Abstract
Background:
Screening for diabetic retinopathy in the community without compromising the routine work of ophthalmologists at hospitals is the essence of teleophthalmology. This study was aimed at investigating the efficacy of teleophthalmology practice for screening diabetic retinopathy from 2012 to 2020. It was also aimed at comparing the 2-year prevalence of camps organized by a district hospital in South India, as well as the footfall, reporting, follow-up, patient response, and diagnostic efficacy at these camps.
Methods:
All patients with diabetes and unexplained vision deterioration attending the mobile camp units underwent non-dilated fundus photography. Patients underwent teleconsultation with the ophthalmologist at the district hospital, and those requiring intervention were called to the district hospital. Trends were studied for the number of patients reporting to the hospital. Patient satisfaction was recorded based on a questionnaire.
Results:
A total of 682 camps were held over 8 years, and 30 230 patients were examined. Teleconsultation was done for 12 157 (40.21%) patients. Patients requiring further investigations, intervention for diabetic retinopathy, or further management of other ocular pathologies were urgently referred to the district hospital (n= 3293 [10.89%] of 30 230 examined patients). The severity and presence of clinically significant macular edema increased significantly with an increased duration of diabetes mellitus (P < 0.001). The percentage of teleconsultations showed an increasing trend over the years (P = 0.001). Similarly, considering trends of patients reporting to the hospital, the attrition rate decreased over the years (P < 0.05). A total of 10 974 of 12 157 (90.27%) patients who underwent teleophthalmic consultation were satisfied with the service.
Conclusions:
Teleconsultations over the years showed an increasing trend, and the attrition rate decreased over the years. Teleophthalmology is achieving success in providing high-quality service, easy access to care, and in increasing patient satisfaction. Future studies on the role of teleophthalmology for other leading preventable causes of blindness seem possible and necessary.
Key Words: diabetic retinopathies, screening, tele-referral, virtual medicine, mobile health, republic of india, satisfaction and patient
INTRODUCTION
Screening for diabetic retinopathy (DR) in the community without compromising the routine work of ophthalmologists at hospitals is the essence of teleophthalmology [1, 2]. In addition to being convenient for the patients, it aids in community screening and reaching out to the remote unserved areas of the country [3-5].
With advancements in technology yielding sensitive and convenient imaging techniques and quick transfer of information online, teleophthalmology has empowered health services to practice medicine remotely. Multiple studies prove teleophthalmology to be an impactful practice [4-6]. The prevalence of teleophthalmology and the patient response has been rising every year. This would directly reflect the acceptance of this novel clinical service for the evaluation and management of patients [6].
The aim of the present study was to evaluate the changing trend of teleophthalmology practice for the screening of DR from 2012 to 2020. We compared the 2 yearly prevalence of camps, as well as the footfall, reporting, follow-up, patient response, and diagnostic efficacy at the camps. The results of this study could provide insight into the trends of using mobile teleophthalmology at DR screening camps in South India.
METHODS
In this descriptive, comparative, retrospective study that was conducted over 8 years from April 2012 to March 2020, we analyzed the trends of mobile teleophthalmology at DR screening camps in South India. The ethics committee of the community medicine department of our university provided ethical approval for the study. The study was performed in accordance with the tenets of the Helsinki Declaration. All participants provided consent.
All patients attending the noncommunicable disease outpatient department of the primary and community health centers in the Palakkad district were directed to visit the mobile teleophthalmology units parked at the gram panchayat office or primary and community healthcare centers. These centers were located in rural communities with low socioeconomic status. The mobile units comprised site administrators trained in acquiring non-dilated fundus pictures, a trained optometrist, and a vision technician.
Four trained optometrists, three vision technicians, and two ophthalmologists were involved in mobile teleophthalmology DR screening programs throughout the study period. Vision evaluation was performed after obtaining their verbal consent using the Snellen chart (Nidek automatic chart projector CP 670; Nidek Co., Ltd., Gamagori, Japan). Dry refraction was tested with static retinoscopy (Welch Allyn Elite Retinoscope, Welch Allyn Inc., NY, USA) and subjectively refined. Torch light examination was performed to rule out conspicuous anterior-segment pathologies, and direct ophthalmoscopy (Welch Allyn Inc., Auburn, NY, USA) was performed to rule out media opacity. Fundus photography was performed using a nonmydriatic ocular fundus camera (Kowa nonmyd D series cameras; Kowa Optimed, Inc., Torrance, CA), and fundus images were sent to the district hospital of Palakkad using e-Nayana software [7] developed by the Centre for Development of Advanced Computing under the Government of India to provide better ophthalmic care in rural areas.
