Abstract
Introduction.
Gastric mucosal biopsies and resections from patients treated with neoadjuvant radiation and/or chemotherapy are frequently encountered. These samples may show histologic features related to therapy including inflammation, ulceration, and epithelial atypia. In some cases, epithelial atypia may be marked, prompting the use of adjunct p53 immunohistochemistry. We examined p53 expression by immunohistochemistry in gastric mucosa following therapy.
Methods.
We evaluated the histology and p53 immunohistochemical expression in gastric mucosa from 57 resections and 3 mucosal biopsies, from 60 patients treated with radiation and/or chemotherapy for gastroesophageal carcinoma (n = 33) or pancreatic carcinoma (n = 27).
Results.
We identified histomorphologic features of therapy-related epithelial changes in 50 of 60 cases (83%). Abnormal p53 expression was present at least focally in nearly half the cases (27 of 60 cases; 45%), all of which showed morphologic evidence of therapy-related epithelial changes. Neuroendocrine cell micronests were present in 37 of 60 cases (62%). Next-generation sequencing (NGS) of foci with therapy-related epithelial changes showing abnormal p53 expression and carcinoma from the same patient was attempted and yielded results in 1 patient. Interestingly, differing TP53 alterations in the patient’s adenocarcinoma and in a histologically benign esophageal submucosal gland with therapy-related epithelial changes and abnormal p53 expression were identified.
Conclusions.
Our results demonstrate that abnormal p53 expression is relatively common in gastric mucosal samples following radiation and/or chemotherapy and suggest that p53 expression should be avoided when distinguishing therapy-related changes from dysplasia or carcinoma. Furthermore, our NGS results raise interesting biological questions, which may warrant further investigation.
Keywords: stomach, esophagus, immunohistochemistry, p53, dysplasia, carcinoma
Introduction
Neoadjuvant chemotherapy and/or radiation are the mainstays of treatment for a variety of malignancies, including upper gastrointestinal (GI) and pancreatic malignancies. GI mucosal injury following radiation and/or chemotherapy is well known, and patients may present clinically with loss of appetite, nausea, vomiting, abdominal pain, diarrhea, ulceration, or bleeding, which may be severe in rare cases.1–3 Samples may be sent for pathologic evaluation in symptomatic patients, as part of endoscopic disease surveillance, or for cancer resections.
The histologic features of gastric mucosal injury associated with radiation and/or chemotherapy include inflammation, ulceration, and epithelial atypia, including cytoplasmic mucin depletion, glandular atrophy, cytoplasmic hypereosinophilia, and nuclear changes (anisonucleosis, hyperchromasia, and pseudostratification, prominent nucleoli).4–9 In some cases, epithelial changes may be pronounced to the point of mimicking dysplasia and/or carcinoma.7–10 Therapy-related changes can usually be distinguished from dysplasia or carcinoma by histomorphology. Morphologic features favoring therapy-related changes include preserved mucosal architecture, bizarre nuclei, epithelial changes accentuated toward the bases of glands with surface maturation, low nuclear:cytoplasmic ratio, cytoplasmic hypereosinophilia, or vacuolization, similar atypia within fibroblasts and endothelial cells, and lack of adjacent intestinal metaplasia.7–11
In rare cases, the distinction between therapy-related atypia and dysplasia or carcinoma may be particularly challenging and the use of adjunct studies including p53 immunohistochemical staining may be considered. TP53 is a tumor suppressor gene that is frequently mutated in gastroesophageal or gastric adenocarcinoma and dysplasia. Evaluation of p53 protein expression by immunohistochemistry can be used as a surrogate marker of TP53 mutation status and is commonly used to confirm a diagnosis of columnar neoplasia.12 Anecdotally, we encountered a few cases of gastric mucosa with therapy-related changes that showed abnormal p53 expression on immunohistochemical staining. Given that this could represent an important pitfall leading to the overdiagnosis of dysplasia or carcinoma, we systematically examined p53 immunohistochemical staining in the gastric mucosa of a series of patients following neoadjuvant radiation and/or chemotherapy.
Materials and Methods
Case Selection
This institutional review board (IRB) approved study included a search of the departmental pathology database for pancreaticoduodenectomy and esophagogastrectomy resection specimens taken between January 1, 2015 and December 31, 2015. Patients who had resections for non-neoplastic disease and those who did not receive chemotherapy or radiation prior to resection were excluded. Three additional cases were identified during routine sign-out, including 2 gastric biopsies and 1 pancreaticoduodenectomy from 2 other contributing institutions.
Fifty-seven patients with resection specimens and 3 patients with biopsy specimens treated with chemotherapy and/or radiation for gastroesophageal adenocarcinoma (n = 30), squamous cell carcinoma of the esophagus (n = 3), or pancreatic ductal adenocarcinoma (n = 27) were included. Demographic and clinical information for each patient were obtained from the electronic medical record.
