Abstract
Background:
Dietary supplement and complementary and alternative medication (CAM) use can contribute to drug interactions, polypharmacy, nonadherence with prescription medications, and healthcare expenses, whereas evidence supporting benefits of using these products is sparse. There is a lack of current published literature describing the patterns or predictors of their use in community-dwelling older adults.
Materials and Methods:
We performed a cross-sectional analysis of community-dwelling adults from Australia and the US, aged 70 years and older (65 years for US minorities), enrolled in the ASPirin in Reducing Events in the Elderly (ASPREE) study. At study enrollment, eligible participants were required to be without concurrent 5-year life-limiting illness and free of documented evidence of cardiovascular disease, dementia, or significant physical disability. During the final study visit, a questionnaire was administered to collect information about supplement/CAM use. Data from 15,729 participants who completed this questionnaire between January 2017 and January 2018 were analyzed. Descriptive statistics were used to report the prevalence and types of products used. Factors associated with use were determined using multivariate regression.
Results:
Mean age of respondents was 79.6 years; 56.4% were female, 88.8% were from Australia, 56.5% reported 12 years of education or less, and 98.7% were living at home. Two-thirds (66.2%) of participants reported use of one or more supplement/CAM in the previous month. Products most commonly used included vitamin D (33.8% of participants), fish oil (22.7%), calcium (20.6%), glucosamine (14.8%), and multivitamin (12.9%). Female sex, US residency, higher education, polypharmacy (prescription medications), and frailty (in women) were significantly associated with higher use of supplements/CAMs.
Conclusions:
Dietary supplement and CAM use is common among community-dwelling older adults in the United States and Australia. Given the high prevalence of use, collaboration between healthcare providers and older adult patients is important to insure safe and optimal use of these products.
Keywords: complementary and alternative medications, dietary supplements, nontraditional medicine
INTRODUCTION
Use of dietary supplements and complementary and alternative medications (CAMs) is common among adults in the United States, with an estimated 57.6% of people aged 20 years and older using at least one dietary supplement or CAM in the past 30 days.1 Trends in use of these products change over time with most recent reports demonstrating an 8% increase in use among adults in the United States in 2017–2018 compared with 2007–2008.1,2 Compared to younger adults, a higher percentage of older adults in the United States use supplements/CAMs, with an estimated 74.3% of adults 60 years and older using one or more dietary supplement or CAM compared to just 59.2% aged 40–59 years and 42.5% aged 20–39 years.1 Similarly, in Australia, older adults are more likely to use supplements/CAMs with 52.7% of adults 71–85 years reporting use compared with just 38.8% among those 70 and younger.3 While supplement/CAM use appears prevalent in older adults, data informing usage rates in this population are nearly a decade old and individuals in the oldest age groups are underrepresented.1,3–5 This poses challenges in understanding contemporary use trends, as specific supplements/CAMs often cycle in and out of popularity. Furthermore, many older adults included in prior analyses have multiple comorbidities and poorer self-reported health, with relatively healthy older adults among the least well studied.4 Finally, information is lacking comparing rates and trends of supplement/CAM use among older adults across countries with rapidly growing populations of older adults such as in the United States and Australia.
Use of supplements and CAMs among older adults is associated with potential benefits and risks. Increased supplement use can signify enhanced interest in health and may be associated with improved overall prescription medication adherence.6 Potential risks, however, include increased drug interactions,4,5 medication burden, nonadherence with prescription medications,7 and increased out-of-pocket expenses.8 Older adults who routinely utilize non-prescription therapies, including supplements/CAMs, are at increased risk of serious drug interactions, a risk that increases with increasing age and use of certain concomitant therapies, including warfarin and aspirin.4,5
Given that older adults are known to be high users of these products, information related to current use patterns among this group is highly relevant. Furthermore, there is great need for involvement of informed healthcare providers in the management of these therapies among this population due to increased risk for side effects, drug interactions, and financial hardship associated with fixed incomes.
This secondary analysis aims to provide updated information regarding use of supplements/CAMs in a community-dwelling, initially healthy older adult population from two large countries (US and Australia) with aging populations. In addition, factors associated with use of these products are explored. Finally, differences in patterns of use between countries are explored as well as differences among men and women given previous reports of sex-specific dietary supplement use patterns9 and the importance, in general, of examining sex as a modifier of health behavior.10
MATERIALS AND METHODS
Study population
We conducted a secondary analysis of quantitative data collected from community-dwelling older adults participating in the ASPirin in Reducing Events in the Elderly (ASPREE) trial. This study enrolled healthy older adults from Australia and the United States aged 70 years and older (65 years and older for US minorities) between 2010 and 2014 and randomized them to low dose daily aspirin 100 mg/day or placebo. The primary outcome of ASPREE was disability-free survival, a composite of death, incident dementia, or persistent physical disability. As a condition of enrollment, eligible participants had to be free of documented evidence of cardiovascular disease, dementia or significant cognitive impairment, severe physical disability, a high risk of bleeding, anemia, any condition that may cause death within 5 years, uncontrolled high blood pressure, and not currently using aspirin for secondary prevention or other antiplatelet or anticoagulant medication.11
Participants attended annual in-person study visits, which included collection of medical diagnoses as well as assessments of cognition, physical function, lifestyle, anthropometric measures, concomitant prescription medications, and other clinical parameters. Supplement/CAM use was included within a one-off questionnaire during the final annual visit (“Milestone” study visit). All Milestone visits were conducted from January 2017 to January 2018, which coincided with the 3rd–7th annual visit for an individual participant, depending on what year they originally enrolled into the study. Trial Registration: ClinicalTrials.gov NCT01038583 and ISRCTN83772183.
