Abstract
Low back pain is one of the leading causes of disability in the world. Regenerative medicine can be one of the novel treatment breakthroughs in patients with low back pain, yet its use is still debatable. We performed a systematic evaluation and meta-analysis to determine the efficacy of platelet-rich plasma (PRP) treatment for patients with chronic low back pain. Comprehensive database searches were performed in four databases. This study was conducted and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses Guideline and registered to PROSPERO. We included and examined randomized controlled trials that looked into research employing PRP for patients with chronic low back pain. Outcomes of interest include clinical enhancement of pain, which is demonstrated in pain scores. Three studies were included comprising 138 patients with chronic low back pain. After 1, 3, and 6 months after injection, there was a substantial reduction in the pain score difference between the PRP and control groups, demonstrating PRP’s superiority over the control group in the treatment of chronic low back pain. PRP injection significantly enhances chronic low back pain in the first, third, and sixth months after injection compared to controls.
Keywords: Platelet-rich plasma, Chronic low back pain, Regenerative medicine, Injections
Introduction
Low back pain (LBP) is defined as pain that lasts at least one day (with/without pain in one or both legs), localized on the posterior aspect from the lower margin of the 12th ribs until the lower gluteal fold [1–3]. The adult population frequently complains about LBP. Worldwide, LBP continues to lead to disability and is now considered a global public health issue. LBP patients require extensive multidisciplinary and multimodal care [1–6]. LBP symptoms can be caused by a variety of anatomical issues, such as nerve roots, muscles, bones, joints, intervertebral discs, fascial structures, and abdominal organs [1,7,8].
Chronic LBP has been successfully treated using regenerative medicine, particularly when platelet-rich plasma (PRP) and mesenchymal stem cells are used [9–13]. PRP was first utilized in open-heart surgery in the 1970s, then 10 years later used in maxillofacial surgery to help in wound healing in reconstructive jaw surgery [14]. The patient’s peripheral blood is spun to produce a high platelet concentration before being used to create PRP. PRP encourages tissue angiogenesis and the mitogenesis of healing cells [13–18] and contains seven essential proteins including platelet-derived growth factors, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor, and adhesive proteins–fibrin, fibronectin, and vitronectin, which can support three phases of tissue healing and their repair cascade (inflammation, proliferation, and remodeling) [12–16,18]. Despite regenerative effects, PRP also has anti-inflammatory and anti-apoptotic by strengthening chondrogenic markers (aggrecan [AGN], collagen type II [COL2], TGF-β1) and inhibiting catabolic pathways (matrix metalloproteinase [MMP]-3, cyclooxygenase-2, interleukin-1 beta (IL-1β), tumor necrosis factor-α, MMP-1, nerve growth factor) [19,20].
PRP can also be used as a supplement to other musculoskeletal treatments such as ligament restoration, joint arthroplasty, joint osteotomy, and treatment for degenerative joint disease [13,16–18,20–22]. In disc degeneration, cytokines and growth factors work on maintaining disc homeostasis by switching from a catabolic state to an anabolic state, and improving the production of COL2 protein and proteoglycan synthesis [13,20,22,23]. Numerous research, including in vitro, in vivo, preclinical animal, and human clinical trials, have examined the relationship between PRP and intervertebral disc degeneration [20,22–29]. Nevertheless, the articles considering the PRP effect on chronic LBP have not been systematically evaluated, and meta-analyzed. The purpose of this study was to conduct a comprehensive review of the literature and a meta-analysis of the efficacy of PRP therapy for persistent LBP.
Methods
1. Search strategy
This study was conducted and reported based the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guideline [30]. The PICO of this study was P (chronic LBP), I (PLP), C (other treatments), and O (pain). A comprehensive literature search was carried out on PubMed, ScienceDirect, ProQuest, and Medline (via EBSCO) in April 2022 with the following search string: platelet-rich plasma and low back pain. The study protocol was registered on PROSPERO (ID: CRD42022322723).
2. Study selection
All included studies contained original data published in English language within 20 years. Randomized controlled trials (RCTs) that analyzed the use of PRP in treating LBP were included. The inquiry did not include any animal experiments, review articles, commentary, or letters to the editor (Fig. 1).
