Abstract
Objective:
To evaluate the use of faster acting (FIA) and standard insulin aspart (SIA) with hybrid automated insulin delivery (AID) in active youth with type 1 diabetes.
Research Design and Methods:
In this double-blind multinational randomized crossover trial, 30 children and adolescents with type 1 diabetes (16 females; aged 15.0 ± 1.7 years; baseline HbA1c 7.5% ± 0.9% [58 ± 9.8 mmol/mol]) underwent two unrestricted 4-week periods using hybrid AID with either FIA or SIA in random order. During both interventions, participants were using the hybrid AID (investigational version of MiniMed™ 780G; Medtronic). Participants were encouraged to exercise as frequently as possible, capturing physical activity with an activity monitor. The primary outcome was the percentage of sensor glucose time above range (180 mg/dL [10.0 mmol/L]) measured by continuous glucose monitoring.
Results:
In an intention-to-treat analysis, mean time above range was 31% ± 15% at baseline, 19% ± 6% during FIA use, and 20% ± 6% during SIA use with no difference between treatments: mean difference = −0.9%; 95% CI: −2.4% to 0.6%; P = 0.23. Similarly, there was no difference in mean time in range (TIR) (78% and 77%) or median time below range (2.5% and 2.8%). Glycemic outcomes during exercise or postprandial periods were comparable for the two treatment arms. No severe hypoglycemia or diabetic ketoacidosis events occurred.
Conclusions:
FIA was not superior to SIA with hybrid AID system use in physically active children and adolescents with type 1 diabetes. Nonetheless, both insulin formulations enabled high overall TIR and low time above and below ranges, even during and after documented exercise.
Trial Registration clinicaltrials.gov:NCT04853030.
Keywords: Exercise, Faster acting insulin, Children, Adolescents, Automated insulin delivery, Type 1 diabetes
Introduction
Automated insulin delivery (AID), with its glucose-responsive approach and mimicking the physiological response of a healthy beta cell, has revolutionized the management of type 1 diabetes. Numerous clinical studies evaluating various AID systems have unequivocally demonstrated safe improvements in glycemic outcomes in individuals with type 1 diabetes of different ages, genders, and diabetes durations.1–6 As such, current clinical guidelines recommend offering AID systems to all youth and adults with type 1 diabetes.7,8
All current commercially available AID systems adopt the hybrid approach, requiring prior meal and exercise announcements by the user to achieve recommended glycemic targets.9,10 Part of the challenge with current AID technology is due to the pharmacokinetic and pharmacodynamic delay, and comparatively slow absorption time from the subcutaneous administration of insulin.11 Faster insulin formulations are continuously being developed that have the potential to further advance the efficacy and safety of AID systems, with the goal of becoming fully AID technology in the future.12
Faster acting insulin aspart (FIA) is a recent aspart (Novo Nordisk, Denmark) formulation, to which two excipients have been added to increase the early absorption (nicotinamide) and to optimize the stability (l-arginine) of the formulation.13 Studies have shown that administration of FIA bolus, either with subcutaneous injections or with an insulin pump, are associated with earlier insulin exposure and action, and earlier offset of exposure than with standard insulin aspart (SIA).14–16
In a first double-blind randomized clinical trial evaluating glycemic outcomes using AID with either FIA or SIA in adults with type 1 diabetes, time in range (TIR; 70–180 mg/dL) was comparable between the two arms.17 Noteworthy, this study was conducted in a supervised inpatient setting, with a fully AID approach involving unannounced/uncovered meals and an unannounced afternoon exercise protocol.17 Recently, randomized clinical trials including adults with type 1 diabetes have demonstrated a modest improvement in glycemic outcomes with FIA compared with SIA using various hybrid AID systems over several weeks.18–20 However, there is currently limited evidence from randomized controlled trials enrolling children and adolescents with type 1 diabetes.21
We aimed to evaluate the use of FIA in a second-generation hybrid AID system in youth with type 1 diabetes over a longer period that included frequent physical activity. Based on available data, we hypothesized that AID with FIA would improve glucose time above range (>180 mg/dL) due to improved postprandial glycemic outcomes compared with SIA.
Methods
Study design
This double-blind, multinational, two-period randomized crossover trial was conducted at two locations: University Children's Hospital Ljubljana in Slovenia and Medical University of Graz in Austria. Written informed consent was obtained from all participants before any study-related activities. The protocol and informed consent or assent forms were approved by the appropriate institutional review boards and ethics committees, and regulatory approval to conduct the study was obtained in both countries. The study is listed on clinicaltrials.gov under registration number NCT04853030.
During the two unrestricted 4-week periods, each participant used one of the two insulin formulations with the MiniMed™ 670G 4.0 (investigational version of MiniMed 780G with equivalent algorithm but without Bluetooth connectivity and with two glucose set points 100 and 120 mg/dL) Medtronic AID system: FIA or SIA, assigned in random order. Randomization was done using a computer-generated sequence with a permuted block size of four. Study participants and investigators were blinded to the treatment allocation.
Eligible participants were children and adolescents aged 10–18 years, diagnosed with type 1 diabetes for at least 6 months, using an insulin pump for at least 3 months, and had a screening HbA1c <11% (97 mmol/mol). Key exclusion criteria were untreated celiac or thyroid disease, current treatment with drugs known to interfere with glucose metabolism, and diabetes management using an ultrarapid acting insulin analogue. A full list of the inclusion and exclusion criteria is given in Supplementary Table S1.