Data definitions
Data obtained from the Milestone Visit Questionnaire concerning medication use was linked with demographic and other health-related data collected during the ASPREE trial. New diagnoses were self-reported at annual visits, and any that were primary or secondary endpoints of interest for the trial (e.g., dementia, cardiovascular disease, bleeding) were adjudicated as part of the main trial procedures as previously outlined.11 For some nonadjudicated conditions (e.g., diabetes, depression, and hypertension) a combination of participant selfreport, medication use, and in-trial objective measures were used to determine the presence of the condition. All conditions analyzed in this paper reflect the participants’ status at the Milestone visit.
Supplement/CAM Use
A user of a dietary supplement or CAM was defined as any participant who selected a supplement or CAM option from the question “Will you take, or have you taken, any of the following in the past month?” An option of fish oil, glucosamine, ginkgo, Coenzyme Q10 (CoQ10), multivitamins, calcium, vitamin D, zinc, vitamin B, vitamin C, vitamin E, and “other supplements, herbal or complementary medicines” was provided, a list developed by a group of investigators with clinical pharmacology, geriatric, and nutritional epidemiological expertise informed by commonly reported CAM use from previous population studies. Participants who selected any of the options or the “other” option were classified as supplement/CAM users.
Outcomes
We examined predictors of supplement/CAM among the full cohort, by sex, and by country. In addition, differences in patterns of supplement/CAM use by country were explored.
Data analysis
Data were analyzed using Stata v16.1 software (College Station, TX, USA). Alpha was set at 0.05. Continuous data were described by mean and 95% confidence intervals (CIs). Categorical data were described as proportions. For univariate analyses comparing groups, χ2 tests were used for categorical variables, and t-tests used for age. A binary logistic regression was used as a predictive model for use of supplements/CAMs. As data could not be determined to be missing at random, multiple imputation was not performed. Potential model covariates were evaluated in age-, sex-, and country-adjusted models as part of model development. The χ2 statistic was used to examine overall significance of the model. Multicollinearity was examined using coefficient of contingence.
Covariates
Potential model covariates were identified from previous literature4,12 and evaluated in univariate logistic regression analyses. Multivariable logistic regression was used to examine the demographic and health-related characteristics associated with CAM use in multivariate models which included sex and age. As sex and country of residence showed the strongest association with CAM use, models stratifying for these variables were also examined. We report odds ratios and 95% CIs.
RESULTS
Overall supplement/CAM use
Data on use of supplements/CAMs from the Milestone Visit questionnaire were available for 15,732 ASPREE participants. Table 1 shows the characteristics of the cohort at the Milestone Visit year. The majority of participants (mean age 79.6 years) were female, living in Australia, received 12 years of education or less, and lived at home with others. Although hypertension and dyslipidemia were present in greater than 80% of the cohort, only 14.4% were diagnosed with diabetes and only 15.7% were categorized as being frail. Two thirds of participants (66.2%) reported use of supplements/CAMs within the past month. Although most participants reported some use of these products, high daily burden was less common with just 12.2% of the entire cohort reporting use of four or more CAM products per day (Figure 1).
TABLE 1.
Characteristics (at Milestone year) of ASPirin in Reducing Events in the Elderly (ASPREE) supplement/complementary and alternative medication (CAM) users and non-users (n[%]).h
| Overall, n = 15,732 | Users of supplements/ CAMa, n = 10,412 (66.2) | Non-users of supplements/CAM, n = 5320 | ||
|---|---|---|---|---|
|
| ||||
| Sexb | Female | 8871 (56.4) | 6619 (63.6) | 2252 (42.3) |
| Male | 6861 (43.6) | 3793 (36.4) | 3068 (57.7) | |
| Age, years | Mean (95% CI)c | 79.6 (79.5–79.7) | 79.5 (79.4–79.6) | 79.7 (79.5–79.8) |
| Age groupc | <75 years | 1927 (12.3) | 1312 (12.6) | 615 (11.6) |
| 75–<80 years | 7698 (48.9) | 5046 (48.5) | 2652 (49.8) | |
| ≥80 years | 6107 (38.8) | 4054 (38.9) | 2053 (38.6) | |
| Educationb | ≤12 years | 8883 (56.5) | 5703 (54.8) | 3180 (59.8) |
| ≥13 years | 6848 (43.5) | 4708 (45.2) | 2140 (40.2) | |
| Countryb | Australia | 13,966 (88.8) | 9001 (86.4) | 4965 (93.3) |
| United States | 1766 (11.2) | 1411 (13.6) | 355 (6.7) | |
| Ethnicityb | Caucasian | 14,748 (94.7) | 9645 (93.6) | 5103 (96.8) |
| African American | 590 (3.8) | 467 (4.5) | 123 (2.3) | |
| Other | 242 (1.5) | 196 (1.9) | 46 (0.9) | |
| Living statusc | Living at home alone | 5536 (35.2) | 3928 (37.8) | 1608 (30.2) |
| Living at home with others | 9983 (63.5) | 6356 (61.1) | 3627 (68.2) | |
| Assisted living setting | 205 (1.3) | 122 (1.1) | 83 (1.6) | |
| BMI, kg/m2 | Mean (95% CI)c | 27.7 (27.6–27.8) | 27.7 (27.6–27.8) | 27.7 (27.6–27.8) |
| Polypharmacyc,d | 4849 (32.0) | 3387 (33.6) | 1462 (28.7) | |
| Morbidities and health conditions | History of cancerf Hypertensione,f | 3841 (24.4) 14,197 (90.2) | 2529 (24.3) 9356 (89.9) | 1312 (24.7) 4841 (91.0) |
| Diabetese,f | 2266 (14.4) | 1439 (13.8) | 827 (15.5) | |
| Dyslipidemiae,f | 12,719 (80.8) | 8504 (81.7) | 4215 (79.2) | |
| In-study MACEe | 420 (2.7) | 245 (2.4) | 175 (3.3) | |
| History of depressionf | 5807 (36.9) | 3979 (38.2) | 1828 (34.4) | |
| Frailtyc,g | 2474 (15.7) | 1741 (16.8) | 733 (13.8) | |
| Pre-frailtyc,g | 6191 (39.4) | 4192 (40.3) | 1999 (37.6) | |
Note: Values are n (% of reporting population) except for age and body mass index (BMI) which are given as mean (95% CI).