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow chart.
3. Quality appraisal and risk of bias assessment
Identification, selection, data extraction, and quality assessment were carried out independently by two authors (L.C.S. and S.A.K.). Different opinions between the two reviewers were settled through conversation and reevaluation with another author (E.K.). The quality analysis of the literature was evaluated with the Jadad Scale [31]. To assess the risk of bias, reviewers should rate each of the five items from the scale into dichotomous variables. An overall score is determined by adding all the item’s scores. Each article will be given a grade to indicate whether it is a high-quality study or one of low quality. Assessment of the level of evidence was performed using the Oxford Centre for Evidence-Based Medicine Guideline 2011 [32].
4. Data extraction and analysis
All data were extracted from the text, figures, tables, and related additional files from each included study. These details comprised (1) article and demographic information, (2) the kind, amount, and length of PRP intervention, and (3) clinical outcomes. The primary clinical outcome was the mean difference in pain score between PRP and control over the period.
Statistical analysis was performed using the Review Manager software (RevMan ver. 5.3; Cochrane Collaboration, Oxford, UK) to do statistical analysis using the Mantel-Haenszel technique. A dichotomous variable and risk ratio with a 95% confidence interval was used to determine the effect. The heterogeneity between trials was found by the I2 value. The results were also shown using forest plots on the data. Additionally, a funnel plot was employed to evaluate the publication bias significance was determined as p-values <0.05.
Results
1. Study selection
A total of 144 studies were obtained from the initial screening (Fig. 1). A total of 79 studies were added after excluding duplication. Seventy articles were removed after the abstract screening. Of the remaining nine studies [24–28,33–35], one study was not published in English [36]. Only three studies with appropriate outcome measurements could be incorporated into the current investigation after full-text reading [24–26].
2. Study quality assessment
Assessment of the level of evidence was evaluated using Oxford Centre for Evidence-Based Medicine Guideline 2011, which discovered that all three studies had level 2 of evidence [32]. Based on quality assessment, all of the studies were labeled as high-level studies (Table 1).
Table 1.
Study quality assessment with Jadad Scale
| Study (year) | Was the study described as random? | Was the randomization scheme described and appropriate? | Was the study described as double blind? | Was the method of double blinding appropriate? (The patient and assessor appropriately blinded) | Was there a description of dropouts and withdrawal? | Score |
|---|---|---|---|---|---|---|
| Won et al. [24] (2022) | V | V | V | V | V | 5 |
| Ruiz-Lopez et al. [25] (2020) | V | V | V | V | V | 5 |
| Tuakli-Wosornu et al. [26] (2016) | V | V | V | V | V | 5 |
Jadad Scale, score quality: 0–2 (low) and 3–5 (high).
3. Article and demographic characteristics
The characteristics of the three studies included in our study are demonstrated in Tables 2 and 3. The investigations, with sample sizes ranging from 34 to 58 participants, were published between 2016 and 2022.
Table 2.
Characteristics of the included studies
| Author (year) | No. of patients | Age (yr) | Sex (male/female) | ||
|---|---|---|---|---|---|
|
|
|
||||
| PRP | Control | PRP | Control | ||
| Won et al. [24] (2022) | 30 | 51±18.1 | 50.5±17 | 6/8 | 6/10 |
|
| |||||
| Ruiz-Lopez et al. [25] (2020) | 50 | 68±13.06 | 61±12.6 | 11/14 | 10/15 |
|
| |||||
| Tuakli-Wosornu et al. [26] (2016) | 58 | 41.4±8.08 | 43.8±8.91 | 14/15 | 3/16 |
Values are presented as mean±standard deviation or number of patients.
PRP, platelet-rich plasma.
Table 3.