Before randomization, participants completed a run-in period of at least 1 week using the study insulin pump (without AID mode) and continuous glucose monitoring (CGM; Guardian™ Sensor 3). Capillary fingerstick blood glucose testing was performed using a Contour Next Link 2.4 blood glucose meter (Ascensia Diabetes Care), and blood ketone testing was performed using the Abbott Precision Xtra meter (Abbott Laboratories). Successful completion of the run-in period required at least 80% CGM wear time during the prior 7 days and an average of at least three blood glucose meter tests per day.
Randomization occurred on the same day that the run-in period was completed. Participants received AID system training at the beginning of each crossover period. Participants were contacted 6–10 days into each study period to initialize AID mode, and then continued to use the AID system for 4 weeks. All participants started with an AID mode target glucose set point of 100 mg/dL and active insulin time set at 2 h. At the AID mode initiation contact, participants were reminded to obtain an overnight fingerstick blood glucose measurement (between 2 and 3 AM) for one night after AID initiation, and if blood glucose was <70 mg/dL, to treat with fast-acting carbohydrates, discontinue AID mode, and notify the investigators the next day for advice to increase the glucose set point and/or active insulin time.
During both periods after AID mode was initialized, participants had telephone contacts at 24 h, 3 days, and 2 weeks, and clinic visits at 1, 3, and 4 weeks. Participants were asked to upload device data for study staff review before each contact, and device data were collected during each follow-up visit. Participants were encouraged to engage in exercise frequently to meet current exercise guidelines of at least 60 min of moderate-to-vigorous exercise per day.22
In line with current consensus recommendations, participants were also advised to set a higher (exercise) glucose target (150 mg/dL) at least 1 h before exercise11,23 and to capture the exercise on a commercially available physical activity monitor (Garmin Venu Sq, Garmin). In the second half of each study period, participants were invited to participate in a 1-day supervised sports camp (∼5 h of exercise per day).
Adverse events were recorded throughout the trial. An adverse event was defined as any untoward medical occurrence in a study participant, irrespective of the relationship between the adverse event and the treatments under investigation. Reportable adverse events were any serious adverse event, an adverse device effect, an adverse event occurring in association with a study procedure, an adverse event that leads to discontinuation of a study device for 2 or more hours, severe hypoglycemia, or diabetic ketoacidosis.24,25
Statistical methods
The primary outcome of the trial was time above range (>180 mg/dL) as measured by CGM tested for superiority. Secondary outcomes included mean glucose concentration, glucose coefficient of variation, TIR (70–180 mg/dL), and the percentage of time >250 mg/dL, time below range (<70 mg/dL), and time <54 mg/dL. CGM and insulin metrics were calculated over a 24-h period, and separately for daytime (6 AM–11:59 PM), nighttime (12 AM–5:59 AM), postmeal periods (3 h after carbohydrates were entered), and exercise periods (from start of exercise to 2 h after exercise cessation).
Postprandial periods began when carbohydrates were first entered and stopped either 3 h after a meal, when additional carbohydrates were entered, or exercise began. Postexercise periods began when the exercise session was initiated and stopped either 2 h after the exercise session ended or when carbohydrates were entered or another exercise session began. Data from the sports camp days were analyzed separately and were not included in the main analysis. CGM and insulin outcomes were calculated in each respective period if a participant had at least 3 days of CGM data for the 24-h period, 2 days for daytime period, 1 day for nighttime and postmeal periods, and 4 h for exercise periods.
Additional secondary outcomes for postmeal periods included peak glucose concentration, glucose excursion, time to peak glucose concentration, and postprandial area under the curve. These additional outcomes were only calculated if each individual postmeal period lasted at least 2 h and then were averaged over all meals for each participant. All CGM and insulin outcomes were summarized appropriately to their distribution. A post hoc analysis was conducted examining postprandial excursions by time of day: breakfast (4 AM–<11 AM), lunch (11 AM–<4 PM), and dinner (4 PM–<9 PM).
Participants completed various quality-of-life surveys including the Diabetes Distress Scale, the Glucose Monitoring Satisfaction Survey, Hypoglycemia Confidence Survey, the Diabetes Technology Attitudes Survey, and Insulin Dosing Systems: Perceptions, Ideas, Reflections, and Expectations (INSPIRE) Survey.
A sample size of 30 participants was selected to provide 80% power with a two-sided type 1 error rate of 5% to reject the null hypothesis of no between-group difference in percentage time >180 mg/dL, under the assumption that the mean percentage time >180 mg/dL in the FIA group would be 6.7% lower than that in the SIA group, with a standard deviation of paired differences of 12.7%.
Statistical analyses were performed on an intention-to-treat basis, and all randomized participants were included in the primary and secondary analyses. A per-protocol analysis was conducted including participants who used the CGM and AID feature at least 80% of the time in both periods. Subgroup analyses were conducted to examine the relationship between the primary outcome and several baseline factors, including age, baseline HbA1c, type 1 diabetes duration, gender, and random C-peptide level. For the primary outcome, the treatments were compared using a repeated measures least squares regression model with an unstructured covariance structure adjusting for study period and HbA1c at randomization as fixed effects.
