Abstract
Aims
The ongoing Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction [OCEAN(a)]-Outcomes trial is evaluating whether Lp(a) lowering can reduce the incidence of cardiovascular events among patients with prior myocardial infarction (MI) or percutaneous coronary intervention (PCI) and elevated Lp(a) (≥200 nmol/L). The purpose of this study is to evaluate the association of elevated Lp(a) with cardiovascular outcomes in an observational cohort resembling the OCEAN(a)-Outcomes trial main enrolment criteria.
Methods and results
This study included patients aged 18–85 years with Lp(a) measured as part of their clinical care between 2000 and 2019. While patients were required to have a history of MI, or PCI, those with severe kidney dysfunction or a malignant neoplasm were excluded. Elevated Lp(a) was defined as ≥200 nmol/L consistent with the OCEAN(a)-Outcomes trial. The primary outcome was a composite of coronary heart disease death, MI, or coronary revascularization. Natural language processing algorithms, billing and ICD codes, and laboratory data were employed to identify outcomes and covariates. A total of 3142 patients met the eligibility criteria, the median age was 61 (IQR: 52–73) years, 28.6% were women, and 12.3% had elevated Lp(a). Over a median follow-up of 12.2 years (IQR: 6.2–14.3), the primary composite outcome occurred more frequently in patients with versus without elevated Lp(a) [46.0 vs. 38.0%, unadjHR = 1.30 (95% CI: 1.09–1.53), P = 0.003]. Following adjustment for measured confounders, elevated Lp(a) remained independently associated with the primary outcome [adjHR = 1.33 (95% CI: 1.12–1.58), P = 0.001].
Conclusion
In an observational cohort resembling the main OCEAN(a)-Outcomes Trial enrolment criteria, patients with an Lp(a) ≥200 nmol/L had a higher risk of cardiovascular outcomes.
Keywords: Coronary artery disease, Lipoprotein(a), Olpasiran, Cardiovascular outcomes
Introduction
Lipoprotein(a) [Lp(a)] is a presumed causal independent risk factor for atherosclerotic cardiovascular disease (ASCVD) in both the general population1,2 and among those with prior coronary artery disease (CAD).3,4 This has prompted the development of new targeted treatments that effectively lower Lp(a). Olpasiran is a small interfering RNA molecule that significantly reduces Lp(a) production in hepatocytes. The recent OCEAN(a)-DOSE trial demonstrated that olpasiran significantly reduced Lp(a) concentrations in patients with established ASCVD.5 As a result, the ongoing Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction [OCEAN(a)]-Outcomes trial is evaluating whether Lp(a) lowering can reduce the incidence of cardiovascular (CV) events among patients with prior myocardial infarction (MI) or percutaneous coronary intervention (PCI) and elevated Lp(a) (≥200 nmol/L).6 We aimed to evaluate the association of elevated Lp(a) with CV outcomes in an observational cohort resembling the OCEAN(a)-Outcomes trial main enrolment criteria.
Methods
Study population
The study population was derived from a large retrospective Lp(a) registry from two large academic medical centers in Boston, MA (Brigham and Women’s Hospital and Massachusetts General Hospital). The design of the Mass General Brigham (MGB) Lp(a) Registry has been previously described.7 The registry included patients aged ≥18 years with Lp(a) measured as part of their clinical care between 2000 and 2019. To simulate the OCEAN(a)-Outcomes trial, patients were included in this analysis if they had a history of MI or PCI. We excluded patients with severe kidney dysfunction (estimated glomerular filtration rate < 15 mL/min/m2), those with a renal transplant, or those on dialysis. Finally, individuals with a malignant neoplasm were excluded. The MGB Institutional Review Board approved the study protocol.
Lipoprotein(a) assays
Lipoprotein(a) [Lp(a)] values were extracted from MGB data repositories. All Lp(a) laboratory testing was performed as part of clinical care at commercial laboratories using locally available assays. Patients underwent testing with either the Lp(a)-particle assay (measured in nmol/L) or the Lp(a)-mass assay (measured in mg/dL). All Lp(a) results reported in mg/dL were converted to nmol/L using the following conversion formula Lp(a) nmol/L = (2.18×Lp(a) mg/dL)−3.83.8,9 Percentile distributions were established separately for each assay to mitigate the potential biases resulting from variations in Lp(a) testing methods during the study period. Lp(a) percentile groups were then aggregated across different assay types. Elevated Lp(a) was defined as ≥ 200 nmol/L (≥93.5 mg/dL) consistent with the OCEAN(a)-Outcomes trial inclusion criteria.
