Abstract
Background:
Renal cell carcinoma (RCC) with sarcomatoid and/or rhabdoid (S/R) dedifferentiation is a highly aggressive tumor with a poor prognosis. Immune checkpoint therapy (ICT) has shown significant treatment efficacy in this subtype. There remains uncertainly regarding the role of cytoreductive nephrectomy (CN) for patients with metastatic RCC (mRCC) with S/R who received ICT.
Objective:
Here, we report the outcomes with ICT for patients with mRCC and S/R dedifferentiation by CN status.
Design, setting, and participants:
A retrospective review was conducted of 157 patients with sarcomatoid, rhabdoid, or sarcomatoid plus rhabdoid dedifferentiation who received an ICT-based regimen at two cancer centers.
Intervention:
CN performed at any time point; nephrectomy with curative intent was excluded.
Outcome measurements and statistical analysis:
ICT treatment duration (TD) and overall survival (OS) from ICT initiation were recorded. To address the immortal time bias, a time-dependent Cox regression model was generated that accounted for confounders identified by a directed acyclic graph as well as a time-dependent nephrectomy variable.
Results and limitations:
A total of 118 patients underwent CN, and of them, 89 underwent upfront CN. The results did not contradict the supposition that CN does not improve ICT TD (hazard ratio [HR] 1.01, 95% confidence interval [CI] 0.67–1.53, p = 0.94) or OS from ICT initiation (HR 0.79, 95% CI 0.47–1.33, p = 0.37). In patients who underwent upfront CN compared with those who did not undergo CN, there was no association with ICT duration or OS (HR 0.61, 95% CI 0.35–1.06, p = 0.08). A detailed clinical summary of 49 patients with mRCC and rhabdoid dedifferentiation is provided.
Conclusions:
In this multi-institutional cohort of mRCC with S/R dedifferentiation treated with ICT, CN was not significantly associated with improved TD or superior OS when accounting for the lead time bias. There appears to be a subset of patients who derive meaningful benefit from CN, so improved tools for stratification prior to CN are needed to optimize outcomes.
Patient summary:
Immunotherapy has improved outcomes for patients with metastatic renal cell carcinoma (mRCC) who have sarcomatoid and/or rhabdoid (S/R) dedifferentiation, which is an aggressive and uncommon feature; yet, the utility of a nephrectomy in this setting is unclear. We found that a nephrectomy did not significantly improve survival or time on immunotherapy for these patients with mRCC and S/R dedifferentiation; yet, there may be a subset of patients who benefit from this surgical approach.
Keywords: Cytoreductive nephrectomy, Sarcomatoid, Rhabdoid, Renal cell carcinoma, CARMENA
1. Introduction
Sarcomatoid and rhabdoid dedifferentiation may occur within the context of most histological subtypes of renal cell carcinoma (RCC), and the dedifferentiation can occur heterogeneously throughout the tumor with an epithelial component and a sarcomatoid or rhabdoid component present [1,2]. Sarcomatoid and rhabdoid dedifferentiation are considered World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grade 4 tumors, in addition to tumors with nuclear pleomorphism present, and these grade 4 tumors are associated with a poor prognosis [2–5]. Prior to the immune checkpoint therapy (ICT) era, sarcomatoid dedifferentiation was associated with inferior survival when analyzed in the context of 406 patients with any WHO/ISUP grade 4 RCC [6]. Alternatively, rhabdoid dedifferentiation had similar cancer-specific survival to other grade 4 RCC without dedifferentiation in a separate series of 264 patients [7].