Missing data were handled by means of direct likelihood. Analyses for the secondary outcomes, per-protocol analysis, and subgroup analyses paralleled the primary analysis. For secondary analyses, the false discovery rate was controlled using the adaptive two-stage Benjamini–Hochberg procedure. All P values are two tailed. Analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Between April 21, 2021, and June 28, 2021, 30 participants were screened and enrolled. University Children's Hospital Ljubljana enrolled 24 participants and the Medical University of Graz enrolled 6 participants. Participants ranged in age from 11 to 18 years, and HbA1c at screening ranged from 5.9% to 9.9% (Table 1). All participants enrolled were non-Hispanic White.
Table 1.
Participant Characteristics at Enrollment
| Overall N = 30 | FIA first N = 15 | SIA first N = 15 | |
|---|---|---|---|
| Age at randomization (years) | |||
| 10–13 | 6 (20%) | 2 (13%) | 4 (27%) |
| 14–18 | 24 (80%) | 13 (87%) | 11 (73%) |
| Mean ± SD | 15.0 ± 1.7 | 15.1 ± 1.6 | 15.0 ± 1.7 |
| Range | 11.8–18.3 | 12.2–18.3 | 11.8–17.9 |
| Gender—Male | 14 (47%) | 7 (47%) | 7 (47%) |
| Race—White non-Hispanic | 30 (100%) | 15 (100%) | 15 (100%) |
| Diabetes duration at randomization (years) | |||
| Mean ± SD | 7.8 ± 3.8 | 8.8 ± 4.2 | 6.8 ± 3.3 |
| Range | 1.3–14.8 | 1.3–14.8 | 2.9–12.6 |
| HbA1c at screening (%) | |||
| ≤8.5 | 24 (80%) | 12 (80%) | 12 (80%) |
| ≥8.6 | 6 (20%) | 3 (20%) | 3 (20%) |
| Mean ± SD | 7.5 ± 0.9 | 7.3 ± 0.9 | 7.7 ± 0.9 |
| Range | 5.9 to 9.9 | 5.9 to 8.9 | 6.8 to 9.9 |
| C-Peptide at screening (ng/mL) | |||
| Mean ± SD | 0.10 ± 0.06 | 0.10 ± 0.05 | 0.11 ± 0.06 |
| <0.1 | 13 (43%) | 8 (53%) | 5 (33%) |
| Baseline % time in range (70–180 mg/dL) | |||
| Mean ± SD | 66% ± 14% | 68% ± 10% | 63% ± 17% |
FIA, faster acting insulin aspart; SIA, standard insulin aspart.
All participants who were randomized completed both periods using each treatment arm and visit and phone completion rates were 100% (Fig. 1). CGM and AID use were high with a median percentage time spent using CGM of 91% during the FIA arm and 89% during the SIA arm, and a median percentage time spent using AID mode of 89% during the FIA arm and 87% during the SIA arm (Supplementary Table S2).
FIG. 1.
Visit completion flowchart.
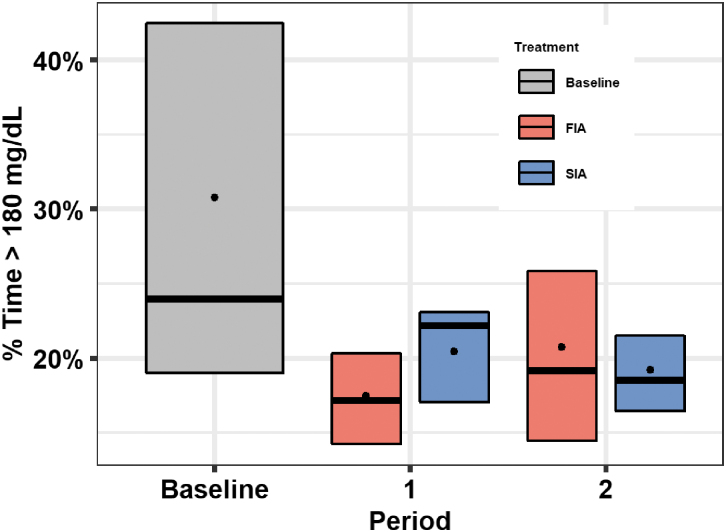
Mean percentage of time above range (>180 mg/dL) was 31% ± 15% at baseline, 19% ± 6% during the FIA arm, and 20% ± 6% during the SIA arm (mean difference [FIA-SIA] = −0.9%; 95% CI −2.4% to 0.6%; P = 0.23; Table 2 and Fig. 2). There was no evidence of a treatment effect by period carryover effect (P = 0.89; Supplementary Fig. S1). The per-protocol analysis showed results similar to the primary analysis (Supplementary Table S3).
Table 2.
