Abstract
Aging is the most significant risk factor for neurodegenerative disorders such as Alzheimer’s disease (AD) associated with profound socioeconomic and personal costs. Consequently, there is an urgent need for animal models that recapitulate the age-related spatial and temporal complexity and patterns of pathology identical to human AD. Our research in aging nonhuman primate models involving rhesus macaques has revealed naturally occurring amyloid and tau pathology, including the formation of amyloid plaques and neurofibrillary tangles comprising hyperphosphorylated tau. Moreover, rhesus macaques exhibit synaptic dysfunction in association cortices and cognitive impairments with advancing age, and thus can be used to interrogate the etiological mechanisms that generate neuropathological cascades in sporadic AD. Particularly, unique molecular mechanisms (eg, feedforward cyclic adenosine 3ʹ,5ʹ-monophosphate [cAMP]-Protein kinase A (PKA)-calcium signaling) in the newly evolved primate dorsolateral prefrontal cortex are critical for persistent firing required for subserving higher-order cognition. For example, dendritic spines in primate dorsolateral prefrontal cortex contain a specialized repertoire of proteins to magnify feedforward cAMP-PKA-calcium signaling such as N-methyl-d-aspartic acid receptors and calcium channels on the smooth endoplasmic reticulum (eg, ryanodine receptors). This process is constrained by phosphodiesterases (eg, PDE4) that hydrolyze cAMP and calcium-buffering proteins (eg, calbindin) in the cytosol. However, genetic predispositions and age-related insults exacerbate feedforward cAMP-Protein kinase A-calcium signaling pathways that induce a myriad of downstream effects, including the opening of K+ channels to weaken network connectivity, calcium-mediated dysregulation of mitochondria, and activation of inflammatory cascades to eliminate synapses, thereby increasing susceptibility to atrophy. Therefore, aging rhesus macaques provide an invaluable model to explore novel therapeutic strategies in sporadic AD.
Keywords: Aging, Alzheimer’s disease, Calcium, Pyramidal cells, Tau
Aging represents the primary risk factor for neurodegenerative disorders such as Alzheimer’s disease (AD). Epidemiological studies have revealed that ~10% of individuals over the age of 65 develop AD, and the prevalence increases significantly with advancing age and is associated with cognitive impairment. The rising prevalence of AD is compounded by a rapidly aging population and is predicted to impose a huge financial burden on healthcare systems globally. As a result, there is an urgent need to develop effective preventative therapeutic strategies to ameliorate cognitive decline with advancing age to augment health span and quality of life.
The neuropathological hallmarks of AD include extracellular deposits of amyloid Aβ plaques and intracellular neurofibrillary tangles (NFTs) comprising hyperphosphorylated tau. Current hypotheses postulate that these pathological phenomena are interconnected, as Aβ oligomers can drive tau phosphorylation (1–3), and accumulations of phosphorylated tau may increase the production of Aβ (4,5), thus establishing vicious cycles that lead to the hallmark toxic phenotypes and the destruction of synapses that mediate memory and cognition. Human AD studies have revealed that cognitive deficits correlate with NFTs, but not Aβ plaques (6), suggesting that understanding the etiology of tau phosphorylation and how it emerges in the aging association cortex is particularly critical in elucidating the pathogenesis of AD. Tau normally serves to assemble and stabilize microtubules (7,8), but with increasing phosphorylation, tau detaches from microtubules and aggregates, and with hyperphosphorylation, fibrillates within dendritic shafts to form NFTs that then proceed to invade the cell soma. The neuron eventually dies from autophagic degeneration, leaving a “ghost tangle” (reviewed in (5)).
Classic neuroanatomical studies have revealed that tau pathology in AD shows a stereotypic progression across the cortical hierarchy. Tau pathology preferentially affects glutamatergic neurons in the limbic and association cortices, beginning in the perirhinal and entorhinal limbic cortices, and proceeding to the neocortical association areas with advanced age, but only afflicting neurons in the primary sensory cortex at end-stage disease (9–12). A growing body of literature supports the notion that phosphorylated tau in AD and other tauopathies can traffic between neurons to propagate along anatomically connected cortical networks via excitatory synapses (13–22). Understanding the selective vulnerability of these highly interconnected glutamatergic neurons remains an ongoing area of investigation in the field and might provide critical signs regarding cell-type specificity of neuropathology in AD.
Nonhuman primates (NHPs) have provided an invaluable model system to probe the neurobiology underlying amyloid and tau pathology in AD. Particularly, aging NHPs provide an opportunity to examine the generation of tau pathology in its native course in sporadic AD in the absence of familial, autosomal dominant mutations. There are striking differences in the magnitude of AD-related neuropathology across NHP species (eg, marmosets, vervets, rhesus macaques, chimpanzees) along the evolutionary lineage, and it is intriguing to note that the extent of pathology correlates with the expansion of the association cortex across species reaching a pinnacle in humans (4,12,23–27). Extensive research has been conducted in rhesus macaques, which provide an indispensable model to illuminate the early etiological mechanisms mediating amyloid and tau pathology. For example, aging rhesus monkeys naturally develop Aβ plaques (28,29) and NFTs (28) resulting in age-related cognitive deficits (30) without having to introduce the mutations that cause autosomal dominant disease, and thus are ideal for studying the changes in aging association cortex that lead to early-stage pathology. In addition to amyloid and tau pathology, aged macaques also show additional signs of AD-like degeneration and age-related phenotypes, including large autophagic vacuoles in dendrites, mitochondrial dysfunction, activation of inflammatory cascades and microglial engulfment, synapse loss, argyrophilia, profound aggregation of late-phase lysosomes, and dystrophic neurites (28). These questions require NHPs, as rodents have a rudimentary association cortex and require transgenic mutations to induce neuropathology with limited tau pathology. Furthermore, rodents are limited in their ability to perform complex cognitive operations that are unique to primates. The use of perfusion-fixed tissue, not possible in humans, also provides remarkable clarity for observing phosphorylated proteins in their native location and interaction with subcellular organelles with nanometer resolution, including multiple key epitopes of phosphorylated tau. This article will review research in rhesus macaques conducted by multiple research groups and provide evidence of how aging monkeys can be leveraged to explore the pathophysiology of AD, various cellular and molecular phenotypes of aging, and age-related cognitive decline to enhance our discovery of novel therapeutic targets. Rhesus macaques have also been invaluable in elucidating the contribution of multiple factors (eg, dietary factors, sexual dimorphism, nutrition, exercise, immune activation) in impacting trajectories with advancing age, although these topics have been extensively reviewed by other groups and are beyond the purview of the Review (31–34).
