Abstract
Background:
Although in the early pandemic period COVID-19 pathology among young children and infants was typically less severe compared with that observed among adults, this has not remained entirely consistent as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have emerged. There is an enormous body of evidence demonstrating the benefits of human milk antibodies (Abs) in protecting infants against a wide range of enteric and respiratory infections. It is highly plausible that the same holds true for protection against SARS-CoV-2 as this virus infects cells of the gastrointestinal and respiratory mucosae. Understanding the durability of a human milk Ab response over time after infection is critical.
Objective:
Previously, we examined the Abs present in milk of those recently infected with SARS-CoV-2 and concluded that the response was secretory immunoglobulin A (sIgA) dominant and that these titers were highly correlated with neutralization potency. The present study aimed to monitor the durability of the SARS-CoV-2 IgA and secretory Ab (sAb) response in milk from COVID-19-recovered lactating individuals over 12 months in the absence of vaccination or reinfection.
Results:
This analysis revealed a robust and durable spike-specific milk sIgA response, and at 9–12 months after infection, 88% of the samples exhibited titers above the positive cutoff for IgA and 94% were above the cutoff for sAb. Fifty percent of participants exhibited less than twofold reduction of spike-specific IgA through 12 months. A strong, significant positive correlation between IgA and sAb against spike persisted throughout the study period. Nucleocapsid-specific Abs were also assessed, which revealed significant background or cross-reactivity of milk IgA against this immunogen, as well as limited/inconsistent durability compared with Spike titers.
Conclusion:
These data suggest that lactating individuals are likely to continue producing spike-specific Abs in their milk for 1 year or more, which may provide critical passive immunity to infants against SARS-CoV-2 throughout the lactation period.
Keywords: COVID-19, SARS-CoV-2, secretory IgA, human milk, lactation, antibodies
Introduction
As of March 2023, there have been over 760 million confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, causing 6.8 million deaths.1 Although in the early pandemic period, COVID-19 pathology among young children and infants was typically less severe compared with that observed among adults, this has not remained entirely consistent as SARS-CoV-2 variants have emerged.
For example, hospitalization rates among children <4 years old increased 5 × during the USA Omicron (B.1.1.529) variant wave (December 19, 2021,–February 19, 2022) compared with during the Delta variant period (B.1.617.2, June 27–December 18, 2021). During this Omicron wave, infants <6 months old accounted for 44% of all COVID-19-related hospitalizations and 21% required ICU admission.2
Especially as variants emerge that are more transmissible, children are at similar risk of SARS-CoV-2 infection as adults,3 and during the 2021–2022 waves of infections in the United States (largely comprising Delta [B.1.617.2] and Omicron [B.1.1.529] variants, respectively), new cases in children aged 0–4 years surpassed those among adults aged 65+ years, an epidemiological feature not previously observed.4
Notably, even asymptomatic infection can lead to multisystem inflammatory syndrome in children (MIS-C), a rare, but potentially deadly, inflammatory condition, and young children are responsible for a significant amount of SARS-CoV-2 dissemination.5,6 Clearly, protecting this population from infection remains essential.7–9
COVID-19 vaccines are available for infants >6 months old, but those <6 months old are not eligible. Although this is, in part, due to the potential for infants to receive vaccine- or infection-induced maternal IgG in utero, optimal placental IgG transfer requires timely maternal infection or vaccination, as well as surmounting the significant vaccine hesitancy among pregnant people.
Importantly, maternal antibodies (Abs) wane from birth until they are undetectable by 6–12 months, with the kinetics of this decline being highly dependent on the initial titers transferred.10 Even for infants receiving high titers of maternal IgG in utero, milk Abs can provide critical complementary mucosal protection.11,12 As such, the passive immunity of the Abs acquired through human milk feeding remains a highly relevant mechanism to protect infants from SARS-CoV-2.
Human milk contains ∼0.7 g/L IgA, which composes ∼90% of the total immunoglobulin (Ig) in milk.13,14 Approximately 2% of Ig in milk is IgG, as humans rely on placentally transferred IgG for systemic immunity.14 Milk-derived Abs are likely most active in the oral/nasal cavity and upper gastrointestinal (GI) tract of infants, although ∼30–50% of these Abs have been shown to resist degradation in the stomach for as long as 2 hours, suggesting that they are functional throughout the GI tract and possibly beyond.15
Milk IgG is derived primarily from serum, with a minority arising from local mammary production. B cells in the mammary gland, which ultimately produce IgA (and to a lesser extent, IgM) that becomes secretory (s)IgA, predominantly originate from the gut-associated lymphoid tissue (GALT), exemplifying a critical entero-mammary link, wherein the secretory Abs (sAbs) found in human milk echo the immunogens identified in the maternal GI tract (and airways).16,17
This IgA is polymerized (mostly dimerized) with a joining (J) chain within the B cell before secretion and then bound by the polymeric Ig receptor (pIgR) on mammary epithelial cells. PIgR is cleaved as it transports Abs into the milk, leaving the secretory component (SC) attached and resulting in sAbs.16,18 Determining whether or not sAbs are elicited in milk after infection or vaccination is critical as this Ab class is highly stable and resistant to enzymatic degradation in milk and all mucosae—not only in the infant oral/nasal cavity but also in the airways and GI tract.14,19
Previously, we examined the Abs present in milk of those recently infected with SARS-CoV-2 and concluded that the response was secretory IgA (sIgA) dominant and that the sIgA titer was highly correlated with neutralization potency.20,21 In the present study, milk samples from 16 COVID-19-recovered study participants were collected longitudinally for up to 12 months postinfection to determine the durability of the sIgA response in milk over time. Specific reactivity against SARS-CoV-2 spike and nucleocapsid was measured.