Teleophthalmology software e-Nayana [7] is used to collect, store, transfer, and retrieve patient records with a unique patient ID. WiMAX connectivity of BSNL and KSWAN (State Wide Area Network of the Kerala government) is strengthening the unit to be technically operated from anywhere in Kerala. Sunayanam is the first e-health application in India utilizing WiMAX connectivity for video conferencing and transferring electronic case sheets. The mobile units were fit with generators and could draw power from local electric lines [8].
All patients with diabetes with unexplained vision deterioration and relatively clear media underwent fundus photography. For ease in recording and calculating data, we divided the duration of diabetes into three categories: ≤ 5 years, 5–10 years, and > 10 years. Teleconsultation was performed with the ophthalmologist at the district hospital after virtually sharing the fundus photographs. Patients were counseled through teleconferencing or telephonically. Those requiring intervention or further investigations were asked to visit the hospital. The cost of one visit (in Indian rupee) by bus from the camp to the hospital was calculated for each camp site. We recorded diagnoses of DR (with grades based on the Early Treatment Diabetic Retinopathy Study) and clinically significant macular edema (CSME) [9].
Self-reported patient satisfaction was recorded based on a questionnaire. In the questionnaire, responses were recorded as “yes” or “no” for the following questions: “Are you satisfied with teleophthalmic screening?”; “Are you satisfied with video conferencing with the ophthalmologist?”; “Are you satisfied with the information and guidance provided by the staff in the mobile unit?”; “Would you prefer teleophthalmology consultation in the future?”; “Are you satisfied enough to recommend teleophthalmologic consultation to others?”; “Were you able to attend the camp without help/support?”; “Was the camp convenient?”; “Was the camp economical?”, and “Was the camp time-saving?”
The study period was divided into four time periods: from April 2012 to March 2014, from April 2014 to March 2016, from April 2016 to March 2018, and from April 2018 to March 2020. Intergroup comparisons were performed in all aspects of the collected data. The percentage of patients accurately diagnosed among those who visited the clinic and that of patients being managed remotely were also analyzed. Trends in patient satisfaction were also studied.
Data were analyzed using SPSS for Windows, version 20.0 (IBM Corp., Armonk, N.Y., USA). Descriptive analyses were performed for all variables. Quantitative variables are expressed as mean (standard deviation [SD]). Count data are expressed as numbers (percentages). The chi-square test was used to identify associations and significance in changing trends. The equality of several population proportions was tested to calculate any significant increase in the severity or presence of CSME with an increased duration of diabetes mellitus. A P-value < 0.05 was considered to indicate statistical significance.
RESULTS
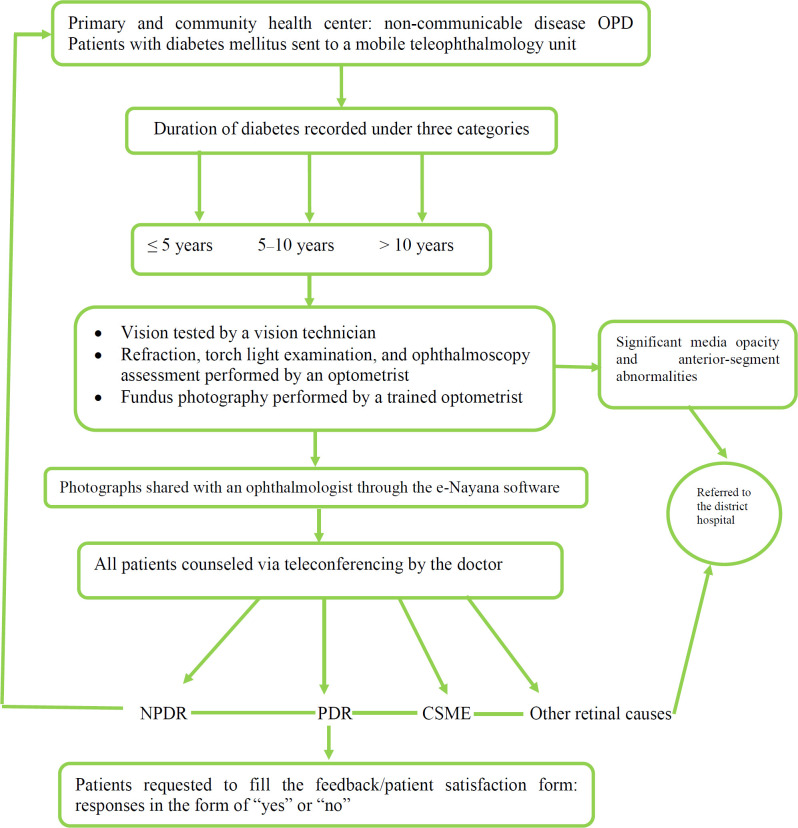
A total of 682 camps were held over the study period, and 30 230 patients were examined in the mobile teleophthalmology units. Figure 1 illustrates the study flowchart. Fundus photographs were acquired, and teleconsultation was conducted for 12 157 (40.22%) patients. Out of 12 157 patients, 5162 (42.46%) were men, and 6995 (57.54%) were women. Most patients had diabetes for > 10 years at the time of camp visit (n = 4375, 35.99%). Out of the 12 157 patients who received teleconsultations, 3293 (27.09%) were asked to report to the hospital for further evaluation and/or treatment. However, only 2438 (74.04%) patients visited the hospital as directed.