Risk factors and baseline characteristics
The primary data source for this registry was the Research Patient Data Registry (RPDR),7 a centralized data registry at MGB. To determine the presence of CV risk factors in each individual, validated natural language processing (NLP) modules,10 laboratory data, as well as International Classification of Diseases (ICD)-9, ICD-10, and Current Procedural Terminology codes were utilized, as previously described.7 The baseline covariate assessment period encompassed 12 months before and 30 days after the Lp(a) test date. For individuals with multiple Lp(a) tests, the baseline covariate assessment period was assigned to the first test. Hyperlipidaemia was defined as treatment with a cholesterol-lowering medication, having a diagnosis of dyslipidemia, or having any of the following laboratory abnormalities during the baseline covariate period: (i) total cholesterol ≥240 mg/dL, (ii) low density lipoprotein cholesterol ≥160 mg/dL, (iii) HDL cholesterol <40 mg/dL (males), (iv) HDL cholesterol <50 mg/dL (females), or total triglycerides ≥175 mg/dL.
Outcomes
The primary outcome was a composite of death from coronary heart disease (CHD), MI, or coronary revascularization. Secondary outcomes included: (i) MI, ii) ischaemic stroke, (iii) coronary revascularization, (iv) CHD mortality, (v) CV mortality, (vi) all-cause mortality, (vii) composite: CHD mortality and MI, (viii) composite: CV mortality, MI and ischaemic stroke, (ix) composite: CV mortality, MI, ischaemic stroke and coronary revascularization. The diagnosis of acute MI and acute ischaemic stroke (non-hemorrhagic) relied on a diagnostic ICD code in the primary hospital discharge position. Coronary revascularization was identified using a combination of ICD diagnostic and procedural codes.
To ascertain the cause of death for patients who were reported as deceased, the National Death Index (NDI) and the Massachusetts Office of Vital Statistics were searched. The underlying and proximal causes of death during the study follow-up period were determined by analysing the ICD-10 codes associated with each deceased patient.
Statistical analysis
Comparisons were made with χ2 or Fisher’s exact tests for categorical variables and with Student t-tests or Mann–Whitney U-(Wilcoxon) tests for continuous variables, as appropriate. Kaplan–Meier survival curves were compared using the log-rank tests. Cox proportional hazard regressions were used to assess the association of Lp(a) groups on outcomes and obtain corresponding unadjusted and adjusted hazard ratios and 95% confidence intervals.
Results
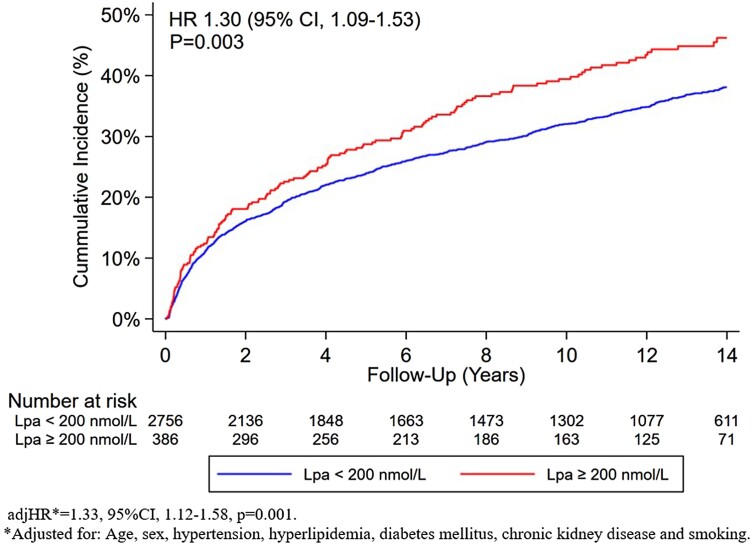
A total of 3142 patients met the established eligibility criteria. Among this group, the median age was 61 [interquartile range (IQR): 52–73] years, 28.6% were women, and 12.3% had elevated Lp(a). Patients with elevated Lp(a), vs. non-elevated Lp(a), were more likely to be women (37.6% vs. 27.3%, P < 0.001) and to have hyperlipidaemia (90.2% vs. 86.0%, P = 0.024). The proportion of patients receiving statin therapy was similar between the two groups (90.7% vs. 88.9%, P = 0.294), whereas patients with elevated Lp(a) had a higher prevalence of non-statin lipid-lowering therapies (24.9% vs. 14.4%, P < 0.001). Over a median follow-up of 12.2 years (IQR: 6.2–14.3), the primary composite outcome occurred more frequently in patients with vs. without elevated Lp(a) [46.0% vs. 38.0%, hazard ratio (HR) = 1.30 (95% confidence interval (CI): 1.09–1.53), P = 0.003; Figure 1]. Following adjustment for measured confounders (age, sex, hypertension, hyperlipidaemia, diabetes mellitus, chronic kidney disease, and smoking), elevated Lp(a) remained independently associated with the primary outcome [adjHR = 1.33 (95%CI: 1.12–1.58), P = 0.001]. Elevated Lp(a) was also associated with increased risk for all secondary outcomes except ischaemic stroke and all-cause mortality (Table 1).