Review of Histopathology
For each case, 2 authors (D.A.H. and E.AM) reviewed all available hematoxylin and eosin (H&E) stained sections containing gastric mucosa and representative tumor sections. The presence or absence of therapy-related epithelial changes in columnar mucosa was noted. Features of therapy-related epithelial changes included nuclear changes (pseudostratification, hyperchromasia, nucleomegaly, anisonucleosis, and prominent nucleoli), increased mitoses, cytoplasmic mucin depletion, hypereosinophilia, or clear cell changes, glandular atrophy, and/or irregular microcystic change as previously described by Brien et al.7 The presence or absence of neuroendocrine cell micronests, defined as scattered, non-confluent clusters of 5 to 15 neuroendocrine cells in the lamina propria or muscularis mucosae was also noted.13, 14
Immunohistochemistry
Immunohistochemistry was performed using standard automated immunohistochemistry systems at each institution with commercially available anti-p53 mouse monoclonal antibodies (Clone BP-53-11, Ventana Medical Systems Inc., Oro Valley, AZ for the Johns Hopkins cases; Clone DO-7, Leica Biosystems, Buffalo Grove, IL for the University of Miami cases; Clone DO-7, Ventana Medical Systems Inc., Oro Valley, AZ for the Cedars Sinai Medical Center case) and was interpreted by 2 authors (D.A.H. and E.A.M) using criteria previously described by Redston et al. for esophageal samples.12–15 Briefly, the percentage of nuclei with positive staining was scored on an intensity scale of 0 to 3+, with 0 + representing no staining and 3 + representing very strong staining. p53 staining was considered abnormal if there was (1) 2 to 3 + nuclear positivity in >50% of cells in at least 1 glandular base or profile or within a contiguous focus of at least 20 surface epithelial cells; (2) total absence of staining (“null”; 0+) in all epithelial cells of at least 1 pit base or granular profile; and (3) strong cytoplasmic staining with complete loss of nuclear staining. p53 staining was considered normal if there was 2 to 3 + nuclear positivity in <50% of epithelial cells in all individual crypts or glandular profiles, and all contiguous stretches of surface epithelium.
Immunohistochemistry for chromogranin A (Clone LK2H10, Roche) was performed on the 57 Johns Hopkins cases to confirm the presence or absence of neuroendocrine cell micronests.
Next-Generation Sequencing
Paired foci of gastric mucosa with therapy-related epithelial changes showing abnormal p53 expression and carcinoma from the same case were designated by D.A.H. and E.A.M. Formalin-fixed paraffin-embedded (FFPE) tissues were macro dissected from the designated areas using Pinpoint reagents (ZymoResearch), followed by DNA extraction using Tissue Preparation System (Siemens) as previously described by Zheng et al.16 Next-generation sequencing (NGS) was conducted using AmpliSeq Cancer Hotspot Panel (v2) and Ion S5 XL sequencer (Life Technologies) for targeted multi-gene amplification as previously described by Zheng et al.16 Briefly, mutations were identified and annotated through both Torrent Variant Caller (Life Technologies) and direct visual inspection of the binary sequence alignment/map file using the Broad Institute’s Integrative Genomics Viewer (IGV) (http://www.broadinstitute.org/igv/).17 The limit of detection of the assay was at 2% allele frequency (AF). Mutations were evaluated and confirmed to be pathogenic/likely pathogenic using genomic databases (ClinVar, OncoKB™, COSMIC) and primary literature sources, including Xu et al.18–21
Statistical Analysis
Statistical analyses were performed using R statistical programming language (R Foundation). Clinicopathologic variables were compared using Pearson’s chi-squared test.
Results
Clinical Characteristics
The clinical characteristics are summarized in Table 1. Of the 60 patients, two-thirds (40 of 60) were male and most patients (88%) self-reported as White. The median age was 64 years (range: 47–83 years). The underlying malignancy was gastroesophageal adenocarcinoma in 30 patients (50%), esophageal squamous cell carcinoma in 3 (5%), and pancreatic ductal adenocarcinoma in 27 (45%). Fifty-four patients (90%) were treated with chemotherapy and radiation, including 1 patient treated with chemotherapy and yttrium radioembolization. Six patients (10%) were treated with chemotherapy alone. None were treated with radiation alone.
Table 1.
