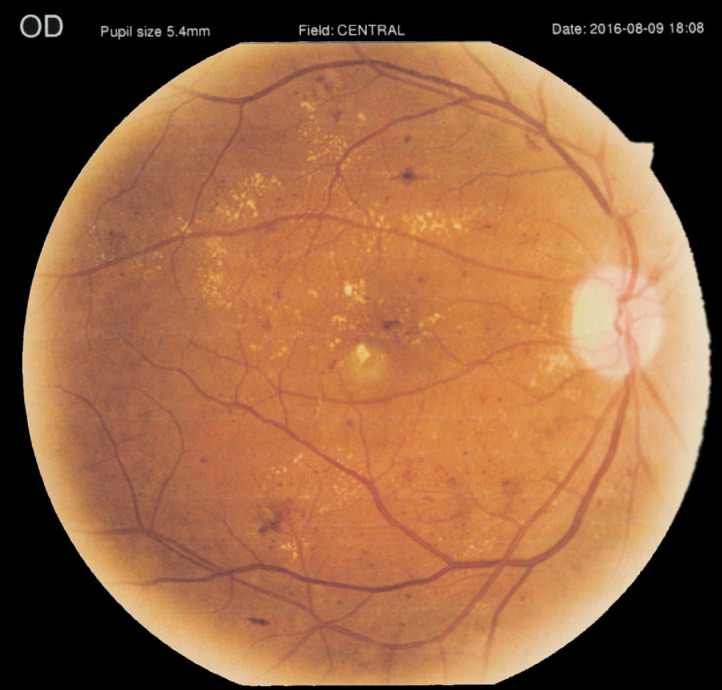
Severe right eye non-proliferative diabetic retinopathy and clinically significant macular edema in a 65-year-old man with corrected distance visual acuity of 40/200 in both eyes. This figure has been reused with the permission of Med Hypothesis Discov Innov Ophthalmol [79].
Figure 5.
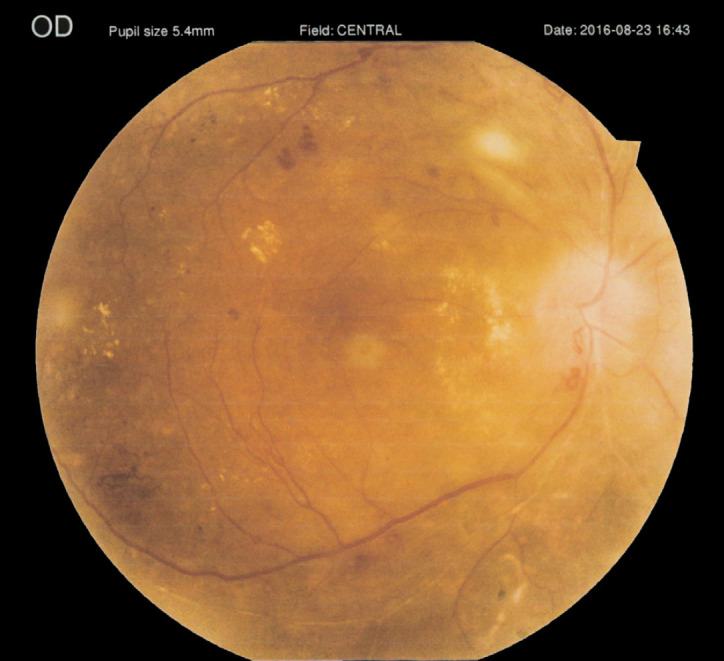
A 68-year-old man with active proliferative diabetic retinopathy and history of previous pan-retinal photocoagulation in his right eye. This figure has been reused with the permission of Med Hypothesis Discov Innov Ophthalmol [79].
This literature review included an up-to-date discussion of the most prevalent causes of VI among the elderly and of the practical applications for this pivotal subject. However, the limitations of the study are that it was performed by a single author, and only PubMed/MEDLINE was searched, which might have resulted in bias. A future review article focusing on the most common geriatric syndromes related to VI could provide practical guidelines for health policymakers to confront this globally challenging health issue.
CONCLUSIONS
VI in most ocular diseases is more prevalent in women due to their longer lifespan. Cataracts and uncorrected refractive errors are the leading causes of VI among the elderly. AAs over the age of 80 years have the highest prevalence of glaucoma, and Hispanics over the age of 80 years have the highest prevalence of DR. Depression has been linked to uncorrected refractive error in the elderly. The overall prevalence of the main causes of VI is expected to increase, which should be considered by health policymakers when planning for the health enhancement of the population.