Continuous Glucose Monitoring Outcomes over 24-Hour Period and After Meals
| Baseline (N = 30) | FIA (N = 30) | SIA (N = 30) | Adjusted difference FIA minus SIA (95% CI) [P]a | |
|---|---|---|---|---|
| Primary outcome: 24-h period | ||||
| % Time >180 mg/dL, mean ± SD | 31% ± 15% | 19% ± 6% | 20% ± 6% | −0.9% (−2.4% to 0.6%) [0.23] |
| Secondary outcomes: 24-h period | ||||
| Hours of data, median (quartiles) | 148 (140, 161) | 609 (577, 622) | 599 (571, 612) | |
| Mean glucose (mg/dL), mean ± SD | 158 ± 24 | 140 ± 10 | 141 ± 8 | −1.2 (−4.9 to 2.5) [0.45] |
| % TIR 70–180 mg/dL, mean ± SD | 66% ± 14% | 78% ± 6% | 77% ± 7% | 1.4% (−0.8% to 3.6%) [0.18] |
| Glucose CV (%), median (quartiles) | 36% (33%, 39%) | 35% (33%, 37%) | 35% (33%, 39%) | −0.8% (−2.2% to 0.5%) [0.18] |
| % Time >250 mg/dL, median (quartiles) | 5.0% (2.2%, 15.8%) | 3.0% (1.7%, 5.2%) | 3.1% (1.8%, 6.0%) | −0.1% (−0.8% to 0.5%) [0.63] |
| % Time <70 mg/dL, median (quartiles) | 2.1% (1.4%, 4.2%) | 2.5% (1.2%, 4.0%) | 2.8% (1.8%, 4.1%) | −0.5% (−1.1% to 0.1%) [0.11] |
| % Time <54 mg/dL, median (quartiles) | 0.36% (0.06%, 0.96%) | 0.41% (0.14%, 1.05%) | 0.42% (0.24%, 1.27%) | −0.11% (−0.33% to 0.08%) [0.23] |
| Secondary outcomes: postmealb | ||||
| Hours of CGM data, median (quartiles) | 93 (78, 101) | 303 (253, 343) | 289 (269, 344) | |
| Mean glucose (mg/dL), mean ± SD | 168 ± 25 | 153 ± 15 | 155 ± 13 | −2.3 (−8.1 to 3.5) [0.53] |
| % TIR 70–180 mg/dL, mean ± SD | 60% ± 15% | 69% ± 9% | 67% ± 9% | 2.0% (−1.9% to 6.0%) [0.44] |
| Peak glucose (mg/dL), mean ± SDc | 210 ± 31 | 199 ± 19 | 202 ± 16 | −4.0 (−10.1 to 2.1) [0.34] |
| Time to peak glucose (min), mean ± SDc | 59 ± 11 | 74 ± 12 | 70 ± 12 | 3.5 (−3.3 to 10.2) [0.44] |
| Excursion (mg/dL), mean ± SDc | 46 ± 23 | 60 ± 12 | 65 ± 14 | −4.7 (−11.9 to 2.6) [0.34] |
| Postprandial AUC (mg/dL), mean ± SDc | 23 ± 14 | 30 ± 8 | 31 ± 9 | −0.7 (−6.1 to 4.6) [0.72] |
| Glucose CV (%), median (quartiles) | 34% (31%, 39%) | 35% (33%, 37%) | 35% (32%, 39%) | −0.4% (−2.3% to 1.6%) [0.69] |
| % Time >180 mg/dL, mean ± SD | 37% ± 16% | 28% ± 9% | 29% ± 9% | −1.8% (−5.8% to 2.2%) [0.48] |
| % Time >250 mg/dL, median (quartiles) | 7.0% (3.8%, 19.1%) | 4.4% (2.7%, 7.3%) | 4.9% (3.0%, 10.0%) | −0.3% (−2.0% to 1.1%) [0.69] |
| % Time <70 mg/dL, median (quartiles) | 2.1% (1.1%, 3.7%) | 2.9% (1.4%, 4.4%) | 2.9% (1.9%, 4.3%) | −0.2% (−1.1% to 0.8%) [0.69] |
| % Time <54 mg/dL, median (quartiles) | 0.22% (0.00%, 0.86%) | 0.68% (0.18%, 1.24%) | 0.50% (0.38%, 0.97%) | −0.07% (−0.42% to 0.30%) [0.69] |
P values and 95% CIs are from a repeated measures least squares regression model with an unstructured covariance structure adjusting for period and HbA1c at randomization as fixed effects. Owing to a skewed distribution, % time >250, <70, and <54 mg/dL were transformed using a rank normal transformation. Multiple comparisons for secondary outcomes were adjusted using the two-stage Benjamini–Hochberg adaptive false discovery rate procedure. The carryover effect for % time >180 mg/dL was tested by adding a period by treatment interaction term to the model. The carryover effect P value was 0.89.
Postmeal periods begin when carbohydrates are first entered, lasting up to 3 h after a meal or until another carbohydrate is entered or exercise begins.
Metric is calculated for each meal, and then averaged across all meals for each participant.
AUC, area under the curve; CGM, continuous glucose monitoring; CV, coefficient of variation; TIR, time in range (70–180 mg/dL).
FIG. 2.
Boxplots for % time >180 mg/dL by treatment group and period. Box plots of the % time >180 mg/dL during baseline, period 1, and period 2 are shown. Baseline is represented by the grey box, FIA period is represented by red boxes, and SIA period is represented by blue boxes. Black dots indicate the mean values, horizontal bars in the boxes indicate the medians, and the bottom and top of each box represent the 25th and 75th percentiles. FIA, faster acting insulin aspart; SIA, standard insulin aspart.