Materials and Methods
Study participants and milk sampling
This study was approved by the Institutional Review Board (IRB) at Mount Sinai Hospital (IRB 19-01243). Individuals were eligible to have their milk samples included in this analysis if they were: (a) lactating; (b) free of any health conditions affecting the immune system; (c) were infected with SARS-CoV-2 (confirmed by FDA-approved COVID-19 test) 3–8 weeks before the first milk sample available; and (d) could also provide a milk sample at least 9 months post-infection.
Participants were recruited nationally through social media in April–June of 2020 and subjected to an informed consent process. Certain participants contributed milk that they had previously frozen for personal reasons, while most participants pumped samples specifically for this research project. All participants were either asymptomatic or experienced mild–moderate symptoms of COVID-19 that were managed at home. The demographic information on participant milk samples is shown in Table 1.
Table 1.
Participant Information
| Sample ID | Age, years | Months postpartum (first sample) | Race/ethnicity |
|---|---|---|---|
| COV120 | 32 | 1 | White or Caucasian |
| COV157 | NA | NA | NA |
| COV204 | 36 | 8 | White or Caucasian |
| COV217 | 35 | 4 | White or Caucasian |
| COV256 | 35 | 7 | White or Caucasian |
| COV270 | 29 | <1 | White or Caucasian |
| COV271 | 33 | 1 | White or Caucasian |
| COV292 | NA | NA | NA |
| COV296 | 32 | 1 | White or Caucasian |
| COV307 | NA | 4 | White or Caucasian |
| COV314 | 32 | 2 | White or Caucasian |
| COV362 | 30 | NA | NA |
| COV364 | 39 | 5 | White or Caucasian |
| COV418 | NA | 1 | White or Caucasian |
| COV434 | 34 | 1 | White or Caucasian |
| COV450 | 36 | 15 | White or Caucasian |
NA, not available.
Participants were asked to collect ∼30 mL of milk per sample into a clean container using electronic or manual pumps and, if able and willing, to continue to pump and save monthly milk samples. Milk samples were frozen at −20°C in the participant's home until samples were picked up and stored at −80°C until testing.
Luminex assay
Milk samples were thawed and centrifuged at 800 g for 15 minutes at room temperature, fat was removed, and the defatted milk was transferred to a new tube. Centrifugation was repeated 2 × to ensure removal of all cells and fat. Skimmed acellular milk was aliquoted and frozen at −80°C until use. SARS-CoV-2 Ab levels were measured through an optimized Luminex assay. SARS-CoV-2 spike trimer (wild-type [WT] Wuhan-1 strain, gifted by the Florian Krammer Laboratory) and nucleocapsid protein (WT; ProSci) were coupled to the beads using the xMAP Ab coupling kit according to the manufacturer's instructions (Luminex).
SARS-CoV-2 spike was coupled to the xMAP beads at 2.5 μg per million beads and nucleocapsid was coupled at 0.5 μg per million beads to account for differences in protein size. The coupled beads were stored in PBS solution with 0.5% casein (Thermo Scientific) and 0.05% sodium azide (Bicca) at 4°C until use.
Milk samples were serially diluted from neat to 1:2,187 with 0.5% casein buffer in a 96-well sample plate. Fifty microliters of milk was then transferred to a black 96-well microplate (Greiner). Coupled xMAP beads were then added to each well at 2,500 beads/region in a total 50-μL solution. The plate was covered with a black lid and shaken at room temperature for 60 minutes at 3 mm. The beads were then captured by the magnetic plate holder and washed twice with 0.5% casein.
One hundred microliters of 2 μg/mL biotin-labeled goat anti-human IgA (Thermo Fisher) or 6 μg/mL biotin-labeled sheep anti-human SC (Nordic MUbio) was added to the plates and shaken for 30 minutes at 3 mm. Beads were washed twice after removing the supernatant, followed by addition of 100 μL of streptavidin-PE (BioLegend) at 1 μg/mL, and shaken for 30 minutes.
Beads were washed 2 × and resuspended in 100 μL of casein buffer and read by a Luminex FlexMAP 3D device with xPONENT 4.2 software. Median fluorescence intensity (MFI) was measured for each well and exported for analysis.
Analytical methods
Control milk samples obtained before December 2019 were used to establish positive cutoff values for each assay. Milk was defined as positive at an optimized screening dilution of 1:18 if the sample exhibited MFI values 3 standard deviations (SDs) above the mean MFIs of COVID-naïve control samples. Endpoint binding titers were calculated from log-transformed titration curves using 4-parameter nonlinear regression, using MFI cutoff values of 650 and 150 for the IgA and sAb assays, respectively.