Figure 1.
Study flowchart. Abbreviations: OPD, out-patient department; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; CSME, clinically significant diabetic macular edema
Table 1 shows 2-yearly trends of DR grades as diagnosed based on teleconsultation. Out of 12 157 patients, 6232 (51.26%) had no evidence of DR. Mild to moderate non-proliferative DR (NPDR) was found in 3229 (26.56%) patients, while severe NPDR was found in 1696 (13.95%) patients. Proliferative DR (PDR) and CSME were noted in 402 (3.31%) and 598 (4.92%) patients, respectively (Table 1). The severity of NPDR and the presence of CSME increased significantly with an increased duration of diabetes mellitus (P = 0.001 and P < 0.001, respectively; Table 1).
Table 1.
Two-yearly trend of patient distribution based on the diabetic retinopathy grade and clinically significant macular edema diagnosed through teleconsultation
| Year | No DR | Mild to Moderate NPDR | Severe NPDR | PDR | CSME |
|---|---|---|---|---|---|
| 2012 – 14 | 801 | 394 | 252 | 54 | 86 |
| 2014 – 16 | 1360 | 491 | 333 | 78 | 136 |
| 2016 – 18 | 1983 | 1120 | 523 | 111 | 181 |
| 2018 – 20 | 2088 | 1224 | 588 | 159 | 195 |
| Total | 6232 | 3229 | 1696 | 402 | 598 |
| * P -value | 0.001 | 0.001 | 0.001 | 0.001 | < 0.001 |
Abbreviations: DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; CSME, clinically significant diabetic macular edema. Note: P-value < 0.001 calculated with the test for equality of several population proportions
Patients requiring further investigations, intervention for DR, or management of other ocular pathologies were urgently referred to the district hospital (n= 3293 [10.89%] out of 30 230 examined patients). These patients were counseled to visit the district hospital within 30 days. We referred 402 (12.20%) patients for treatment of PDR, 321 (9.74%) for management of CSME, and 277 (8.41%) for further evaluation of clinically suspicious macular imaging findings. The cause of decreased vision could not be determined at the mobile teleophthalmology units in 146 (4.43%) of the urgently referred patients. Good-quality fundus photographs could not be captured in 119 (3.61%) patients. These patients were also referred to the district hospital. Other referrals were provided for visually significant cataract (n = 1684, 51.14%), age-related macular degeneration (n = 256, 7.77%), glaucoma (n = 68, 2.06%), and other reasons (n = 20, 0.61%). Re-screening guidelines of the Early Treatment Diabetic Retinopathy Study classification were used to advise follow-up to all other patients [9, 10-12].
The mean cost of travel by bus in the district was Rs. 8 for 10 km. The distance to the hospital from the camps ranged from 4 to 204 km, with a mean (SD)distance of 80.22 (43.85) km. The mean (SD) cost of traveling from the camp site to the hospital was Rs. 61.48 (29.11). Most examined patients visited the hospital with an attendant, thereby doubling the cost of travel per visit. This traveling cost was saved when the patients underwent teleophthalmologic consultations.