ETHICAL DECLARATIONS
Ethical Approval:
This study was a review, and no ethical approval was required.
Conflict of Interest:
None
FUNDING
None
ACKNOWLEDGMENTS
None
References
- 1.Orimo H. [Reviewing the definition of elderly] Nihon Ronen Igakkai Zasshi. 2006;43(1):27–34. doi: 10.3143/geriatrics.43.27. [DOI] [PubMed] [Google Scholar]
- 2.Owsley C. Aging and vision. Vision Res. 2011;51(13):1610–22. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 4.Tong XW, Zhao R, Zou HD, Zhu JF, Wang J, Yu J, et al. [A prevalence investigation of blindness and vision impairment in 2009 in older adults of Dachang Blocks of Baoshan District, Shanghai, China] Zhonghua Yan Ke Za Zhi. 2011;47(9):785–90. [PubMed] [Google Scholar]
- 5.Larsen PP, Thiele S, Krohne TU, Ziemssen F, Krummenauer F, Holz FG, et al. Visual impairment and blindness in institutionalized elderly in Germany. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):363–370. doi: 10.1007/s00417-018-4196-1. [DOI] [PubMed] [Google Scholar]
- 6.Cypel MC, Salomão SR, Dantas PEC, Lottenberg CL, Kasahara N, Ramos LR, et al. Vision status, ophthalmic assessment, and quality of life in the very old. Arq Bras Oftalmol. 2017;80(3):159–164. doi: 10.5935/0004-2749.20170039. [DOI] [PubMed] [Google Scholar]
- 7.National Institutes of Health. National Eye Institute. ‘All vision impairment’. 2020. [Accessed: April 29, 2021]. Available at : https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/all-vision-impairment-data-and-statistics.
- 8.GBD 2019 Blindness. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan S, Zhou X, Yan J, Liu X, Yi J, Zhou X, et al. The prevalence and risk factors of visual impairment among rural residents aged 50 years and above in Yugan county, China. Ophthalmic Epidemiol. 2018;25(5-6):331–337. doi: 10.1080/09286586.2018.1476557. [DOI] [PubMed] [Google Scholar]
- 10.Baldev VF, Chopra R, Batra N, Singh S. Pattern of Ocular Morbidity in the Elderly Population of Northern India. J Clin Diagn Res. 2017;11(8):NC20–NC23. doi: 10.7860/JCDR/2017/27056.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai, Taiwan. Ophthalmology. 2003;110(6):1089–95. doi: 10.1016/S0161-6420(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Wang X, Wang J, Huang W, Gao Y, Luo Y, et al. Prevalence and Causes of Visual Impairment in a Chinese Adult Population: The Taizhou Eye Study. Ophthalmology. 2015;122(7):1480–8. doi: 10.1016/j.ophtha.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Nowak MS, Smigielski J. The prevalence and causes of visual impairment and blindness among older adults in the city of Lodz, Poland. Medicine (Baltimore). 2015;94(5):e505. doi: 10.1097/MD.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varma R, Kim JS, Burkemper BS, Wen G, Torres M, Hsu C, et al. Prevalence and Causes of Visual Impairment and Blindness in Chinese American Adults: The Chinese American Eye Study. JAMA Ophthalmol. 2016;134(7):785–93. doi: 10.1001/jamaophthalmol.2016.1261. [DOI] [PubMed] [Google Scholar]
- 15.Thapa R, Bajimaya S, Paudyal G, Khanal S, Tan S, Thapa SS, et al. Prevalence and causes of low vision and blindness in an elderly population in Nepal: the Bhaktapur retina study. BMC Ophthalmol. 2018;18(1):42 . doi: 10.1186/s12886-018-0710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen H, Zhang H, Gong W, Qian T, Cheng T, Jin L, et al. Prevalence, Causes, and Factors Associated with Visual Impairment in a Chinese Elderly Population: The Rugao Longevity and Aging Study. Clin Interv Aging. 2021;16:985–996. doi: 10.2147/CIA.S304730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Huang TL, Tsai RK, Sheu MM. Prevalence and causes of visual impairment in elderly Amis aborigines in Eastern Taiwan (the Amis Eye Study) Jpn J Ophthalmol. 2012;56(6):624–30. doi: 10.1007/s10384-012-0178-8. [DOI] [PubMed] [Google Scholar]