Intervention and outcomes of the included studies
| Author (year) | Intervention | Site of injection | Method of injection | Dose | Outcome | |
|---|---|---|---|---|---|---|
| PRP | Control | |||||
| Won et al. [24] (2022) | PRP (14 patients) vs. 0.5% lidocaine (16 patients) | Lumbopelvic ligaments | Preparation → local anesthesia administered → injection | 5–6 mL | 6 mL | Improvement of pain score in 1, 3, and 6 months |
| Ruiz-Lopez et al. [25] (2020) | PRP (25 patients) vs. corticosteroid (triamcinolone) (25 patients) | Epidural | Preparation → light sedation of midazolam → injection | 16.5 mL leucocyte rich-PRP and 3.5 mL contrast | 16.5 mL triamcinolone and 3.5 mL contrast | Improvement of pain score in 1, 3, and 6 months |
| Tuakli-Wosornu et al. [26] (2016) | PRP (29 patients) vs. contrast agent (18 patients) | Intradiscal | Preparation → local anesthesia administered → injection | 3–4 mL | 3–4 mL | Improvement of pain score in 1, 4, and 8 weeks |
PRP, platelet-rich plasma.
4. The mean differences of pain score between platelet-rich plasma and control groups
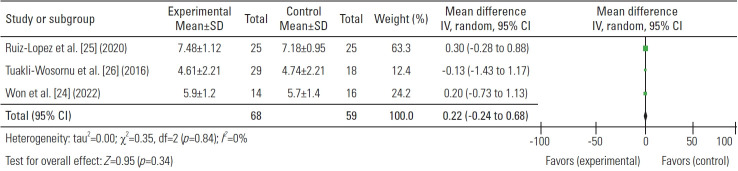
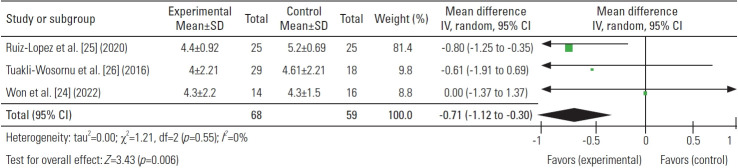
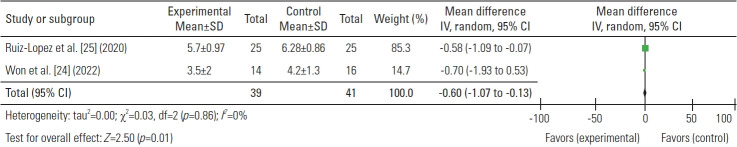
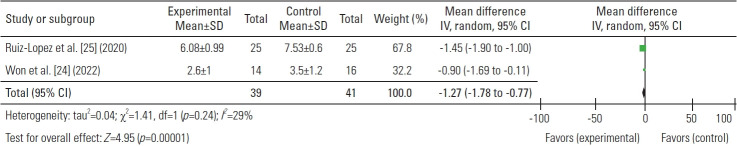
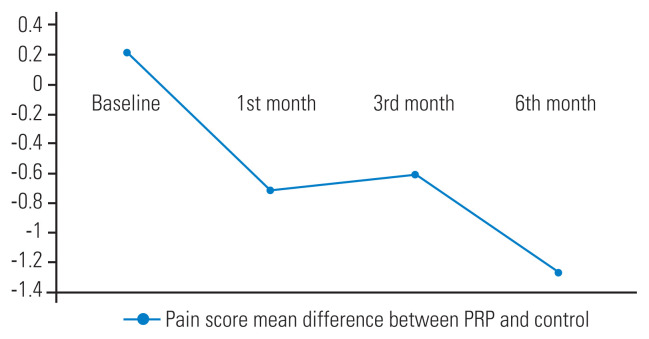
The mean pain score was assessed in three investigations at 1, 3, and 6 months following treatments. There was a significant difference in the pain score (mean) at the baseline between the PRP and control group which was 0.22, higher in the PRP group (Fig. 2). The difference in the pain score between the PRP and control group considerably increased after 1, 3, and 6 months of injection (−0.71, −0.60, and −1.27, respectively) (Figs. 3–6). These findings demonstrated PRP’s advantage over the control group in the management of chronic LBP.
Fig. 2.
Baseline mean difference of pain score. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Fig. 3.
Mean differences in 1 month. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Fig. 4.
Mean differences in 3 months. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Fig. 5.