Mean glucose was 158 ± 24 mg/dL at baseline, 140 ± 10 mg/dL during the FIA arm, and 141 ± 8 mg/dL during the SIA arm (mean difference [FIA-SIA] = −1.2 mg/dL; 95% CI −4.9 to 2.5 mg/dL; P = 0.45; Table 2). Mean percentage of TIR was 66% ± 14% during baseline, 78% ± 6% during the FIA arm, and 77% ± 7% during the SIA arm (mean difference [FIA-SIA] = 1.4%; 95% CI −0.8% to 3.6; P = 0.18). Median percentage of time with glucose levels <54 mg/dL was 0.36% (0.06%, 0.96%) during baseline, 0.41% (0.14%, 1.05%) during the FIA arm, and 0.42% (0.24%, 1.27%) during the SIA arm (mean difference [FIA-SIA] = −0.11%; 95% CI −0.33% to 0.08%; P = 0.23).
During postmeal periods, mean percentage time >180 mg/dL was 37% ± 16% during baseline, 28% ± 9% during the FIA arm, and 29% ± 9% during the SIA arm (mean difference [FIA-SIA] = −1.8%; 95% CI −5.8% to 2.2%; P = 0.48; Table 2). Other postprandial CGM metrics including peak glucose and postprandial area under the curve were not significantly different for the two treatment arms. Postprandial excursion for breakfast meals was 42 ± 38 mg/dL during baseline, 64 ± 21 mg/dL during the FIA arm, and 77 ± 21 mg/dL during the SIA arm (mean difference [FIA-SIA] = −12 mg/dL; 95% CI −20 to −3 mg/dL; P = 0.004; Supplementary Table S13). There was no significant difference in postprandial excursion during the lunch or dinner meals.
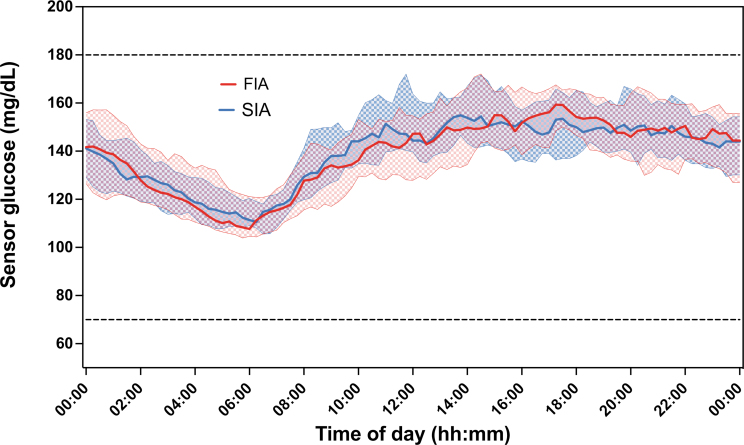
The mean glucose over the 24 h was largely similar for the two treatment arms (Fig. 3). Other CGM outcomes by time of day were also similar for the two treatment arms (Supplementary Table S4). There were also no treatment group differences for CGM outcomes during exercise (Supplementary Table S5).
FIG. 3.
Mean glucose by 15-min period over 24-h day. The dots and solid curve represent the median value, the shaded region represents the 25th and 75th percentiles, and the dashed curves represent the 10th and 90th percentiles.
Mean total daily insulin dose was 1.19 ± 0.63 units/kg per day during baseline, 0.75 ± 0.12 units/kg per day during the FIA arm, and 0.77 ± 0.15 units/kg per day during the SIA arm (mean difference [FIA-SIA] = −0.02 units/kg per day; 95% CI −0.08 to 0.05 units/kg per day; P = 0.80; Supplementary Table S6). Total, basal, and bolus daily insulin were similar for the two treatment arms during daytime, nighttime, and postmeal periods.
Treatment group differences between FIA and SIA on percentage time >180 mg/dL did not differ by age, HbA1c, diabetes duration, gender, or C-peptide level (Supplementary Table S7). The treatment effect by age group and by baseline HbA1c group is shown in Supplementary Tables S8 and S9.
Exploratory outcomes for the sports camp days and meal challenge test are given in Supplementary Tables S10 and S11. TIR and time >180 mg/dL for the sports camp days were similar to the 24-h outcomes. Insulin use for the sports camp days was similar to that of the rest of the study. TIR (70–180 mg/dL) was 41% ± 21% for FIA and 55% ± 28% for SIA for the missed bolus test and 51% ± 29% for FIA and 54% ± 29% for SIA for the delayed bolus test. Questionnaire results are reported in Supplementary Table S12.
One adverse event was reported during baseline, two adverse events during the FIA period, and two adverse events during the SIA period. One of the adverse events in the FIA period was ketonemia, and the rest were viral infections. None of these adverse events were serious, and there were no cases of severe hypoglycemia nor diabetic ketoacidosis during the study.
Discussion
In this randomized controlled trial investigating the use of a faster acting insulin analogue with hybrid AID in youth with type 1 diabetes, we demonstrate comparable glycemic outcomes between FIA and SIA. The primary outcome of this study, the superiority of FIA compared with SIA in time above range (>180 mg/dL), was not met. However, given that the achieved glycemic outcomes in both study arms, which exceeded the current consensus clinical recommendations (time above range 19% and 20% and TIR 78% and 77%, respectively), an additional significant improvement in glycemic outcomes with FIA could be challenging to achieve.
The performance of the hybrid AID system during the study was noteworthy, with a reduction in time above range from 31% during the baseline period to ∼19% during the observational period, and a concomitant improvement in TIR from 66% during the baseline to ∼78% during the observational period, irrespective of insulin formulation.
