Abstract
The objective of this study was the in vitro differentiation of isolates of Salmonella enteritidis whose virulences differed in a chick model. A total of 14 strains of S. enteritidis were isolated from either the environment, dairy products, or infected patients. The isolates could be divided into two groups on the basis of their virulence (50% lethal dose) in chickens infected intraperitoneally. When the strains were incubated in adherence test medium (Spanish patent 9700408), only the virulent strains produced aggregates and formed visible filaments attached to the glass tube. These results suggest, although for a limited number of strains, that aggregation in such a medium could be used as a diagnostic tool to discriminate virulent strains of S. enteritidis.
Salmonella enteritidis infection is a major cause of food-borne illness (11) and remains an important cause of gastroenteritis in humans worldwide. It is usually acquired by ingestion of contaminated water or food, and poultry products are a major source in many developed countries.
During its passage through the body, Salmonella must be able to tolerate several environments with hostile conditions such as low gastric pH and the antimicrobial actions of peptides secreted by the enterocytes. During this journey an intimate interaction takes place between the bacteria and the host cells through a series of biochemical signals. In fact, the factors initiating this communication proceed primarily from the environment at the intestinal lumen. Later, during invasion within both epithelial and phagocytic cells significant molecular changes occur. These changes involve many different inducing signaling processes that result in an array of phenotypes (2). Thus, it is generally assumed that virulence in Salmonella and many other microorganisms is an induced property (8). Accordingly, osmolarity, oxygen tension, pH, the concentrations of free iron and magnesium, and many other factors significantly influence the phenotype and the expression of invasion genes (4, 6, 13).
Differences in the virulence and invasiveness of different strains of S. enteritidis have already been found with a variety of animal models (7, 9, 12, 14, 18), although as Humphrey et al. (12) point out, there is a need to be able to differentiate between virulent and avirulent strains of Salmonella without necessarily resorting to the use of animal models.
Because our objective was the in vitro differentiation of virulent strains of S. enteritidis, it was necessary to identify environmental signals with which we could induce the in vitro expression of some specific genes involved in pathogenesis. S. enteritidis strains with different levels of virulence were therefore incubated in several media in order to detect such a new phenotype expressed only by virulent strains.
MATERIALS AND METHODS
Bacteria.
A total of 14 strains of S. enteritidis were studied. They were isolated in Navarra-Spain from either the environment, dairy products, or infected patients, and they were randomly selected from the respective groups (Table 1). The fresh isolates obtained were used to prepare a stock suspension in skim milk that was stored at −85°C. They were identified as S. enteritidis by biochemical and serological procedures (1, 9, 12; g, m −). The phage types and plasmid profiles of all isolates were determined. The typing results are presented in Table 1.
TABLE 1.
Properties of the bacterial strains used in this study
| Bacterial strain | Origin | Plasmid profilea | Phage type |
|---|---|---|---|
| 20 | River | 37.6 | PT4 |
| 27 | River | 37.6 | PT4 |
| 33 | River | 37.6 | PT4 |
| 57 | River | 37.6, 3.2 | PT6 |
| 942 | River | 37.6 | PT4 |
| 45 | River | 37.6 | PT4 |
| 4839 | Cream | 37.6 | PT4 |
| 5161 | Cake | 37.6, 5.6 | PNR |
| 3934 | Human | 37.6 | PT1 |
| 1344 | Human | 63, 6, 4.2 | PT4 |
| 4271 | Human | 37.6 | PT1 |
| 5507 | Human | 37.6, 32.5 | PT6A |
| 5996 | Human | 37.6 | PT4 |
| 6268 | Human | 37.6, 32.5 | PT6A |
Plasmid size (in megadaltons).
Virulence studies.
Newly hatched layer ISA Brown chicks were held in a safety cabinet at constant humidity and temperature and received food and water ad libitum. The chicks originated from Salmonella-free flocks. The infective doses of the strains were estimated by plating the appropriate dilution of the stock suspension in sterile saline on tryptic soy agar (TSA). Colonies were counted after incubation for 1 day at 37°C. The chickens were randomized into groups of six birds and were infected intraperitoneally (i.p.) with 2 × 101 to 2 × 106 CFU of the corresponding strain. To calculate the 50% lethal dose (LD50) of each strain, the number of dead chickens was recorded every 24 h. The LD50 was calculated at day 3 postinfection by using the Grafit computer program (version 3.0; Erithacus Software Limited).
