Abstract
Introduction
In recent years, primary surgical treatment of older women with non-metastatic breast cancer has decreased in favor of primary endocrine therapy (PET). PET can be considered in women with a remaining life expectancy of less than five years. The aim of this study was to (1) assess the risk of distant metastases and other cause mortality over ten years in women aged 65 and older with stage I-III breast cancer treated with PET, (2) whether this was associated with geriatric characteristics and comorbidities and to (3) describe the reasons on which the choice for PET was made.
Methods
Women were included from the retrospective FOCUS cohort, which comprises all incident women diagnosed with breast cancer aged 65 or older between January 1997 and December 2004 in the Comprehensive Cancer Center Region West in the Netherlands. We selected women (N = 257) with stage I-III breast cancer and treated with PET from this cohort. Patient characteristics (including comorbidity, polypharmacy, walking, cognitive and sensory impairment), treatment and tumor characteristics were retrospectively extracted from charts. Outcomes were distant metastasis and other cause mortality. Cumulative incidences were calculated using the Cumulative Incidence for Competing Risks method (CICR); and subdistribution hazard ratios (SHR) were tested between groups based on age, geriatric characteristics and comorbidity with the Fine and Gray model.
Results
Women treated with PET were on average 84 years old and 41% had one or more geriatric characteristics. Other cause mortality exceeded the cumulative incidence of distant metastasis over ten years (83 versus 5.6%). The risk of dying from another cause further increased in women with geriatric characteristics (SHR 2.06, p < 0.001) or two or more comorbidities (SHR 1.72, p < 0.001). Often the reason for omitting surgery was not recorded (52.9%), but if recorded surgery was omitted mainly at the patient’s request (18.7%).
Discussion
This study shows that the cumulative incidence of distant metastasis is much lower than other cause mortality in older women with breast cancer treated with PET, especially in the presence of geriatric characteristics or comorbidities. This confirms the importance of assessment of geriatric characteristics to aid counseling of older women.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-023-07029-4.
Keywords: Breast cancer, Geriatric oncology, Primary endocrine therapy
Background
Around thirty per cent of women diagnosed with breast cancer is older than seventy [1]. In general, older individuals are highly variable with respect to comorbidities, functional and cognitive capabilities and social support system. Also, older individuals with cancer tend to value quality of life over longevity [2]. These factors can influence treatment decisions in older women with breast cancer. In the last decade, surgery has been more frequently omitted in older women with operable breast cancer and treatment with primary endocrine therapy (PET) has increased [3–5].
There are several reasons for choosing PET over primary surgery [3]. Women with more comorbidities have a higher rate of non-breast cancer related causes [6–8] and may not live long enough to develop distant metastasis. This holds especially true for those with low-risk breast cancer and a high other cause mortality risk, for example women with stage I breast cancer and severe dementia. This risk of dying from another cause than breast cancer (other cause mortality) can influence both patient and doctor preferences in breast cancer treatment. Current recommendations for treating older women with breast cancer (SIOG/EUSOMA) state that PET should only be offered to older women with estrogen receptor (ER) positive tumors who have an estimated life expectancy of less than five years, and are unfit for or refuse surgery [9]. Prediction tools are available to predict risk of recurrence and other cause mortality in older women with cancer to aid informing and advising older women in this treatment decisions [10–12].
In this study we aim to assess the ten year risk of distant metastases and other cause mortality in women aged 65 and older with stages I-III breast cancer treated with PET, and whether this was associated with geriatric characteristics and comorbidities. We further aim to describe treatment preferences.
Methods
Study population
Data was used from the retrospective FOCUS cohort study (Female breast cancer in the elderly; Optimizing Clinical guidelines Using clinic-pathological & molecular data), which comprises all incident women diagnosed with breast cancer aged 65 or older between January 1997 and December 2004 in the Comprehensive Cancer Center Region West in the Netherlands (N = 3672). This cohort, which was previously described in detail [13], was derived of the National Cancer Registry in the Netherlands containing all newly diagnosed tumors. The study was approved by the scientific board of the Netherlands Cancer Registry. For this study, we included women who had stage I-III breast cancer. We defined PET as not receiving primary surgery but being treated with endocrine therapy (tamoxifen or an aromatase inhibitor). In this time period, patients were not treated with neoadjuvant endocrine therapy, so we believe that this definition covers all patients who were treated with PET.