Results of this study observed in active children and adolescents with type 1 diabetes are complementing results observed in adults using different insulin delivery modalities,17,26–29 including three recent studies evaluating FIA with different AID systems in an unsupervised environment.18–20 Lee et al.20 reported small (−1.4%) improvements in time above range and in TIR (+1.9%) with FIA use over 6 weeks, and Beck et al.18 reported similar improvements in time above range (−2%) and TIR (2%) with FIA use over 13 weeks. Importantly, both the above-mentioned studies were open label, hence participants' knowledge of the insulin formulation used could have influenced their decision making when administering insulin.
On the contrary, Boughton et al.19 reported unchanged time above range and TIR with double-blinded FIA use in another AID system over 8 weeks and reporting a slight improvement in time below range (−0.3%) with FIA use. In our study, there was no difference between study interventions in hypoglycemia metrics, and both time <70 mg/dL and time <54 g/dL values were within the consensus clinical recommendations.9
To our knowledge, this is the first free-living study to evaluate glycemic outcomes in children and adolescents with type 1 diabetes using AID during unsupervised exercise. During exercise and recovery periods, AID was able to maintain near-normoglycemia (TIR 78% and 81%, respectively) regardless of insulin formulation used. Although time below range was moderately higher than overall results (7.2% with FIA and 5.9% with SIA), time in clinically relevant hypoglycemia <54 mg/dL was low in both study arms (0.00% with FIA and 0.12% with SIA).
A previous free-living study estimated glycemic outcomes associated with exercise indirectly through setting a higher (exercise) target (150 mg/dL) in two different AID systems.30 The study demonstrated that the use of a higher target, recommended to be initiated before exercise, maintained the same TIR and time in clinically relevant hypoglycemia as in matched periods when a higher target was not used. Notably, without activity monitor data, no temporal association between exercise intensity, duration, and timing of higher glucose target initiation could be made.30
Ekhlaspour et al.31 demonstrated improved TIR with hybrid AID use during a 48-h ski camp with prolonged physical activity in children and adolescents with type 1 diabetes. Under real-time remote monitoring, daytime (62.4%) and overall (66.4%) TIR achieved with hybrid AID use were comparable with our observations. Recently, Morrison et al.32 showed no difference in TIR between FIA and SIA (81% for both insulin formulations) with AID use over 24 h after exercise in adults with type 1 diabetes.
We evaluated postprandial glycemic outcomes, and TIR approached the recommended clinical consensus target of >70% (i.e., 69% with FIA and 67% with SIA) in this period with no difference between the arms. However, a post hoc analysis assessing postprandial excursion by type of meal found FIA has a lower excursion than SIA during breakfast. Previous studies have demonstrated that FIA provides modest improvements in postprandial glycemic outcomes20,28 or no differences at all27 when used within AID.
It might be possible that due to the highly adaptable AID control algorithm that readjusts insulin delivery based on glycemic profiles, differences in pharmacokinetics and pharmacodynamics of currently approved insulin analogues are not of sufficient extent to provide a clinically meaningful advantage.11 To further improve postprandial glycemic outcomes, significantly faster insulin analogues33 or adjunctive therapies are likely needed.34
The second-generation AID systems have been adapted to further improve glycemic management and usability, with several algorithmic advancements, including adjustable glucose targets, automated correction boluses, factory-calibrated CGM technology, and updated controllers that ensure a more robust personalization of the therapy and increased time in AID mode.1 Certain AID systems also have a meal detection feature that, if prompted, can alert the system to deliver more aggressive automated-correction doses.29,35,36
Importantly, during the entire study duration, participants maintained a high level of AID use (∼90%), reflecting the usability of the system, a key element to fulfilling the glycemic benefits of AID use also in children and adolescents.
The strengths of our study include the multinational, double-blind, crossover design and including children and adolescents. In addition, there was no remote monitoring or close supervision, hence the assessment of glycemic outcomes with AID use, including during free-living exercise captured with activity monitors, supports generalizability of these findings.
Our study also has limitations worth noting. Our study cohort included only participants who were already using an insulin pump and/or CGM (including first-generation AID system), which does not necessarily represent the broader type 1 diabetes population. Furthermore, despite the relatively favorable baseline HbA1c levels (mean HbA1c 7.5%, range 5.9%–9.9%), the participants consistently achieved recommended glycemic outcomes throughout the study period, irrespective of their diverse socioeconomic backgrounds and baseline glycemic status. Finally, each study period was only 4 weeks, so the change in HbA1c could not be compared between the two treatment arms.
However, CGM metrics may provide a more comprehensive assessment of glycemic control.37 In addition, CGM metrics calculated over 4 weeks of CGM use have been shown to be strongly correlated with their 3-month CGM metric, so we expect these results to be similar over a longer study duration.38
In conclusion, this randomized, double-blind clinical trial did not demonstrate superiority of FIA over SIA in physically active children and adolescents with type 1 diabetes using an advanced hybrid AID system. Both insulin formulations enabled high TIR and low time above range and time below range, including during periods with documented physical activity.
Supplementary Material
Acknowledgments
The authors thank all the participants, nurses, and nurse educators for their dedication and commitment to this study.