Sarcomatoid dedifferentiation has poor outcomes with angiogenesis targeted therapy and cytotoxic chemotherapy, but ICT significantly improved outcomes for patients with metastatic RCC (mRCC) and sarcomatoid dedifferentiation [8–10]. In a post hoc analysis of CheckMate 214, patients with sarcomatoid dedifferentiation receiving nivolumab plus ipilimumab had significantly improved overall survival (OS; (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.30–0.70] and progression-free survival (HR 0.54, 95% CI 0.33–0.86) compared with those randomized to sunitinib, and 19% experienced a complete response [10]. Combinations of ICT and angiogenesis targeted therapy, such as nivolumab plus cabozantinib or lenvatinib plus pembrolizumab, also improved outcomes significantly when compared with sunitinib for patients with sarcomatoid dedifferentiation [11,12]. These improvements with ICT are an exciting opportunity for oncologists to reconsider treatment strategies for patients with sarcomatoid dedifferentiation.
Prior to 2018, cytoreductive nephrectomy (CN) was a standard of care treatment option for patients with mRCC. The utility and timing of CN was questioned by results from the CARMENA and SURTIME trials, respectively [13,14]. The CARMENA trial randomized 450 patients with intermediate- or poor-risk mRCC to CN followed by sunitinib versus sunitinib alone [13]. Sunitinib alone was noninferior to the CN arm for OS (HR 0.89, 95% CI 0.71–1.10); yet, these findings were critiqued for issues related to poor adherence to the study protocol and were before the ICT era [15]. In the SURTIME trial, deferred CN produced longer median OS, and more patients were able to receive systemic therapy than upfront CN [14]. The presence of sarcomatoid dedifferentiation may have a significant impact in decision-making for CN given the aggressive course of these infrequent variants [16,17]. Retrospective data from the targeted therapy era suggest that CN can safely be performed and improve outcomes for patients with sarcomatoid dedifferentiation [18,19]. The role of CN for sarcomatoid and/or rhabdoid (S/R) dedifferentiation in the ICT era is unknown.
The objective of this study is to assess whether CN improves clinical outcomes in patients with S/R dedifferentiation phenotypes who receive ICT compared with patients who did not undergo CN. Additionally, we describe a large cohort of patients with mRCC and rhabdoid dedifferentiation who received ICT.
2. Patients and methods
We conducted a retrospective review of patients with mRCC and sarcomatoid, rhabdoid, or sarcomatoid plus rhabdoid dedifferentiation who had primary renal tumor in situ and received an ICT-based regimen at the University of Texas MD Anderson Cancer Center (MDACC) or Memorial Sloan Kettering Cancer Center (MSKCC). The institutional review boards approved this study at both respective institutions. Clinical data were collected by individual chart reviews from each institution’s electronic medical record system. Demographic characteristics, histological subtype, International Metastatic RCC Database Consortium (IMDC) risk score at the initiation of ICT, sites of disease, nephrectomy status, reason for not performing CN, Charlson comorbidity index, prior systemic therapies, ICT, and subsequent systemic therapies were recorded. Patients were managed as per the best practice at MDACC and MSKCC. Clinical endpoints of interest were treatment duration (TD) on ICT and OS. TD was calculated as the time from ICT initiation until discontinuation for any reason, and patients who remained on ICT at the time of analysis were censored using the date of the last follow-up. OS was calculated as the time from ICT initiation until death or the last follow-up, if a patient was still living at the time of data collection. A directed acyclic graph (DAG) was used to identify potential confounders to be adjusted in regression models (Supplementary Fig. 1). For this adjustment, we used outcome regression models as opposed to propensity score matching because outcome regression yields higher power and precision by not discarding data in order to better fit the model. Our analysis is at risk of an immortal time bias because patients who underwent CN are guaranteed to live from ICT initiation until the time of CN, whereas patients who did not have deferred CN may discontinue treatment any time after ICT initiation. In our primary analysis, we accounted for the immortal time bias by generating a time-dependent Cox regression model that included five confounders identified by the DAG (comorbidity index, number of prior therapies, IMDC score, dedifferentiation type, and epithelial histology) as well as a time-dependent nephrectomy variable that tracked whether nephrectomy has occurred during the estimation process. This approach eliminates the immortal time bias while avoiding wasting data by using all follow-up data since ICT initiation [20–22]. We performed secondary analyses excluding patients who underwent deferred CN, which addresses the immortal time bias, and separately by not accounting for the immortal time bias. For these analyses, HRs and 95% CIs were calculated using a multivariable Cox regression model. Median survival times were calculated using the Kaplan-Meier method. Median follow-up was estimated by the reverse Kaplan-Meier method.