Abbreviations: CI, confidence interval; MACE, major adverse cardiovascular events.
Reported use of one or more herbal, supplement or complementary medication in the past month.
Collected at baseline of the ASPREE trial.
Collected on the day of the Milestone visit.
Polypharmacy was defined as intake of four or more different prescribed medication types on the day of the Milestone clinic visit.
Occurrence at any time between ASPREE trial baseline and the day of the Milestone clinic visit Milestone.
History of cancer and depression at Milestone visit was obtained from pre-trial self-reported history + occurrence of adjudicated cancer/depression event in trial. History of hypertension, dyslipidemia and diabetes used the definitions previously described in the ASPirin in Reducing Events in the Elderly (ASPREE) trial (Study design of ASPREE: a randomized, controlled trial. Contemp Clin Trials 2013;36 (2):555–64. doi: 10.1016/j.cct.2013.09.014).
Frailty/pre-frailty: participants were classified as frail if they met at least three of the following criteria and pre-frail if they met one or two of the criteria: (1) BMI < 20 kg/m2; (2) lowest 20% of grip strength taking into account sex and BMI; (3) the participant endorsed “I felt that everything I did was an effort” and/or “I could not get going” for three or more days during the last week, according to the Center for Epidemiological Studies-Depression 10 (CES-D10) scale; (4) time to walk 3 meters (10 feet) was in the lowest 20% taking into account sex and height, and (5) no walking outside the home in the last 2 weeks, or the longest amount of time walking outside without sitting down to rest was less than 10 min, according to LIFE Disability questionnaire responses.
Row numbers may not reflect full cohort due to missing responses. No variable exceeded 4% missing data.
FIGURE 1.
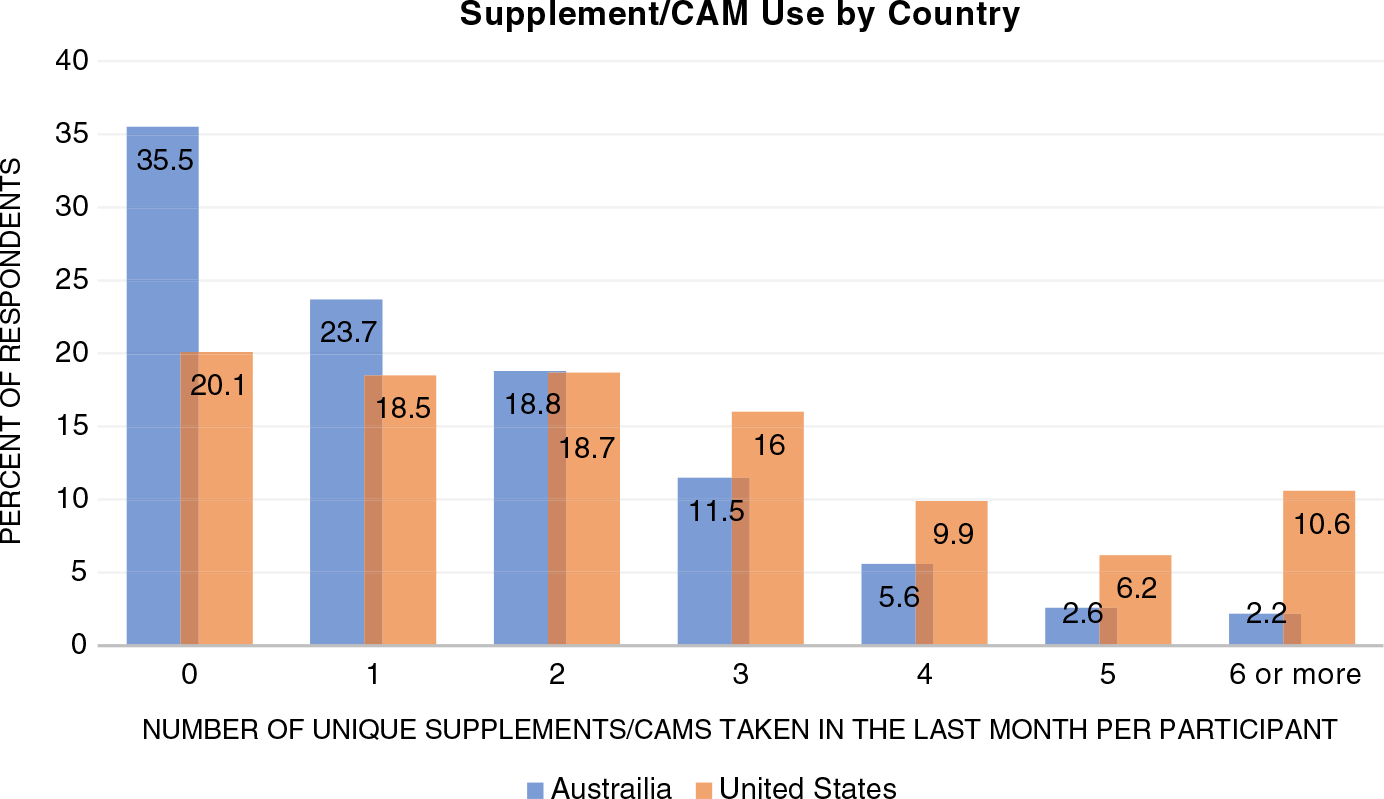
Supplement/(CAM) Use (in the last month) by country. p-value <0.001 indicating that distribution is not equal for this variable when comparing Australia residents to US residents.