Significance of longitudinal changes in Ab titers was analyzed by a paired one-way ANOVA test. Correlation analyses were performed using the Pearson correlation test.
Neutralization assay
A proxy neutralization assay that detects Abs specific for the spike receptor-binding domain (RBD), which can prevent RBD-ACE2 binding, was performed using milk samples, following the manufacturer's protocol (GenScript). Results were read on a PowerWave plate reader, and the percentage of virus neutralization was determined compared with the provided positive and negative controls.
Results
Longitudinal spike-specific Ab profiles in milk after SARS-CoV-2 infection
Sixteen sets of milk samples obtained at sequential time points from COVID-19-recovered lactating individuals were examined (Table 1). Samples were analyzed for IgA and sAb reactivities against SARS-CoV-2 spike and nucleocapsid antigens using a Luminex-based immunoassay, as described in the Materials and Methods section. All milk samples obtained from COVID-19-recovered participants exhibited positive MFIs, indicating spike-specific IgA and sAb 3–8 weeks postinfection (Fig. 1A, B).
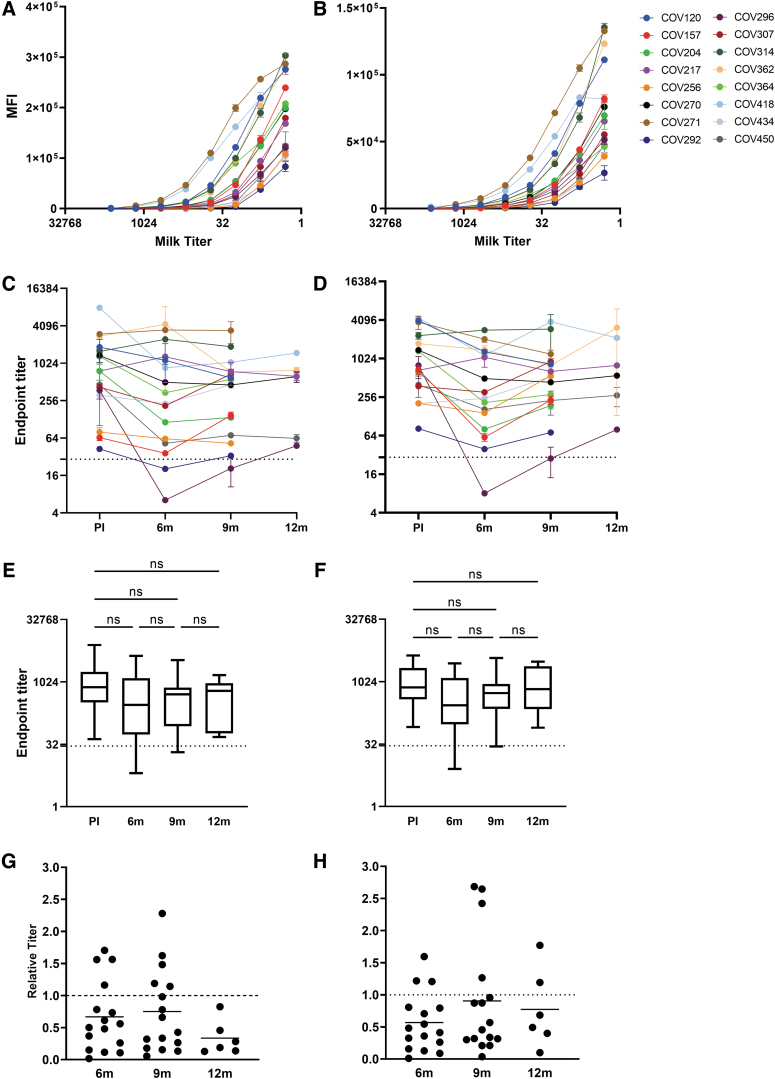
FIG. 1.
Longitudinal human milk IgA and sAb reactivity against SARS-CoV-2 spike demonstrates significant durability over time. SARS-CoV-2 Ab levels were measured using an optimized Luminex assay, as described in the Materials and Methods section. (A, B) Titration curves of IgA and sAbs 3–8 weeks postinfection; (A) IgA and (B) sAbs. (C, D) Individual endpoint titers over time; (C) IgA and (D) sAbs. Dotted lines: positive cutoff value. (E, F) Grouped endpoint titers with mean values and ANOVA for change over time; (E) IgA and (F) sAbs. Dotted lines: positive cutoff value. (G, H) Relative endpoint titers over time compared with 3–8 weeks postinfection; (G) IgA and (H) sAbs. Dotted line indicates relative titer of 1, meaning no change. PI, postinfection; sAbs, secretory antibodies; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Longitudinal milk samples were similarly assessed, and endpoint titers determined for each assay. It was found that at the last time point available (9–12 months), 14/16 participants (88%) continued to exhibit positive IgA endpoint titers against spike (Fig. 1C) and 15/16 participants (94%) continued to exhibit positive sAb endpoint titers against spike (Fig. 1D).