Clinical and Histopathologic Features with Abnormal or Normal p53 Immunohistochemistry.
| p53 expression by immunohistochemistry |
||||
|---|---|---|---|---|
| All patients (n = 60) | Abnormal (n = 27) | Normal (n = 33) | ||
|
| ||||
| n (%) | n (%) | n (%) | P value | |
|
| ||||
| Age, median (range), years | 64 (range: 47–83) | 66 (range: 50–83) | 62 (range: 47–75) | |
| Sex | ||||
| M | 40 (67%) | 19 (48%) | 21 (52%) | .78 |
| F | 20 (33%) | 8 (40%) | 12 (60%) | |
| Race | ||||
| White | 53 (88%) | 23 (43%) | 30 (57%) | .47 |
| Black or African American | 5 (8%) | 3 (60%) | 2 (40%) | |
| Asian | 1 (2%) | 1 (100%) | 0 (0%) | |
| Hispanic | 1 (2%) | 0 (0%) | 1 (100%) | |
| Underlying malignancy | ||||
| Gastroesophageal adenocarcinoma | 30 (50%) | 17 (57%) | 13 (43%) | .09 |
| Esophageal squamous cell carcinoma | 3 (5%) | 2 (67%) | 1 (33%) | |
| Pancreatic ductal adenocarcinoma | 27 (45%) | 8 (30%) | 19 (70%) | |
| Treatment | ||||
| Chemoradiationa | 54 (90%) | 27 (50%) | 27 (50%) | .06 |
| Chemotherapy only | 6 (10%) | 0 (0%) | 6 (100%) | |
| Interval from last treatment to specimen collection | ||||
| < = 30 days | 12 (20%) | 6 (50%) | 6 (50%) | .62 |
| 3–60 days | 35 (58%) | 17 (49%) | 18 (51%) | |
| >60 days | 12 (20%) | 4 (25%) | 8 (75%) | |
| Unknown | 1 (2%) | 0 (0%) | 1 (100%) | |
| Location | ||||
| Gastroesophageal junction/gastric cardia | 32 (53%) | 18 (56%) | 14 (44%) | .25 |
| Gastric body | 2 (3%) | 1 (50%) | 1 (50%) | |
| Gastric antrum | 24 (40%) | 7 (29%) | 17 (71%) | |
| Pylorus | 2 (3%) | 1 (50%) | 1 (50%) | |
| Therapy-related epithelial changes | ||||
| Present | 50 (83%) | 27 (54%) | 23 (46%) | .005 |
| Absent | 10 (17%) | 0 (0%) | 10 (100%) | |
| Neuroendocrine cell micronests | ||||
| Present | 37 (62%) | 21 (57%) | 16 (43%) | .04 |
| Absent | 23 (38%) | 6 (26%) | 17 (74%) | |
Bold values indicate statistical significance, P value < .05
Includes 1 patient treated with chemotherapy and yttrium radioembolization.
Histopathologic and Immunohistochemical Findings
The histopathologic and immunohistochemical findings are summarized in Table 1. Features of therapy-related epithelial changes were present at least focally in specimens from 50 of 60 patients (83%), including all patients with gastroesophageal carcinoma and in the gastric mucosa of 17 of 27 (63%) of patients with pancreatic ductal adenocarcinoma. In all cases, therapy-related epithelial changes were morphologically distinct from the patient’s underlying carcinoma and dysplasia, when present.
In all cases, p53 immunohistochemical staining was performed on representative sections of gastric mucosa with therapy-related epithelial changes (n = 50) or non-neoplastic gastric mucosa (n = 10). Abnormal p53 expression was at least focally present in columnar epithelium in 27 of 60 (45%) cases, all of which showed morphologic features of therapy-related epithelial changes (Figure 1) and was absent in areas lacking therapy-related epithelial changes. All cases with abnormal p53 expression showed 2 to 3 + nuclear positivity in >50% of cells in at least 1 glandular profile or base. Other abnormal patterns of p53 expression, including increased nuclear positivity in contiguous foci of surface epithelial cells, complete loss of staining (null), and/or cytoplasmic staining patterns, were not observed. Abnormal p53 expression was associated with the presence of therapy-related epithelial changes (P = .005) and neuroendocrine cell micronests (P = .04) and showed a trend toward association with treatment with combined chemoradiation therapy versus chemotherapy alone (P = .06). Abnormal p53 expression was also more frequent in samples from patients with gastroesophageal carcinomas (19 of 33; 58%) versus those with pancreatic ductal adenocarcinoma (8 of 27; 30%), though this did not reach statistical significance.
Figure 1.