Mean differences in 6 months. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Fig. 6.
The mean difference of pain score between platelet-rich plasma (PRP) and control.
Discussion
The results of the current meta-analysis discovered that PRP demonstrated superiority in pain reduction in patients with LBP, compared to control which was shown by the enhancement in the pain score in the first, third, and sixth months of the procedure.
There are several underlying methods by which PRP injections might lessen pain and enhance function. Platelet concentration in PRP is 3 to 8 times that in serum. It can be a result of the effect of PRP that has anti-inflammation, angiogenesis, cell migration, and anabolic metabolism for tissue repair and regeneration. When platelets are activated in PRP, numerous growth factors, such as TGF-β, platelet-derived growth factor, insulin-like growth factor, basic fibroblast growth factor, VEGF, and many more, can be released. They can stimulate chondrocytes and collagen II synthesis, prevent chondrocyte and mesenchymal stem cell apoptosis, and avoid catabolic effects of inflammatory cytokines such as IL-1β and MMPs. PRP also contains a plasma protein, which is recognized to play a vital role in healing processes in connective tissue such as Sox9, AGN, COL I, and COL II. Due to their impact on increased osmotic pressure, aggregans play a function in water absorption as well as providing tensile strength and anchoring the tissue to the bone through increased collagen. PRP injection surpasses the nuclear factor-kB signaling pathway and modifies this pathological condition to an anabolic and anti-inflammatory state [11,13,17,19,20,22].
Won et al. [24] conducted an RCT and demonstrated PRP injection improvement of The Oswestry Disability Index and Roland Morris Disability Questionnaire (RMDQ) in the third and sixth months. According to the study, PRP injection offered pain alleviation that lasted longer than corticosteroid, contrast agent, or lidocaine injection, which served as the control arm [24–26]. Akeda et al. [28] discovered that the PRP group revealed better improvement in pain-related disability which was demonstrated by the enhancement of RMDQ score at 26 weeks and walking ability at the 4th and 8th weeks.
Results of PRP injection for LBP with sacroiliac joint (SIJ) pathology also provide a considerable improvement in pain compared to lidocaine injection. According to the findings of Singla et al. [29], PRP injection offers higher pain relief in SIJ pathology commencing at 6 weeks after therapy and is sustained until the third month. The study reported that the percentage of pain-free patients in PRP injection at 3 months was 90% compared to group lidocaine which reduced to only 25% [29].
In a meta-analysis of animal studies, PRP injection is obtained and expected to regenerate intervertebral discs, which can be observed through changes in disc height index (DHI) and Pfirmann grade in magnetic resonance imaging (MRI) examination [37]. But in a study by Akeda et al. [28], radiologic evaluation in human intervertebral discs reveals that PRP injection does not give change in DHI or corticosteroid injection. A similar result was determined from MRI analysis for disc degeneration grade which did not reveal any change from baseline in the PRP or corticosteroid group [28]. One hypothesis is that an intervertebral disc’s low capacity for self-repair results from its avascularity. In mild circumstances, PRP injection may be able to slow down or even stop the degeneration of the intervertebral disc [12,38,39].
Contrary to the results of the current study, Zielinski et al. [33] discovered that PRP injection did not significantly reduce pain compared to saline injection in patients with chronic lumbar discogenic back pain. Schepers et al. [34] similarly came to a similar conclusion, concluding that during a 12-month follow-up period, intradiscal PRP injection did not significantly enhance the average pain score or worst pain score compared to the control. The cause of these discrepancies is perhaps due to the variation in the PRP concentration [14,18,40].
Compared to PRP, corticosteroids have a lesser risk of allergic responses or rejection [12,20]. Complications of corticosteroid injection might be a pain at the injection site, skin discoloration, weakening of the adjacent tendon, fat atrophy, osteoporosis, and osteonecrosis, and when utilized as the epidural injection can cause an epidural abscess, septic meningitis or Tachon syndrome [41–44]. Increased pain at the injection site, which can go away in a week, and pelvic itching that went away after taking diphenhydramine are complications of PRP injection [25]. Schepers et al. [34] discovered one severe complication after PRP injection without prophylactic antibiotic, spondylodiscitis. However, our included studies did not determine any serious complications in the PRP group [24–26].