Figure 1.
Cumulative incidence for the primary composite outcome of coronary heart disease, myocardial infarction, or coronary revascularization by level of Lp(a).
Table 1.
Hazard ratio for secondary cardiovascular outcomes associated with Lipoprotein(a) ≥ 200 vs. <200 nmol/L
| Secondary outcome | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| MI | 1.41 | (1.12–1.78) | 0.004 | 1.40 | (1.11–1.78) | 0.005 a |
| Ischaemic stroke | 1.19 | (0.83–1.72) | 0.336 | 1.13 | (0.77–1.66) | 0.536b |
| Coronary revascularization | 1.40 | (1.13–1.72) | 0.002 | 1.42 | (1.14–1.75) | 0.001 a |
| CHD death | 1.40 | (1.07–1.83) | 0.013 | 1.52 | (1.16–2.01) | 0.003 c |
| CHD death and MI | 1.31 | (1.08–1.60) | 0.007 | 1.35 | (1.10–1.66) | 0.004 a |
| CV death | 1.27 | (1.02–1.58) | 0.029 | 1.39 | (1.10–1.74) | 0.005 a |
| CV death, MI, and ischaemic stroke | 1.27 | (1.07–1.51) | 0.005 | 1.31 | (1.09–1.56) | 0.003 a |
| CV death, MI, ischaemic stroke, and coronary revascularization | 1.26 | (1.08–1.47) | 0.003 | 1.29 | (1.10–1.51) | 0.002 a |
| All-cause mortality | 1.04 | (0.89–1.22) | 0.622 | 1.10 | (0.93–1.30) | 0.255a |
Bold text indicates statistical significance at an alpha level of 0.05.
CI, confidence interval; CHD, coronary heart disease; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction.
Adjusted for: Age, sex, hypertension, hyperlipidaemia, diabetes mellitus, chronic kidney disease, and smoking.
Adjusted for: Age and sex.
Adjusted for: Age, sex, hypertension, hyperlipidaemia, diabetes mellitus, and chronic kidney disease.
Discussion
In an observational cohort of patients with prior MI or PCI—thus, resembling the main OCEAN(a)-Outcomes Trial enrolment criteria—those with Lp(a) ≥ 200 nmol/L had a higher long-term risk of the primary composite outcome, as well as individual and secondary composite outcomes such as MI, coronary revascularization, and CV mortality.
These results are consistent with previous studies indicating that increased Lp(a) levels are associated with worse outcomes among patients with previous CAD.3,4 Furthermore, the study supports the selection criteria of the OCEAN(a)-Outcomes Trial and underscores the importance of examining the impact of treatments that aim to lower CV risk by reducing Lp(a) levels in this patient population.
This study has limitations, including its retrospective design, potential selection bias, and enrolment of patients from a single geographic region, which could result in reduced generalizability. We utilized various Lp(a) assays and the conversion formula employed to change mg/dL to nmol/L may not be precise. Nevertheless, our results reinforce the importance of the ongoing OCEAN(a)-Outcomes Trial and suggest that lowering Lp(a) may impact numerous different CV endpoints.
Contributor Information
Arthur Shiyovich, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Adam N Berman, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Stephanie A Besser, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
David W Biery, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Daniel M Huck, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Brittany Weber, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Christopher Cannon, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
James L Januzzi, Cardiology Division, Massachusetts General Hospital, Harvard Medical School, and Baim Institute for Clinical Research, Boston, MA 02115, USA.
John N Booth, III, Center for Observational Research, Amgen Inc., Thousand Oaks, CA 91320, USA.
Khurram Nasir, Department of Cardiovascular Medicine, Division of Cardiovascular Prevention and Wellness, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX 77030, USA.
Marcelo F Di Carli, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
J Antonio G López, Global Development, Amgen Inc., Thousand Oaks, CA 91320, USA.
Shia T Kent, Center for Observational Research, Amgen Inc., Thousand Oaks, CA 91320, USA.