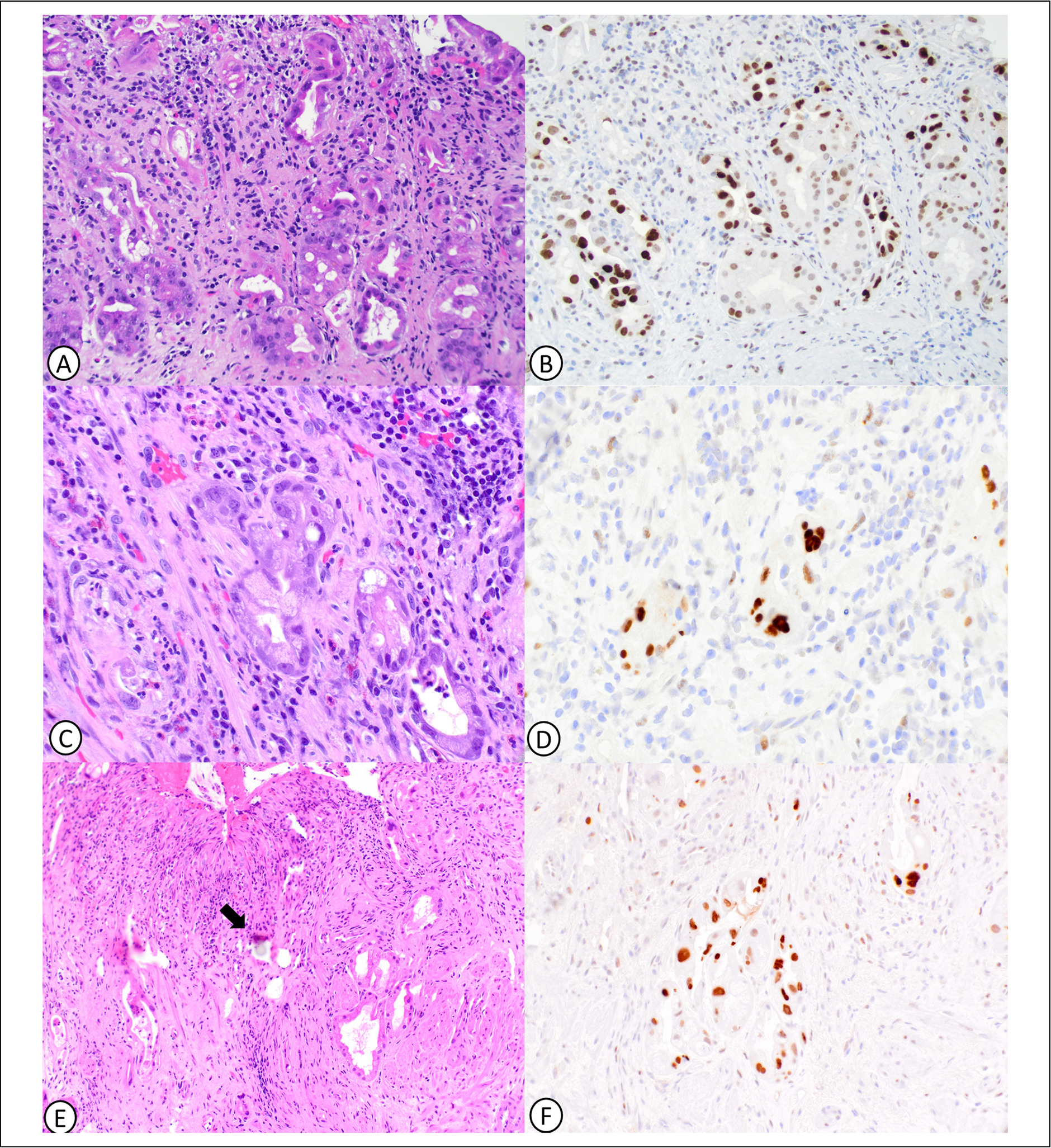
Therapy-related epithelial changes and abnormal p53 expression in gastric mucosa. Gastric oxyntic mucosa with deep glands showing microcystic change with attenuated epithelial lining (A) and abnormally increased p53 expression (B). Gastric cardia mucosa with glands showing nuclear pleomorphism and nucleoli but low nuclear: cytoplasmic ratio (C) and abnormally increased p53 expression (D). Gastric mucosa with yttrium radioembolization beads (arrow) with associated bizarre epithelial atypia (E) and abnormally increased p53 expression (F).
Neuroendocrine cell micronests were present in 37 of 60 cases (62%) by H&E and/or chromogranin immunohistochemical staining (Figure 2, Table 2). Chromogranin staining was performed in 57 cases and confirmed the presence of neuroendocrine cell micronests in 35 cases. Of the remaining 3 cases without chromogranin staining, neuroendocrine cell micronests were identifiable on H&E in 2 cases. Neuroendocrine cell micronests were more common in samples from the gastroesophageal junction or gastric cardia (P = .006) and showed a trend toward association with the presence of therapy-related epithelial changes (P = .06).
Figure 2.