Aging Rhesus Monkeys Recapitulate Amyloid and Tau Pathology in AD
Histological examination of postmortem human AD brain has revealed how cortical tau pathology in AD originates in layer pre-α of the perirhinal cortex and the layer pre-α (Layer II) cell islands of the entorhinal cortex (ERC; (9,12,36). Cortical tau pathology then emerges in pyramidal cells in deeper layers of the ERC, in the hippocampus, and in the association cortex, with pyramidal cells in the primary sensory and motor cortex only impacted in terminal stages of the illness (11,12,35). Rhesus monkeys with advanced age intrinsically recapitulate AD-like early-stage cortical tau pathology with the same qualitative pattern and sequence observed in human AD patients, with neuropathology emerging in the ERC Layer II cell islands (4). Similar to humans, tau pathology later develops in pyramidal cells in the association cortices, whereas the primary visual cortex (V1) remains unaffected until the end stages of the disease (4,23). With significantly advanced age, rhesus macaques exhibit classic NFTs in the ERC and dorsolateral prefrontal cortex (dlPFC; [4]), comprising paired helical filaments with periodicity and blunt ends identical to those in human AD patients, and which are labeled by the AT8 antibody used to clinically diagnose AD. Similar patterns of tau pathology with advancing age have also been seen in marmosets (25), vervet monkeys (27), baboons (36), and chimpanzees (26), where the degree of pathology corresponds with the extent of evolutionary expansion of the association cortex. Furthermore, the tremendous elaboration of glutamatergic synapses across evolutionary phylogeny could be a critical factor in mediating the generation of tau pathology to ultimately manifest in degenerative cascades in humans (37,38). NHPs also naturally develop amyloid plaques with advancing age, which are qualitatively identical to human AD patients (4,26,29,39–41). However, current hypotheses in the field purport that tau phosphorylation is a critical precipitating factor in the etiology of AD as longstanding neuroanatomical studies across human life span show that tau pathology begins about a decade before the formation of Aβ (42) and tau pathology, but not Aβ, correlates with progressive gray matter loss (43) and cognitive impairment (44). Furthermore, the recent case study of an AD patient with a rare, combined PS1 and Christchurch ApoE3 mutation, who did not develop dementia in spite of the extensive formation of Aβ, but very restricted tau pathology, supports the prevailing idea that aberrant tau is a key disease-inducing mechanism (45).
A tremendous advantage of research involving NHPs is the prospect of interrogating neuropathology using perfusion-fixed brains with negligible postmortem interval, which allows the detection of early-stage tau phosphorylation that is often lost in human brains due to rapid dephosphorylation by phosphatases and membrane degradation postmortem (46). Perfusion fixation allows ultrastructural visualization of early-stage, soluble phosphorylated tau epitopes that are lost in postmortem human tissue, as well as more advanced, fibrillated tau species. We have taken advantage of this opportunity to implement high-spatial-resolution immunoelectron microscopy (immunoEM) to examine the earliest stages of tau pathology in situ, and to investigate the molecular and cellular mechanisms in aging association cortex that mediate tau hyperphosphorylation. For example, we have visualized early-stage phosphorylated pS214-tau aggregating on microtubules, within glutamate synapses, and on the calcium-containing smooth endoplasmic reticulum (SER), beginning in middle age in ERC, and at later ages in dlPFC (23,28,47). Protein kinase A (PKA)-mediated phosphorylation of tau at S214 is a particularly important step in the cascade of tau pathology in AD, as it causes tau to detach from microtubules and aggregate in dendrites (4,23,48), and primes tau for hyperphosphorylation by GSK3β (49,50). ImmunoEM has been particularly instrumental in revealing pS214-tau trafficking between neurons within omega bodies in ERC layer II in middle-aged macaques and in layer III dlPFC in aged macaques (28). pS214-tau trafficking was only seen near excitatory (28), but not inhibitory synapses, consistent with the notion of tau spreading, uptake, and aggregation occurring in highly interconnected glutamatergic circuits, leading to tau-induced toxicity (15,17,18,51–53). Our ongoing studies suggest that rhesus macaques can be used to examine the emergence of tau phosphorylated at threonine 217 (pT217-tau) in aging association cortex (38,47). pT217-tau is a particularly important phosphorylation epitope on tau as it is emerging as a promising new in vivo biomarker for AD in cerebrospinal fluid (CSF) and plasma, superior to pT181-tau in correlations with PET measures of tau and Aβ (54–58), correlating with disease stage and progression (59), and allowing early identification of at-risk presymptomatic individuals (54,59–61). In the oldest monkeys, we find AT8-labeled fibrils in dendrites, which eventually invade the perisomatic compartment, paralleling the degenerative process in humans (28). Intriguingly, across the primate lineage, rhesus macaques express both 3R and 4R isoforms in the brain (62), identical to human AD, but markedly different from rodents and even marmosets, and therefore provide an ideal opportunity to understand the contribution of 3R and 4R tau isoforms in the generation of neurofibrillary tangle pathology.
Cellular and Molecular Mechanisms Underlying Tau Phosphorylation in AD
A critical question that is central to understanding the pathophysiology of AD lies in elucidating why tau pathology preferentially afflicts glutamatergic neurons in association cortices. Based on a large body of work, we have hypothesized that glutamatergic neurons in association cortices have unique molecular features that allow these cells to partake in higher-order cognition, yet predispose these cells to neurodegeneration with advanced age (63). Our aging research has focused on the rhesus macaque dlPFC, which mediates top-down regulation of higher-order cognition, including working memory, executive function, abstract thought, and regulation of emotion. The seminal work from Goldman-Rakic, Arnsten, Fuster, and colleagues has revealed how neurons in the rhesus macaque dlPFC represent position in visual space across the delay period of a working memory task, maintaining neuronal firing without bottom-up sensory stimulation (64). These “Delay cells” are spatially tuned and are involved in persistent firing for their preferred spatial position (65). This persistent firing arises from extensive, recurrent excitatory circuits in deep layer III of the dlPFC with NMDAR synapses on dendritic spines, with lateral inhibition to sculpt the information held in working memory stores (66–70). The dendrites of dlPFC layer III pyramidal cells greatly expand during primate evolution, including significant increases in dendritic spine density for integration of excitatory inputs (71,72) and these cortical circuits are particularly vulnerable with advancing age showing profound atrophy of dendritic spines and dendrites (73–75), which is associated with cognitive decline (76).
What confers heightened vulnerability of dlPFC microcircuits to neurodegeneration with advancing age? Decades of in vivo physiology and cell-type-specific molecular characterization from Arnsten and colleagues in rhesus macaques have revealed how glutamatergic synapses on dendritic spines in deep layer III dlPFC exhibit evidence of magnified intracellular calcium release (38,77,78), where cyclic adenosine 3ʹ,5ʹ-monophosphate [cAMP] signaling increases calcium release from the SER (called the spine apparatus when it elaborates in the dendritic spine) into the cytosol (23). Calcium is released from the SER through multiple calcium channels such as IP3 receptors and ryanodine receptors (eg, RyR2). The data support the idea that dlPFC dendritic spines, particularly in layer III, contain the molecular machinery for cAMP-PKA signaling to enhance the release of calcium from the SER, which in turn can increase cAMP production, creating feedforward signaling (reviewed in [77]). At a functional level, the local generation of intracellular calcium release near the glutamatergic synapse may help to maintain the PSD in a depolarized state needed for NMDAR-dependent persistent firing. However, exacerbated levels of cAMP-calcium signaling induce detrimental effects, opening nearby potassium channels (eg, HCN, KCNQ) to reduce firing (79–81). This constraining mechanism might provide necessary negative feedback in a recurrent excitatory circuit to suppress the generation of seizures, to dynamically gate network inputs, and to take the PFC “offline” during uncontrollable stress when elevated levels of stress-induced catecholamines significantly increase cAMP-calcium signaling (80,82,83).