- 18.Pan CW, Qian DJ, Sun HP, Ma Q, Xu Y, Song E. Visual Impairment among Older Adults in a Rural Community in Eastern China. J Ophthalmol. 2016;2016:9620542. doi: 10.1155/2016/9620542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan SH, Deng LL, Zhou XJ, Yi JL, Zhou QQ, Li YP, et al. [Prevalence status and influencing factors of visual impairment in the elderly people in rural areas of Yugan County, Jiangxi Province] Zhonghua Yan Ke Za Zhi. 2018;54(8):605–610. doi: 10.3760/cma.j.issn.0412-4081.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Yuan Y, Zhang X, Guan HJ. [A prevalence investigation of blindness and low vision in 2008 among adults aged 60 years or above in 2 villages of Nantong] Zhonghua Yan Ke Za Zhi. 2012;48(10):908–14. [PubMed] [Google Scholar]
- 21.Lamoureux E, Pesudovs K. Vision-specific quality-of-life research: a need to improve the quality. Am J Ophthalmol. 2011;151(2):195–7. doi: 10.1016/j.ajo.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Tetteh J, Fordjour G, Ekem-Ferguson G, Yawson AO, Boima V, Entsuah-Mensah K, et al. Visual impairment and social isolation, depression and life satisfaction among older adults in Ghana: analysis of the WHO’s Study on global AGEing and adult health (SAGE) Wave 2. BMJ Open Ophthalmol. 2020;5(1):e000492 . doi: 10.1136/bmjophth-2020-000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crews JE; DPA, Chou CF, Stevens JA, Saaddine JB. Falls Among Persons Aged ≥65 Years With and Without Severe Vision Impairment - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(17):433–7. doi: 10.15585/mmwr.mm6517a2. [DOI] [PubMed] [Google Scholar]
- 24.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308(5):493–501. doi: 10.1001/jama.2012.9014. [DOI] [PubMed] [Google Scholar]
- 25.Rovner BW, Casten RJ, Hegel MT, Massof RW, Leiby BE, Ho AC, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2014;121(11):2204–11. doi: 10.1016/j.ophtha.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age Ageing. 2006;35(6):560–5. doi: 10.1093/ageing/afl074. [DOI] [PubMed] [Google Scholar]
- 27.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 28.Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. 2008;19(1):55–9. doi: 10.1097/ICU.0b013e3282f154bd. [DOI] [PubMed] [Google Scholar]
- 29.Congdon NG. Prevention strategies for age related cataract: present limitations and future possibilities. Br J Ophthalmol. 2001;85(5):516–20. doi: 10.1136/bjo.85.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoneim EM. Modified capsulorhexis for fluid-filled mature cataracts. Med Hypothesis Discov Innov Ophthalmol. 2021 Summer;10(2):59–66. doi: 10.51329/mehdioptometry1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni W, Li X, Hou Z, Zhang H, Qiu W, Wang W. Impact of cataract surgery on vision-related life performances: the usefulness of Real-Life Vision Test for cataract surgery outcomes evaluation. Eye (Lond). 2015;29(12):1545–54. doi: 10.1038/eye.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West SK. Looking forward to 20/20: a focus on the epidemiology of eye diseases. Epidemiol Rev. 2000;22(1):64–70. doi: 10.1093/oxfordjournals.epirev.a018025. [DOI] [PubMed] [Google Scholar]
- 33.Delcourt C, Cristol JP, Tessier F, Léger CL, Michel F, Papoz L. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: the POLA study. Pathologies Oculaires Liées à l’Age. Am J Epidemiol. 2000;151(5):497–504. doi: 10.1093/oxfordjournals.aje.a010235. [DOI] [PubMed] [Google Scholar]
- 34.Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11(6):478–83. doi: 10.1097/00055735-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related nuclear and cortical cataract : a case-control study in the Age-Related Eye Disease Study, AREDS Report No 5. Ophthalmology. 2001;108(8):1400–8. doi: 10.1016/s0161-6420(01)00626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoh N, Jonasson F, Sasaki H, Kojima M, Ono M, Takahashi N, et al. Cortical lens opacification in Iceland. Risk factor analysis -- Reykjavik Eye Study. Acta Ophthalmol Scand. 2001;79(2):154–9. doi: 10.1034/j.1600-0420.2001.079002154.x. [DOI] [PubMed] [Google Scholar]
- 37.Vashist P, Talwar B, Gogoi M, Maraini G, Camparini M, Ravindran RD, et al. Prevalence of cataract in an older population in India: the India study of age-related eye disease. Ophthalmology. 2011;118(2):272–8. doi: 10.1016/j.ophtha.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institutes of Health. ‘Cataract Data and Statistics’. 2019. [Accessed: April 29, 2021)]. Available at : https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics.