3. Results
Of the 159 patients who met our study criteria, two were excluded from analyses because the IMDC score was unavailable. For the cohort analyzed, 91 patients were from MDACC and 66 from MSKCC. Patients initiated ICT between March 2013 and February 2021. Sarcomatoid was the most common type of dedifferentiation (n = 78), followed by 49 patients with rhabdoid dedifferentiation alone and 30 patients with sarcomatoid plus rhabdoid dedifferentiation. The breakdown of histological subtype by dedifferentiation is provided in Table 1. Sarcomatoid dedifferentiation was observed in clear cell, papillary, chromophobe, unclassified, and mucinous tubular and spindle cell carcinoma, whereas rhabdoid dedifferentiation was observed only in clear cell RCC (ccRCC) and unclassified RCC (n = 1). Of the patients, 52% had two or three metastases prior to the initiation of ICT, and the breakdown of IMDC favorable-, intermediate-, and poor-risk disease was 9.6%, 54.1%, and 36.3%, respectively.
Table 1 –
Baseline characteristics and treatment information for all patients and by type of dedifferentiation present
| Met. sRCC (n = 78) | Met. rRCC (n = 49) | Met. S + R RCC (n = 30) | Total (n = 157) | Upfront CN (n = 89) | Deferred CN (n = 29) | No CN (n = 39) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age at diagnosis (yr), median (range) | 57 (35–73) | 60 (36–84) | 56 (37–74) | 58 (35–84) | 57 (37–74) | 57 (35–84) | 57 (36–75) |
| Sex, n (%) | |||||||
| Male | 64 (82.1) | 37 (75.5) | 21 (70) | 122 (77.7) | 66 (74.2) | 25 (86.2) | 31 (79.5) |
| Female | 14 (17.9) | 12 (24.5) | 9 (30) | 35 (22.3) | 23 (25.8) | 4 (13.8) | 8 (20.5) |
| Primary epithelial histology, n (%) | |||||||
| Clear cell | 62 (79.5) | 48 (98.0) | 29 (96.7) | 139 (88.5) | 79 (88.8) | 27 (93.1) | 33 (84.6) |
| Papillary | 1 (1.3) | 0 (0) | 0 (0) | 1 (0.6) | 1 (1.1) | 0 | 0 |
| Chromophobe | 5 (6.4) | 0 (0) | 0 (0) | 5 (3.2) | 4 (4.5) | 0 | 1 (2.6) |
| Unclassified | 9 (11.5) | 1 (2.0) | 1 (3.3) | 11 (7.0) | 4 (4.5) | 2 (6.9) | 5 (12.8) |
| MTSC | 1 (1.3) | 0 (0) | 0 (0) | 1 (0.6) | 1 (1.1) | 0 | 0 |
| No. of metastases at ICT, n (%) | |||||||
| 1 | 11 (14.1) | 9 (18.4) | 6 (20.0) | 26 (16.5) | 13 (14.6) | 7 (24.1) | 6 (15.4) |
| 2–3 | 31 (39.7) | 38 (77.5) | 13 (43.3) | 82 (52.2) | 45 (50.6) | 16 (55.2) | 21 (53.8) |
| ≥4 | 36 (46.2) | 2 (4.1) | 11 (36.7) | 49 (31.2) | 31 (34.8) | 6 (20.7) | 12 (30.8) |
| Systemic therapies before ICT, n (%) | |||||||
| 0 | 47 (60.2) | 34 (69.4) | 21 (70.0) | 102 (65.0) | 45 (50.6) | 25 (86.2) | 32 (82.0) |
| 1 | 23 (29.5) | 12 (24.5) | 7 (23.3) | 42 (26.8) | 32 (36.0) | 4 (13.8) | 6 (15.4) |