Patient characteristics and treatments
Charts were reviewed by trained personnel and data was retrospectively extracted on tumor characteristics (histology, TNM stage), treatments, comorbidity (using ICD10 coding) and geriatric characteristics (walking impairment – defined as walking difficulties or use of walking aid noted in the chart, cognitive impairment – defined as history of dementia or cognitive impairment noted in the chart, sensory impairment - defined as positive if using a hearing aid or if poor vision was noted in the chart, and polypharmacy – defined 5 or more medications in use). If data were missing, they were classified as “missing” category within the variable. Age categories were defined as: 65 to 79 years (N = 81), and 80 years and older (N = 177). Geriatric characteristics were classified as having no geriatric characteristic present or 1 or more. Comorbidities were grouped in 0–1 and 2 or more comorbidities. Two additional categories combined the age groups with geriatric characteristic or comorbidities categories.
Outcomes
Longitudinal outcomes were occurrence of distant metastasis and mortality from other causes than breast cancer defined as death without a distant metastasis. Follow-up data on mortality was acquired through linking the data from the National Cancer Registry with municipal population registries, until January 1st 2013. Data on the outcome occurrence of distant metastasis was extracted by trained personnel from medical charts. We give the outcome data over a time period of ten years, because a substantial number of women in this cohort survived past the recommended five years life expectancy to consider PET. Reasons for omitting surgery were recorded in categories (mental condition, physical condition, both mental and physical condition, patient preference, age, inoperable, other diagnosis determines prognosis) or in free text for other reasons. In case e.g. “mental condition” was mentioned together with “wish patient” the first reason was used.
Statistics
The cumulative incidences of distant metastasis and other cause mortality (death in absence of distant metastasis) were calculated using the Cumulative Incidence for Competing Risks method (CICR) [14, 15]. We calculated cumulative incidences for the whole cohort, and separate cumulative incidences for different groups based on age categories, geriatric characteristics and comorbidities. The cumulative incidences between groups based on age, geriatric characteristics and comorbidities were tested using the Fine and Gray model. Analyses were adjusted for TNM stage and age. Analyses were performed in IBM SPSS Statistics version 25 and STATA version 14.0.
Results
Table 1 shows the characteristics of the women in our study. Women treated with PET were on average 84 years old (to compare, the average age of women in this cohort that did choose surgery was 75.7 years). Polypharmacy was present in 14.4%. 14.8% of the women had cognitive impairment, 14.8% had a walking impairment, 18.7% a sensory impairment. Cumulating these geriatric characteristics results in 41.2% of the women treated with PET that have at least one geriatric characteristic present. Furthermore two or more comorbidities were present in 42.0% of the women. The TNM stages were distributed as follows: 0–1.2%, I − 19.8%, II- 34.2%, III – 16.3% and also 28.4% missing a TNM classification in their chart.
Table 1.