Patient satisfaction was recorded as “yes” or “no” as answered by the patient. A total of 10 974 out of 12 157 (90.27%) patients who underwent teleophthalmic consultation were satisfied with the service. Table 2 shows the detailed responses with respect to patient satisfaction to various questions. Out of all the questions asked, maximum positive responses (71%) were received for the following question: “Were you able to attend the camp without help/support?” Table 3 shows increasing trends in the number of camps held, with the footfall in these camps and teleconsultations held every 2 years from 2012 to 2020. The percentage of teleconsultations showed an increasing trend over the years (P = 0.001; Table 3). Similarly, trends were studied for the number of patients reporting to the hospital (Table 4), and the attrition rate decreased over the years (P = 0.019).
Table 2.
Patient response to teleophthalmology service
| Variable | Satisfied (%) |
|---|---|
| Cost-effectiveness | 37 |
| Convenience | 31 |
| Time-saving | 33 |
| Ability to attend camps without help | 71 |
| Sufficient satisfaction to recommend teleophthalmology to others | 63 |
| Preference for teleophthalmology consultation in the future | 24 |
| Information provided by the staff in the mobile bus | 69 |
| Video conferencing with the ophthalmologist | 79 |
| Teleophthalmology screening | 36 |
Table 3.
Number of camps held, footfall in these camps, and number of teleconsultations conducted every 2 years from 2012 to 2020: changing trends
| Year | Camp Places, n (%) | *Number of Patients examined by Optometrist, n (%) | Total fundus photo taken, n | *Tele Consultation done, n (%) |
|---|---|---|---|---|
| 2012 – 14 | 52 (7.62) | 3350 (11.08) | 1587 | 1587 (13.05) |
| 2014 – 16 | 249 (36.51) | 5700 (18.86) | 2398 | 2398 (19.73) |
| 2016 – 18 | 204 (29.91) | 10 993 (36.36) | 3918 | 3918 (32.23) |
| 2018 – 20 | 177 (25.95) | 10 187 (33.70) | 4254 | 4254 (34.99) |
| Total | 682 (100) | 30 230 (100) | 12 157 | 12 157 (100) |
Abbreviations: n, number; %, percentage. Note: *P-value = 0.001 calculated with the chi-square test.
Table 4.
Proportion of patients reporting to the hospital every 2 years and change in the attrition rate over the years
| Year | Teleconsultation done , n | Patients asked to report to a hospital , n (%) | Patients who reported to the hospital, n (%) | *Attrition rate, % |
|---|---|---|---|---|
| 2012 – 14 | 1587 | 445 (28.04) | 311 (69.89) | 30.11 |
| 2014 – 16 | 2398 | 676 (28.19) | 473 (69.97) | 30.03 |
| 2016 – 18 | 3918 | 1065 (27.18) | 799 (75.02) | 24.98 |
| 2018 – 20 | 4254 | 1107 (26.02) | 855 (77.24) | 22.77 |
| Total | 12 157 | 3293 (27.09) | 2438 (74.04) | 25.97 |
Abbreviations: n, number; %, percentage. Note: *P-value = 0.019 calculated by Chi-square test.
DISCUSSION
The present study found that teleconsultations showed an increasing trend over the years and that the attrition rate decreased over the years. Teleophthalmology is achieving success in providing high-quality service, easy access to care, and high patient satisfaction.
Approximately 285 million people are visually impaired worldwide, out of which 39 million are blind. By 2030, 552 million people are estimated to develop diabetes mellitus, causing an increased drift of diabetes by 69% in developing countries [13]. Approximately one-third of the patients with diabetes develop DR, which is the most frequent cause of blindness in the working population aged 20 – 74 years [14]. The prevalence of DR is expected to be doubled by 2050, involving 14.6 million individuals [15, 16]. The asymptomatic nature of DR in its early stages necessitates early detection and management before the onset of visual symptoms and the worsening of visual acuity [17].
Teleophthalmology is a dependable method for recognizing patients requiring interventions for CSME and PDR [18]. A study from diabetes clinics in the United States of America reported that one in five patients with diabetes developed DR detected via teleophthalmology [19]. In our experience, the total number of patients screened with diabetes progressively increased, reaching the figure of 10 187 patients in 2018 – 2020 from 3350 patients in 2012 – 2014. This might be due to less awareness and availability of teleophthalmology in 2012 compared to now. Over 20% increase in teleconsultation for DR showed that teleophthalmology is a good complement to existing services. It was easy to screen normal eyes with no DR at the primary level only and follow-up annually without a referral [20]. In the present study, approximately 50% of the patients showed normal ocular examination findings, with no features of DR. This approach provided a cost-effective, door-to-door service, which was convenient for both patients and ophthalmologists [21]. Moreover, it reduced the workload of ophthalmologists and allowed them to spend more time on patients with severe diseases at the primary health level [22].