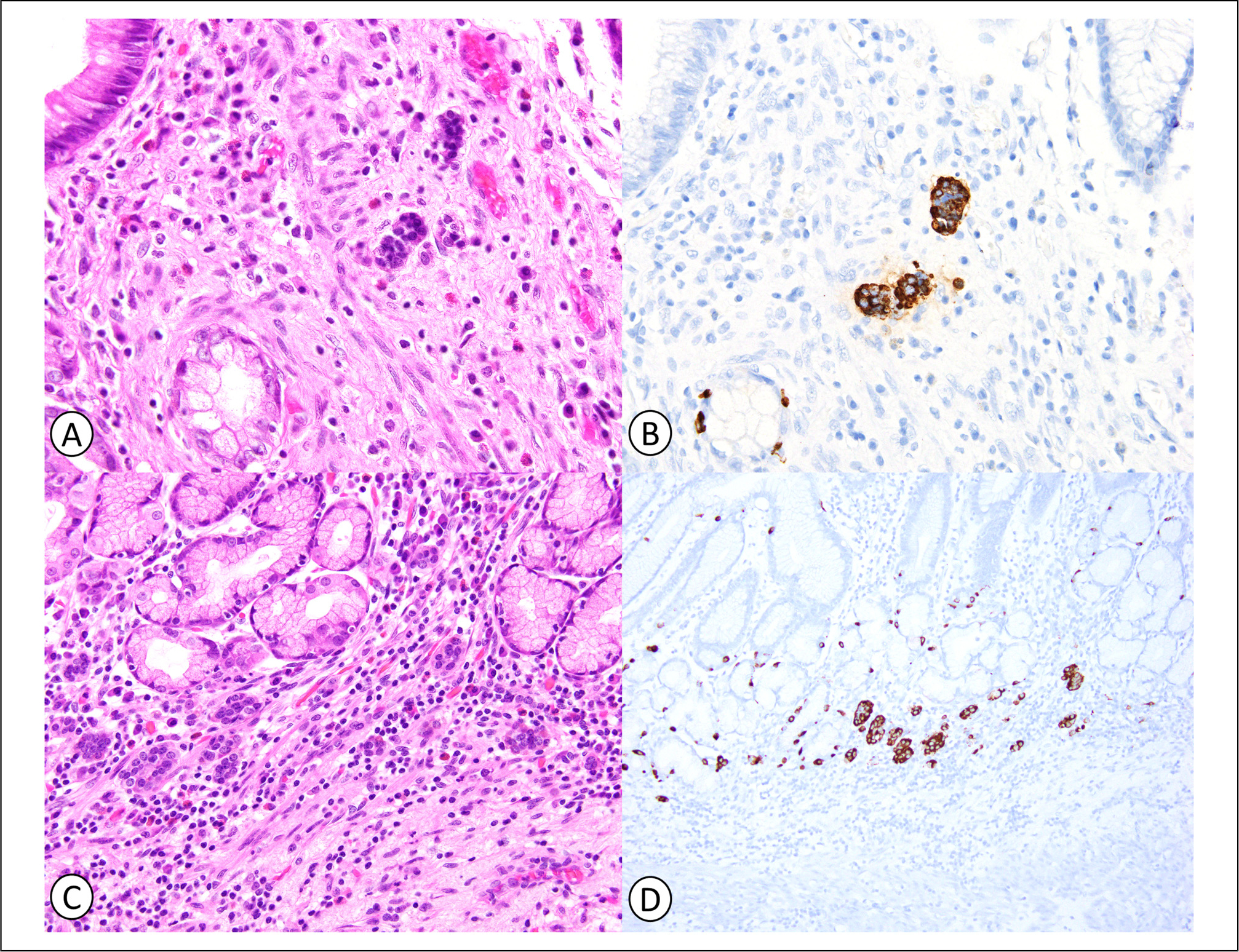
Neuroendocrine cell micronests in gastric mucosa with therapy-related changes. Neuroendocrine cell micronests consist of small clusters of round, monomorphic cells with finely granular chromatin on hematoxylin and eosin (H&E) (A and C), and are confirmed by positive chromogranin immunostaining (B and D).
Table 2.
Clinical and Histopathologic Features with Presence or Absence of Neuroendocrine Cell Micronests.
| Neuroendocrine cell micronests |
P value | ||
|---|---|---|---|
| Present (n = 37) | Absent (n = 23) | ||
|
| |||
| n (%) | n (%) | ||
|
| |||
| Treatment | |||
| Chemoradiationa (n = 54) | 34 (63%) | 20 (37%) | .86 |
| Chemotherapy only (n = 6) | 3 (50%) | 3 (50%) | |
| Interval from last treatment to specimen collection | |||
| < = 30 days (n = 12) | 5 (42%) | 7 (58%) | .33 |
| 3–60 days (n = 35) | 24 (69%) | 11 (31%) | |
| >60 days (n = 12) | 7 (58%) | 5 (42%) | |
| Unknown (n = 1) | 1 (100%) | 0 (0%) | |
| Location | |||
| Gastroesophageal junction/gastric cardia (n = 32) | 26 (81%) | 6 (19%) | .006 |
| Gastric body (n = 2) | 1 (50%) | 1 (50%) | |
| Gastric antrum (n = 24) | 10 (42%) | 14 (58%) | |
| Pylorus (n = 2) | 0 (0%) | 2 (100%) | |
| Therapy-related epithelial changes | |||
| Present (n = 50) | 34 (68%) | 16 (32%) | .06 |
| Absent (n = 10) | 3 (30%) | 7 (70%) | |
Bold values indicate statistical significance, P value < .05
Includes 1 patient treated with chemotherapy and yttrium radioembolization.