In vitro aggregation and adherence.
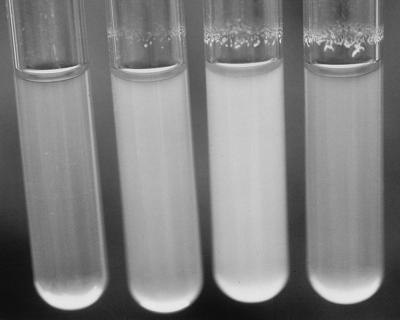
Organisms were retrieved from suspensions stored at −85°C, plated onto TSA plates, and incubated overnight at 37°C. Several colonies were then transferred to 50 ml of Trypticase soy broth (TSB) and incubated at 37°C on an orbital shaker (150 rpm) for 3 h to the logarithmic phase (optical density at 590 nm [OD590], 0.4). After centrifugation, the bacteria were washed and resuspended in the test medium. The suspension was adjusted to an OD590 of 0.125 (approximately 108 CFU/ml on the basis of viable-cell counts on TSA) before use. Four milliliters of the bacterial suspension was then incubated in glass tubes at 37°C at 200 rpm by using an orbital shaker. The tubes were placed in a rack in order to gain an extra lateral movement during shaking. The composition of the adherence test medium (ATM) for these aggregation studies was 60 mM NaCl, 30 mM NaHCO3, 20 mM KCl, and 111 mM glucose (pH 8.4) (5). This medium was supplemented and modified in order to study the conditions of the synthesis of the matrix. All the assessments of the biofilm, except the optical and electronic microscope studies, were monitored visually by subjective quantification (see Fig. 1) after 3 h of incubation in ATM.
FIG. 1.
Subjective quantification of the variability in the amount of biofilm. From left to right, subjective amounts of −, +, ++, and +++, respectively.
SDS-PAGE (LPS analysis).
Analysis of lipopolysaccharide (LPS) was performed by the procedure of Hitchcock and Brown (10). Briefly, the strains stored at −85°C were incubated in TSB overnight at 37°C. After centrifugation, the bacteria were washed and resuspended in phosphate-buffered saline (pH 7.2) to an OD525 of 0.5 to 0.6. A total of 1.5 ml of this suspension was centrifuged in a microcentrifuge for 3 min. The pellet was resuspended in 50 μl of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) lysis buffer (2% SDS, 4% 2-mercaptoethanol, 10% glycerol, and 0.002% bromophenol blue in 1 M Tris-HCl buffer [pH 6.8]) and boiled at 100°C for 10 min. A total of 25 μg of proteinase K (Merck) was added, and the suspension was incubated at 60°C for 1 h. Five microliters of the digested sample was mixed with 10 μl of lysis buffer, and 5 μl of this dilution was loaded onto each lane of SDS-polyacrylamide gels comprising 11% separation gels. Following electrophoresis, the bands were stained with silver (20).
Statistical analysis.
The significance of the differences in the levels of virulence of the isolates was assessed initially by cluster analysis and then by a Mann-Whitney two-tailed test.
Electron microscopy.
S. enteritidis 5996 was incubated for 3 h in ATM, and the supernatant of the culture was removed. The biofilm that formed was collected from the glass wall by using a syringe with distilled water. The cells were fixed with 4% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 1 h at 4°C, washed twice in 125 mM sucrose–50 mM cacodylate buffer, and postfixed in 1% OsO4–100 mM cacodylate at 4°C for 3 h. After two washes with 340 mM Veronal sodium (pH 7.4), the culture was embedded in 4% molten Noble agar (Difco Laboratories). The gel was embedded in Epon-812, and ultrathin sections were examined with a Zeiss EM10CR electron microscope (Carl Zeiss Germany, Oberkochen, Germany).
RESULTS
Challenge of chicks with S. enteritidis strains.
The results obtained after i.p. challenge of the layer chicks demonstrated that the clinical strains of S. enteritidis showed a similar intragroup virulence (log10 LD50, 2.65 ± 0.52), in contrast to the strains isolated from environmental and dairy products isolates, which constituted a much more heterogeneous group (log10 LD50, 3.14 ± 1.37). Table 2 presents the LD50s of each strain. When a statistical cluster analysis of the variable LD50 data for all strains tested was adopted, two groups with cluster centers in log10 LD50s of 2.47 (high level of virulence) and 4.63 (low level of virulence) were found. These differences were demonstrated to be highly significant (P < 0.01) when a Mann-Whitney U test was applied.