More US participants (79.9%) reported supplement/CAM use (“any”) compared to Australian participants (64.4%) (p < 0.001) (Figure 1). Furthermore, there was a tendency for US participants to report taking higher numbers of supplements/CAMs compared with Australian participants, with only 21.9% of Australian participants reporting use of >2 supplements/CAMs compared to 42.7% of US participants (Figure 1).
Specific product use
The proportion of overall participants taking each type of supplement/CAM varied significantly (Figure 2), with the products used most commonly including vitamin D (33.8% of participants), fish oil (22.7%), calcium (20.6%), glucosamine (14.8%), and multivitamins (12.9%). An additional 25.5% reported taking an “other” (nonspecified) product.
FIGURE 2.
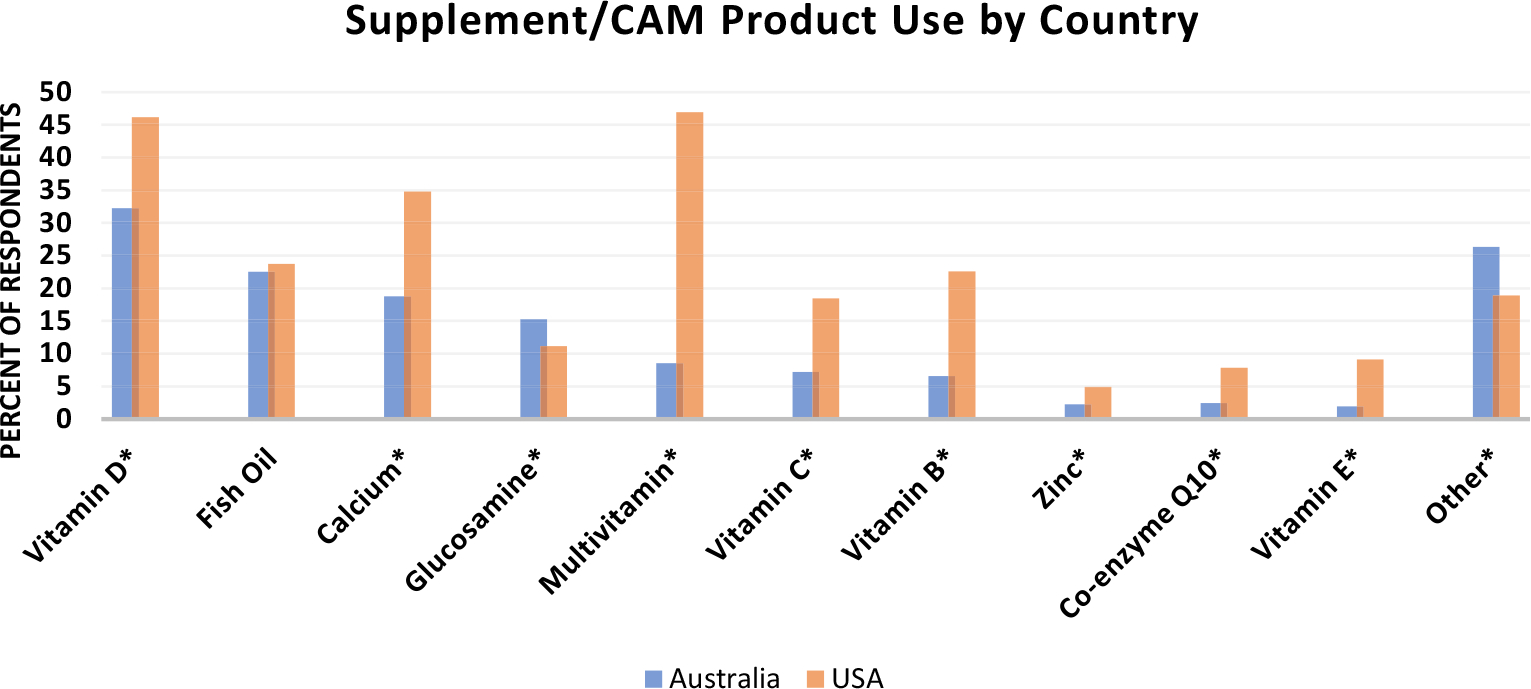
Proportion of the Cohort using specific supplement/complementary and alternative medication (CAM) products. Country differences significant for every supplement/CAM (p < 0.001) except for fish oil (no significant difference in proportion taking fish oil). Gingko and ginseng excluded from figure given that <2% of respondents used these supplements
Furthermore, there was variation in use between US and Australian participants with a significantly higher percentage of US participants using CoQ10 (5.5% more), multivitamins (38.3% more), calcium (16.0% more), vitamin D (13.9% more), zinc (2.6% more), vitamin B (16.0% more), vitamin C (11.3% more), and vitamin E (7.2% more) (p < 0.001 for all comparison). A higher percentage of Australian participants reported use of glucosamine and “other” (nonspecified) herbals and supplements (Figure 2).