Endpoint titers were grouped by time point to determine if significant changes had occurred over the study period (Fig. 1E, F). Values were compared by the paired ANOVA test. At the initial 3–8-week postinfection time point, samples exhibited a mean IgA endpoint titer of 1354 (range 43–3,465) against spike. At the following time point examined, 5–6 months postinfection, samples exhibited a mean IgA endpoint titer of 960 (range 20–4,323) against spike, which was not significantly different from the initial time point (Fig. 1E).
At 9 months, samples exhibited a mean IgA endpoint titer of 740 (range 33–3,437) against spike, which was also not significantly different compared with either the initial time point or the 5–6-month time point. Following a highly similar trend, mean sAb titers against spike at 3–8 weeks (mean 1,436, range 82–4,000), 5–6 months (mean 721, range 8–2,835), and 9–12 months (mean 1,007, range 28–3,816) were not significantly different (Fig. 1F).
To evaluate the relative change in IgA and sAb levels over time, endpoint titers for each longitudinal milk sample were normalized by the individual's endpoint titer of their initial milk sample obtained 3–8 weeks postinfection (Fig. 1G, H). Notably, 94% of participants exhibited <10-fold reduction in the IgA endpoint titer 5–6 months postinfection and 56% exhibited <2-fold reduction.
By 9 months, 50% of participants exhibited <2-fold decrease in the IgA titer, having decreased by only 25% on average compared with those at 3–8 weeks (range: 73% decrease to 127% increase). By the end of the study period, IgA had decreased by 64% (17–87%); however, this was not significantly different from the 9-month time point (Fig. 1G).
Examining the durability of spike-specific sAb, COV296, and COV157 exhibited >10-fold decrease in titer at 5–6 months postinfection, with the remaining participants exhibiting <10-fold decreases (titers were 57% compared with those observed at 3–8 weeks). At 9 months postinfection, sAb titers were on average maintained at 90% of those measured at 3–8 weeks postinfection, with only COV295 exhibiting >10-fold decrease, although this titer was also similarly increased compared with that measured at 5–6 months. Fifty percent of participants exhibited <2-fold reduction by the end of the study period (Fig. 1H).
Longitudinal nucleocapsid-specific Ab profiles in milk after SARS-CoV-2 infection
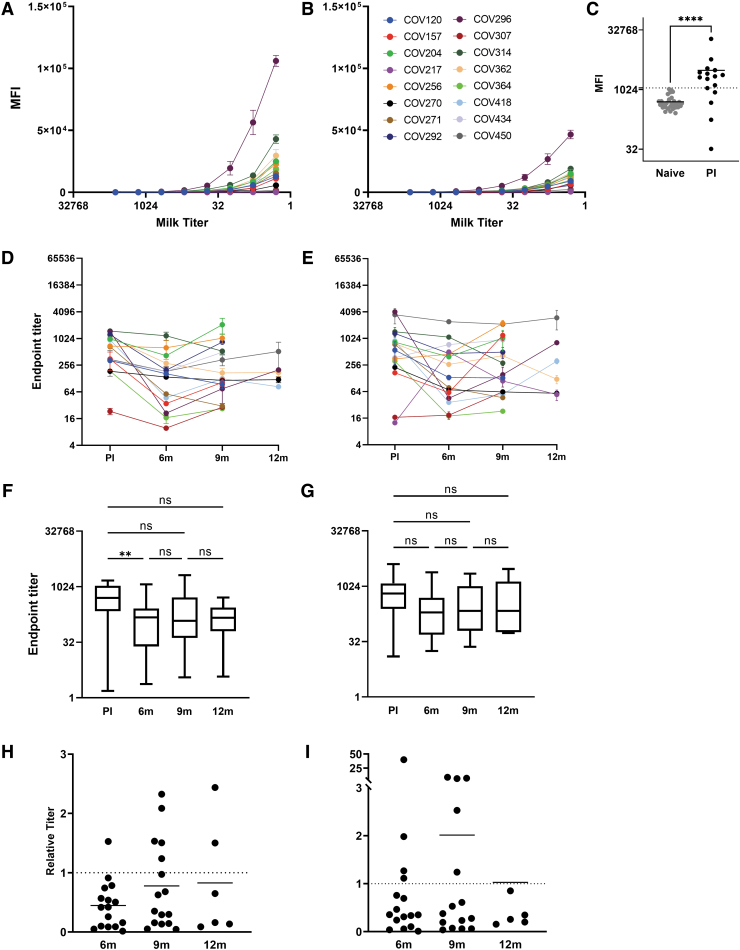
Similar analysis of IgA and sAb reactivity against the SARS-CoV-2 nucleocapsid protein was performed (Fig. 2). Despite the significant difference in IgA reactivity against nucleocapsid exhibited by milk obtained from COVID-recovered donors compared with the COVID-naïve controls (p < 0.0001), a positive MFI cutoff value could not be determined due to high background reactivity of the COVID-naïve control samples (Fig. 2C, dashed line: mean naïve MFI +3SD).
FIG. 2.