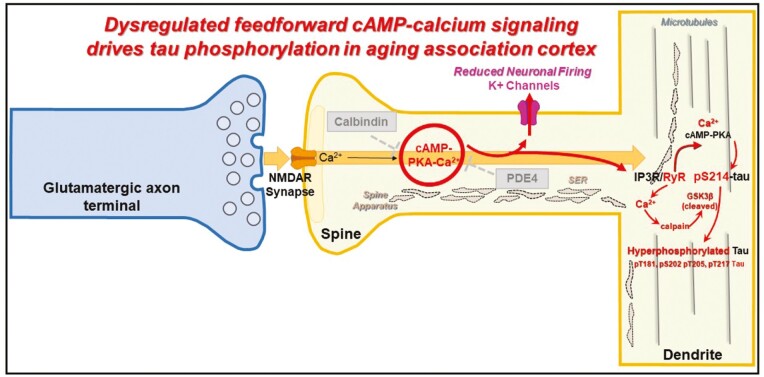
There is a multitude of regulatory mechanisms that can control feedforward cAMP-calcium signaling in aging association cortices. For example, phosphodiesterases (PDE4s) hydrolyze cAMP once it is generated, and calbindin binds cytosolic calcium released within the cell, or when calcium undergoes influx through NMDAR2B-containing dendritic spines (23,79,84). Moreover, noradrenergic alpha-2AR and mGluR3 are positioned in postsynaptic compartments on the plasma membrane of dendritic spines to inhibit cAMP production (23,79,84). Intriguingly, there are molecular gradients in several of these calcium-regulatory components (eg, PDE4D, mGluR3, calbindin) showing increases in transcript and protein expression across cortical hierarchy and during primate evolution (85,86). With advanced age, we have discovered that a decrement in expression or inhibition of these calcium-regulatory mechanisms (87) and/or induced by inflammation (77) manifests in dysregulated cAMP-calcium signaling in the aging association cortex (23,87–89) (Figure 1). This drives calcium “leak” from PKA-phosphorylated RyR2 (pRyR2) from the SER into the cytosol described in AD (4,91), which causes calcium dysregulation (92,93). Furthermore, we have observed reduced calcium binding in the cytosol as the calcium-binding protein, calbindin, which is lost with age from pyramidal cells and associated with increased NFT pathology in AD (88,89) (Figure 1). We have also observed cell-type-specific and subcompartment-specific alterations with aging, as PDE4D immunoreactivity was absent in dendritic spines and shafts of pyramidal cells in aged macaque dlPFC but preserved in astroglial cells (47,94). Protracted calcium dysregulation within the cytosol leads to the activation of the calcium-dependent protease calpain, which disinhibits a critical kinase, GSK3β, normally suppressed by PKA (95,96) to induce hyperphosphorylation of tau and mediate autophagic neurodegeneration (97). Loss of proteostasis and impairments in the autophagy-lysosomal and ubiquitination pathway in aging and AD would further compound the aggregation of hyperphosphorylated tau (98,99).
Figure 1.
Schematic illustrating how dysregulated cAMP-calcium signaling in dorsolateral prefrontal cortex Layer III cortical circuits leads to tau phosphorylation with advancing age. Under normal conditions, feedforward cAMP-calcium signaling is held in check by phosphodiesterases (PDE4s) localized in postsynaptic compartments in dendritic spines, which hydrolyze cAMP, and calcium-buffering protein calbindin, which sequesters intracellular cytosolic calcium. Calcium levels rise through multiple sources, including the calcium conductance of N-methyl-d-aspartic acid (NMDAR) channels (specifically composed of NR2B subunits) as well as release from internal storage within the smooth endoplasmic reticulum (SER), called the spine apparatus once it extends into the dendritic spine. However, age-related decrease in calcium-regulatory proteins, PDE4, and calbindin, leads to exacerbated feedforward cAMP-calcium signaling which induces several downstream effects, including opening of K+ channels (90), and tau phosphorylation. Protein kinase A (PKA) directly phosphorylates tau at the critical S214 residue, which causes tau to detach from microtubules and aggregate in dendrites (4,23,48). Calcium “leak” from PKA-phosphorylated RyR2 (pRyR2) from the SER into the cytosol in dendritic spines and shafts further drives dysregulated cAMP-calcium signaling and leading to activation of calcium-dependent protease calpain which cleaves the N-terminus of GSK3β kinase to induce hyperphosphorylation at multiple phosphorylation epitopes, including T181, S202/T205 (labeled by AT8), and T217.
Aberrant Mitochondrial Dynamics in Aging Association Cortex
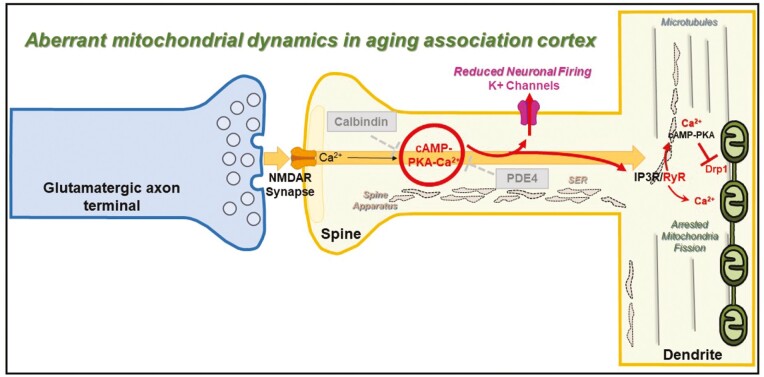
Exacerbated cAMP-calcium signaling can produce a myriad of deleterious effects, including in organelles such as mitochondria (87) (Figure 2). Mitochondria are crucial organelles that sustain neuronal function by controlling energy metabolism, cellular respiration, reactive oxygen species (ROS) generation and elimination, and modulating calcium flux. They are highly dynamic organelles, undergoing fission and fusion in an activity-dependent fashion (105). We have reported marked changes in mitochondrial morphology in dlPFC pyramidal neurons from aged rhesus macaques (106). Specifically, immunoEM paired with 3D reconstruction from serial sections revealed mitochondria with different size profiles characterized by thin segments that intermingle with enlarged segments, a phenotype we described as mitochondria-on-a-string (MOAS), indicative of impaired mitochondrial fission (Figure 2). Identical MOAS phenotypes have been demonstrated in the postmortem hippocampus of human subjects with AD, and in mice with human autosomal dominant genetic mutations (107). Similarly, presynaptic mitochondrial abnormalities in aging macaque dlPFC, indicative of pathology, have been shown to contribute to synaptic and cognitive impairment (108). Mitochondrial dysfunction and pathology have been identified in the dlPFC and other brain regions as part of the progression in postmortem human AD brains, suggesting compromised mitochondrial bioenergetics is a key driver of disease progression (109–111).
Figure 2.
Schematic illustrating how dysregulated cAMP-calcium signaling in dorsolateral prefrontal cortex layer III cortical circuits leads to aberrant mitochondrial dynamics. Our data have revealed how calcium overload with advanced age leads to mitochondrial dysfunction (eg, incomplete fission), resulting in a phenotype called “Mitochondria-on-a-string” (MOAS). Within the dendritic shafts of glutamatergic-like pyramidal neurons of layer III of the dlPFC of aged rhesus macaques, we have observed MOAS with pinched constricted regions next to the calcium-containing smooth endoplasmic reticulum (SER). Fission is initiated by the dynamin-like GTPase, dynamin-related protein 1 (Drp1; also known as DLP1; [115]), which translocates from the cytosol into the outer mitochondrial membrane (OMM), where it interacts with its primary receptor—mitochondrial fission protein 1 (Fis1). Drp1 oligomers assemble into rings and spirals around the OMM, leading to the final membrane constriction and scission (100–102). The efficacy of Drp1 in fission is determined by its GTPase activity, which is inhibited by elevated PKA signaling with advancing age (103,104).
The morphological alterations in mitochondria indicate that MOAS may arise from impairments in mitochondrial fission due to dysregulation of the mitochondrial fission machinery. Our findings suggest that mitochondrial division is initiated, producing constricted segments in the mitochondrial body, but that the process of fission is unable to proceed to completion, ie, resulting in “unfinished fission” (Figure 2). This hypothesis is consistent with the findings that “pinched” segments were associated with (a) calcium-associated SER cisterns, which have been shown to encircle mitochondria to initiate constriction and are thought to play an active role in defining the positions of mitochondrial division sites (112,113) and (b) Drp1, the GTPase that is recruited to constriction sites and subsequently cuts the mitochondrial membrane, and Fis1, its primary receptor on the outer mitochondrial membrane (114–116).