Characteristics of CAM users
In the overall cohort (n = 15,732), age, sex, education level, country of residence, living situation, polypharmacy, history of diabetes, frailty, and development of major adverse cardiovascular events (MACE) during the trial were significantly associated with reporting supplement/CAM use in a multivariate regression analysis (Table 2). When predictors of supplement/CAM use were examined by country, factors associated with supplement CAM use were directionally the same with similar effect sizes in each country; however, the significance of the associations in the US cohort were lost (except for female sex and history of dyslipidemia) due to the proportionally smaller number of US participants enrolled in the ASPREE trial (Table S1).
TABLE 2.
Multivariate logistic regression modeling for odds of supplement/complementary and alternative medication use.a
| Univariate regression |
Multivariate regressionb |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | Adj OR (95% CI) | P |
|
| ||||
| Age (per year) | 0.99 (0.99–1.00) | 0.105 | 0.99 (0.98–1.00) | 0.013 |
| Sex (ref male) | 2.38 (2.22–2.54) | <0.001 | 2.16 (2.00–2.33) | <0.001 |
| Education (ref ≤ 12 year education) | 1.23 (1.15–1.31) | <0.001 | 1.18 (1.10–1.27) | <0.001 |
| Country (ref Aus) | 2.19 (1.94–2.48) | <0.001 | 1.99 (1.69–2.33) | <0.001 |
| Living situation (ref living alone) | 0.73 (0.69–0.78) | <0.001 | 0.87 (0.81–0.94) | <0.001 |
| Polypharmacy | 1.26 (1.17–1.36) | <0.001 | 1.25 (1.15–1.36) | <0.001 |
| Hypertension | 0.88 (0.78–0.98) | 0.023 | 0.92 (0.81–1.04) | 0.258 |
| Diabetes | 0.87 (0.79–0.96) | 0.004 | 0.84 (0.76–0.93) | 0.001 |
| Frailtyc (ref not frail) | 1.18 (1.13–1.24) | <0.001 | 1.06 (1.00–1.12) | 0.064 |
| Dyslipidemia | 1.17 (1.08–1.27) | <0.001 | 1.04 (0.95–1.15) | 0.352 |
| Racial identification (ref white) | 1.16 (1.12–1.20) | <0.001 | 1.03 (0.99–1.08) | 1.42 |
| History of depression | 1.18 (1.10–1.27) | <0.001 | 1.02 (0.94–1.10) | 0.641 |
| Development of MACEd during trial | 0.71 (0.58–0.86) | 0.001 | 0.70 (0.57–0.87) | 0.001 |
Note: Values given as odds ratios (ORs) (95% CI) or Adjusted ORs (95% CI) for univariate and multivariable regression, respectively. N = 15,732 for univariate regression (small amounts of missing data depending upon the variable), whereas for the multivariable model n = 14,990.
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Reported use of one or more herbal, supplement or comp med in the past month.
Variables selected for multivariate model were age and sex, plus those identified as significant in univariate regression.
Frailty: participants were classified as frail if they met at least three of the following five criteria and pre-frail if they met one or two of the criteria: (1) BMI < 20 kg/m2 (“Shrinking”); (2) lowest 20% of grip strength taking into account sex and BMI (“Weakness”); (3) the participant endorsed “I felt that everything I did was an effort” and/or “I could not get going” for three or more days during the last week, according to the Center for Epidemiological Studies-Depression 10 (CES-D10) scale (“Exhaustion”); (4) time to walk 3 meters (10 feet) was in the lowest 20% taking into account sex and height (“Slowness”), and (5) no walking outside the home in the last 2 weeks, or the longest amount of time walking outside without sitting down to rest was less than 10 min, (“Low activity”) according to LIFE Disability questionnaire responses.
MACE: major adverse cardiovascular events including nonfatal myocardial infarction and fatal or nonfatal ischemic stroke.
When women and men were examined separately (Table 3), there were key differences in the factors associated with CAM use. In both men and women, polypharmacy and country of residence remained a significant predictor in multivariate models, with greater likelihood of supplement/CAM use in those taking five or more prescription medications and those with residence in the United States. In men, higher level of education and history of dyslipidemia were significant predictors of supplement/CAM use. In women, increased age, living with others, history of diabetes, and development of MACE during the trial were associated with reduced odds of reporting CAM use, whereas frailty was associated with an increase in reported supplement/CAM use.
TABLE 3.