Longitudinal human milk IgA and sAbs against SARS-CoV-2 nucleocapsid are maintained less consistently compared with spike Abs and subject to significant background reactivity. (A–C) Titration curves of IgA and sAbs 3–8 weeks postinfection; (A) IgA, (B) sAbs, and (C) the high background IgA of COVID-19-naïve milk, which hinders determination of a positive cutoff value. MFI at 1/18 dilution (screening dilution). (D, E) Individual endpoint titers over time; (D) IgA and (E) sAbs. (F, G) Grouped endpoint titers with mean values and ANOVA for change over time; (F) IgA and (G) sAbs. Relative endpoint titers over time compared with 3–8 weeks postinfection. (H) IgA and (I) sAbs. Dotted line indicates relative titer of 1, meaning no change. MFI, median fluorescence intensity; PI, postinfection.
Endpoint titers were calculated as described above. At the initial 3–8-week postinfection time point, samples exhibited a mean IgA endpoint titer of 649 (range 1–1,098) against nucleocapsid. By 5–6 months postinfection, samples exhibited a mean IgA endpoint titer of 233 (range 2–1,175) against nucleocapsid, which was significantly decreased from the initial time point (Fig. 2, p = 0.0066).
By 9 months, samples exhibited a mean IgA endpoint titer of 383 (range 4–1,042) against nucleocapsid, which was not significantly different compared with either the initial time point or the 5–6-month time point. The mean sAb titers against nucleocapsid at 3–8 weeks (mean 981, range 13–4,100), 5–6 months (mean 427, range 18–2,450), and 9–12 months (mean 590, range 23–2,150) were not significantly different (Fig. 2F).
In contrast to the spike data detailed above, the longitudinal changes observed for sAb titers (Fig. 2C, D) were inconsistent with changes observed for IgA. Specifically, COV217, COV314, COV362, and COV418 exhibited divergent IgA versus sAb titer changes over time.
Compared with 3–8 weeks postinfection, the IgA titers decreased by an average of 22% at 9 months, while sAb titers increased by an average of 100% (Fig. 2E, F). ANOVA determined no significant changes in IgA or sAb titers across most time points, with the exception of a significant IgA decrease from 3–8 weeks to 5–6 months postinfection.
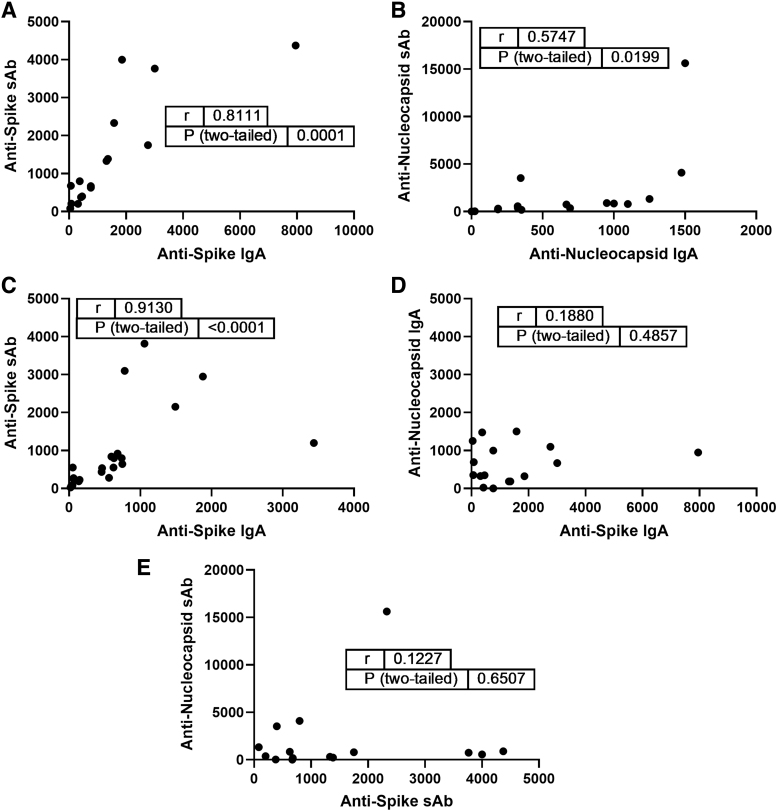
IgA and sAb titers against spike and nucleocapsid at each time point were compared using Pearson correlation analysis (Fig. 3). As shown in our previous work, a strong, significant positive correlation was found between IgA and sAb spike titers 3–8 weeks postinfection (Fig. 3A, r = 0.81; p = 0.0001). The correlation between IgA and sAb against spike was maintained throughout the study period (Fig. 3C, r = 0.91, p < 0.0001).
FIG. 3.
Spike-specific human milk IgA and sAbs remain highly correlated throughout the study period, while nucleocapsid Abs exhibit moderate correlation and spike versus nucleocapsid Ab reactivities are unrelated. (A) Spike IgA versus sAbs 3–8 weeks postinfection. (B) Nucleocapsid IgA versus sAbs 3–8 weeks postinfection. (C) Correlation between spike IgA and sAbs at the end of the study period. (D) Spike versus nucleocapsid IgA 3–8 weeks postinfection. (E) Spike versus nucleocapsid sAbs 3–8 weeks postinfection. Endpoint titers were compared using Pearson correlation analysis.
In contrast, the correlation between IgA and sAb titers against nucleocapsid at 3–8 weeks postinfection was only moderate (Fig. 3B, r = 0.57; p = 0.0199), and no correlation was found in the samples 5–6 months postinfection or beyond (data not shown). No correlation was found between antispike and antinucleocapsid IgA or sAb titers (Fig. 3D, E).