Mitochondrial fission is part of a quality-control mechanism whereby damaged mitochondrial components are segregated from healthy components, followed by mitochondrial division and mitophagy (105). A balance between mitochondrial fusion versus fission is also necessary for limiting the production of toxic ROS and for normal cellular metabolism, whereas disruptions in these processes affect the cell and may be implicated in neurodegenerative diseases (117–119). In vitro studies have also shown that irregular mitochondrial fission may be a part of a pathological process that impairs mitochondrial membrane permeability (ie, opens mitochondrial permeability transition pores), resulting in the release of cytochrome c in cytoplasm and activation of caspases that, in turn, initiate apoptotic or necrotic cell death pathways (120–122). Impairments in mitochondrial dysfunction are associated with decreased mitochondrial respiration and increased oxidative stress along with lipid peroxidation and glycolysis (110,111). Rodent and human AD studies show that mitochondrial oxidative stress occurs early in the disease process in AD (123–125), and mitochondrial oxidative stress is associated with increased phosphorylation of tau (126). Based on multiple studies, it has been suggested that MOAS may arise from excessive calcium flux and bioenergetic stress leading to dysregulated mitochondrial fission (127,128). Furthermore, calcium overload of mitochondria can indirectly initiate the generation of pro-inflammatory cytokines, such as activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome (129,130), ultimately leading to synaptic loss with advanced age. In sum, these findings are consistent with the idea that mitochondrial dysfunction is an early signature of pathology in neurodegeneration resulting in hypometabolism and cognitive deficits (131).
Activation of Inflammatory Cascades to Induce Atrophy of Glutamatergic Synapses in Aging Association Cortex
A defining feature of aging involves significant changes to both the innate and adaptive immune systems. Recent evidence suggests that activation of inflammatory cascades in brain aging contributes significantly to atrophy of cortical circuits that are critical for higher-order cognition and ensuing risk for neurodegeneration. For example, although glial cells play an important physiological role in supporting network activity, aberrant activation of astrocytes and microglia has been shown to induce inappropriate elimination of synapses under pathological conditions (132,133).
The molecular mechanisms that drive the activation of inflammatory pathways with aging are an important arena of discovery. Particularly, the complement cascade signaling pathway is an important mechanism that has garnered extensive attention. Complement signaling is one of the key arms of the innate immune system, allowing the immune system to rapidly recognize and eradicate foreign antigens (134). The classical pathway of complement activation is initiated by C1q, which leads to the activation of downstream complement components, importantly C3 and C4, which can recruit microglia through their cognate receptors to “tag” vulnerable synaptic elements (135). Rodent and human studies have revealed a dramatic upregulation of synapse-associated C1q transcript and protein during aging and in AD, which plays a role in age-related memory dysfunction (136,137). Aberrant reactivation of complement cascade signaling pathways also has been implicated in various neurodegenerative disorders including Parkinson’s disease (138,139). In mouse models of AD, C1q is necessary for soluble β-amyloid oligomers to induce synapse elimination prior to plaque formation (140). Likewise, prominent accumulation of C1q has been observed near the postsynaptic density (PSD) of Tau-P301S mice and in postmortem AD brain, changes that are associated with the microglial engulfment of synaptic components (141). C3 and C3a receptors (C3aR1) are also positively correlated with cognitive decline and Braak tau staging in human AD brains (142). Furthermore, in mouse models of frontotemporal dementia caused by progranulin deficiency, there is a remarkable upregulation in C1q expression in microglia, resulting in concomitant tagging of dysfunctional synapses by C3 and phagocytosis (143). On the contrary, the reduction of complement cascade signaling pathways using genetic and/or antibody-mediated inhibition of C1q leads to rescue of synaptic alterations, neuroinflammation, and degenerative signatures (140–142).
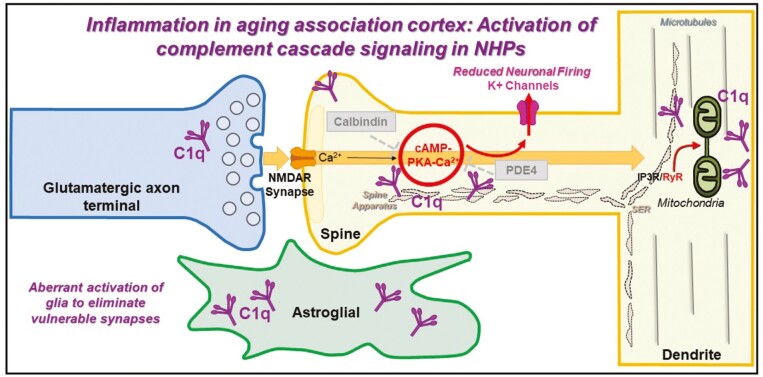
We have characterized the expression and subcellular localization of the initiating complement signaling protein, C1q, in the aging macaque dlPFC and rat medial PFC (mPFC), with a focus on the Layer III circuits known to exhibit age-related loss of dendritic spines (144). We found a large increase in the expression of C1q with advancing age in the rhesus monkey dlPFC, and corroborated this finding in rat mPFC. At the anatomical level, we confirmed dense glial localization of C1q. These findings are consistent with previous RNA-sequencing and immunohistochemistry studies in rodent and human brain (136,145–147), suggesting C1q in glial cells, indicating that the protein may be inherently synthesized in this cell type. In addition, we observed C1q localization within pyramidal neurons, particularly in dendritic spines and shafts (144). Specifically, C1q was located near the synaptic membrane in dendritic spines and near dysmorphic MOAS within shafts (Figure 3). These findings support observations in hippocampal neurons, where calcium overload of mitochondria has been shown to activate inflammatory caspase-3 actions (148), which may be associated with increased levels of C1q (149,150). Intriguingly, C1q was evident on the calcium-storing spine apparatus and near or within glutamatergic synapses (144). The subcompartment-specific localization of C1q within neurons might engage other immune pathway receptors, such as major histocompatibility complex I (151,152). Our findings lend credence to the notion that C1q may signal from within pyramidal neurons to initiate phagocytosis by reactive glia and that aberrant reactivation of inflammatory cascades with aging may lead to neurodegeneration and ensuing cognitive deficits. Glial cells such as microglia and astrocytes might be particularly susceptible to cellular senescence, which leads to the inhibition of key intracellular signaling pathways, further driving the release of pro-inflammatory cytokines with aging (153,154). Senescent glial cells have been shown to be particularly important in driving AD pathology by precluding the ability of glial cells to encapsulate Aβ plaques, ultimately contributing toward cognitive decline (153). In fact, clearance of senescent glial cells with first-generation senolytics has been shown to prevent gliosis, hyperphosphorylation of tau, and NFT pathology in a tau-transgenic mouse model of AD (155). Genetic risk factors for sporadic AD such as ApoE4 can further exacerbate the development of neuroinflammation and tau pathology (156), suggesting the intersection between inflammatory pathways and hyperphosphorylation of tau (157), and these phenotypes may be amplified in primates as opposed to rodents (158). Rhesus macaques all carry the ApoE4 risk allele and provide an invaluable opportunity to dissect the contribution of this genetic risk factor in the native course of the illness, and how it intersects with amyloid and tau pathology (159).
Figure 3.