- 39.Prasad M, Malhotra S, Kalaivani M, Vashist P, Gupta SK. Gender differences in blindness, cataract blindness and cataract surgical coverage in India: a systematic review and meta-analysis. Br J Ophthalmol. 2020;104(2):220–224. doi: 10.1136/bjophthalmol-2018-313562. [DOI] [PubMed] [Google Scholar]
- 40.Lai K, Cui J, Ni S, Zhang Y, He J, Yao K. The effects of postmenopausal hormone use on cataract: a meta-analysis. PLoS One. 2013;8(10):e78647. doi: 10.1371/journal.pone.0078647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein BE, Klein R, Lee KE, Knudtson MD, Tsai MY. Markers of inflammation, vascular endothelial dysfunction, and age-related cataract. Am J Ophthalmol. 2006;141(1):116–22. doi: 10.1016/j.ajo.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Brundle C, Waterman HA, Ballinger C, Olleveant N, Skelton DA, Stanford P, et al. The causes of falls: views of older people with visual impairment. Health Expect. 2015;18(6):2021–31. doi: 10.1111/hex.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lepcha NT, Sharma IP, Sapkota YD, Das T, Phuntsho T, Tenzin N, et al. Changing trends of blindness, visual impairment and cataract surgery in Bhutan: 2009-2018. PLoS One. 2019;14(5):e0216398. doi: 10.1371/journal.pone.0216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song P, Wang H, Theodoratou E, Chan KY, Rudan I. The national and subnational prevalence of cataract and cataract blindness in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010804 . doi: 10.7189/jogh.08-010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papudesu C, Clemons TE, Agrón E, Chew EY Age-Related Eye Disease Study 2 Research Group. Association of Mortality with Ocular Diseases and Visual Impairment in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 13. Ophthalmology. 2018;125(4):512–521. doi: 10.1016/j.ophtha.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou CF, Frances Cotch M, Vitale S, Zhang X, Klein R, Friedman DS, et al. Age-related eye diseases and visual impairment among U S adults. Am J Prev Med. 2013;45(1):29–35. doi: 10.1016/j.amepre.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aljied R, Aubin MJ, Buhrmann R, Sabeti S, Freeman EE. Prevalence and determinants of visual impairment in Canada: cross-sectional data from the Canadian Longitudinal Study on Aging. Can J Ophthalmol. 2018;53(3):291–297. doi: 10.1016/j.jcjo.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 48.Screening for Impaired Visual Acuity in Older Adults: Recommendation Statement. Am Fam Physician. 2016;93(12) [PubMed] [Google Scholar]
- 49.Chou R, Dana T, Bougatsos C, Grusing S, Blazina I. Screening for Impaired Visual Acuity in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(9):915–33. doi: 10.1001/jama.2016.0783. [DOI] [PubMed] [Google Scholar]
- 50.Clarke EL, Evans JR, Smeeth L. Community screening for visual impairment in older people. Cochrane Database Syst Rev. 2018;2(2):CD001054. doi: 10.1002/14651858.CD001054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferraz FH, Corrente JE, Opromolla P, Schellini SA. Influence of uncorrected refractive error and unmet refractive error on visual impairment in a Brazilian population. BMC Ophthalmol. 2014;14:84. doi: 10.1186/1471-2415-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvi SM, Akhtar S, Currie Z. Ageing changes in the eye. Postgrad Med J. 2006;82(971):581–7. doi: 10.1136/pgmj.2005.040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph S, Krishnan T, Ravindran RD, Maraini G, Camparini M, Chakravarthy U, et al. Prevalence and risk factors for myopia and other refractive errors in an adult population in southern India. Ophthalmic Physiol Opt. 2018;38(3):346–358. doi: 10.1111/opo.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92(3):258–66. doi: 10.1097/OPX.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 55.National Institutes of Health. National Eye Institute. ‘Nearsightedness (Myopia) Data and Statistics’. 2019. [Accessed: April 29, 2021]. Available at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/nearsightedness-myopia-data-and-statistics.