Durability of the SARS-CoV-2 neutralization capacity of milk from COVID-19-recovered donors over time
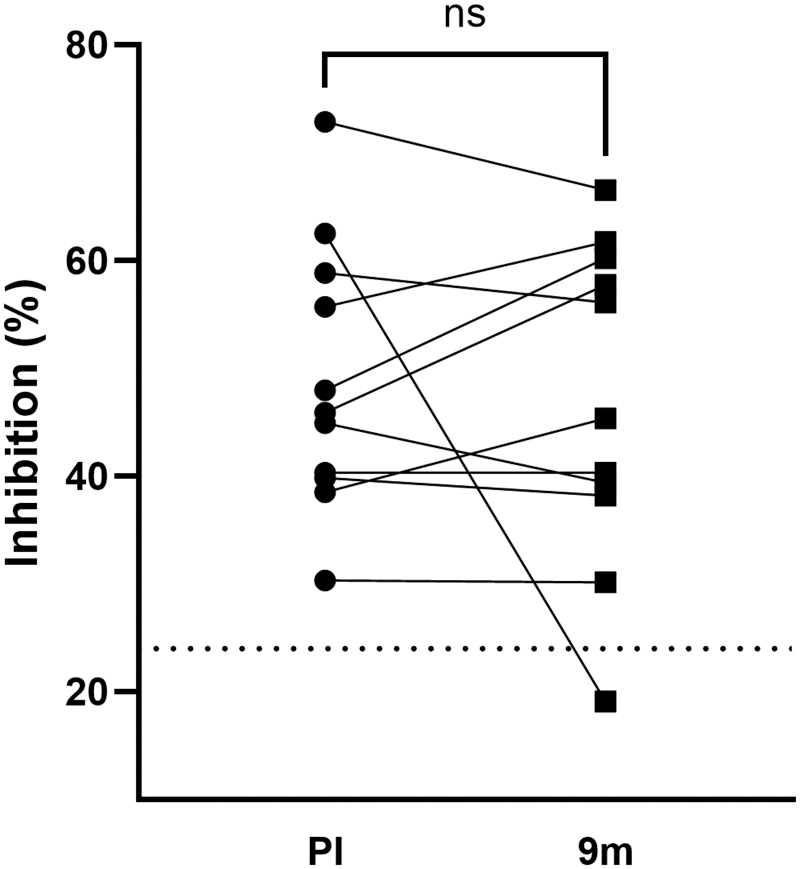
A proxy neutralization assay that measures Abs specific for the spike RBD, which block the RBD-ACE2 interaction, was performed using a premade kit (GenScript). Eleven pairs of samples analyzed above were examined for changes in neutralization capacity from 3 to 8 weeks postinfection to 9 months postinfection. At 3–8 weeks postinfection, the mean % neutralization of neat milk was 49% (range 30 − 73%), with all samples exhibiting neutralization activity that was significantly greater than that exhibited by naïve milk (p = 0.0029).
At 9 months, the mean % neutralization was 47% (range 30 − 67%) (Fig. 4). However, one sample's neutralization capacity dropped below that of the naïve controls by 9 months (COV364, from 63% to 19%). Notably, COV364 dropped 67% in the IgA titer and 89% in the sAb titer at 9 months postinfection. It was found that no significant change in neutralization was detected between the early and late groups.
FIG. 4.
SARS-CoV-2-neutralizing Ab in human milk is maintained for at least 9 months after infection. A proxy neutralization assay that measures Abs specific for the spike RBD, which block the RBD-ACE2 interaction, was performed using a premade kit according to instructions (GenScript). Dotted line: background neutralization level of COVID-19-naïve milk. Paired t-test was used for the analysis. PI, postinfection; RBD, receptor-binding domain.
Discussion
Nearly all sAbs are derived from the GALT, via the entero-mammary link, which involves homing of Ab-secreting B cells from the gut to the mammary gland. Various animal studies have demonstrated this migration and homing during late pregnancy and lactation. Homing appears to be controlled hormonally as well as by various adhesion factors on B cells and the maternal vasculature, including MadCAM-1, integrin α4β7, CCL28, and CCR10.22
This link is an evolutionarily critical mechanism facilitating specific protection to a vulnerable infant against pathogens in the maternal/infant environment, sampled by the maternal GALT, and providing key immunological training for the infant.22 Infants benefit greatly from the sAbs provided in human milk, as the neonate mucosal immune system is relatively deficient in sAb production as well as other key immune factors.