Different studies have used different parameters to gauge the usefulness of teleophthalmology as a screening tool for DR [23], including improvement in unnecessary referrals, improved interpretation of retinographs by family doctors, and cost-effectiveness [24, 25]. This makes it difficult to compare the results of these studies, although all of them prove teleophthalmology to be promising in the diagnosis of DR. In the present study, we evaluated the efficacy of this tool based on changing trends with time and patient satisfaction.
Similar to the present study, changing trends of teleophthalmology screening in DR were evaluated in Pareja-Rios et al.’s study from Spain [25]. Over 8 years, from 2007 to 2015, the number of patients screened with this tool increased, easing the workload of specialists and enabling better interpretation of images by family physicians. However, they partially attributed this improvement to a change in the policy during the study period that allowed the use of tropicamide [25]. In the present study, we consistently evaluated fundus photographs of the non-dilated eye, interpreted by ophthalmologists instead of family doctors. The duration of diabetes mellitus was associated with the severity of DR.
Patient satisfaction is a growing responsibility of the healthcare system. Patient satisfaction with teleophthalmology ranges from 88% to 99.8% [26, 27]. In the present study, a creditable response was provided by 90.27% of the patients who received teleconsultation. However, we included multiple factors influencing patient satisfaction to achieve a more comprehensive response. Patients’ difficulties in receiving regular examinations nearby and attending multiple hospital visits, adequate follow-up, and timely referral were addressed by this program. Over the study period, more patients preferred teleophthalmology owing to the advantages of saving time, no requirement of traveling, cost-effectiveness, and convenience. Increased quality of assistance and patient accessibility to telemedicine prompt it to be a preferred modality.
Approximately 27% of patients with moderate-to-severe NPDR and PDR were referred for further evaluation in the present study, while < 20% of patients received this referral in previous studies. This might be due to differences in the inclusion criteria, screening methods, and ethnicity of the study population [28-30]. Massin et al. reported that 25% of the patients had been referred to the hospital, including those with cataract and poor-quality photographs in addition to diabetes [31]. In the present study, an ophthalmologic referral was provided to patients with severe disease, and > 70% of patients reached the hospital for further assessment. The decreased attrition rate over the study period indicates good patient satisfaction with this program, strengthening their belief in teleconsultation services.
The conventional method for DR screening was fundus examination under mydriasis, with an immediate check-up for type 2 diabetes mellitus and a check-up after 5 years of the diagnosis of type 1 diabetes mellitus [32, 33]. The annual requirement of follow-ups for DR and the increasing global diabetic population suggest that examinations under mydriasis would require over 4.5 million h/year [34]. Therefore, screening methods involving the use of non-mydriatic cameras are showing an increasing trend [35-37].
Similarly, in the present study, we used non-mydriatic fundus imaging and led to on-time screening of patients with DR. Moreover, screening via teleophthalmology has a sensitivity of > 80% and specificity of > 90%, thereby making it an effective technique. However, its application has been challenging because of the requirement for high-definition digital images [38, 39].
The outcomes of the present study are consistent with those of other previous studies, which showed a satisfaction rate of around 90% or more with tele-consultation [40-42]. To the best of our knowledge, this is the first study from India involving patients with diabetes to report the patient satisfaction rate over 8 years. The present findings are supported by the findings of Kumari et al.’s study from India, which reported a satisfaction rate of 94% with telemedicine at a diabetes clinic [41]. However, theirs was a comparative study involving 132 patients attending a single camp in South India. In the present study, the lack of requirement for help or support was an advantage of teleconsultation for most (71%) people. People were ready to undergo follow-ups via teleconsultation, consistent with the findings of Kujri et al.’s study, which reported over 50% of patients preferred teleophthalmology for follow-ups [26]. The accessibility, reduced examination time, and convenience were the supporting factors.
Teleophthalmology, as a collateral benefit, facilitates easy maintenance of records/documentation [42]. Since it is a screening program, a high sensitivity helps in earlier diagnosis and prevents progression. Asymptomatic patients who missed their annual fundus evaluation were also examined via teleconsultation when they visited the non-communicable disease camp.