TABLE 2.
Correlation between biofilm formation of S. enteritidis strains and virulence
| S. enteritidis strain | Biofilm formationa | Log10 LD50 at day 3 postinfectionb |
|---|---|---|
| 20 | ++ | 2.61 |
| 27 | +++ | 2.83 |
| 33 | +++ | 2.25 |
| 57 | +++ | 2.45 |
| 942 | − | 5.29 |
| 45 | − | 4.00 |
| 4839 | − | 4.60 |
| 5161 | +++ | 1.14 |
| 3934 | +++ | 1.79 |
| 1344 | +++ | 2.87 |
| 4271 | +++ | 2.26 |
| 5507 | ++ | 3.18 |
| 5996 | ++ | 2.89 |
| 6268 | ++ | 2.94 |
Subjective quantification of biofilm formation after incubation in the test medium.
LD50 for day-old chicks following i.p. challenge.
Effect of starvation on S. enteritidis strains.
In vitro incubation of the individual strains in ATM revealed the ability of some of them to adhere to the glass wall at the interphase between the medium and the air, and a visible biofilm was formed (Fig. 1). On examination by phase-contrast microscopy, autoaggregation of the cells was observed even when they were not attached to the glass. The three strains which constituted the group with a low level of virulence showed neither such special aggregation nor the biofilm formation (Table 2).
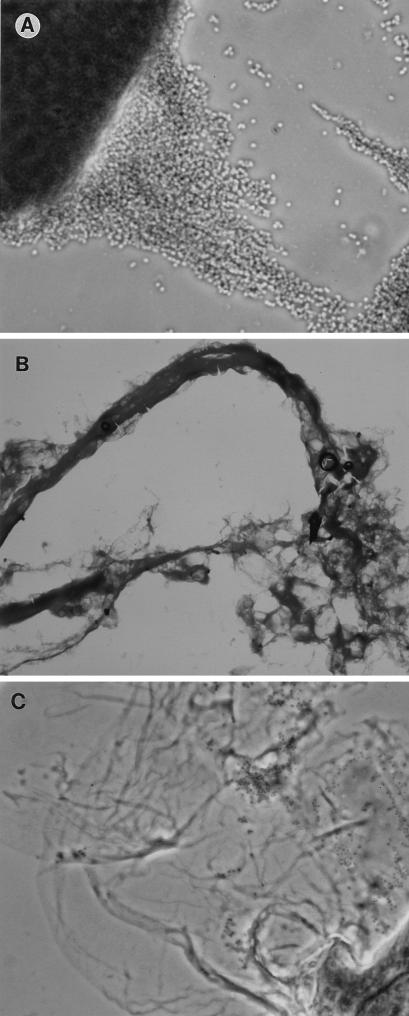
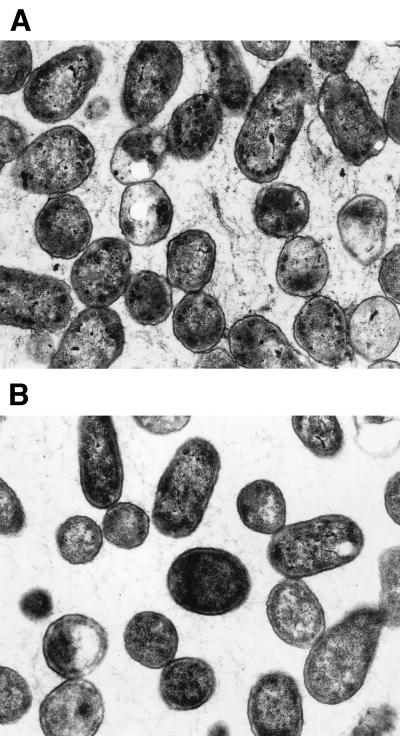
The phenomenon described above became visible after only 40 min of incubation, and after 2 h, a biofilm was obvious (Fig. 1). This was stable and remained attached even after vigorous shaking. Light and electron microscopy studies of the biofilm revealed that the bacteria were present in an extracellular matrix (Fig. 2 and 3).
FIG. 2.