Characteristics of women with non-metastatic breast cancer treated with PET
| Number (%) / Median (IQR) | |
|---|---|
| N = 257 | |
| Patient related | |
| Age | 84.2 (77.7; 89.1) |
| Age categories | |
| 65–69 | 18 (7.0) |
| 70–74 | 22 (8.6) |
| 75–79 | 41 (16.0) |
| 80–84 | 57 (22.2) |
| 85–89 | 71 (27.6) |
| 90+ | 48 (18.6) |
| Geriatric characteristics | |
| Walking impairment | 38 (14.8) |
| Polypharmacy | 37 (14.4) |
| Cognitive impairment | 38 (14.8) |
| Sensory impairment | 48 (18.7) |
| 1 or more geriatric characteristics | 106 (41.2) |
| Comorbidities | |
| 0–1 comorbidities | 149 (58.0) |
| 2 or more comorbidities | 108 (42.0) |
| Tumor related | |
| TNM stage | |
| 0 | 3 (1.2) |
| I | 51 (19.8) |
| II | 88 (34.2) |
| III | 42 (16.3) |
| Missing | 73 (28.4) |
| Grade | |
| 1 | 5 (1.9) |
| 2 | 19 (3.9) |
| 3 | 5 (1.9) |
| Missing | 237 (92.2) |
| Morphology | |
| Ductal | 122 (47.5) |
| Lobular | 23 (8.9) |
| Other/missing | 112 (43.6) |
| ER/PR | |
| Positive ER or PR | 86 (33.5) |
| Negative ER and PR | 12 (4.7) |
| Missing | 159 (61.9) |
Frequencies in no. (%), no. (%), or median (interquartile range, IQR). N/a = not applicable
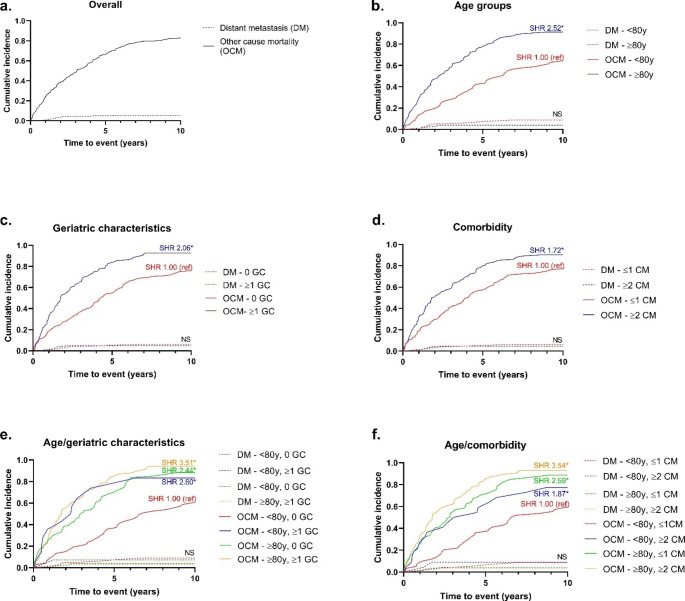
Figure 1 shows the cumulative incidences (over ten years) of distant metastasis and of other cause mortality (death without distant metastasis), comparing different age groups, geriatric characteristics and corresponding subdistribution hazards (SHR’s) are presented in Table 2. Overall, the cumulative incidence of distant metastasis was 5.55% (95%CI 3.16–8.80%) whereas the incidence of other cause mortality was 82.7% (95%CI 77.3–87.0%) (Fig. 1a). Cumulative incidences of distant metastasis were not associated with age, number of geriatric characteristics or comorbidities.
Fig. 1.
Ten years risk of distant metastasis and other cause mortality in women with non-metastatic breast cancer treated with PET, dependent on age, geriatric characteristics and comorbidity
SHR: subdistribution hazard ratio. Ref: reference. DM: distant metastasis. OCM: other cause mortality. GC: geriatric characteristic, y: years. *: P ≤ 0.05, NS: not significant. Fine and Gray analyses were used for calculation of SHRs, results from the crude model are shown
Table 2.