Age-related alterations in complement cascade C1q signaling in dorsolateral prefrontal cortex Layer III. C1q expression accumulates in glia and postsynaptically in dendritic spines and dendritic shafts, with a sparser expression in axon terminals. Within dendritic spines, C1q aggregates in perisynaptic and extrasynaptic subcompartments in association with the spine apparatus of glutamatergic synapses. Within dendritic shafts, C1q aggregates in close proximity to dysmorphic mitochondria. We hypothesize that the rise in complement C1q signaling in the aged dlPFC may be due to age-related dysregulation of feedforward cAMP-PKA-calcium signaling but may also cause calcium overload of mitochondria and the initiation of inflammatory actions to eliminate dysfunctional neuronal elements and synapses by microglia-mediated phagocytosis (144).
Role of Stress Signaling in Aggravating Feedforward, cAMP-Calcium Signaling with Advancing Age
Multiple studies have provided convincing evidence of how physiological and psychological stress with advancing age can ultimately lead to the loss of synapses on dendritic spines and the weakening of higher cognitive abilities. The association cortices that mediate higher-order cognition are particularly modulated by catecholamines, norepinephrine (NE), and dopamine (DA), which can rapidly activate intracellular stress signaling pathways to weaken synaptic connections and impair cognition (78,82,160). Acute psychological stress results in significantly reduced working memory-related activity in the dlPFC and less deactivation of the default mode network due to supraoptimal levels of catecholamines (161). Similarly, chronic stress exposure leads to sustained weakening of network connections by calcium-cAMP-PKA-K+ signaling, leading to the removal of spines and dendrites (162–165), findings validated in humans (166). Both acute and chronic stress can potently drive cAMP-calcium signaling via catecholamines acting through NE alpha-1 adrenergic receptors (α1-AR) and DA-1 receptors to mediate Gq-IP3R-mediated calcium-protein kinase C signaling and Gs-cAMP-PKA signaling, respectively.
Chronic stress signaling pathways also initiate and propagate inflammatory cascades. Dysregulated stress and inflammation can mediate the release of the enzyme glutamate carboxypeptidase II (GCPII), which hydrolyzes N-acetylaspartylglutamate (NAAG) to glutamate and N-acetylaspartate (NAA), and therefore elevates ambient glutamate levels at excitatory synapses (167). GCPII suppresses NAAG-induced activation of mGluR3, which is located in postsynaptic subcompartments in dlPFC Layer III microcircuits, further exacerbating feedforward, cAMP-calcium signaling locally within dendritic spines (84,168,169). We have recently shown that systemic administration and local infusion of 2-(3-mercaptopropyl) pentanedioic acid (2-MPPA), which inhibits GCPII, improved working memory performance in aged rats (170). In parallel studies conducted in rhesus macaques, systemic administration of 2-MPPA, significantly improved working memory performance without any toxic side effects, with the greatest enhancement in the oldest animals (169). Furthermore, inflammation can induce the generation and release of kynurenic acid from astrocytes (171), an endogenous metabolite that blocks NMDAR (172,173) and impairs PFC working memory function (174). In fact, various components of the kynurenine pathway are currently under investigation for therapeutic development in cognitive disorders, including aging and neurodegeneration (175). These studies highlight how multiple cellular and molecular mechanisms interact, converging ultimately in inducing the atrophy of dendritic spines and dendrites leading to cognitive impairment with advancing age.
Conclusions and Future Directions
These findings provide evidence of how NHPs can be used to probe the cellular, molecular, and circuit alterations in higher-order association cortices that mediate cognition and are particularly susceptible to undergoing atrophy with aging. Particularly, NHPs such as rhesus macaques provide an unprecedented opportunity to elucidate the natural course of tau pathology in aging association cortex in the absence of autosomal dominant mutations and assess novel disease-modifying pharmacological strategies to ameliorate cognitive deficits with advancing age. NHPs recapitulate cardinal features of AD pathophysiology, including synapse loss, mitochondrial dysfunction, and microglial and astrocytic activation in vulnerable brain regions.
The tremendous advances in molecular and genetic tools have paved the way for great strides in future NHP research. For example, exogenous injection of Aβ oligomers in adult rhesus macaques produces pathological features reminiscent of preclinical AD, with synaptic dysfunction, neuroinflammation, and even NFT pathology (40,41,176). Recent studies highlight the possibility of using genetic delivery of mutated tau in a region-specific manner in rhesus macaque brain to induce misfolded tau propagation and templating and the possibility of testing biomarkers in CSF and blood (177). Structural investigations involving cryo-EM and mass spectrometry-based proteomics of tau filaments, including detailed mapping of posttranslational modifications in AD, are revealing how tau fibril structure influences the diversity of tauopathy strains, and these studies will be particularly important in understanding the 3D architecture of tau propagation (178–180). The generation of genetically engineered transgenic NHPs, including marmosets and rhesus macaques, offers the possibility of introducing germline mutations and exogenous gene expression changes to evaluate how genetic risk factors in neurodegenerative diseases impact higher-order cortical circuits present in the primate brain (181–183). Innovations in optogenetics in NHPs, which use genetically coded light-gated ion channels, offer the unique opportunity to selectively activate or silence cell types and neural pathways to study cognitive operations (184,185). Finally, refinement of single-cell transcriptomics with RNA-sequencing across the evolutionary lineage in primates is offering clues regarding species-specific molecular differences across homologous neuronal, glial, and nonneuronal cell types (186–188), particularly relevant to illuminating why specific cortical circuits are vulnerable in neurological diseases such as AD (189). The undertaking and successful implementation of these multidisciplinary approaches to elucidate the neurobiology of neurodegenerative disorders will require a concerted effort from research institutions, funding agencies, and pharmaceutical industries to advance scientific discovery. These technical and conceptual developments might provide unique insight into understanding the underlying cellular and molecular basis of devastating disorders like AD, to augment the development of intervention strategies.
Acknowledgments
I thank Dr. Amy F.T. Arnsten for her outstanding mentorship, intellectual guidance, invaluable insight for various research directions, and feedback on this manuscript, including members of the Arnsten laboratory at Yale University for their support of this research.
Funding
This work was supported by National Institutes of Health grants Pioneer Award DP1AG047744-01 and R01AG061190 (AFTA), Alzheimer’s Association Research Fellowship AARF-17-533294 (DD), American Federation for Aging Research/Diamond Postdoctoral Fellowship (DD), National Institute of Aging 1R21AG079145-01 (DD), and support from the Alzheimer’s Disease Research Unit from Christopher H. van Dyck.
Conflict of Interest
None.