Factors associated with supplement/complementary and alternative medication use in older women and men in the ASPirin in Reducing Events in the Elderly cohort.a
| Women (n = 8477) |
Men (n = 6507) |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P |
|
| ||||
| Age (per year) | 0.98 (0.97–0.99) | <0.001 | 1.00 (0.99–1.01) | 0.998 |
| Education (ref ≤ 12 years education) | 1.03 (0.93–1.14) | 0.609 | 1.39 (1.26–1.54) | <0.001 |
| Country (ref Aus) | 2.04 (1.71–2.44) | <0.001 | 1.89 (1.55–2.31) | <0.001 |
| Living situation (ref living alone) | 0.83 (0.76–0.91) | <0.001 | 0.92 (0.82–1.03) | 0.158 |
| Polypharmacy | 1.30(1.16–1.46) | <0.001 | 1.14 (1.01–1.29) | 0.033 |
| Diabetes | 0.70 (0.60–0.81) | <0.001 | 0.97 (0.85–1.11) | 0.656 |
| Dyslipidemia | 0.88 (0.75–1.02) | 0.097 | 1.14 (1.01–1.27) | 0.029 |
| Frailty | 1.10(1.02–1.18) | 0.016 | 1.04 (0.96–1.13) | 0.331 |
| Development of MACE in-trialb | 0.60 (0.43–0.83) | 0.002 | 0.78 (0.60–1.02) | 0.072 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Multivariate regression analysis stratified by sex. Values given as adjusted odds ratios (95% CI).
MACE: major adverse cardiovascular events including nonfatal myocardial infarction and fatal or nonfatal ischemic stroke.
DISCUSSION
We found that self-reported supplement/CAM use was high in this post-hoc analysis of community-dwelling cohort of older adults participating in a primary prevention aspirin trial. ASPREE enrolled a primarily healthy cohort of older adults, and at the time of the Milestone Visit when supplement/CAM use was queried, most had remained healthy, with low prevalence of frailty and chronic diseases such as diabetes. Our findings suggest that many older adults, even those who are generally healthy, utilize supplements/CAMs marketed toward preserving health.
Two thirds of the ASPREE participants at the Milestone Visit reported use of at least one supplement/CAM in the past 30 days. Multivitamins, fish oil, calcium, and vitamin D were among the highest utilized products. When comparing countries, US participants were more likely to be users of supplements/CAMs than Australian participants and more likely to use multiple supplement/CAM products. Beyond US residence, other predictors of supplement/CAM use included female sex, higher education level, polypharmacy, and frailty. Living with someone else, history of diabetes, and development of MACE during the trial were associated with a decreased chance of reporting supplement/CAM use. Interestingly, when examining men and women separately, different factors predicted supplement/CAM use among each sex, with more education and a diagnosis of dyslipidemia predicting use in men but not women and frailty predicting use in women but not men. Future research investigating supplement/CAM usage patterns among generally healthy, older men versus women may help better understand these differences.
The use of multivitamins in US participants in this study was notably high at 46.9% which is similar to a rate of 57.7% reported previously based on 2013–2014 survey data in US adults 50 years and older.12 Large-scale clinical trials have demonstrated no benefit in overall mortality or for specific morbidities with use of multivitamins13,14 and health experts have consistently highlighted the lack of proven benefit when multivitamins are taken in addition to, or in place of, a healthy diet.15–17 Recently, the US Preventive Services Task Force issued a statement concluding that current evidence is insufficient to assess the risk vs benefit of multivitamin use for the prevention of cardiovascular disease or cancer.18 Multivitamin use is not promoted by any US government agency or any national health organization; however, use rates continue to be high, with an estimated one third of all Americans reporting use of a multivitamin.15 Fish oil, utilized by one fifth of the participants in this study cohort, has been studied for use in multiple conditions, including cardiovascular disease, cancer prevention, cognitive function, and ophthalmic conditions.19–22 While evidence demonstrating the benefits associated with consumption of omega-3 fatty acids in foods is strong, evidence supporting use of fish oil supplements for treatment/prevention of adverse health outcomes is conflicting.23 Similarly, vitamin D has been investigated in an array of conditions, and while deficiency of vitamin D is often linked to adverse outcomes, supplementation with vitamin D in many cases is not linked to improved outcomes.24–26 Finally, calcium supplementation for prevention of facture is supported by national organizations,27 however, it is important to note that total calcium intake should not exceed upper limits and this total should include dietary sources. Supplementing with calcium at a level that exceeds the upper recommended intake can lead to adverse effects, such as increased risk for kidney stones.28 Given sparse and conflicting evidence related to risks versus benefits of supplement/CAM use in older adults,18,20,22,24,28,29 decisions related to use of specific products should ideally be tailored to an individual patient as a result of shared decision making between a patient and their healthcare team.
Cost is an additional consideration when using supplements/CAMs given lack of health insurance coverage leading to complete out of pocket expense. This is especially concerning in older adults given the likelihood for this population to have a fixed income. The mean out of pocket annual expenditure in the US for supplements/CAMs in 2012 was estimated to be $368 per person.30 The high utilization of supplements/CAMs among older adults in the setting of unclear benefit, potential risks, and high out-of-pocket costs allows the opportunity for healthcare providers to assist in optimal utilization of these products.
While the motivation to use supplements/CAMs among this population in the face of uncertain benefit and possible risk is unclear, one theory may be that older adults are inclined to take action to preserve their health during the process of aging rather than do nothing, a phenomenon known as action bias which occurs when people choose the risks associated with doing something over those of doing nothing.31 This theory may be supported by the fact that, in this study, frailty was a predictor of use among female participants. The signs of aging experienced among those with frailty may prompt the decision to take action by pursuing supplement/CAM use. Irrespective of motivation, such high use of these products as found in our cohort indicates investment by patients in maintaining their health. Engaging patients seeking supplements/CAMs in discussion is an important opportunity for healthcare professionals to establish rapport, earn trust, and make an impact in the care of patients. On the other hand, the marketing of dietary supplements and CAM products without a prescription and with the suggestion that they are “natural” can lead to uninformed use. The high utilization of supplements/CAMs among older adults demonstrated in this study reinforces the need for involvement of pharmacists and other healthcare professionals in these decisions given the high risk of adverse effects associated with supplement/CAM use in this population.29 An ethical framework for pharmacists has been proposed in Australia to help guide pharmacist-patient interactions related to the use of dietary supplements and CAM.32 Within this framework, pharmacies are encouraged to train pharmacists and staff to provide evidence-based recommendations regarding CAM. In addition, there is training to encourage setting up the pharmacy in a way that encourages discourse between pharmacists and patients when patients are seeking CAMs and to ensure pharmacists are prepared to identify harms associated with CAM use. Given that utilization rates of supplements/CAMs in our study was even higher among United States compared with Australian participants, a finding not previously reported, future research could focus on adaptation of the proposed Australian framework to US pharmacies.