Subdistribution hazard ratios (SHR) of distant metastasis and other cause mortality in women with non-metastatic breast cancer treated with PET, dependent on age, geriatric characteristics and comorbidity
| Distant metastatasis - SHR (95% CI) | Competing event - SHR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Age | ||||||
| 65–79 years | 1.00 (ref) | 1.00 (ref) | na | 1.00 (ref) | 1.00 (ref) | n/a |
| ≥80 years | 0.46 (0.16–1.31) | 0.39 (0.14–1.12) | n/a | 2.52 (1.83–3.46) | 2.39 (1.70–3.34) | n/a |
| Geriatric characteristics | ||||||
| No walking impairment | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Walking impairment | 0.95 (0.21–4.24) | 0.93 (0.20–4.27) | 1.10 (0.22–5.56) | 1.74 (1.22–2.50) | 1.72 (1.17–2.53) | 1.58 (1.07–2.33) |
| No cognitive impairment | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Cognitive impairment | 0.43 (0.06–3.28) | 0.45 (0.06–3.59) | 0.57 (0.06–5.05) | 2.15 (1.47–3.15) | 2.09 (1.42–3.06) | 1.87 (1.30–2.68) |
| <5 medications per dag | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥5 medications per day | 1.67 (0.46–6.05) | 1.76 (0.47–6.64) | 2.09 (0.55–7.94) | 1.52 (1.05–2.22) | 1.43 (0.96–2.11) | 1.24 (0.81–1.90) |
| No sensory impairment | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Sensory impairment | 0.73 (0.16–3.31) | 0.78 (0.17–3.63) | 0.93 (0.75) | 1.60 (1.15–2.24) | 1.51 (1.07–2.13) | 1.34 (0.95–1.90) |
| Total geriatric characteristics 0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Total geriatric characteristics ≥ 1 | 0.79 (0.26–2.37) | 0.81 (0.25–2.61) | 1.05 (0.31–3.54) | 2.06 (1.55–2.74) | 1.97 (1.46–2.63) | 1.66 (1.23–2.25) |
| Comorbidities | ||||||
| 0–1 comorbidities | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥2 comorbidities | 0.76 (0.25–2.27) | 0.74 (0.24–2.28) | 0.88 (0.24–3.21) | 1.72 (1.31–2.28) | 1.60 (1.20–2.12) | 1.47 (1.11–1.96) |
| Age/geriatric characteristics | ||||||
| 65–79 years and 0 geriatric characteristics | 1.00 (ref) | 1.00 (ref) | n/a | 1.00 (ref) | 1.00 (ref) | n/a |
| 65–79 years and ≥ 1 geriatric characteristics | 0.83 (0.10–7.03) | 0.91 (0.19–8.48) | n/a | 2.60 (1.21–5.60) | 2.46 (1.16–5.22) | n/a |
| >80 years and 0 geriatric characteristics | 0.40 (0.10–1.60) | 0.34 (0.08–1.37) | n/a | 2.44 (1.68–3.56) | 2.32 (1.56–3.45) | n/a |
| >80 years and ≥ 1 geriatric characteristics | 0.49 (0.14–1.72) | 0.43 (0.11–1.65) | n/a | 3.51 (2.41–5.12) | 3.35 (2.24–5.01) | n/a |
| Age/comorbidities | ||||||
| 65–79 years and 0–1 comorbidities | 1.00 (ref) | 1.00 (ref) | n/a | 1.00 (ref) | 1.00 (ref) | n/a |
| 65–79 years and ≥ 2 comorbidities | 1.10 (0.21–5.67) | 0.98 (0.16–5.99) | n/a | 1.87 (1.03–3.39) | 1.76 (0.95–3.26) | n/a |
| >80 years and 0–1 comorbidities | 0.53 (0.14–1.97) | 0.44 (0.12–1.60) | n/a | 2.59 (1.75–3.83) | 2.49 (1.63–3.80) | n/a |
| >80 years and ≥ 2 comorbidities | 0.41 (0.10–1.71) | 0.34 (0.08–1.52) | n/a | 3.54 (2.37–5.29) | 3.11 (2.14–5.12) | n/a |
SHR: subdistribution hazard ratio. 95% CI: 95% confidence interval. Ref: reference. N/a: not applicable. Fine and Gray analyses: model 1 - crude model; model 2 - as model 1 plus TNM stage; model 3 - as model 2 plus age
The cumulative incidence of other cause mortality was 91.2% (95%CI 85.7–94.7%) for women aged 80 and older, versus 64.3 (95%CI 52.6–73.9%) for women aged 65–79 years (Fig. 1b. Model 1: SHR 2.52, 95%CI 1.83–3.46, p < 0.001). This effect remains also after adjusting for TNM stage (model 2: SHR 2.39, 95%CI 1.70–3.34, p < 0.001). Women with 1 or more geriatric characteristics had a cumulative incidence of other cause mortality of 90.7% (95%CI 83.9–94.7%) compared to those without geriatric characteristics: 74.6% (95%CI 65.7–81.5%) (Fig. 1c. Model 1: SHR 2.06, 95%CI 1.55–2.24), p < 0.001). After adjusting for TNM stage (Model 2: SHR 1.97, 95%CI 1.46–2.63, p < 0.001) and additionally for age (Model 3: SHR 1.66, 95%CI 1.23–2.25, p < 0.001) similar effects were seen. With 2 or more comorbidities the cumulative incidence of other cause mortality was 90.3% (95%CI 82.7–94.7%) versus 77.2% (95%CI 69.2–83.3%) in with 0–1 comorbidities (Fig. 1.d. Model 1: SHR 1.72, 95%CI 1.31–2.28, p < 0.001). Also after adjusting for TNM stage (Model 2: SHR 1.60, 95%CI 1.20–2.12, p = 0.001) and additionally for age (Model 3: SHR 1.47, 95%CI 1.11–1.96, p = 0.007) similar effects were seen.