Deepak L Bhatt, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Mount Sinai Heart, Icahn School of Medicine at Mount Sinai Health System, New York, NY 10029, USA.
Ron Blankstein, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Lead author biography
 Dr. Arthur Shiyovich is an advanced CV imaging fellow at the Brigham and Women’s Hospital and Harvard Medical School in Boston, MA. He completed his training in Internal Medicine and Cardiology at the Rabin Medical Center in Tel-Aviv, Israel. Following his training, he stayed as a senior cardiologist at Rabin Medical Center and was appointed as an assistant professor in the Faculty of Medicine at Tel-Aviv University. His research focuses on the use of CV imaging for CAD and structural heart disease, as well as novel methods for improved risk assessment in CV disease. Dr. Shiyovich has authored over 100 publications and serves as a reviewer and an editor in several medical journals in the field of CV medicine.
Dr. Arthur Shiyovich is an advanced CV imaging fellow at the Brigham and Women’s Hospital and Harvard Medical School in Boston, MA. He completed his training in Internal Medicine and Cardiology at the Rabin Medical Center in Tel-Aviv, Israel. Following his training, he stayed as a senior cardiologist at Rabin Medical Center and was appointed as an assistant professor in the Faculty of Medicine at Tel-Aviv University. His research focuses on the use of CV imaging for CAD and structural heart disease, as well as novel methods for improved risk assessment in CV disease. Dr. Shiyovich has authored over 100 publications and serves as a reviewer and an editor in several medical journals in the field of CV medicine.
Data availability
The raw data that underlie the findings of this study cannot be shared at this time due to legal/ ethical reasons. However, interested parties may contact the authors to inquire about accessing the data.
Funding
The study was funded, in part, by Amgen Inc.
References
- 1. Thomas PE, Vedel-Krogh S, Kamstrup PR, Nordestgaard BG. Lipoprotein(a) is linked to atherothrombosis and aortic valve stenosis independent of C-reactive protein. Eur Heart J 2023;44:1449–1460. [DOI] [PubMed] [Google Scholar]
- 2. Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jørgensen T, Linneberg A, Niiranen T, Salomaa V, Jousilahti P, Yarnell J, Ferrario MM, Veronesi G, Brambilla P, Signorini SG, Iacoviello L, Costanzo S, Giampaoli S, Palmieri L, Meisinger C, Thorand B, Kee F, Koenig W, Ojeda F, Kontto J, Landmesser U, Kuulasmaa K, Blankenberg S. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J 2017;38:2490–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, Ezhov MV, Jukema JW, Jensen HK, Tokgözoğlu SL, Mach F, Huber K, Sever PS, Keech AC, Pedersen TR, Sabatine MS. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019;139:1483–1492. [DOI] [PubMed] [Google Scholar]
- 4. Zhang H, Zhang Y, Tian T, Wang T, Chen J, Yuan J, Qian J, Hu F, Dou K, Qiao S, Wu Y, Guan C, Xu B, Yang W, Song L. Association between lipoprotein(a) and long-term outcomes after percutaneous coronary intervention for lesions with in-stent restenosis. J Clin Lipidol 2023. [DOI] [PubMed] [Google Scholar]
- 5. O'Donoghue ML, Rosenson RS, Gencer B, López JAG, Lepor NE, Baum SJ, Stout E, Gaudet D, Knusel B, Kuder JF, Ran X, Murphy SA, Wang H, Wu Y, Kassahun H, Sabatine MS; OCEAN(a)-DOSE Trial Investigators . Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med 2022;387:1855–1864. [DOI] [PubMed] [Google Scholar]
- 6.https://clinicaltrials.gov/ct2/show/NCT05581303
- 7. Berman AN, Biery DW, Ginder C, Hulme OL, Marcusa D, Leiva O, Wu WY, Singh A, Divakaran S, Hainer J, Turchin A, Januzzi JL, Natarajan P, Cannon CP, Di Carli MF, Bhatt DL, Blankstein R. Study of lipoprotein(a) and its impact on atherosclerotic cardiovascular disease: design and rationale of the mass general Brigham Lp(a) registry. Clin Cardiol 2020;43:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J 2019;40:2760–2770. [DOI] [PubMed] [Google Scholar]
- 9. Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: A population-based study. Arterioscler Thromb Vasc Biol 2020;40:255–266. [DOI] [PubMed] [Google Scholar]
- 10. Iyengar PV. Regulation of ubiquitin enzymes in the TGF-β pathway. Int J Mol Sci 2017;18:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that underlie the findings of this study cannot be shared at this time due to legal/ ethical reasons. However, interested parties may contact the authors to inquire about accessing the data.