Light micrographs of the biofilms formed by strain 5996 after incubation in ATM and staining with Gram stain. Light microscopy shows large clusters of bacteria (A) forming long filaments (B) and an extracellular matrix (C). Magnifications, ×830 (A), ×83 (B), and ×332 (C).
FIG. 3.
Electron micrographs of the biofilm formed by strain 5996. The presence of an extracellular matrix is observed only after incubation of the strain in ATM (A) but not after incubation in TSB (B). Magnification, ×18,750.
The expression of fimbrial antigens SEF 14, SEF 21, and SEF 17 (19) by the S. enteritidis strains was examined by enzyme-linked immunosorbent assay. All the strains examined expressed SEF 14, SEF 21, and SEF 17 when they were grown under appropriate conditions (data not shown). No significant differences among the LPS pattern profiles for the different isolates were detected (data not shown).
ATM solution does not support bacterial growth. When the medium was enriched with fetal calf serum (10%) or yeast extract (3 mg/ml), the bacteria were able to multiply, but then the phenomenon was inhibited. Following this observation, the effect of medium supplementation was studied. The results demonstrated that when the solution was supplemented with an inorganic source of phosphorus, nitrogen, sulfur, magnesium, calcium, or iron (Fe3+) the adherence was inhibited (Table 3).
TABLE 3.
MICs of salts for biofilm formation
| Supplementing salt | Concn (mg/ml) in normal mediuma | MIC (mg/ml) | Ratio of MIC/concn in normal medium |
|---|---|---|---|
| NH4Cl | 1 | 10 | 10 |
| Na2HPO4 | 10 | 10 | 1 |
| (NH4)+H2PO4 | 5 | 5 | 1 |
| CaCl2 | 0.01 | 5 | 500 |
| MgCl2 | 0.02 | 0.018 | 1 |
| FeCl3 | 0.014 | 0.28 | 20 |
| SO4Na2 | 5 | 10 | 2 |
Minimum concentration required in a medium for heterotrophic bacteria.
Other sugars that Salmonella is able to utilize for growth could be substituted for glucose, but the phenomenon did not change. However, when the substituting sugar was either lactose or sucrose, which Salmonella cannot use, the biofilm was not formed (Table 4).
TABLE 4.
Effect of concentration of glucose and substituting sugar on biofilm formation
| Substituting sugar (concn [mM])a | Metabolic capacityb | Biofilm formationc |
|---|---|---|
| Glucose (111) | + | +++ |
| Glucose (55) | + | + |
| Glucose (222) | + | + |
| Lactose (111) | − | − |
| Lactose (55) | − | − |
| Saccharose (111) | − | − |
| Saccharose (55) | − | − |
| Maltose (111) | + | − |
| Maltose (55) | + | +++ |
| Mannose (111) | + | +++ |
| Galactose (111) | + | +++ |
Either the concentration of glucose in standard ATM was changed or another sugar was substituted for glucose and the bacteria were incubated in the different solutions as described in Materials and Methods.
Capacity of S. enteritidis to metabolize sugar.
Biofilm formation was subjectively quantified from + to +++.
Modulation of biofilm formation.
Significant biofilm formation was not observed upon regular orbital shaking and was inhibited in static cultures. The phenomenon was temperature dependent and was enhanced at 42°C, was less pronounced at 22°C, and was not produced at 4°C.
The addition of an inhibitor of protein synthesis (tetracycline at 200 μg/ml) reduced biofilm production, while inhibitors of RNA synthesis (chloramphenicol at 200 μg/ml and rifampin at 200 μg/ml) and DNA synthesis (nalidixic acid at 200 μg/ml) had no effect.
The levels of glucose in the medium were found to be critical (Table 4). An equivalent osmolarity created by the addition of 55 mM NaCl did not affect the biofilm production, although when the concentration of NaCl was increased to 300 mM, complete inhibition was observed.
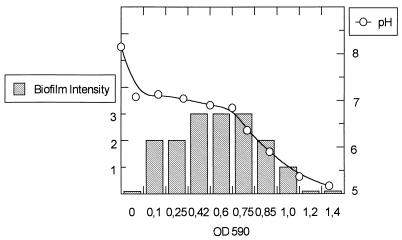
Figure 4 shows how the biofilm production increases as the inoculum rises, reaching the maximum peak when the OD590 is 0.8. Above that density, the level of biofilm production decreases until the complete inhibition of the biofilm production phenotype corresponding to the acidification of the medium below pH 6. Accordingly, biofilm production was also inhibited when standard ATM was acidified to pH 6 by the addition of HCl.