This study has some limitations. First, the study design was retrospective. Second, the questionnaire used for patient satisfaction was not checked for internal consistency or reliability. However, this was an Indian study on DR screening using a non-mydriatic method with a large sample size that reported the 8-year experience in mobile teleophthalmology. Multicentric studies involving more ophthalmologists and patients from different parts of the country with details of follow-ups at the screening camps for disease monitoring may be useful in promoting teleophthalmology as a routine screening tool in the future.
CONCLUSIONS
Teleconsultations showed an increasing trend over the years, and the attrition rate decreased over the years. Teleophthalmology is achieving success in providing high-quality service, easy access to care, and high patient satisfaction. Teleophthalmology is a feasible option for screening numerous patients with diabetes and can become the screening system of choice, primarily in underserviced rural regions. Additionally, during the coronavirus disease pandemic, teleophthalmology is a potential innovation with high patient satisfaction and widespread adoption. Future studies on the role of teleophthalmology for other leading preventable causes of blindness should be conducted.
ETHICAL DECLARATIONS
Ethical approval:
The ethics committee of the community medicine department of our university provided ethical approval for the study. The study was performed in accordance with the tenets of the Helsinki Declaration. All participants provided consent.
Conflict of interest:
None.
FUNDING
This work was funded by the Hospital Development Committee of the district hospital of Palakkad. The initial capital investment was made by the National Prevention of Control of Blindness (NPCB) fund from Delhi (Rs. 65 L) and Palakkad Jilla Panchayat (Rs. 25 L). The bus and equipment were purchased with this investment. The e-Nayana teleophthalmology software developer was the Center for Development of Advanced Computing (CDAC) under the Government of India.
ACKNOWLEDGMENTS
None.
References
- 1.Sharma M, Jain N, Ranganathan S, Sharma N, Honavar SG, Sharma N, et al. Tele-ophthalmology: Need of the hour. Indian J Ophthalmol. 2020;68(7):1328–1338. doi: 10.4103/ijo.IJO_1784_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Driver DD. Teleophthalmology for first nations clients at risk of diabetic retinopathy: a mixed methods evaluation. JMIR Med Inform. 2015;3(1):e10. doi: 10.2196/medinform.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Board of Governors in supersession of the Medical Council of India (2020) ‘Telemedicine Practice Guidelines’. [Accessed: May 14, 2020]. Available at: https://www.mohfw.gov.in/pdf/Telemedicine.pdf.
- 4.John S, Sengupta S, Reddy SJ, Prabhu P, Kirubanandan K, Badrinath SS. The Sankara Nethralaya mobile teleophthalmology model for comprehensive eye care delivery in rural India. Telemed J E Health. 2012;18(5):382–7. doi: 10.1089/tmj.2011.0190. [DOI] [PubMed] [Google Scholar]
- 5.Azzolini C, Torreggiani A, Eandi C, Donati S, Oum MA, Vinciguerra R, et al. A teleconsultation network improves the efficacy of anti-VEGF therapy in retinal diseases. J Telemed Telecare. 2013;19(8):437–42. doi: 10.1177/1357633X13501760. [DOI] [PubMed] [Google Scholar]
- 6.Prathiba V, Rema M. Teleophthalmology: a model for eye care delivery in rural and underserved areas of India. Int J Family Med. 2011;2011:683267. doi: 10.1155/2011/683267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kartha MC, Sudhamony S, Elizabeth Thomas T, Dinu D, Sudalaimani C. 2013 IEEE Global Humanitarian Technology Conference. South Asia Satellite (GHTC-SAS): IEEE; 2013. Mobile tele-ophthalmology units for early detection and treatment of serious eye ailments among rural population; pp. 187–190. [Google Scholar]
- 8.Sheheersha SK, Saravanabavan V. ‘An analysis of the role of sunayanam mobile ophthalmology unit for preventing blindness among rural poor in Thiruvantahapuram district’. In: Saravanabavan V, editor. Geospatial Technologies for Resource Evaluation and Management . Madurai, India: Jayalakshmi Publication; 2015. pp. 412–414. ISBN : 978-93-84193-36-2 . [Google Scholar]