References
- 1. Um JW, Kaufman AC, Kostylev M, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- 3. Metaxas A, Thygesen C, Kempf SJ, et al. Ageing and amyloidosis underlie the molecular and pathological alterations of tau in a mouse model of familial Alzheimer’s disease. Sci Rep. 2019;9:15758. doi: 10.1038/s41598-019-52357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paspalas CD, Carlyle B, Leslie S, et al. The aged rhesus macaque manifests Braak-stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 2018;14:680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnsten AFT, Datta D, Del Tredici K, Braak H.. Hypothesis: tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement. 2021;17:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barbier P, Zejneli O, Martinho M, et al. Role of tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci. 2019;11:204. doi: 10.3389/fnagi.2019.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venkatramani A, Panda D.. Regulation of neuronal microtubule dynamics by tau: implications for tauopathies. Int J Biol Macromol. 2019;133:473–483. doi: 10.1016/j.ijbiomac.2019.04.120 [DOI] [PubMed] [Google Scholar]
- 9. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL.. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172 [DOI] [PubMed] [Google Scholar]
- 10. Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS.. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis DA, Campbell MJ, Terry RD, Morrison JH.. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 13. Vogels T, Leuzy A, Cicognola C, et al. Propagation of tau pathology: integrating insights from postmortem and in vivo studies. Biol Psychiatry. 2020;87:808–818. doi: 10.1016/j.biopsych.2019.09.019 [DOI] [PubMed] [Google Scholar]
- 14. Adams JN, Maass A, Harrison TM, Baker SL, Jagust WJ.. Cortical tau deposition follows patterns of entorhinal functional connectivity in aging. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmed Z, Cooper J, Murray TK, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Busche MA, Hyman BT.. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat Neurosci. 2020;23:1183–1193. doi: 10.1038/s41593-020-0687-6 [DOI] [PubMed] [Google Scholar]
- 17. Calafate S, Buist A, Miskiewicz K, et al. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep. 2015;11:1176–1183. doi: 10.1016/j.celrep.2015.04.043 [DOI] [PubMed] [Google Scholar]
- 18. de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeVos SL, Miller RL, Schoch KM, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufman SK, Del Tredici K, Thomas TL, Braak H, Diamond MI.. Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 2018;136:57–67. doi: 10.1007/s00401-018-1855-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeda S, Wegmann S, Cho H, et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat Commun. 2015;6:8490. doi: 10.1038/ncomms9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vasconcelos B, Stancu IC, Buist A, et al. Heterotypic seeding of tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of tau-pathology in vivo. Acta Neuropathol. 2016;131:549–569. doi: 10.1007/s00401-015-1525-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carlyle BC, Nairn AC, Wang M, et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A. 2014;111:5036–5041. doi: 10.1073/pnas.1322360111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leslie SN, Datta D, Christensen KR, van Dyck CH, Arnsten AFT, Nairn AC.. Phosphodiesterase PDE4D is decreased in frontal cortex of aged rats and positively correlated with working memory performance and inversely correlated with PKA phosphorylation of tau. Front Aging Neurosci. 2020;14:578483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez-Callejas JD, Fuchs E, Perez-Cruz C.. Evidence of tau hyperphosphorylation and dystrophic microglia in the common marmoset. Front Aging Neurosci. 2016;8:315. doi: 10.3389/fnagi.2016.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edler MK, Sherwood CC, Meindl RS, et al. Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol Aging. 2017;59:107–120. doi: 10.1016/j.neurobiolaging.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latimer CS, Shively CA, Keene CD, et al. A nonhuman primate model of early Alzheimer’s disease pathologic change: implications for disease pathogenesis. Alzheimers Dement. 2019;15:93–105. doi: 10.1016/j.jalz.2018.06.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paspalas CD, Carlyle BC, Leslie S, et al. The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 2018;14:680–691. doi: 10.1016/j.jalz.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uno H, Walker LC.. The age of biosenescence and the incidence of cerebral beta-amyloidosis in aged captive rhesus monkeys. Ann N Y Acad Sci. 1993;695:232–235. doi: 10.1111/j.1749-6632.1993.tb23058.x [DOI] [PubMed] [Google Scholar]
- 30. Rapp PR, Amaral DG.. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiou KL, Montague MJ, Goldman EA, et al. Rhesus macaques as a tractable physiological model of human ageing. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190612. doi: 10.1098/rstb.2019.0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM.. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schramm RD, Paprocki AM, Bavister BD.. Features associated with reproductive ageing in female rhesus monkeys. Hum Reprod. 2002;17:1597–1603. doi: 10.1093/humrep/17.6.1597 [DOI] [PubMed] [Google Scholar]
- 34. Stonebarger GA, Bimonte-Nelson HA, Urbanski HF.. The rhesus macaque as a translational model for neurodegeneration and Alzheimer’s disease. Front Aging Neurosci. 2021;13:734173. doi: 10.3389/fnagi.2021.734173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braak E, Braak H, Mandelkow EM.. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315 [DOI] [PubMed] [Google Scholar]
- 36. Schultz C, Dehghani F, Hubbard GB, et al. Filamentous tau pathology in nerve cells, astrocytes, and oligodendrocytes of aged baboons. J Neuropathol Exp Neurol. 2000;59:39–52. doi: 10.1093/jnen/59.1.39 [DOI] [PubMed] [Google Scholar]
- 37. Arnsten AFT, Datta D, Leslie S, Yang ST, Wang M, Nairn AC.. Alzheimer’s-like pathology in aging rhesus macaques: unique opportunity to study the etiology and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arnsten AFT, Datta D, Preuss TM.. Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: an evolutionary perspective. Am J Primatol. 2021:e23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mufson EJ, Benzing WC, Cole GM, et al. Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: colocalization with amyloid plaques. Neurobiol Aging. 1994;15:621–627. doi: 10.1016/0197-4580(94)00064-6 [DOI] [PubMed] [Google Scholar]
- 40. Forny-Germano L, Lyra e Silva NM, Batista AF, et al. Alzheimer’s disease-like pathology induced by amyloid-beta oligomers in nonhuman primates. J Neurosci. 2014;34:13629–13643. doi: 10.1523/JNEUROSCI.1353-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yue F, Feng S, Lu C, et al. Synthetic amyloid-beta oligomers drive early pathological progression of Alzheimer’s disease in nonhuman primates. iScience. 2021;24:103207. doi: 10.1016/j.isci.2021.103207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braak H, Del Tredici K.. Neuroanatomy and pathology of sporadic Alzheimer’s disease. Adv Anat Embryol Cell Biol. 2015;215:1–162. [PubMed] [Google Scholar]
- 43. La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [11C]PIB-PET centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimers Dement. 2019;15:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01 [DOI] [PubMed] [Google Scholar]
- 45. Arboleda-Velasquez JF, Lopera F, O’Hare M, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med. 2019;25:1680–1683. doi: 10.1038/s41591-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Zhang Y, Hu W, et al. Rapid alteration of protein phosphorylation during postmortem: implication in the study of protein phosphorylation. Sci Rep. 2015;5:15709. doi: 10.1038/srep15709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Datta D, Leslie SN, Wang M, et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 2021;17:920–932. doi: 10.1002/alz.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jicha GA, Weaver C, Lane E, et al. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu F, Liang Z, Shi J, et al. PKA modulates GSK-3β- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006;580:6269–6274. doi: 10.1016/j.febslet.2006.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu SJ, Zhang JY, Li HL, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200 [DOI] [PubMed] [Google Scholar]
- 51. Colin M, Dujardin S, Schraen-Maschke S, et al. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020;139:3–25. doi: 10.1007/s00401-019-02087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dujardin S, Begard S, Caillierez R, et al. Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathol Commun. 2018;6:132. doi: 10.1186/s40478-018-0637-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu H, Hardy J, Duff KE.. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21:1350–1358. doi: 10.1038/s41593-018-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janelidze S, Stomrud E, Smith R, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun. 2020;11:1683. doi: 10.1038/s41467-020-15436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barthelemy NR, Bateman RJ, Hirtz C, et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimers Res Ther. 2020;12:26. doi: 10.1186/s13195-020-00596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive tau PET in Alzheimer’s disease. Sci Adv. 2020;6:eaaz2387. doi: 10.1126/sciadv.aaz2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78:149–156. doi: 10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17:1353–1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barthelemy NR, Li Y, Joseph-Mathurin N, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med. 2020;26:398–407. doi: 10.1038/s41591-020-0781-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mattsson-Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Janelidze S, Palmqvist S, Leuzy A, et al. Detecting amyloid positivity in early Alzheimer’s disease using combinations of plasma Abeta42/Abeta40 and p-tau. Alzheimers Dement. 2021. [DOI] [PubMed] [Google Scholar]