Even past the neonatal period, these Abs can supplement the infant's own immunity to provide protection against pathogens, against which the infant does not yet have immunological protection. sAbs are critical due to their relative durability in harsh environments, including the milk itself, and all mucosal fluids, including the oral/nasal cavity and GI tract.14,15
SARS-CoV-2 does not transmit through human milk. Although viral RNA has been identified in a small minority of milk samples studied,23–25 infectious SARS-CoV-2 particles have not been observed. Several meta-analyses to date have failed to find evidence of worsened health outcomes in infants (of any age) chest/breastfed by a SARS-CoV-2-infected person or increased rates of infection for these infants compared with those who are formula fed.26–28
We and others have reported SARS-CoV-2-specific Abs in milk obtained from donors with previous infection.21,29–31 Our prior work determined that infection elicits a robust spike-specific milk IgA and sAb response in ∼90% of cases, which is highly neutralizing. In the present study, we aimed to longitudinally monitor the SARS-CoV-2 IgA and sAb response in the milk from COVID-19-recovered individuals over 1 year. Unlike the observed decay over time of the serum response that has been widely cited in other reports,32–35 we observed little change in spike-specific milk titers over time.
Notably, 50% of participants exhibited <2-fold reduction of spike-specific IgA, and 94% of participants sustained positive sAb titers against spike through 12 months. IgA and sAb titers maintained a strong, significant positive correlation throughout the study period. Abs against the nucleocapsid protein have not been intensively studied because these Abs would be non-neutralizing; however, these Abs are useful as indicators of prevaccine or breakthrough infections and likely play a key role in clearing virus-infected cells or modulating immune response.36
As such, the longevity of the nucleocapsid Ab response remains highly informative and worth investigating. Previous studies have demonstrated cross-reactivities among CoV-229E, CoV-OC43, MERS, and SARS nucleocapsid in serum samples of COVID-19-recovered patients,37 which may explain the high level of background observed among the COVID-naïve samples, as well as the erratic titers measured over time relative to those against spike.
The serological response against SARS-CoV-2 varies by variant, disease severity, and pre-existing conditions.38 Generally, the decay rate of spike/RBD-specific serum IgA is high. The half-life of RBD-specific IgA has been found to be ∼71 days, with certain reports finding serum IgA levels to be undetectable after 60 days.32,39,40
The serum IgG half-life has been reported to be >3 months in multiple studies, with 57% of individuals who were asymptomatically infected being IgG seropositive after 8 months and 71% of those who experienced mild symptoms maintaining positive IgG titers.32,39,40 In the present study, spike-specific sIgA titers in milk were shown to be significantly more durable over time.
The highly durable titers observed in the present study may be reflective of long-lived plasma cells in the GALT and/or mammary gland, as well as continued antigen stimulation in these compartments, possibly by other human coronaviruses or repeated exposures to SARS-CoV-2.
Consistent with the present data, it has been shown in general that although an initial IgA plasmablast response declines quickly, IgA-producing plasma cells can persist for decades in the human GALT, even when not measurable in the periphery.41 Given the established entero-mammary link, the highly durable spike-specific sIgA in milk observed herein strongly suggests similarly long-lived plasma cells in the mammary gland.
The present data suggest that lactating individuals who recover from COVID-19 are likely to continue producing specific sIgA in their milk for 1 year or more. Based on studies of other mucosal pathogens, milk sIgA likely provides critical passive immunity to infants against SARS-CoV-2 infection and COVID-19 pathology. The significant durability of the spike-specific sIgA response in milk highlights the importance of continued chest/breastfeeding after SARS-CoV-2 infection.
Given the present lack of knowledge concerning not only the potency, function, durability, and variation of the human milk immune response to SARS-CoV-2 infection but also across this understudied field in general, the present data contribute greatly to filling immense knowledge gaps and further our work toward in vivo efficacy testing of extracted milk Abs in the COVID-19 pandemic context and beyond.
Acknowledgments
As always, the authors are indebted to the milk donors who made this work possible. Spike protein was generously gifted by the Krammer Laboratory.
Authors' Contributions
X.Y., A.F., and C.D. performed the experimental work and prepared raw data. X.Y. drafted the manuscript. N.P. coordinated participant interactions and sample pickups. R.L.R.P. conceived of and designed the study and experiments, analyzed and formatted experimental data, and revised the manuscript. All authors have read and approved the manuscript.
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Information
This study was funded by the NIH/NIAID, grant number R01 AI158214.