When combining both age and geriatric characteristics, we observed higher cumulative incidences of other cause mortality in those aged over 80 years without geriatric characteristics (cumulative incidence 87.9%, 95%CI 77.4–93.8%. Model 2: SHR 2.32, 95%CI 1.56–3.45, p < 0.001), those aged 65–79 years with 1 or more geriatric characteristic (cumulative incidence 79.7, 95% CI 57.9–91.0%. Model 2: SHR 2.46, 95%CI 1.16–5.22, p = 0.019) and those aged over 80 years with 1 or more geriatric characteristics (cumulative incidence 92.8%, 95%CI 85.6–96.4%. Model 2: SHR 3.35, 95%CI 2.24–5.01, p < 0.001) compared to those aged 65–79 years without geriatric characteristics (cumulative incidence 57.5%, 95%CI 43.1–69.5. SHR 1.00 reference) (Fig. 1e).
When combining age and comorbidity, we observed higher cumulative incidences of other cause mortality in those aged 65–79 years with 2 or more comorbidities (cumulative incidence 77.3, 95% CI 53.7–89.8%. Model 2: SHR 1.76, 95%CI 0.95–3.26, p = 0.071), those aged over 80 years with 0–1 comorbidities (cumulative incidence 88.7%, 95%CI 79.7–93.9%. Model 2: SHR 2.49, 95%CI 1.63–3.80, p < 0.001), and those aged over 80 years with 2 or more comorbidities (cumulative incidence 93.0%, 95%CI 85.1–96.8%. Model 2: SHR 3.11, 95%CI 2.14–5.12, p < 0.001) compared to those aged 65–79 years with 0–1 comorbidities (cumulative incidence 59.5%, 95%CI 45.5–71.0. SHR 1.00 reference) (Fig. 1f).
Table 3 shows the reasons for omitting surgery in women treated with PET. For roughly half of the women (52.9%) no information was found in medical charts. In 17.1% it was stated in the medical chart that the patient had a preference for PET over primary surgery. In 11.7% surgery was omitted due to the patient’s physical condition and in 4.2% the patient’s mental condition was the reason for omission of surgery. In 5.1% of women inoperability was noted, but it was not specified whether this was due to the women’ condition or due to inoperability of the tumour. Age alone was given as a reason in 3.5% of women.
Table 3.
Reasons to omit surgery in women with non-metastatic breast cancer treated with ET.
| N= | % | |
|---|---|---|
| No information | 136 | 52.9 |
| Wish patient | 44 | 17.1 |
| Physical conditon | 30 | 11.7 |
| Mental condition | 11 | 4.2 |
| Mental and physical condition | 2 | 0.8 |
| Inoperable | 13 | 5.1 |
| Age only | 9 | 3.5 |
| Other diagnosis determines prognosis | 4 | 1.6 |
| Other (reasons noted: tumor was chance finding, due to surgery, no primary tumor, not opportune according to surgeon, pleural effusion/axillary nodes) | 8 | 3.2 |
In case another reason e.g. “mental condition” was mentioned together with “wish patient” this was counted as the first reason in this table
Discussion
In this study, we showed the real-life trajectories of women that were treated with PET between 1997 and 2004. We showed that the ten year cumulative incidence of distant metastasis in women treated with PET is very low and other cause mortality is high. As expected, our data confirm that in addition to increasing age, geriatric characteristics and comorbidity are predictors for other-cause mortality in older women with stage I-III breast cancer treated with PET.