The large sample size, interview methodology (structured visits which followed standardized operating procedures), and few missing data were strengths of this study. Notable limitations include a lack of racial diversity within the cohort which prohibits generalizing these findings to more diverse populations. The majority of the cohort was Australian, limiting the ability to draw further conclusions about supplement/CAM use by country. By nature of volunteering to participate in a prospective randomized controlled trial, especially one that was focused on a common nonprescription product (aspirin) as a health preventive, the cohort may have been subject to selection bias in that participants who are comfortable with the concept of taking medications may also be more health conscious—this may have led to an artificially higher rate of self-reported supplement/CAM use than that of the general older adult population. Although this study was able to provide information regarding use of specific supplement/CAM products in the past 30 days, we did not have information regarding frequency or duration, or dose of products used. Future research replicating this work with a more diverse population and more detailed data collection related to medication is certainly warranted.
We utilized a targeted list of supplements/CAMs in the questionnaire to capture specific product use but over one in five participants also reported using “other” when responding to type of supplements/CAMs used. It is not possible to draw conclusions about what specific products in the “other” category were used, but this finding itself highlights the need for ongoing research in this area and how rapidly products cycle in and out of popularity. Given the population queried, we could speculate that some of the “other” products used may be products with alleged nootropic effects. Use of these products appears to be dramatically rising, with more than 1/3 of adults aged 74 years and older taking a supplement for brain health currently, a market predicted to grow to 5.8 billion by 2023.33 This subtype of supplement is of particular concern given the lack of evidence-based prescription therapies for dementia-related conditions, leaving patients more prone to seek out novel or alternative treatments. Studies have demonstrated that labels for these products do not always match actual ingredients, posing the potential for drug–drug, drug-diet, and drug-disease interactions without the ability to fully prevent or monitor due to lack of specific information on drug and dose recieved.34 An additional supplement gaining popularity is melatonin. A recent analysis found an over 5-fold increase in use of this supplement from 1999–2000 to 2017–2018.35 Although generally safe at lower doses, excessive doses of melatonin can lead to an increased risk of adverse events including fatigue, headache, daytime drowsiness, glucose intolerance, increased blood pressure, and tachycardia.36 Cannabis usage trends are also on the rise and in an evaluation specific to adults 65 years and older, a two-fold increase in use was seen in 2018 compared to 2015.37 Future inclusion of these products in surveys of healthy older adults would provide useful insight allowing for targeted interventions to ensure safe use.
CONCLUSIONS
A high proportion of participants enrolled in the large Australian- and US-conducted ASPREE trial self-reported use of CAM or supplements. Use of these products was highest among patients who were female, from the United States, less educated, or with polypharmacy. Within women specifically, frailty was also a predictor of use. Although prevalence of use was high, overall burden of supplement/CAM use was low with only 12.2% of participants reporting the use of more than three unique products. Products most commonly utilized included vitamin D, fish oil, calcium, and multivitamin. Our findings emphasize the importance of healthcare providers actively assessing use of supplements/CAMs in healthy older adults.
Supplementary Material
Table S1. Factors associated with odds of supplement/CAM use by country in the ASPREE cohort.
Key points
In a cohort of 15,732 community-dwelling older adults from Australia and the US, 66.2% reported use of one or more dietary supplement or complementary and alternative medication (CAM) in the past month.
Female sex, US residency, higher education, polypharmacy, and frailty were associated with self-reported use of supplements/CAMs.
Why does this paper matter?
This paper demonstrates that, currently, supplement/CAM utilization is prevalent among initially healthy older adults, indicating engagement in their health. This presents opportunities for healthcare professionals to educate older adult patients regarding potential benefits and harms of nontraditional medicine use and screen for untoward effects.
ACKNOWLEDGMENTS
We thank the steering committee of the ASPREE trial for making the dataset available for this study.
FUNDING INFORMATION
The ASPREE trial was supported by the National Institute on Aging (NIA) and the National Cancer Institute at the National Institutes of Health (NIH) (grant numbers U01AG029824, U19AG062682); the National Health and Medical Research Council of Australia (NHMRC) (grant numbers 334047, 1127060), Monash University and the Victorian Cancer Agency
Footnotes
CONFLICT OF INTEREST
All the authors declare that they have no conflicts of interest with regard to this manuscript.
SPONSOR’S ROLE
The sponsors had no role in the design, methods, data collection, analysis, or preparation of the manuscript.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Dietary Supplement Use Among Adults: United States, 2017–2018. National Health and Nutrition Examination Survey. Accessed June 13, 2022. https://www.cdc.gov/nchs/products/databriefs/db399.htm.