In terms of the cost-effectiveness of the injection treatments used, PRP injections may be more expensive than corticosteroid injections, which are one-third the price of PRP injections. However, corticosteroid injection only temporarily alleviates symptoms, which necessitates the purchase of additional treatments, such as surgical procedures, and may result in higher costs [45].
The strength of the study was the availability of similar quantitative scores over three periods of time. Additionally, different database searches from four databases improved the quality of our study’s search. However, the small number of samples among included studies became our limitation. Future RCT with large samples is required to deliver a better quality of data.
Conclusions
PRP injection considerably reduces chronic LBP in the first, third, and sixth months after injection compared to controls, which provides a more favorable clinical results without significant differences in adverse events.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: LCS, SAK, EK; data acquisition: LCS, SAK; analysis of data: LCS; drafting of the manuscript: LCS, SAK, EK; critical revision: LCS, SAK, IS, EK; and supervision: IS, EK.
References
- 1.Allegri M, Montella S, Salici F, et al. Mechanisms of low back pain: a guide for diagnosis and therapy. F1000Res. 2016;5:1530. doi: 10.12688/f1000research.8105.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–4. doi: 10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–67. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 4.Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8:299. doi: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nees TA, Riewe E, Waschke D, Schiltenwolf M, Neubauer E, Wang H. Multidisciplinary pain management of chronic back pain: helpful treatments from the patients’ perspective. J Clin Med. 2020;9:145. doi: 10.3390/jcm9010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 7.Casser HR, Seddigh S, Rauschmann M. Acute lumbar back pain. Dtsch Arztebl Int. 2016;113:223–34. doi: 10.3238/arztebl.2016.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barros G, McGrath L, Gelfenbeyn M. Sacroiliac joint dysfunction in patients with low back pain. Fed Pract. 2019;36:370–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Sanapati J, Manchikanti L, Atluri S, et al. Do regenerative medicine therapies provide long-term relief in chronic low back pain: a systematic review and metaanalysis. Pain Physician. 2018;21:515–40. [PubMed] [Google Scholar]
- 10.Navani A, Manchikanti L, Albers SL, et al. Responsible, safe, and effective use of biologics in the management of low back pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2019;22(1S):S1–74. [PubMed] [Google Scholar]
- 11.Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD. Treatment of chronic low back pain: new approaches on the horizon. J Pain Res. 2017;10:1111–23. doi: 10.2147/JPR.S132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SZ, Jin JY, Guo YD, et al. Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: a preliminary investigation. Mol Med Rep. 2016;13:3475–81. doi: 10.3892/mmr.2016.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchini M, Cruciani M, Mengoli C, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus. 2019;17:357–67. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain NK, Gulati M. Platelet-rich plasma: a healing virtuoso. Blood Res. 2016;51:3–5. doi: 10.5045/br.2016.51.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JY, Fabricant PD, Ishmael CR, Wang JC, Petrigliano FA, Jones KJ. Utilization of platelet-rich plasma for musculoskeletal injuries: an analysis of current treatment trends in the United States. Orthop J Sports Med. 2016;4:2325967116676241. doi: 10.1177/2325967116676241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins T, Alexander D, Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. 2021;6:225–35. doi: 10.1302/2058-5241.6.200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akeda K, Yamada J, Linn ET, Sudo A, Masuda K. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res. 2019;12:753–67. doi: 10.2147/JPR.S153085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed S, Yu J. Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain. J Spine Surg. 2018;4:115–22. doi: 10.21037/jss.2018.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le AD, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624–34. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang Y, Yang M, Ke S, Zhang Y, Xu G, Li Z. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid Med Cell Longev. 