References
- 1. Centers for Disease Control and Prevention. COVID Data Tracker. April 18 ed. US Department of Health and Human Services, CDC: Atlanta, GA, USA; 2023. [Google Scholar]
- 2. Hamid S, Woodworth K, Pham H, et al. COVID-19-associated hospitalizations among US infants aged <6 months—COVID-NET, 13 States, June 2021–August 2022. MMWR Morb Mortal Wkly Rep 2022;71(45):1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawood FS, Porucznik CA, Veguilla V, et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. JAMA Pediatr 2021;176(1):59–67; doi: 10.1001/jamapediatrics.2021.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marks KJ, Whitaker M, Agathis NT, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR Morb Mortal Wkly Rep 2022;71(11):429–436; doi: 10.15585/mmwr.mm7111e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: Novel virus and novel case. Hosp Pediatr 2020;10(6):537–540; doi: 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 6. Kwak JH, Lee SY, Choi JW, et al. Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Clin Exp Pediatr 2020;64(2):68–75; doi: 10.3345/cep.2020.01900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69(14):411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science 2020;368(6490):489–493; doi: 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang A, Tong ZD, Wang HL, et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis 2020;26(6); doi: 10.3201/eid2606.200301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niewiesk S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 2014;5:446; doi: 10.3389/fimmu.2014.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013;4:185; doi: 10.3389/fimmu.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henkle E, Steinhoff MC, Omer SB, et al. The effect of exclusive breast-feeding on respiratory illness in young infants in a maternal immunization trial in Bangladesh. Pediatr Infect Dis J 2013;32(5):431–435; doi: 10.1097/INF.0b013e318281e34f [DOI] [PubMed] [Google Scholar]
- 13. Weaver LT, Arthur HM, Bunn JE, et al. Human milk IgA concentrations during the first year of lactation. Arch Dis Child 1998;78(3):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011;3(4):442–474; doi: 10.3390/nu3040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demers-Mathieu V, Underwood MA, Beverly RL, et al. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients 2018;10(5); doi: 10.3390/nu10050631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr 2010;156(2 Suppl):S8–S15; doi: 10.1016/j.jpeds.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 17. Brandtzaeg P. Mucosal immunity: Integration between mother and the breast-fed infant. Vaccine 2003;21(24):3382–3388. [DOI] [PubMed] [Google Scholar]
- 18. Low EN, Zagieboylo L, Martino B, et al. IgA ASC accumulation to the lactating mammary gland is dependent on VCAM-1 and alpha4 integrins. Mol Immunol 2010;47(7–8):1608–1612; doi: 10.1016/j.molimm.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fouda GG, Eudailey J, Kunz EL, et al. Systemic administration of an HIV-1 broadly neutralizing dimeric IgA yields mucosal secretory IgA and virus neutralization. Mucosal Immunol 2017;10(1):228–237; doi: 10.1038/mi.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox A, Marino J, Amanat F, et al. The IgA in milk induced by SARS-CoV-2 infection is comprised of mainly secretory antibody that is neutralizing and highly durable over time. PLoS One 2022;17(3):e0249723; doi: 10.1371/journal.pone.0249723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA against SARS-CoV-2 detected in human milk. iScience 2020:101735; doi: 10.1016/j.isci.2020.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez JM, Fernandez L, Verhasselt V. The GutBreast Axis: Programming health for life. Nutrients 2021;13(2); doi: 10.3390/nu13020606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gross R, Conzelmann C, Muller JA, et al. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020;395(10239):1757–1758; doi: 10.1016/S0140-6736(20)31181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chambers C, Krogstad P, Bertrand K, et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA 2020;324(13):1347–1348; doi: 10.1001/jama.2020.15580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu C, Liu W, Su H, et al. Breastfeeding risk from detectable severe acute respiratory syndrome coronavirus 2 in breastmilk. J Infect 2020;81(3):452–482; doi: 10.1016/j.jinf.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centeno-Tablante E, Medina-Rivera M, Finkelstein JL, et al. Transmission of SARS-CoV-2 through breast milk and breastfeeding: A living systematic review. Ann N Y Acad Sci 2021;1484(1):32–54; doi: 10.1111/nyas.14477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez-Bermejo M, Peris-Ochando B, Murillo-Llorente MT. COVID-19: Relationship and impact on breastfeeding—A systematic review. Nutrients 2021;13(9); doi: 10.3390/nu13092972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large Medical Center in New York City. JAMA Pediatr 2021;175(2):157–167; doi: 10.1001/jamapediatrics.2020.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pace RM, Williams JE, Jarvinen KM, et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio 2021;12(1); doi: 10.1128/mBio.03192-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lebrao CW, Cruz MN, Silva MHD, et al. Early identification of IgA Anti-SARSCoV-2 in milk of mother with COVID-19 infection. J Hum Lact 2020;36(4):609–613; doi: 10.1177/0890334420960433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Favara DM, Ceron-Gutierrez ML, Carnell GW, et al. Detection of breastmilk antibodies targeting SARS-CoV-2 nucleocapsid, spike and receptor-binding-domain antigens. Emerg Microbes Infect 2020;9(1):2728–2731; doi: 10.1080/22221751.2020.1858699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5(12):1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruel T, Stéfic K, Nguyen Y, et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA. 2, BA. 4, and BA. 5 in patients receiving monoclonal antibodies. Cell Rep Med 2022;3(12):100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muecksch F, Wise H, Templeton K, et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: A serological analysis. Lancet Microbe 2022;3(7):e493–e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan Y, Jiang X, Yang L, et al. SARS-CoV-2-specific immune response in COVID-19 convalescent individuals. Signal Transduct Target Ther 2021;6(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang S, Yang M, He S, et al. A SARS-CoV-2 antibody curbs viral nucleocapsid protein-induced complement hyperactivation. Nat Commun 2021;12(1):2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Facciuolo A, Scruten E, Lipsit S, et al. High-resolution analysis of long-term serum antibodies in humans following convalescence of SARS-CoV-2 infection. Sci Rep 2022;12(1):9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Röltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol 2020;5(54):eabe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol 2020;5(52):eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021;13(577); doi: 10.1126/scitranslmed.abd2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landsverk OJ, Snir O, Casado RB, et al. Antibody-secreting plasma cells persist for decades in human intestine. J Exp Med 2017;214(2):309–317; doi: 10.1084/jem.20161590 [DOI] [PMC free article] [PubMed] [Google Scholar]