NGS Results
In one case, DNA was isolated from a submucosal gland with therapy-related epithelial changes and abnormal p53 expression and paired invasive adenocarcinoma from the same patient. NGS revealed TP53 p.R306* in the adenocarcinoma, and a TP53 p.P250L alteration in the histologically benign submucosal glands (Figure 3). No other variants were detected. NGS was attempted in several additional cases but the foci of gastric mucosa with therapy-related epithelial changes were quantitatively insufficient for analysis.
Figure 3.
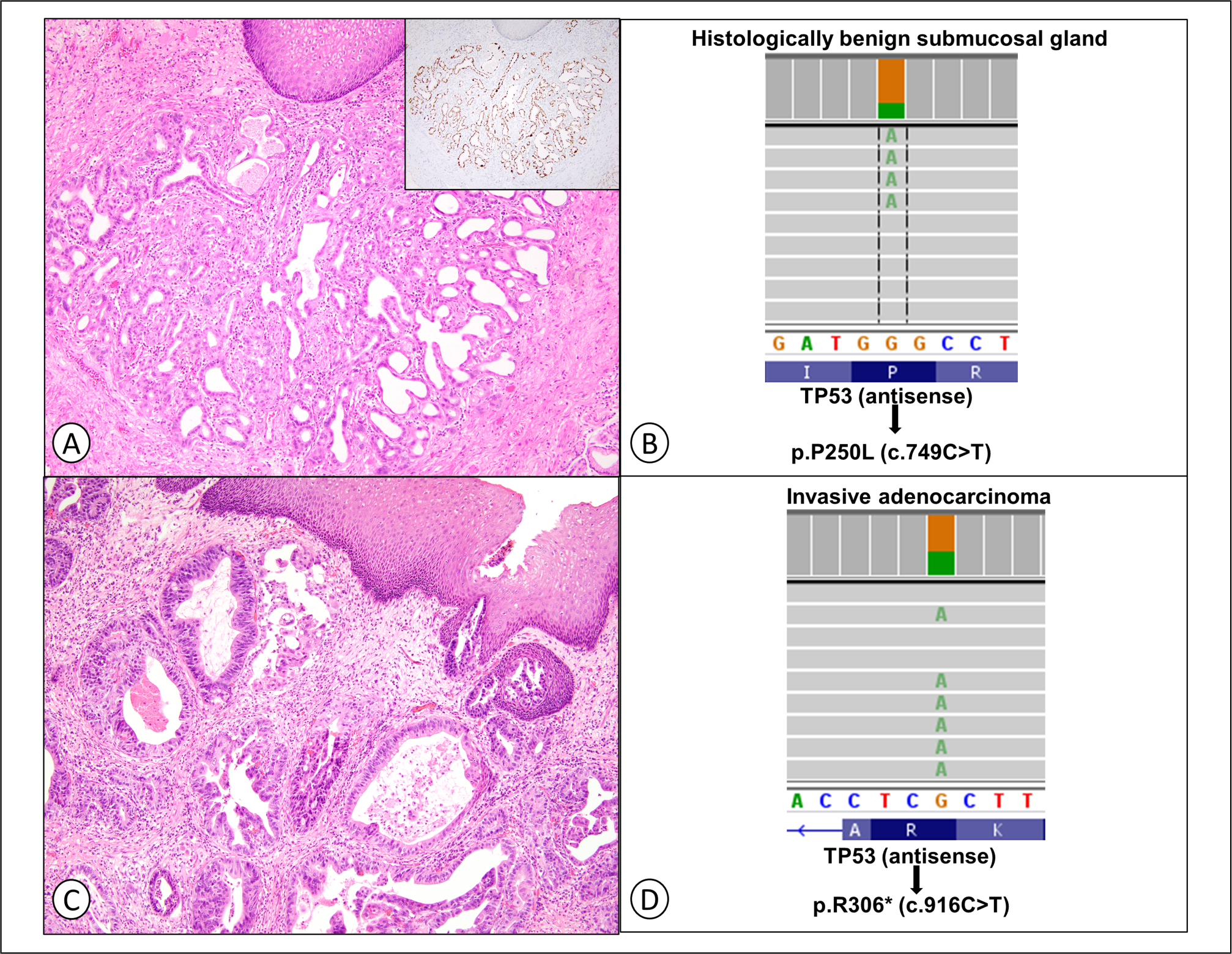
Next-generation sequencing (NGS) was performed on a histologically benign esophageal submucosal gland with therapy-related epithelial changes (A and B) and abnormally increased p53 expression (A, inset) and paired invasive adenocarcinoma (C and D) from the same patient.