- 2.Gahche J, Bailey R, Burt V, et al. Dietary Supplement Use among U.S. Adults Has Increased since NHANES III (1988–1994). NCHS Data Brief, no 61. National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 3.Burnett AJ, Livingstone KM, Woods JL, McNaughton S. Dietary supplement use among Australian adults: findings from the 2011–2012 National Nutrition and Physical Activity Survey. Nutrients. 2017;9(11):1248. doi: 10.3390/nu9111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agbabiaka TB, Spencer NH, Khanom S, Goodman C. Prevalence of drug-herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711–e717. doi: 10.3399/bjgp18X699101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesen C, Harbig P, Barat I, Damsgaard EM. Correlation between the use of ‘over-the-counter’ medicines and adherence in elderly patients on multiple medications. Int J Clin Pharmacol. 2014;36(1):92–97. doi: 10.1007/s11096-013-9892-7 [DOI] [PubMed] [Google Scholar]
- 7.Açıkgöz SK, Açıkgöz E, Topal S, et al. Effect of herbal medicine use on medication adherence of cardiology patients. Complement Ther Med. 2014;22(4):648–654. doi: 10.1016/j.ctim.2014.05.013. Epub 2014 Jun 6. [DOI] [PubMed] [Google Scholar]
- 8.Nahin RL, Barnes PM, Stussman BJ. Expenditures on complementary health approaches: United States, 2012. Natl Health Stat Rep. 2016;95:1–11. [PubMed] [Google Scholar]
- 9.Rontogianni MO, Kanellopoulou A, Markozannes G, et al. Prevalence and determinants of sex-specific dietary supplement uin a greek cohort. Nutrients. 2021;13(8):2857. doi: 10.3390/nu13082857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020; 396(10250):565–582. doi: 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36(2):555–564. doi: 10.1016/j.cct.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan ECK, Eshetie TC, Gray SL, Marcum ZA. Dietary supplement use in middle-aged and older adults. J Nutr Health Aging. 2022; 26(2):133–138. doi: 10.1007/s12603-022-1732-9. [DOI] [PubMed] [Google Scholar]
- 13.Lim JE, Weinstein SJ, Liao LM, Sinha R, Huang J, Albanes D. Multivitamin use and overall and site-specific cancer risks in the National Institutes of Health-AARP diet and health study. J Nutr. 2022;152(1):211–216. doi: 10.1093/jn/nxab322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol. 2011; 173(8):906–914. doi: 10.1093/aje/kwq447. Epub 2011 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Multivitamin/mineral Supplements. National Institutes of Health. Accessed June 13 2022. https://ods.od.nih.gov/factsheets/MVMS-HealthProfessional/.
- 16.White PL. Vitamin preparations as dietary supplements and as therapeutic agents. JAMA. 1959;169(1):41–45. doi: 10.1001/jama.1959.03000180043010 [DOI] [PubMed] [Google Scholar]
- 17.Council on Sicentific Affiars. Vitamin preparations as dietary supplements and as therapeutic agents. JAMA. 1987;257(14):1929–1936. doi: 10.1001/jama.1987.03390140099035 [DOI] [PubMed] [Google Scholar]
- 18.US preventative Task Force. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. US Preventative Task Force Recommendation Statement. JAMA. 2022;327:2326–2333. [DOI] [PubMed] [Google Scholar]
- 19.Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst Rev. 2016;4:CD009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrenson JG, Evans JR, Cochrane Eyes and Vision Group. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2015;2015(4):CD10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, et al. n-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omega-3 Fatty Acids. National Institutes of Health. Accessed June 13 2022. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer/.
- 24.Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitamin D Fact Sheet for Health Professionals. National Institutes of Health. Accessed June 13 2022. https://ods.od.nih.gov/factsheets/vitamind-healthprofessional/.
- 26.LeBoff MS, Chou SH, Ratliff KA, et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299–309. doi: 10.1056/NEJMoa2202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33:2049–2102. doi: 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson RD, LaCroix AZ, Gass M, Women’s Health Initiative Investigators, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. [DOI] [PubMed] [Google Scholar]
- 29.Gellar AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373:1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahin RL, Barnes PM, Stussman BJ. Expenditures on Complementary Health Approaches: United States, 2012. (433KB PDF) National Health Statistics Reports. National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 31.Ubel PA. Why too many vitamins feels just about right. JAMA Intern Med. 2022;182(8):791–792. doi: 10.1001/jamainternmed.2022.0119. [DOI] [PubMed] [Google Scholar]
- 32.Popattia AS, Hattingh L, La Caze A. Improving pharmacy practice in relation to complementary medicines: a qualitative study evaluating the acceptability and feasibility of a new ethical framework in Australia. BMC Med Ethics. 2021;22(1):3. doi: 10.1186/s12910-020-00570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe AL, Venkataraman A. The safety and efficacy of botanicals with nootropic effects. Curr Neuropharmacol. 2021;19(9):1442–1467. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford C, Boyd C, Avula B, Wang YH, Khan IA, Deuster PA. A public health issue: dietary supplements promoted for brain health and cognitive performance. J Altern Complement Med. 2020;26(4):265–272. doi: 10.1089/acm.2019.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Somers VK, Xu H, Lopez-Jimenez F, Covassin N. Trends in use of melatonin supplements among US adults, 1999–2018. JAMA. 2022;327(5):483–485. doi: 10.1001/jama.2021.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuehn BM. Climbing melatonin use for insomnia raises safety concerns. JAMA. 2022;328(7):605–607. [DOI] [PubMed] [Google Scholar]
- 37.Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med. 2020;180:609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Factors associated with odds of supplement/CAM use by country in the ASPREE cohort.