These results underline the importance of estimating the risk of other cause mortality when counselling older women with non-metastatic breast cancer about treatment options. A full comprehensive geriatric assessment, has been shown to be strongly predictive of other-cause mortality in many previous studies [16–18]. Therefore, our results, based on a limited set of retrospectively obtained geriatric characteristics, might even underestimate the actual predictive capacity of these factors for other cause mortality.
It could be argued that PET is a good treatment option for frail older women with a short life expectancy. Previous randomized clinical trials have shown that PET is non-inferior to surgery with regard to overall survival for women over the age of 70, but a significant proportion of the study participants did require secondary surgery during follow-up. These trials were however performed with tamoxifen while in recent years, aromatase inhibitors have been shown superior to tamoxifen in both (neo)adjuvant and metastatic settings [19, 20] and more lines of endocrine therapy have become available. PET may therefore be more effective than what has been previously published. However, an observational study of women aged 80 years and older with stage I-II breast cancer showed that omission of surgery negatively impacted overall mortality after five years [21]. Furthermore, breast cancer surgery is generally regarded as minor surgery with a small risk of severe complications. However, in one of our previous studies of women aged 65 years or older, the risk of less severe post-operative complications, such as wound infections, hematomas and seromas was 19%, and was increased with older age and comorbidities. There were no severe life-threatening complications and mortality was not higher in the group of older women with complications [13]. Furthermore, endocrine therapy also comes with side-effects, with 31 to 73% of women discontinuing adjuvant endocrine therapy within 5 years after initiation, mostly because of toxicity [22]. Our group recently investigated toxicity of adjuvant endocrine therapy in older patients with breast cancer and its impact on quality of life. The results showed that patients who discontinued therapy due to toxicity had a worse quality of life compared to patients who continued therapy. However, discontinuation of treatment did not positively affect quality of life after discontinuation, which implies that the poorer quality of life is not caused by toxicity [23]. Although these studies have not been performed in women treated with PET, is it likely that side-effects occur as frequently in this group of women. Knowledge on these real-life treatment trajectories, side-effects and complications might aid older women with breast cancer and their physicians, so the impact of primary surgery versus PET (with possible side-effects and the need for chronic medication use) can be weighed for their preferred treatment.
Recently, a prediction tool was developed in the British Age Gap study to aid decisions for primary treatment in women with breast cancer aged 70 and older [10]. This model could help determine which older women might benefit most from PET or from primary surgery by determining other cause mortality and breast cancer-specific mortality. The researchers also investigated the impact of decision tools itself on quality of life in a randomized trial, but no difference in quality of life was seen between women that used the decision tool versus those receiving usual care. Interestingly, the fraction of treatment with PET was higher in those using the decision tool than those receiving usual care (21.0 versus 15.4 per cent) [24]. Furthermore, the British Age Gap study showed that quality of life dropped significantly between baseline and the first six weeks in both treatment groups, which did not recover within the first two years [25]. As for the functional status, a gradual decline was observed for women treated with PET, while women who received primary surgery showed an early sharp fall between baseline and 6 weeks. Both groups failed to recover to their baseline functional status. In contrast, a prospective cohort study from our group (in preparation) into older women with early-stage breast cancer with questionnaires including the Groningen Activity Rating Scale (GARS) and the EORTC-QLQC30 and BR23 showed that functional status and quality of life did not decline after breast surgery, irrespective of the occurrence of postoperative complications, which suggests that the impact of postoperative complications on these outcomes are in fact minimal. Hence, the decision for PET or surgical treatment remains a personal decision but the risks of surgery appear to be low, even in women with geriatric characteristics present. It is noteworthy that in more than half of our patients no information was recorded on why primary endocrine therapy was chosen over surgery, which is comparable to the results of Hamaker et al. [3]. Probably there are several reasons to choose PET within this group, which might be or not be related to frailty. Recording reasons for treatment decisions more often would aid future research and ultimately provide information to aid personal treatment decisions for older patients. Also, for a large proportion of our patients that were treated with PET no information on hormone receptor status was available, and around 5% were treated with PET despite negative hormone receptor expression. This partly reflects the time period in which hormone receptor expression was not routinely assessed, but another hypothesis would be that the omission of hormone receptor testing might be associated with frailty. However, with the data from this cohort these hypotheses cannot be adequately tested.