- 56.Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The Complications of Myopia: A Review and Meta-Analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi: 10.1167/iovs.61.4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tideman JW, Snabel MC, Tedja MS, van Rijn GA, Wong KT, Kuijpers RW, et al. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016;134(12):1355–1363. doi: 10.1001/jamaophthalmol.2016.4009. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health. National Eye Institute. ‘Farsightedness (Hyperopia) Data and Statistics’. 2019. [Accessed: April 29, 2021]. Available at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/farsightedness-hyperopia-data-and-statistics.
- 59.Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I European Glaucoma Prevention Study (EGPS) Group. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114(1):3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health. National Eye Institute. ‘Glaucoma Data and Statistics’. 2019. [Accessed: April 29, 2021]. Available at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/glaucoma-data-and-statistics.
- 61.Restrepo NA, Laper SM, Farber-Eger E, Crawford DC. Local genetic ancestry in CDKN2B-AS1 is associated with primary open-angle glaucoma in an African American cohort extracted from de-identified electronic health records. BMC Med Genomics. 2018;11(Suppl 3):70 . doi: 10.1186/s12920-018-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aziz K, Friedman DS. Tonometers-which one should I use? Eye (Lond). 2018;32(5):931–937. doi: 10.1038/s41433-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. Vision Health Initiative. ‘Keep an Eye on Your Vision Health’. 2020. [Accessed: April 29, 2021]. Available at: https://www.cdc.gov/visionhealth/resources/features/keep-eye-on-vision-health.html.
- 64.Salouti R, Alishiri AA, Gharebaghi R, Naderi M, Jadidi K, Shojaei-Baghini A, et al. Comparison among Ocular Response Analyzer, Corvis ST and Goldmann applanation tonometry in healthy children. Int J Ophthalmol. 2018;11(8):1330–1336. doi: 10.18240/ijo.2018.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108(11):1954–65. doi: 10.1016/s0161-6420(01)00874-0. [DOI] [PubMed] [Google Scholar]
- 66.Al-Namaeh M. Pharmaceutical treatment of primary open angle glaucoma. Medical hypothesis, discovery & innovation in optometry. 2021;2(1):8–17. [Google Scholar]
- 67.Ramin S, Soheilian M, Habibi G, Ghazavi R, Gharebaghi R, Heidary F. Age-Related Macular Degeneration: A Scientometric Analysis. Med Hypothesis Discov Innov Ophthalmol. 2015;4(2):39–49. [PMC free article] [PubMed] [Google Scholar]
- 68.National Institutes of Health. National Eye Institute. ‘Age-Related Macular Degeneration (AMD) Data and Statistics’. 2019. [Accessed April 29, 2021]. Available at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics.
- 69.AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1) Ophthalmology. 2012;119(11):2282–9. doi: 10.1016/j.ophtha.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sin HP, Liu DT, Lam DS. Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration. Acta Ophthalmol. 2013;91(1):6–11. doi: 10.1111/j.1755-3768.2011.02357.x. [DOI] [PubMed] [Google Scholar]
- 71.Jonas JB, Bourne RR, White RA, Flaxman SR, Keeffe J, Leasher J, et al. Visual impairment and blindness due to macular diseases globally: a systematic review and meta-analysis. Am J Ophthalmol. 2014;158(4):808–15. doi: 10.1016/j.ajo.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia Pac J Ophthalmol (Phila). 2017;6(6):493–497. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 73.world health organization. ‘Global report on diabetes’. 2016. [Accessed April 29, 2021]. Availbale at: https://www.who.int/publications/i/item/9789241565257.
- 74.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17 . doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wykoff CC. Prevention and Management of Diabetes-Related Eye Disease. American Diabetes Association; 2019 . May, Management of Diabetes-Related Retinopathy . Bookshelf ID: NBK544520. [PubMed] [Google Scholar]
- 77.Zhang C, Wang H, Nie J, Wang F. Protective factors in diabetic retinopathy: focus on blood-retinal barrier. Discov Med. 2014 Sep;18(98):105–12. [PubMed] [Google Scholar]
- 78.Roy S, Kern TS, Song B, Stuebe C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am J Pathol. 2017;187(1):9–19. doi: 10.1016/j.ajpath.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afarid M, Attarzadeh A, Farvardin M, Ashraf H. The Association of Serum Leptin Level and Anthropometric Measures With the Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. Med Hypothesis Discov Innov Ophthalmol. 2018;7(4):156–162. [PMC free article] [PubMed] [Google Scholar]
- 80.National Institutes of Health. National Eye Institute. ‘Diabetic Retinopathy Data and Statistics’. 2020. [Accessed: April 29, 2021]. Availbale at: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/diabetic-retinopathy-data-and-statistics.