FIG. 4.
Effect of inoculum and pH on the biofilm formation. Logarithmic-phase cells of Salmonella enteritidis 5996 were resuspended in ATM to an OD590 of 0.125 to 1.4. The cells were incubated as described in Materials and Methods, and the pH was measured and biofilm formation was monitored visually at 2 h of incubation. Biofilm formation was subjectively quantified from + to +++.
Biofilm formation occurred optimally when cells were in the logarithmic phase (data not shown).
DISCUSSION
Our first objective was to compare in a chick model the levels of virulence of several strains of S. enteritidis isolated from either the environment, dairy products, or human clinical specimens. The results demonstrated that in the chick model (i.p. route) the strains isolated from humans were more virulent than isolates from either the environment or foods. Three strains of these last two groups were significantly less virulent than the rest of the strains. Other investigators have also detected a heterogeneity of virulence among different isolates of S. enteritidis (7, 9, 12, 14, 18).
There is a need to be able to correlate virulence with an in vitro feature. We therefore studied the effect of starvation on the strains, because in many pathogenic bacteria, starvation serves as an environmental signal which triggers the expression of virulence factors (3, 15, 16). We studied the behavior of S. enteritidis strains after incubation in ATM, a starvation medium deficient in several essential elements, such as nitrogen, phosphorus, calcium, magnesium, sulfur, and iron, but with a source of energy such as glucose.
Under these conditions, only the more virulent strains appeared to adhere to the glass wall, forming visible filaments as a biofilm. This biofilm was stable, remaining attached even after vigorous shaking. A variety of attractive forces are probably involved between the biofilm and the glass and include electrostatic and hydrophobic interactions. The high concentration of urea (1.5 M) needed to inhibit the adherence suggests the relatively low level of participation of hydrophobic interactions in the process observed in this study.
This phenotype is independent of plasmid type, LPS structure, or fimbrial expression.
The results obtained after changing the incubation conditions or after supplementation of ATM suggest that two different kinds of signals trigger the formation of a biofilm: starvation and contact. Starvation was demonstrated by adding natural sources of elements such as yeast extract or calf serum. Furthermore, supplementation of individual salts of nitrogen, phosphorus, calcium, magnesium, sulfur, and iron were enough to inhibit the phenomenon, although the MICs were different. Thus, only 0.018 mg of magnesium salt per ml was inhibitory, in contrast to the salts of nitrogen or phosphorus, of which 10 mg/ml was required to produce an equivalent inhibition. Magnesium is essentially an intracellular cation; therefore, it is possible that it could be a signal that indicates to the bacterium that it is in the extracellular environment.
Although starvation is necessary, this was an active process with a requirement for energy. Glucose or another source of energy (metabolizable sugar) was required, and it was also a temperature-dependent process that was accelerated at higher temperatures and that was completely inhibited at 4°C. Biofilm production was optimum for logarithmic-phase cells in an environment above pH 6.
The second signal required for biofilm formation is contact. Biofilm formation was not observed in static cultures or when incubation was performed at low turbulence under normal shaking conditions. The observation that the turbulence of the medium was important could imply that the phenomenon was related to aeration. However, when ATM was incubated in a flask in which a large area of the medium was in contact with air and shaking was at the regular speed, the biofilm was largely absent. In fact, a biofilm was produced in the area of the surface where the shear forces are maximum, in the border of the cone created by shaking.
Although we do not yet know the pathogenic role of this ability to aggregate and adhere to glass surfaces, if there is any, it is possible that this feature is related to the ability of the cells to adhere to epithelial cells. Adherence to intestinal surfaces plays a large role in mucosal colonization for nearly all enteric pathogens. Salmonellae are enteroinvasive pathogens that require attachment to the luminal surface to counter the peristaltic cleansing motion of the intestine and to initiate penetration through the mucus. Several reports suggest that contact with eukaryotic cells or even with glass surfaces could be a signal that triggers the transcription of virulence genes in bacteria (1, 17, 21). The cells must sense whether they are attached to a surface. It is not precisely known, however, how detection of the substratum might be achieved. One suggestion was that the stress set up in the cell membrane caused by the forces involved in adhesion might result in changes in membrane permeability (17). Thus, starving the cell of its essential elements could induce a genetic change toward the expression of a biofilm.