- 62. Sharma G, Huo A, Kimura T, et al. Tau isoform expression and phosphorylation in marmoset brains. J Biol Chem. 2019;294:11433–11444. doi: 10.1074/jbc.RA119.008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arnsten AFT, Datta D, Wang M.. The genie in the bottle-magnified calcium signaling in dorsolateral prefrontal cortex. Mol Psychiatry. 2020;epub Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fuster JM, Alexander GE.. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652 [DOI] [PubMed] [Google Scholar]
- 65. Funahashi S, Bruce CJ, Goldman-Rakic PS.. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331 [DOI] [PubMed] [Google Scholar]
- 66. Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6 [DOI] [PubMed] [Google Scholar]
- 67. González-Burgos G, Barrionuevo G, Lewis DA.. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82 [DOI] [PubMed] [Google Scholar]
- 68. González-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA.. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004 [DOI] [PubMed] [Google Scholar]
- 69. Wang M, Yang Y, Wang CJ, et al. NMDA receptors subserve working memory persistent neuronal firing in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kritzer MF, Goldman-Rakic PS.. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109 [DOI] [PubMed] [Google Scholar]
- 71. Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093 [DOI] [PubMed] [Google Scholar]
- 72. Elston GN, Benavides-Piccione R, Elston A, et al. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:26–35. doi: 10.1002/ar.a.20278 [DOI] [PubMed] [Google Scholar]
- 73. Zhu H, Suk HY, Yu RY, et al. Evolutionarily conserved role of calcineurin in phosphodegron-dependent degradation of phosphodiesterase 4D. Mol Cell Biol. 2010;30:4379–4390. doi: 10.1128/MCB.01193-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dumitriu D, Hao J, Hara Y, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Luebke J, Barbas H, Peters A.. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev. 2010;62:212–232. doi: 10.1016/j.brainresrev.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morrison JH, Baxter MG.. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arnsten AFT, Datta D, Wang M.. The genie in the bottle-magnified calcium signaling in dorsolateral prefrontal cortex. Mol Psychiatry. 2021;26:3684–3700. doi: 10.1038/s41380-020-00973-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang M, Ramos B, Paspalas C, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. [DOI] [PubMed] [Google Scholar]
- 80. Arnsten AFT, Wang M, Paspalas CD.. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Galvin VC, Yang S-T, Paspalas CD, et al. Muscarinic M1 receptors modulate working memory performance and activity via KCNQ potassium channels in primate prefrontal cortex. Neuron. 2020;106:649–661.e4. doi: 10.1016/j.neuron.2020.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arnsten AFT, Jin LE, Gamo NJ, et al. Role of KCNQ potassium channels in stress-induced deficit of working memory. Neurobiol Stress. 2019;11:100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jin LE, Wang M, Galvin VC, et al. mGluR2 vs. mGluR3 in primate prefrontal cortex: postsynaptic mGluR3 strengthen cognitive networks. Cereb Cortex. 2018;28:974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burt JB, Demirtas M, Eckner WJ, et al. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci. 2018;21:1251–1259. doi: 10.1038/s41593-018-0195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Carlyle BC, Kitchen RR, Kanyo JE, et al. A multiregional proteomic survey of the postnatal human brain. Nat Neurosci. 2017;20:1787–1795. doi: 10.1038/s41593-017-0011-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Datta D, Leslie S, Wang M, et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hof PR, Morrison JH.. Neocortical neuronal subpopulations labeled by a monoclonal antibody to calbindin exhibit differential vulnerability in Alzheimer’s disease. Exp Neurol. 1991;111:293–301. [DOI] [PubMed] [Google Scholar]
- 89. Lally G, Faull RL, Waldvogel HJ, Ferrari S, Emson PC.. Calcium homeostasis in ageing: studies on the calcium binding protein calbindin D28K. J Neural Transm. 1997;104:1107–1112. doi: 10.1007/BF01273323 [DOI] [PubMed] [Google Scholar]
- 90. Wang M, Gamo NJ, Yang Y, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lacampagne A, Liu X, Reiken S, et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017;134:749–767. doi: 10.1007/s00401-017-1733-7 [DOI] [PubMed] [Google Scholar]
- 92. Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- 93. Bellinger AM, Reiken S, Dura M, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Datta D, Enwright JF, Arion D, et al. Mapping phosphodiesterase 4D (PDE4D) in macaque dorsolateral prefrontal cortex: postsynaptic compartmentalization in layer III pyramidal cell circuits. Front Neuroanat. 2020;14:578483. doi: 10.3389/fnana.2020.578483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Goñi-Oliver P, Lucas JJ, Avila J, Hernández F.. N-terminal cleavage of GSK-3 by calpain—a new form of GSK-3 regulation. J Biol Chem. 2007;282:22406–22413. [DOI] [PubMed] [Google Scholar]
- 96. Jin N, Yin X, Yu D, et al. Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci Rep. 2015;5:8187. doi: 10.1038/srep08187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yamashima T. Reconsider Alzheimer’s disease by the “calpain-cathepsin hypothesis”—a perspective review. Prog Neurobiol. 2013;105:1–23. doi: 10.1016/j.pneurobio.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 98. Menzies FM, Fleming A, Caricasole A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 99. Orr ME, Oddo S.. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:53. doi: 10.1186/alzrt217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ingerman E, Perkins EM, Marino M, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. James DI, Parone PA, Mattenberger Y, Martinou JC.. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200 [DOI] [PubMed] [Google Scholar]
- 102. Yoon Y, Krueger EW, Oswald BJ, McNiven MA.. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cereghetti GM, Costa V, Scorrano L.. Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ. 2010;17:1785–1794. doi: 10.1038/cdd.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Youle RJ, van der Bliek AM.. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Morozov YM, Datta D, Paspalas CD, Arnsten AFT.. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol Aging. 2017;51:9–18. doi: 10.1016/j.neurobiolaging.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang L, Trushin S, Christensen TA, et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s disease. Sci Rep. 2016;6:18725. doi: 10.1038/srep18725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH.. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111:486–491. doi: 10.1073/pnas.1311310110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ansari MA, Scheff SW.. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Grimm A, Eckert A.. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–431. doi: 10.1111/jnc.14037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Navarro A, Boveris A.. Brain mitochondrial dysfunction in aging, neurodegeneration, and Parkinson’s disease. Front Aging Neurosci. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK.. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Friedman JR, Nunnari J.. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mozdy AD, McCaffery JM, Shaw JM.. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Otsuga D, Keegan BR, Brisch E, et al. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Smirnova E, Griparic L, Shurland DL, van der Bliek AM.. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Santos D, Esteves AR, Silva DF, Januario C, Cardoso SM.. The impact of mitochondrial fusion and fission modulation in sporadic Parkinson’s disease. Mol Neurobiol. 2015;52:573–586. doi: 10.1007/s12035-014-8893-4 [DOI] [PubMed] [Google Scholar]
- 118. Wang DB, Garden GA, Kinoshita C, et al. Declines in Drp1 and parkin expression underlie DNA damage-induced changes in mitochondrial length and neuronal death. J Neurosci. 2013;33:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang X, Su B, Lee HG, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Galluzzi L, Blomgren K, Kroemer G.. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10:481–494. doi: 10.1038/nrn2665 [DOI] [PubMed] [Google Scholar]
- 121. Green DR, Galluzzi L, Kroemer G.. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lin MT, Beal MF.. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 123. Zhu X, Raina AK, Lee HG, et al. Oxidative stress and neuronal adaptation in Alzheimer disease: the role of SAPK pathways. Antioxid Redox Signal. 2003;5:571–576. doi: 10.1089/152308603770310220 [DOI] [PubMed] [Google Scholar]