Discussion
In this study, we examined p53 immunohistochemical staining in gastric mucosal samples from 60 patients treated with radiation and/or chemotherapy. We found abnormal p53 protein expression at least focally in gastric mucosa and/or esophageal submucosal glands in 27 of 60 (45%) cases, all of which showed morphological evidence of therapy-related epithelial changes. From a practical perspective, these findings highlight an important diagnostic pitfall and suggest that therapy-related epithelial changes and columnar neoplasia should be distinguished by histomorphology alone and that p53 staining should be avoided in this setting.
We also found increased p53 protein expression more frequently in patients receiving combined radiation plus chemotherapy versus chemotherapy alone and in patients with gastroesophageal carcinomas, wherein radiation was likely more directly targeting the gastric mucosa, suggesting that radiation may play a central role in this process. Similar findings have been previously reported in normal rectal mucosa adjacent to rectal carcinomas following neoadjuvant radiation, as well as in the intestinal epithelium of mice following irradiation, supporting the role of radiation in the upregulation of p53.22,23
The exact biological mechanism of p53 upregulation in this context remains unclear though. TP53 is known to be upregulated in states of DNA damage and repair, such as in response to hypoxia or oxidative stress, and one explanation could be that p53 protein expression is increased as a repair mechanism in response to injury caused radiation and/or chemotherapy. However, our single case where NGS was successfully performed revealed differing TP53 alterations in a histologically benign esophageal submucosal gland and the patient’s adenocarcinoma. This raises the possibility of an alternative mechanism wherein radiation and/or chemotherapy may induce mutations in TP53, which could then become clonal if progenitor cells are affected. The clinical significance of this is unknown, though GI cancers following radiation therapy have been reported.24–27 On the other hand, oncogenic driver mutations have also been detected in normal colonic crypts in patients not treated with chemoradiation, and so the possibility of a similar phenomenon in our case cannot be excluded.28 NGS was attempted on several additional samples, but it failed due to insufficient cellularity in the foci of abnormal p53 expression. Additional studies are needed to fully elucidate the mechanism and significance of these findings.
Compared to a previous study characterizing gastric dysplasia-like epithelial atypia by Brien et al,. we found histomorphologic features of therapy-related epithelial changes in a significantly higher proportion of cases (83% in our study vs 7.5% in Brien et al.).7 We included cases with any features of therapy-related changes, even those with only mild epithelial changes, which may account for this difference. Similarly, we found abnormal p53 expression in a significantly higher proportion of our cases compared to Brien et al. (45% in our study vs 6.6% in Brien et al.).7 This may be related to the use of differing criteria for interpretation of p53 expression. Differences in sample size and treatment regimens from the study periods may also have contributed.
Lastly, like Stewart and Hillery, we found that neuroendocrine cell micronests are common in injured mucosa.13,14 This has also been reported in GI mucosal damage related to inflammatory bowel disease.13 Knowledge of this feature is important as it may raise concern for autoimmune metaplastic atrophic gastritis or potentially mimic a well-differentiated neuroendocrine tumor, especially on small gastric biopsy specimens. In our series, most samples with neuroendocrine cell micronests were in the vicinity of the gastroesophageal junction, cardia, or antrum arguing against autoimmune metaplastic atrophic gastritis, which is a disease process affecting the gastric body. None of our patients had serologic testing for autoimmune metaplastic atrophic gastritis, however, correlation with serology may be helpful, particularly if neuroendocrine cell micronests are predominantly present in the gastric body.
Limitations of our study include a relatively small overall sample size and limited number of biopsy specimens. Patients were treated with differing radiation and/or chemotherapy regimens and had variable intervals between treatment and pathology specimens. On resection specimens, the amount of gastric mucosa that was sampled was variable between specimens. NGS results were limited due to small size of foci with therapy-related changes and abnormal p53 which had insufficient cellularity for analysis.
In summary, we evaluated gastric columnar mucosa from 60 patients following radiation and/or chemotherapy. We found that histomorphologic evidence of therapy-related epithelial changes and the presence of neuroendocrine cell micronests are common (83% and 62% of cases, respectively). We also found that foci of abnormal p53 expression are relatively common (45% of cases), which highlights an important pitfall in the distinction of therapy-related changes from columnar neoplasia.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Jerry D’Amato Charity Foundation.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent
Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration
Not applicable, because this article does not contain any clinical trials.