Further studies are required to determine if the induction of filaments in ATM is an artifact or if it plays an important role in the in vivo interactions with the epithelial cells. Several studies are now in progress to correlate our findings in vitro with adherence in vivo and the role of this new phenotype during the stage of macrophage survival.
In conclusion, the results obtained suggest, although for a limited number of strains, that the aggregation in ATM could be used as a diagnostic tool to discriminate between strains of S. enteritidis.
ACKNOWLEDGMENTS
We thank Ian McLaren for carrying out the plasmid profiling of the strains used in this study. We also thank R. Díaz, I. Dorronsoro, and J. Leiva for providing us with the clinical strains of S. enteritidis.
This research was supported by the Gobierno de Navarra-Spain (grant 1363-94). Fellowship support for C. Solano from the Departamento de Salud del Gobierno de Navarra is gratefully acknowledged.
REFERENCES
- 1.Davies D G, Geesey G G. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlay B B, Leung K Y, Rosenshine I, García del Portillo F. Salmonella interactions with the epithelial cell. ASM News. 1992;58:486–489. [Google Scholar]
- 3.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 4.Galan J E. Molecular genetic bases of Salmonella entry into the host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 5.Gamazo, C., C. Solano, R. Díaz, B. Sesma, I. Dorronsoro, and M. Alvarez. February 1997. Procedimiento para la diferenciación in vitro de las cepas virulentas de Salmonella enteritidis. Spanish patent 9700408.
- 6.García Véscovi E, Soncini F, Groisman E A. Magnesium as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 7.Gast R K, Benson S T. The comparative virulence for chicks of Salmonella enteritidis phage type 4 isolates and isolates of phage types commonly found in poultry in the United States. Avian Dis. 1995;39:567–574. [PubMed] [Google Scholar]
- 8.Groisman E A, Ochman H. How to become a pathogen. Trends Microbiol. 1994;289:421–427. doi: 10.1016/0966-842x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Hinton M, Threlfall E J, Rowe B. The invasiveness of different strains of Salmonella enteritidis phage type 4 for young chickens. FEMS Microbiol Lett. 1990;70:193–196. doi: 10.1111/j.1574-6968.1990.tb13977.x. [DOI] [PubMed] [Google Scholar]
- 10.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lypopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hook E W. Salmonella (including Salmonella typhi) In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious diseases. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 2013–2033. [Google Scholar]
- 12.Humphrey T J, Williams A, McAlpine K, Lever M S, Guard-Petter J, Cox J M. Isolates of Salmonella enterica enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol Infect. 1996;117:79–88. doi: 10.1017/s0950268800001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C A, Falkow S A. Entry of Salmonella into epithelial cells. In: Cabello F, Hormaeche C, Mastroeni P, Bonina L, editors. Biology of salmonella. New York, N.Y: Plenum Press; 1993. pp. 169–179. [Google Scholar]
- 14.McKee A S, McDermid A S, Williams A, Marsh P D. Virulence and carriage of two S. enteritidis PT4 strains in an in vivo model. In: Colin P, le Goux J M, Clement G, editors. Salmonella and Salmonellosis Proceedings. Ploufragan, France. 1997. [Google Scholar]
- 15.McLeod G I, Spector M P. Starvation- and stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (ςS) independent and occurs through both phoP-dependent and -independent pathways. J Bacteriol. 1996;178:3683–3688. doi: 10.1128/jb.178.13.3683-3688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–921. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 17.Petterson J, Nordfelth R, Dubidina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 18.Poppe C, Demczuk W, McFadden K, Johnson R P. Virulence of Salmonella enteritidis phagetypes 4, 8 and 13 and other Salmonella spp. for day-old chicks, hens and mice. Can J Vet Res. 1993;57:282–287. [PMC free article] [PubMed] [Google Scholar]
- 19.Thorns C J. Salmonella fimbriae: novel antigens in the detection and control of salmonella infections. Br Vet J. 1995;151:643–658. doi: 10.1016/s0007-1935(95)80146-4. [DOI] [PubMed] [Google Scholar]
- 20.Tsai C, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J P, Normar S. Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]