- 124. Zhu X, Su B, Wang X, Smith MA, Perry G.. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yin F, Sancheti H, Patil I, Cadenas E.. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Melov S, Adlard PA, Morten K, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One. 2007;2:e536. doi: 10.1371/journal.pone.0000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Favaro G, Romanello V, Varanita T, et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat Commun. 2019;10:2576. doi: 10.1038/s41467-019-10226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gomes LC, Di Benedetto G, Scorrano L.. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Park S, Won JH, Hwang I, Hong S, Lee HK, Yu JW.. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci Rep. 2015;5:15489. doi: 10.1038/srep15489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rimessi A, Bezzerri V, Patergnani S, Marchi S, Cabrini G, Pinton P.. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat Commun. 2015;6:6201. doi: 10.1038/ncomms7201 [DOI] [PubMed] [Google Scholar]
- 131. Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD.. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chung WS, Barres BA.. The role of glial cells in synapse elimination. Curr Opin Neurobiol. 2012;22:438–445. doi: 10.1016/j.conb.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chung WS, Welsh CA, Barres BA, Stevens B.. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stephan AH, Barres BA, Stevens B.. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810 [DOI] [PubMed] [Google Scholar]
- 135. Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- 136. Stephan AH, Madison DV, Mateos JM, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33:13460–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mathys H, Davila-Velderrain J, Peng Z, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Li Q, Barres BA.. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 139. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. doi: 10.1016/j.immuni.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 140. Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dejanovic B, Huntley MA, De Maziere A, et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron. 2018;100:1322–1336.e7. doi: 10.1016/j.neuron.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 142. Litvinchuk A, Wan YW, Swartzlander DB, et al. Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer’s disease. Neuron. 2018;100:1337–1353.e5. doi: 10.1016/j.neuron.2018.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lui H, Zhang J, Makinson SR, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Datta D, Leslie SN, Morozov YM, et al. Classical complement cascade initiating C1q protein within neurons in the aged rhesus macaque dorsolateral prefrontal cortex. J Neuroinflammation. 2020;17:8. doi: 10.1186/s12974-019-1683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hammond TR, Dufort C, Dissing-Olesen L, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271.e6. doi: 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Li Z, Jo J, Jia JM, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gyorffy BA, Kun J, Torok G, et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc Natl Acad Sci U S A. 2018;115:6303–6308. doi: 10.1073/pnas.1722613115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Nonaka S, Nakanishi H.. Microglial clearance of focal apoptotic synapses. Neurosci Lett. 2019;707:134317. doi: 10.1016/j.neulet.2019.134317 [DOI] [PubMed] [Google Scholar]
- 151. Corriveau RA, Huh GS, Shatz CJ.. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0 [DOI] [PubMed] [Google Scholar]
- 152. Lee H, Brott BK, Kirkby LA, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Baker DJ, Petersen RC.. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–1216. doi: 10.1172/JCI95145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sahu MR, Rani L, Subba R, Mondal AC.. Cellular senescence in the aging brain: a promising target for neurodegenerative diseases. Mech Ageing Dev. 2022;204:111675. doi: 10.1016/j.mad.2022.111675 [DOI] [PubMed] [Google Scholar]
- 155. Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ.. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Serrano-Pozo A, Das S, Hyman BT.. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20:68–80. doi: 10.1016/S1474-4422(20)30412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Souder DC, Dreischmeier IA, Smith AB, et al. Rhesus monkeys as a translational model for late-onset Alzheimer’s disease. Aging Cell. 2021;20:e13374. doi: 10.1111/acel.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Poduri A, Gearing M, Rebeck GW, Mirra SS, Tigges J, Hyman BT.. Apolipoprotein E4 and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. Am J Pathol. 1994;144:1183–1187. [PMC free article] [PubMed] [Google Scholar]
- 160. Arnsten AF, Wang MJ, Paspalas CD.. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G.. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 162. Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104 [DOI] [PubMed] [Google Scholar]
- 163. Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF.. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hains AB, Yabe Y, Arnsten AFT.. Chronic stimulation of alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Moda-Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R.. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116–128. doi: 10.1016/j.nbd.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Woo E, Datta D, Arnsten AFT.. Glutamate metabotropic receptor type 3 (mGlu3) localization in the rat prelimbic medial prefrontal cortex. Front Neuroanat. 2022;16:849937. doi: 10.3389/fnana.2022.849937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yang S, Datta D, Elizabeth W, et al. Inhibition of glutamate-carboxypeptidase-II in dorsolateral prefrontal cortex: potential therapeutic target for neuroinflammatory cognitive disorders. Mol Psychiatry. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Datta D, Leslie SN, Woo E, et al. Glutamate carboxypeptidase II in aging rat prefrontal cortex impairs working memory performance. Front Aging Neurosci. 2021;13:760270. doi: 10.3389/fnagi.2021.760270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cervenka I, Agudelo LZ, Ruas JL.. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794 [DOI] [PubMed] [Google Scholar]
- 172. Pullan LM, Cler JA.. Schild plot analysis of glycine and kynurenic acid at the N-methyl-d-aspartate excitatory amino acid receptor. Brain Res. 1989;497:59–63. doi: 10.1016/0006-8993(89)90969-4 [DOI] [PubMed] [Google Scholar]
- 173. Schwarcz R, Pellicciari R.. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439 [DOI] [PubMed] [Google Scholar]
- 174. Chess AC, Simoni MK, Alling TE, Bucci DJ.. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Stone TW, Darlington LG.. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Beckman D, Ott S, Donis-Cox K, et al. Oligomeric Abeta in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc Natl Acad Sci U S A. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Beckman D, Chakrabarty P, Ott S, et al. A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimers Dement. 2021;17:933–945. doi: 10.1002/alz.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Arakhamia T, Lee CE, Carlomagno Y, et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell. 2021;184:6207–6210. doi: 10.1016/j.cell.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wesseling H, Mair W, Kumar M, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 2020;183:1699–1713.e13. doi: 10.1016/j.cell.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Park JE, Zhang XF, Choi SH, Okahara J, Sasaki E, Silva AC.. Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci Rep. 2016;6:34931. doi: 10.1038/srep34931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhou Y, Sharma J, Ke Q, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature. 2019;570:326–331. doi: 10.1038/s41586-019-1278-0 [DOI] [PubMed] [Google Scholar]
- 183. Park JE, Silva AC.. Generation of genetically engineered non-human primate models of brain function and neurological disorders. Am J Primatol. 2019;81:e22931. doi: 10.1002/ajp.22931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Galvan A, Stauffer WR, Acker L, et al. Nonhuman primate optogenetics: recent advances and future directions. J Neurosci. 2017;37:10894–10903. doi: 10.1523/JNEUROSCI.1839-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rajalingham R, Sorenson M, Azadi R, Bohn S, DiCarlo JJ, Afraz A.. Chronically implantable LED arrays for behavioral optogenetics in primates. Nat Methods. 2021;18:1112–1116. doi: 10.1038/s41592-021-01238-9 [DOI] [PubMed] [Google Scholar]
- 186. Krienen FM, Goldman M, Zhang Q, et al. Innovations present in the primate interneuron repertoire. Nature. 2020;586:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ma S, Skarica M, Li Q, et al. Molecular and cellular evolution of the primate dorsolateral prefrontal cortex. Science. 2022;377:abo7257. doi: 10.1126/science.abo7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhu Y, Sousa AMM, Gao T, et al. Spatiotemporal transcriptomic divergence across human and macaque brain development. Science. 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Franjic D, Skarica M, Ma S, et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022;110:452–469.e14. doi: 10.1016/j.neuron.2021.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]