References
- 1.Monma S, Kato K, Shouji H, et al. Gastric mucosal injury and hemorrhage after definitive chemoradiotherapy for locally advanced esophageal cancer. Esophagus. 2019;16(4):402–407. [DOI] [PubMed] [Google Scholar]
- 2.Lee KJ, Kim HM, Jung JW, et al. Gastrointestinal hemorrhage after concurrent chemoradiotherapy in locally advanced pancreatic cancer. Gut Liver. 2013;7(1):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afifi ANAM, Powerski M, Jechorek D, et al. Radiation-induced damage in the upper gastrointestinal tract: clinical presentation, diagnostic tests and treatment options. Best Pract. Res. Clin. Gastroenterol. 2020;48–49:101711. [DOI] [PubMed] [Google Scholar]
- 4.Doria MIJr Doria LK, Faintuch J, et al. Gastric mucosal injury after hepatic arterial infusion chemotherapy with floxuridine. A clinical and pathologic study. Cancer. 1994;73(8):2042–2047. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa F, Mino-Kenudson M, Shimizu M, et al. Gastroduodenitis associated with yttrium 90–microsphere selective internal radiation: an iatrogenic complication in need of recognition. Arch. Pathol. Lab. Med. 2008;132(11):1734–1738. [DOI] [PubMed] [Google Scholar]
- 6.Crowder CD, Grabowski C, Inampudi S, et al. Selective internal radiation therapy-induced extrahepatic injury: an emerging cause of iatrogenic organ damage. Am. J. Surg. Pathol. 2009;33(7):963–975. [DOI] [PubMed] [Google Scholar]
- 7.Brien TP, Farraye FA, Odze RD. Gastric dysplasia-like epithelial atypia associated with chemoradiotherapy for esophageal cancer: a clinicopathologic and immunohistochemical study of 15 cases. Mod. Pathol. 2001;14(5):389–396. [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Smith JG, LaVanway JM. Peptic ulceration with marked epithelial atypia following hepatic arterial infusion chemotherapy. A lesion initially misinterpreted as carcinoma. Am. J. Surg. Pathol. 1983;7(3):261–268. [DOI] [PubMed] [Google Scholar]
- 9.Jewell LD, Fields AL, Murray CJ, et al. Erosive gastroduodenitis with marked epithelial atypia after hepatic arterial infusion chemotherapy. Am. J. Gastroenterol. 1985;80(6):421–424. [PubMed] [Google Scholar]
- 10.Petras RE, Hart WR, Bukowski RM. Gastric epithelial atypia associated with hepatic arterial infusion chemotherapy. Its distinction from early gastric carcinoma. Cancer. 1985;56(4):745–750. [DOI] [PubMed] [Google Scholar]
- 11.Shih AR, Misdraji J. Drug-induced pathology of the upper gastrointestinal tract. Diagn. Histopathol. 2017;23(2):84–95. [Google Scholar]
- 12.Choi W-T, Lauwers GY, Montgomery EA. Utility of ancillary studies in the diagnosis and risk assessment of Barrett’s esophagus and dysplasia. Mod. Pathol. 2022;35(8):1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong M, Larson BK, Dhall D. Neuroendocrine proliferations in inflammatory bowel disease: differentiating neuroendocrine tumours from neuroendocrine cell micronests. Histopathology. 2019;74(3):415–423. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CJR, Hillery S. Mucosal endocrine cell micronests and single endocrine cells following neo-adjuvant therapy for adenocarcinoma of the distal oesophagus and oesophagogastric junction. J. Clin. Pathol. 2007;60(11):1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redston M, Noffsinger A, Kim A, et al. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology. 2022;162(2):468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Tseng L-H, Haley L, et al. Clinical validation of coexisting driver mutations in colorectal cancers. Hum. Pathol. 2019;86:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14(2):178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information. ClinVar; [VCV000420136.7], https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000420136.7. 2013. Accessed July 10, 2022.
- 19.Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarty D, Gao J, Phillips S, et al. OncoKB: a precision oncology knowledge base. JCO Precision Oncology.2017;1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Reumers J, Couceiro JR, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7(5):285–295. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JW, Pritchard DM, Hickman JA, et al. Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am. J. Pathol. 1998;153(3):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshikawa R, Yanagi H, Kusunoki M, et al. Prognostic values of radiation-induced p53 in adjacent normal mucosa and p21WAF1/CIP1 expression in rectal cancer patients. Int. J. Oncol. 2002;21(6):1223–1228. [PubMed] [Google Scholar]
- 24.Raissouni S, Raissouni F, Rais G, et al. Radiation induced esophageal adenocarcinoma in a woman previously treated for breast cancer and renal cell carcinoma. BMC Res. Notes. 2012;5:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadleigh R, Elsayem A, Hussain M. Esophageal carcinoma following radiotherapy. J. Med. 1998;29(5–6):375–380. [PubMed] [Google Scholar]
- 26.Sasaki K, Ishihara S, Hata K, et al. Radiation-associated colon cancer: a case report. Mol Clin Oncol. 2017;6(6):817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shousha S, Fawcett A, Luqmani YA, et al. Multifocal squamous cell carcinoma of the oesophagus following radiotherapy for bilateral breast carcinoma. J. Clin. Pathol. 2001;54(9):718–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee-Six H, Olafsson S, Ellis P, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574(7779):532–537. [DOI] [PubMed] [Google Scholar]