Authors' Contributions
K.D., S.B., D.P.Z., and T.B. drafted the article. S.B., P.C., and C.K. did the statistical analysis and verified the underlying data. K.D., S.B., E.F.-R, D.P.Z., N.P., A.M., Z.L., P.C., M.F., H.S., N.B., C.K., and T.B. reviewed and edited the article. All contributing authors approved the final version of the article. T.B. is the guarantor of this study and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
K.D. served on advisory boards of Novo Nordisk, Pfizer, and Sanofi. K.D. received honoraria for participation in the speaker's bureau of Abbott, Eli Lilly, Medtronic, Novo Nordisk, and Pfizer. E.F.-R. reports having received speaker honoraria from Eli Lilly, Novo Nordisk, and Merck. E.F.-R. served on advisory boards for Eli Lilly and Sanofi. D.P.Z. has received honoraria for speaking engagements from Ascensia Diabetes, Insulet Canada, and Medtronic Diabetes. D.P.Z. served on an advisory board for Dexcom and has received an ISPAD-JDRF Research Fellowship and research support from Insulet and the Leona M. and Harry B. Helmsley Charitable Trust.
H.S. served on advisory boards of Amarin, Amgen, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, and Sanofi. P.C. reports no personal financial disclosures but reports that his current employer has received consulting payments on his behalf from vTv Therapeutics, Beta Bionics, Dexcom, and Diasome. H.S. received honoraria for participation in the speaker's bureau of Amarin, Amgen, Bayer, Boehringer Ingelheim, Eli Lilly, Daiichi Sankyo, Novo Nordisk, and Sanofi. H.S.'s institution received investigator-initiated grant support from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, MSD, and Sanofi. N.B. received honoraria for participation in the speaker's bureau of Abbott and Medtronic.
T.B. served on advisory boards of Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic, Indigo, and DreaMed Diabetes. T.B. received honoraria for participating in the speaker's bureaux of Eli Lilly, Novo Nordisk, Medtronic, Abbott, Sanofi, Aventis, Astra Zeneca, and Roche. T.B. owns stocks of DreamMed Diabetes. T.B.'s institution received research grant support from Abbott, Medtronic, Novo Nordisk, GluSense, Sanofi, Novartis, Sandoz, and Zealand Pharma. No other financial disclosures were reported.
Funding Information
This was an investigator-initiated study, sponsored by the Faculty of Medicine, University of Ljubljana. The study was funded in part by University Medical Centre Ljubljana Research and Development Grant 20210205 and by Medtronic Research Grant ERP-2019-11958. Medtronic provided study materials. K.D., N.B., and T.B. were funded in part by Slovenian National Research Agency Grants J3-2536, J7-1820, and P3-0343.
Supplementary Material
References
- 1. Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): A multicentre randomised crossover trial. Lancet 2021;397(10270):208–219; doi: 10.1016/s0140-6736(20)32514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boughton CK, Allen JM, Ware J, et al. Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med 2022;387(10):882–893; doi: 10.1056/NEJMoa2203496 [DOI] [PubMed] [Google Scholar]
- 3. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381(18):1707–1717; doi: 10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnside MJ, Lewis DM, Crocket HR, et al. Open-source automated insulin delivery in type 1 diabetes. N Engl J Med 2022;387(10):869–881; doi: 10.1056/NEJMoa2203913 [DOI] [PubMed] [Google Scholar]
- 5. Russell SJ, Beck RW, Damiano ER, et al. Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med 2022;387(13):1161–1172; doi: 10.1056/NEJMoa2205225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ware J, Allen JM, Boughton CK, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386(3):209–219; doi: 10.1056/NEJMoa2111673 [DOI] [PubMed] [Google Scholar]
- 7. ElSayed NA, Aleppo G, Aroda VR, et al. 7. Diabetes technology: Standards of care in diabetes-2023. Diabetes Care 2023;46(Suppl 1):S111–s127; doi: 10.2337/dc23-S007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sherr JL, Schoelwer M, Dos Santos TJ, et al. ISPAD clinical practice consensus guidelines 2022: Diabetes technologies: Insulin delivery. Pediatr Diabetes 2022;23(8):1406–1431; doi: 10.1111/pedi.13421 [DOI] [PubMed] [Google Scholar]
- 9. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes care 2019;42(8):1593–1603; doi: 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ceriello A, Prattichizzo F, Phillip M, et al. Glycaemic management in diabetes: Old and new approaches. Lancet Diabetes Endocrinol 2022;10(1):75–84; doi: 10.1016/s2213-8587(21)00245-x [DOI] [PubMed] [Google Scholar]
- 11. Phillip M, Nimri R, Bergenstal RM, et al. Consensus recommendations for the use of automated insulin delivery (AID) technologies in clinical practice. Endocr Rev 2022; doi: 10.1210/endrev/bnac022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kowalski A. Pathway to artificial pancreas systems revisited: Moving downstream. Diabetes Care 2015;38(6):1036–1043; doi: 10.2337/dc15-0364 [DOI] [PubMed] [Google Scholar]