Strengths of this study include the real-world data with registration data, which includes all consecutive women aged 65 and over diagnosed with breast cancer in the region West in the Netherlands over 8 years. Therefore, there was no selection in women included. Another strength is the analysis of competing other cause mortality and the long-term follow-up data that were available. Limitations of this study include its retrospective design and the associated missing data on cause of death and reasons for treatment decisions. Furthermore, the number of geriatric characteristics measured is limited, and was dependent on what was noted by medical personal in the charts. In addition, the incidence of distant metastasis in women treated with PET may be underreported due to loss to follow up of frail women. Therefore, this study might overestimate the incidence of other cause mortality.
In conclusion, the ten year incidence of other cause mortality in older women treated with PET increases with age, the presence of geriatric characteristics and comorbidity, and is much higher than the risk of distant metastasis. This underlines the importance of predicting other cause mortality when counselling older women with non-metastatic breast cancer in their treatment options. In order to more accurately identify which older women benefit from a certain treatment option, more geriatric-based prognostic factors should be included in decision making in daily practice, for example by including screening tools or a comprehensive geriatric assessment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Comprehensive Cancer Centre Netherlands (Leiden region).
Abbreviations
- PET
primary endocrine therapy
Authors’ contributions
MW and NG conceived the idea of the manuscript, MW and AB conducted the statistical analyses, MW, AL and NG wrote the draft of the manuscript. GJL, EB and NdG contributed to the design of the study and the acquisition of the data. GJL is the principal investigator of the FOCUS cohort. All authors (MW, AL, AdB, EB, FvdB, MD, JK, GJL, JP and NdG) contributed to revising and editing the manuscript and gave approval of the final version to be published.
Funding
This work was supported by the Dutch Cancer Foundation (KWF 2007–3968).
Declarations
Ethics approval and consent to participate
This retrospective observational study was approved by the scientific board of the Netherlands Cancer Registry.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeSantis CE, Ma JM, Gaudet MM, Newman LA, Miller KD, Sauer AG, et al. Breast cancer statistics, 2019. Ca-Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28(7):1367–1380. doi: 10.1002/pon.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamaker ME, Bastiaannet E, Evers D, Water W, Smorenburg CH, Maartense E, et al. Omission of surgery in elderly patients with early stage breast cancer. Eur J Cancer. 2013;49(3):545–552. doi: 10.1016/j.ejca.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 4.de Glas NA, Jonker JM, Bastiaannet E, de Craen AJ, van de Velde CJ, Siesling S, et al. Impact of omission of surgery on survival of older patients with breast cancer. Br J Surg. 2014;101(11):1397–1404. doi: 10.1002/bjs.9616. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg. 2007;94(10):1209–1215. doi: 10.1002/bjs.5834. [DOI] [PubMed] [Google Scholar]
- 6.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Janssen-Heijnen ML, Maas HA, Houterman S, Lemmens VE, Rutten HJ, Coebergh JW. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer. 2007;43(15):2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkler IH et al (2021) Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the european society of breast Cancer specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. [DOI] [PubMed]