2020;2020:8893819. doi: 10.1155/2020/8893819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SZ, Rui YF, Tan Q, Wang C. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15:220. doi: 10.1186/ar4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won SJ, Kim DY, Kim JM. Effect of platelet-rich plasma injections for chronic nonspecific low back pain: a randomized controlled study. Medicine (Baltimore) 2022;101:e28935. doi: 10.1097/MD.0000000000028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Lopez R, Tsai YC. A randomized double-blind controlled pilot study comparing leucocyte-rich platelet-rich plasma and corticosteroid in caudal epidural injection for complex chronic degenerative spinal pain. Pain Pract. 2020;20:639–46. doi: 10.1111/papr.12893. [DOI] [PubMed] [Google Scholar]
- 26.Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R. 2016;8:1–10. doi: 10.1016/j.pmrj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Xuan Z, Yu W, Dou Y, Wang T. Efficacy of platelet-rich plasma for low back pain: a systematic review and meta-analysis. J Neurol Surg A Cent Eur Neurosurg. 2020;81:529–34. doi: 10.1055/s-0040-1709170. [DOI] [PubMed] [Google Scholar]
- 28.Akeda K, Ohishi K, Takegami N, et al. Platelet-rich plasma releasate versus corticosteroid for the treatment of discogenic low back pain: a double-blind randomized controlled trial. J Clin Med. 2022;11:304. doi: 10.3390/jcm11020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singla V, Batra YK, Bharti N, Goni VG, Marwaha N. Steroid vs. platelet-rich plasma in ultrasound-guided sacroiliac joint injection for chronic low back pain. Pain Pract. 2017;17:782–91. doi: 10.1111/papr.12526. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.OCEBM Levels of Evidence Working Group . The Oxford Levels of Evidence 2 [Internet] Oxford: Oxford Centre for Evidence-Based Medicine; 2011. [cited 2022 May 10]. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. [Google Scholar]
- 33.Zielinski MA, Evans NE, Bae H, et al. Safety and efficacy of platelet rich plasma for treatment of lumbar discogenic pain: a prospective, multicenter, randomized, double-blind study. Pain Physician. 2022;25:29–34. [PubMed] [Google Scholar]
- 34.Schepers MO, Groot D, Kleinjan EM, Pol MM, Mylenbusch H, Klopper-Kes AH. Effectiveness of intradiscal platelet rich plasma for discogenic low back pain without Modic changes: a randomized controlled trial. Interv Pain Med. 2021;1:100011. doi: 10.1016/j.inpm.2022.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Zhou J, Liu C, et al. A prospective study comparing platelet-rich plasma and local anesthetic (LA)/corticosteroid in intra-articular injection for the treatment of lumbar facet joint syndrome. Pain Pract. 2017;17:914–24. doi: 10.1111/papr.12544. [DOI] [PubMed] [Google Scholar]
- 36.Byvaltsev VA, Kalinin AA, Okoneshnikova AK. Comparative analysis of the effectiveness of PRP therapy and facetoplasty in older patients with isolated lumbar facet syndrome: long-term results of a randomized controlled trial. Adv Gerontol. 2019;32:804–11. [PubMed] [Google Scholar]
- 37.Li P, Zhang R, Zhou Q. Efficacy of platelet-rich plasma in retarding intervertebral disc degeneration: a meta-analysis of animal studies. Biomed Res Int. 2017;2017:7919201. doi: 10.1155/2017/7919201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciapetti G, Granchi D, Devescovi V, et al. Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J. 2012;21(Suppl 1):S10–9. doi: 10.1007/s00586-012-2234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavakoli J, Diwan AD, Tipper JL. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int J Mol Sci. 2020;21:4889. doi: 10.3390/ijms21144889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Zhang Z, Ma Y, Liu X, Zhu Q. Platelet-rich plasma injection vs corticosteroid injection for conservative treatment of rotator cuff lesions: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24680. doi: 10.1097/MD.0000000000024680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14:203–7. [PMC free article] [PubMed] [Google Scholar]
- 43.Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206. doi: 10.1186/1471-2474-11-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benoist M, Boulu P, Hayem G. Epidural steroid injections in the management of low-back pain with radiculopathy: an update of their efficacy and safety. Eur Spine J. 2012;21:204–13. doi: 10.1007/s00586-011-2007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11. doi: 10.1051/sicotj/2017062. [DOI] [PMC free article] [PubMed] [Google Scholar]