- 13. Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast-acting insulin aspart: The role of niacinamide. Pharm Res 2019;36(3):49; doi: 10.1007/s11095-019-2578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bode BW, Iotova V, Kovarenko M, et al. Efficacy and safety of fast-acting insulin aspart compared with insulin aspart, both in combination with insulin degludec, in children and adolescents with type 1 diabetes: The onset 7 trial. Diabetes Care 2019;42(7):1255–1262; doi: 10.2337/dc19-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heise T, Hövelmann U, Zijlstra E, et al. A comparison of pharmacokinetic and pharmacodynamic properties between faster-acting insulin aspart and insulin aspart in elderly subjects with type 1 diabetes mellitus. Drugs Aging 2017;34(1):29–38; doi: 10.1007/s40266-016-0418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heise T, Stender-Petersen K, Hövelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin Pharmacokinet 2017;56(6):649–660; doi: 10.1007/s40262-016-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dovc K, Piona C, Yeşiltepe Mutlu G, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: A double-blind randomized crossover trial. Diabetes Care 2020;43(1):29–36; doi: 10.2337/dc19-0895 [DOI] [PubMed] [Google Scholar]
- 18. Beck RW, Russell SJ, Damiano ER, et al. A multicenter randomized trial evaluating fast-acting insulin aspart in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24(10):681–696; doi: 10.1089/dia.2022.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boughton CK, Hartnell S, Thabit H, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: A double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab 2021;23(6):1389–1396; doi: 10.1111/dom.14355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee MH, Paldus B, Vogrin S, et al. Fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system in adults with type 1 diabetes: A randomized, open-label, crossover trial. Diabetes Care 2021; doi: 10.2337/dc21-0814 [DOI] [PubMed] [Google Scholar]
- 21. Ware J, Allen JM, Boughton CK, et al. Hybrid closed-loop with faster insulin aspart compared with standard insulin aspart in very young children with type 1 diabetes: A double-blind, multicenter, randomized, crossover study. Diabetes Technol Ther. 2023;25(6):431–436. doi: 10.1089/dia.2023.0042 [DOI] [PubMed] [Google Scholar]
- 22. Adolfsson P, Taplin CE, Zaharieva DP, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in children and adolescents with diabetes. Pediatr Diabetes 2022;23(8):1341–1372; doi: 10.1111/pedi.13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riddell MC, Peters AL. Exercise in adults with type 1 diabetes mellitus. Nat Rev Endocrinol 2023;19(2):98–111; doi: 10.1038/s41574-022-00756-6 [DOI] [PubMed] [Google Scholar]
- 24. Abraham MB, Karges B, Dovc K, et al. ISPAD clinical practice consensus guidelines 2022: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2022;23(8):1322–1340; doi: 10.1111/pedi.13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2022;23(7):835–856; doi: 10.1111/pedi.13406 [DOI] [PubMed] [Google Scholar]
- 26. Avgerinos I, Papanastasiou G, Karagiannis T, et al. Ultra-rapid-acting insulins for adults with diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2021;23(10):2395–2401; doi: 10.1111/dom.14461 [DOI] [PubMed] [Google Scholar]
- 27. Hsu L, Buckingham B, Basina M, et al. Fast-acting insulin aspart use with the MiniMed(TM) 670G system. Diabetes Technol Ther 2021;23(1):1–7; doi: 10.1089/dia.2020.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozer K, Cooper AM, Ahn LP, et al. Fast acting insulin aspart compared with insulin aspart in the Medtronic 670G hybrid closed loop system in type 1 diabetes: An open label crossover study. Diabetes Technol Ther 2021;23(4):286–292; doi: 10.1089/dia.2020.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stamati A, Karagiannis T, Tsapas A, et al. Efficacy and safety of ultra-rapid insulin analogues in insulin pumps in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 2022;193(110144; doi: 10.1016/j.diabres.2022.110144 [DOI] [PubMed] [Google Scholar]
- 30. Dovc K, Battelino T, Beck RW, et al. Impact of temporary glycemic target use in the hybrid and advanced hybrid closed-loop systems. Diabetes Technol Ther 2022;24(11):848–852; doi: 10.1089/dia.2022.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ekhlaspour L, Forlenza GP, Chernavvsky D, et al. Closed loop control in adolescents and children during winter sports: Use of the tandem control-IQ AP system. Pediatr Diabetes 2019;20(6):759–768; doi: 10.1111/pedi.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison D, Zaharieva DP, Lee MH, et al. Comparable glucose control with fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system during exercise. Diabetes Technol Ther 2022;24(2):93–101; doi: 10.1089/dia.2021.0221 [DOI] [PubMed] [Google Scholar]
- 33. Svehlikova E, Mursic I, Augustin T, et al. Pharmacokinetics and pharmacodynamics of three different formulations of insulin aspart: A randomized, double-blind, crossover study in men with type 1 diabetes. Diabetes Care 2021;44(2):448–455; doi: 10.2337/dc20-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsoukas MA, Majdpour D, Yale JF, et al. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: A single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit Health 2021;3(11):e723–e732; doi: 10.1016/s2589-7500(21)00139-4 [DOI] [PubMed] [Google Scholar]
- 35. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368(9):824–833; doi: 10.1056/NEJMoa1206881 [DOI] [PubMed] [Google Scholar]
- 36. Weinzimer SA, Bailey RJ, Bergenstal RM, et al. A comparison of postprandial glucose control in the medtronic advanced hybrid closed-loop system versus 670G. Diabetes Technol Ther 2022;24(8):573–582; doi: 10.1089/dia.2021.0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodbard D. Metrics to evaluate quality of glycemic control: comparison of time in target, hypoglycemic, and hyperglycemic ranges with “risk indices”. Diabetes Technol Ther 2018;20(5):325–334; doi: 10.1089/dia.2017.0416 [DOI] [PubMed] [Google Scholar]
- 38. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther 2018;20(4):314–316; doi: 10.1089/dia.2017.0455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.