- 10.Ward SE, Holmes GR, Morgan JL, Broggio JW, Collins K, Richards PD, et al. Bridging the age gap: a prognostic model that predicts survival and aids in primary treatment decisions for older women with oestrogen receptor-positive early breast cancer. Br J Surg. 2020;107(12):1625–1632. doi: 10.1002/bjs.11748. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Moreno C, Pilleron S, Neuendorff NR, Soto-Perez-de-Celis E. How we use noncancer-specific survival prediction in geriatric oncology: a Young International Society of geriatric oncology and nursing & Allied Health Interest Group initiative. J Geriatr Oncol. 2022;13(4):516–520. doi: 10.1016/j.jgo.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 12.van der Plas-Krijgsman WG, Giardiello D, Putter H, Steyerberg EW, Bastiaannet E, Stiggelbout AM, et al. Development and validation of the PORTRET tool to predict recurrence, overall survival, and other-cause mortality in older patients with breast cancer in the Netherlands: a population-based study. Lancet Health Longev. 2021;2(11):E704–E11. doi: 10.1016/S2666-7568(21)00229-4. [DOI] [PubMed] [Google Scholar]
- 13.de Glas NA, Kiderlen M, Bastiaannet E, de Craen AJ, van de Water W, van de Velde CJ, et al. Postoperative complications and survival of elderly breast cancer patients: a FOCUS study analysis. Breast Cancer Res Treat. 2013;138(2):561–569. doi: 10.1007/s10549-013-2462-9. [DOI] [PubMed] [Google Scholar]
- 14.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 15.Putter H, Schumacher M, van Houwelingen HC. On the relation between the cause-specific hazard and the subdistribution rate for competing risks data: the Fine-Gray model revisited. Biometrical J. 2020;62(3):790–807. doi: 10.1002/bimj.201800274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 17.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in Predicting Treatment Tolerance and all-cause mortality in older patients with Cancer. Oncologist. 2012;17(11):1439–1449. doi: 10.1634/theoncologist.2012-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denewet N, De Breucker S, Luce S, Kennes B, Higuet S, Pepersack T. Comprehensive geriatric assessment and comorbidities predict survival in geriatric oncology. Acta Clin Belg. 2016;71(4):206–213. doi: 10.1080/17843286.2016.1153816. [DOI] [PubMed] [Google Scholar]
- 19.Ruhstaller T, Giobbie-Hurder A, Colleoni M, Jensen MB, Ejlertsen B, de Azambuja E, et al. Adjuvant letrozole and tamoxifen alone or sequentially for Postmenopausal Women with hormone receptor-positive breast Cancer: long-term Follow-Up of the BIG 1–98 trial. J Clin Oncol. 2019;37(2):105–114. doi: 10.1200/JCO.18.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouridsen HT, Lonning P, Beckmann MW, Blackwell K, Doughty J, Gligorov J, et al. Use of aromatase inhibitors and bisphosphonates as an anticancer therapy in postmenopausal breast cancer. Expert Rev Anticancer Ther. 2010;10(11):1825–1836. doi: 10.1586/era.10.160. [DOI] [PubMed] [Google Scholar]
- 21.de Boer AZ, de Glas NA, Marang-van de Mheen PJ, Dekkers OM, Siesling S, de Munck L, et al. Effect of omission of surgery on survival in patients aged 80 years and older with early-stage hormone receptor-positive breast cancer. Br J Surg. 2020;107(9):1145–1153. doi: 10.1002/bjs.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemij AA, de Glas NA, Derks MGM, Bastiaannet E, Merkus JWS, Lans TE et al (2022) Discontinuation of adjuvant endocrine therapy and impact on quality of life and functional status in older patients with breast cancer. Breast Cancer Res Treat. [DOI] [PMC free article] [PubMed]
- 24.Wyld L, Reed MWR, Collins K, Burton M, Lifford K, Edwards A et al (2021) Bridging the age gap in breast cancer: cluster randomized trial of the effects of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg. [DOI] [PMC free article] [PubMed]
- 25.Morgan JL, Shrestha A, Reed MWR, Herbert E, Bradburn M, Walters SJ, et al. Bridging the age gap in breast cancer: impact of omission of breast cancer surgery in older women with oestrogen receptor-positive early breast cancer on quality-of-life outcomes. Br J Surg. 2021;108(3):315–325. doi: 10.1093/bjs/znaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.