- 9.Solomon SD, Goldberg MF. ETDRS Grading of Diabetic Retinopathy: Still the Gold Standard? Ophthalmic Res. 2019;62(4):190–195. doi: 10.1159/000501372. [DOI] [PubMed] [Google Scholar]
- 10.Yonekawa Y, Modi YS, Kim LA, Skondra D, Kim JE, Wykoff CC. American Society of Retina Specialists Clinical Practice Guidelines on the Management of Nonproliferative and Proliferative Diabetic Retinopathy without Diabetic Macular Edema. J Vitreoretin Dis. 2020;4(2):125–135. doi: 10.1177/2474126419893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gračner T. Screening for Diabetic Retinopathy - a Twelve-Month Review. Acta Clin Croat. 2020;59(3):424–430. doi: 10.20471/acc.2020.59.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alali NM, Albazei A, Alotaibi HM, Almohammadi AM, Alsirhani EK, Alanazi TS, et al. Diabetic Retinopathy and Eye Screening: Diabetic Patients Standpoint, Their Practice, and Barriers; A Cross-Sectional Study. J Clin Med. 2022;11(21):6351. doi: 10.3390/jcm11216351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94(3):322–32. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med. 2013;30(4):387–98. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 15.Khattab AAA, Ahmed MM, Hammed AH. Pars plana vitrectomy for tractional diabetic macular edema with or without internal limiting membrane peeling. Med Hypothesis Discov Innov Ophthalmol. 2022;11(3):110–118. doi: 10.51329/mehdiophthal1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Namaeh M. Common causes of visual impairment in the elderly. Med Hypothesis Discov Innov Ophthalmol. 2021;10(4):191–200. doi: 10.51329/mehdiophthal1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale MJ, Scruggs BA, Flaxel CJ. Diabetic eye disease: A review of screening and management recommendations. Clin Exp Ophthalmol. 2021;49(2):128–145. doi: 10.1111/ceo.13894. [DOI] [PubMed] [Google Scholar]
- 18.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology. 2007;114(9):1748–54. doi: 10.1016/j.ophtha.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Owsley C, McGwin G Jr, Lee DJ, Lam BL, Friedman DS, Gower EW, et al. Diabetes eye screening in urban settings serving minority populations: detection of diabetic retinopathy and other ocular findings using telemedicine. JAMA Ophthalmol. 2015;133(2):174–81. doi: 10.1001/jamaophthalmol.2014.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: an overview. Indian J Community Med. 2011;36(4):247–52. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy GVS. Situational analysis of diabetic retinopathy screening in India: How has it changed in the last three years? Indian J Ophthalmol. 2021;69(11):2944–2950. doi: 10.4103/ijo.IJO_1242_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Topp SM. The significance of primary health care for building back better: lessons from COVID-19. WHO South-East Asia Journal of Public Health. 2021;10(3):3–5. [Google Scholar]
- 23.Liu Y, Zupan NJ, Swearingen R, Jacobson N, Carlson JN, Mahoney JE, et al. Identification of barriers, facilitators and system-based implementation strategies to increase teleophthalmology use for diabetic eye screening in a rural US primary care clinic: a qualitative study. BMJ Open. 2019;9(2):e022594 . doi: 10.1136/bmjopen-2018-022594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez Villa S, Alonso Álvarez C, de Dios Del Valle R, Salazar Méndez R, Cuesta García M, Ruiz García MJ, et al. Five-year experience of tele-ophthalmology for diabetic retinopathy screening in a rural population. Arch Soc Esp Oftalmol. 2016;91(9):426–30. doi: 10.1016/j.oftal.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Pareja-Ríos A, Bonaque-González S, Serrano-García M, Cabrera-López F, Abreu-Reyes P, Marrero-Saavedra MD. Tele-ophthalmology for diabetic retinopathy screening: 8 years of experience. Arch Soc Esp Oftalmol. 2017;92(2):63–70. doi: 10.1016/j.oftal.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Kurji K, Kiage D, Rudnisky CJ, Damji KF. Improving diabetic retinopathy screening in Africa: patient satisfaction with teleophthalmology versus ophthalmologist-based screening. Middle East Afr J Ophthalmol. 2013;20(1):56–60. doi: 10.4103/0974-9233.106388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul PG, Raman R, Rani PK, Deshmukh H, Sharma T. Patient satisfaction levels during teleophthalmology consultation in rural South India. Telemed J E Health. 2006;12(5):571–8. doi: 10.1089/tmj.2006.12.571. [DOI] [PubMed] [Google Scholar]
- 28.Martínez Rubio M, Moya Moya M, Bellot Bernabé A, Belmonte Martínez J. Cribado de retinopatía diabética y teleoftalmología [Diabetic retinopathy screening and teleophthalmology] Arch Soc Esp Oftalmol. 2012;87(12):392–5. doi: 10.1016/j.oftal.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Boucher MC, Desroches G, Garcia-Salinas R, Kherani A, Maberley D, Olivier S, et al. Teleophthalmology screening for diabetic retinopathy through mobile imaging units within Canada. Can J Ophthalmol. 2008;43(6):658–68. doi: 10.3129/i08-120. [DOI] [PubMed] [Google Scholar]
- 30.Cavallerano AA, Cavallerano JD, Katalinic P, Blake B, Rynne M, Conlin PR, et al. A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center--the Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139(4):597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 31.Massin P, Chabouis A, Erginay A, Viens-Bitker C, Lecleire-Collet A, Meas T, et al. OPHDIAT: a telemedical network screening system for diabetic retinopathy in the Ile-de-France. Diabetes Metab. 2008;34(3):227–34. doi: 10.1016/j.diabet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Eszes DJ, Szabó DJ, Russell G, Kirby P, Paulik E, Nagymajtényi L, et al. Diabetic Retinopathy Screening Using Telemedicine Tools: Pilot Study in Hungary. J Diabetes Res. 2016;2016:4529824. doi: 10.1155/2016/4529824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal M, Singhal A, Kumar P, Vats S, Kaushik J, Srujana D, et al. Pattern and distribution of neovascularization in proliferative diabetic retinopathy on fundus fluorescein angiography: A growing paradigm. Medical Journal Armed Forces India . 2021 doi: 10.1016/j.mjafi.2021.02.006. doi: 10.1016/j.mjafi.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tayapad JB, Bengzon AU, Valero SO, Arroyo MH, Papa RT, Fortuna EJ, et al. Implementation and pilot data on diabetic retinopathy in a teleophthalmology program at a multispecialty primary care clinic. Philipp J Ophthalmol. 2014;39:90–3. [Google Scholar]
- 35.Yaslam M, Al Adel F, Al-Rubeaan K, AlSalem RK, Alageel MA, Alsalhi A, et al. Non-mydriatic fundus camera screening with diagnosis by telemedicine for diabetic retinopathy patients with type 1 and type 2 diabetes: a hospital-based cross-sectional study. Ann Saudi Med. 2019;39(5):328–336. doi: 10.5144/0256-4947.2019.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Chen R, Lu Y, Dou X, Ye B, Cai Z, et al. Single-Field Non-Mydriatic Fundus Photography for Diabetic Retinopathy Screening: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2019;62(2):61–67. doi: 10.1159/000499106. [DOI] [PubMed] [Google Scholar]
- 37.Upadhyaya S, Agarwal A, Rengaraj V, Srinivasan K, Newman Casey PA, Schehlein E. Validation of a portable, non-mydriatic fundus camera compared to gold standard dilated fundus examination using slit lamp biomicroscopy for assessing the optic disc for glaucoma. Eye (Lond). 2022;36(2):441–447. doi: 10.1038/s41433-021-01485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liesenfeld B, Kohner E, Piehlmeier W, Kluthe S, Aldington S, Porta M, et al. A telemedical approach to the screening of diabetic retinopathy: digital fundus photography. Diabetes Care. 2000;23(3):345–8. doi: 10.2337/diacare.23.3.345. [DOI] [PubMed] [Google Scholar]
- 39.John S, Srinivasan S, Sundaram N. Validation of computer-aided diagnosis of diabetic retinopathy from retinal photographs of diabetic patients from Telecamps. Tele health and Medicine Today. 2021;6(4):1–11. [Google Scholar]
- 40.Sreelatha OK, Ramesh SV. Teleophthalmology: improving patient outcomes? Clin Ophthalmol. 2016;10:285–95. doi: 10.2147/OPTH.S80487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumari Rani P, Raman R, Manikandan M, Mahajan S, Paul PG, Sharma T. Patient satisfaction with tele-ophthalmology versus ophthalmologist-based screening in diabetic retinopathy. J Telemed Telecare. 2006;12(3):159–60. doi: 10.1258/135763306776738639. [DOI] [PubMed] [Google Scholar]
- 42.Nuzzi R, Bovone D, Maradei F, Caselgrandi P, Rossi A. Teleophthalmology Service: Organization, Management, Actual Current Applications, and Future Prospects. Int J Telemed Appl. 2021;2021:8876957. doi: 10.1155/2021/8876957. [DOI] [PMC free article] [PubMed] [Google Scholar]