| ≥2 | 8 (10.3) | 3 (6.1) | 2 (6.7) | 13 (8.2) | 12 (13.4) | 0 | 1 (2.6) |
| Type of ICT received, n (%) | |||||||
| Nivolumab + ipilimumab | 32 (41.0) | 19 (38.8) | 13 (43.3) | 64 (40.8) | 26 (29.2) | 17 (58.6) | 23 (59.0) |
| ICT + TKI | 19 (24.4) | 15 (30.6) | 9 (30.0) | 43 (27.4) | 28 (31.5) | 7 (24.1) | 9 (23.0) |
| ICT monotherapy | 26 (33.3) | 11 (22.4) | 7 (23.3) | 44 (28.0) | 32 (36.0) | 5 (17.3) | 7 (18.0) |
| ICT + other | 1 (1.3) | 4 (8.2) | 1 (3.3) | 6 (3.8) | 3 (3.3) | 0 | 0 |
| IMDC risk score at ICT initiation, n (%) | |||||||
| Favorable | 8 (10.3) | 2 (4.1) | 5 (16.7) | 15 (9.6) | 13 (14.6) | 1 (3.5) | 1 (2.6) |
| Intermediate | 44 (56.4) | 30 (61.2) | 11 (36.7) | 85 (54.1) | 47 (52.8) | 17 (58.6) | 19 (48.7) |
| Poor | 26 (33.3) | 17 (34.7) | 14 (46.6) | 57 (36.3) | 29 (32.6) | 11 (37.9) | 19 (48.7) |
| Nephrectomy, n (%) | |||||||
| Yes | 55 (70.5) | 35 (71.4) | 28 (93.3) | 118 (75.2) | |||
| No | 23 (29.5) | 14 (28.6) | 2 (6.7) | 39 (24.8) | |||
| Time of nephrectomy, n (%) | |||||||
| Upfront CN | 43 (78.2) | 27 (77.1) | 19 (67.9) | 89 (75.4) | |||
| Deferred CN | 12 (21.8) | 8 (22.9) | 9 (32.1) | 29 (24.6) | |||
| Charlson comorbidity index, n (%) | |||||||
| 6 | 17 (21.8) | 7 (14.3) | 5 (16.7) | 29 (18.5) | 17 (19.1) | 7 (24.1) | 5 (12.8) |
| 7–8 | 43 (55.1) | 29 (59.2) | 16 (53.3) | 88 (56.0) | 53 (59.6) | 15 (51.8) | 20 (51.3) |
| ≥9 | 18 (23.1) | 13 (26.5) | 9 (30.0) | 40 (25.5) | 19 (21.3) | 7 (24.1) | 14 (35.9) |
CN = cytoreductive nephrectomy; ICT = immune checkpoint therapy; IMDC = International Metastatic RCC Database Consortium; Met. = metastatic; MTSC = mucinous tubular and spindle cell carcinoma; RCC = renal cell carcinoma; rRCC = rhabdoid renal cell carcinoma; S + R = sarcomatoid plus rhabdoid; sRCC = sarcomatoid renal cell carcinoma; TKI = tyrosine kinase inhibitor.
Most patients received ICT as the first-line treatment of mRCC, but 35% received at least one treatment prior to ICT. Nivolumab plus ipilimumab was the most frequently used ICT regimen (40.8%), followed by ICT monotherapy (28.0%) and ICT + tyrosine kinase inhibitor combinations (27.4%). The study entry criterion was an in situ primary tumor, and CN was frequently performed in our cohort (75.2%). Of those who underwent CN, the majority had upfront CN (75.4%). The most cited reasons for not performing CN were symptomatic primary site or high-risk location of distant metastatic disease (35.9%) followed by a “wait and watch” approach without a subsequent response to ICT (25.6%). There was no difference in median Charlson comorbidity index scores between upfront CN, deferred CN, and no CN.
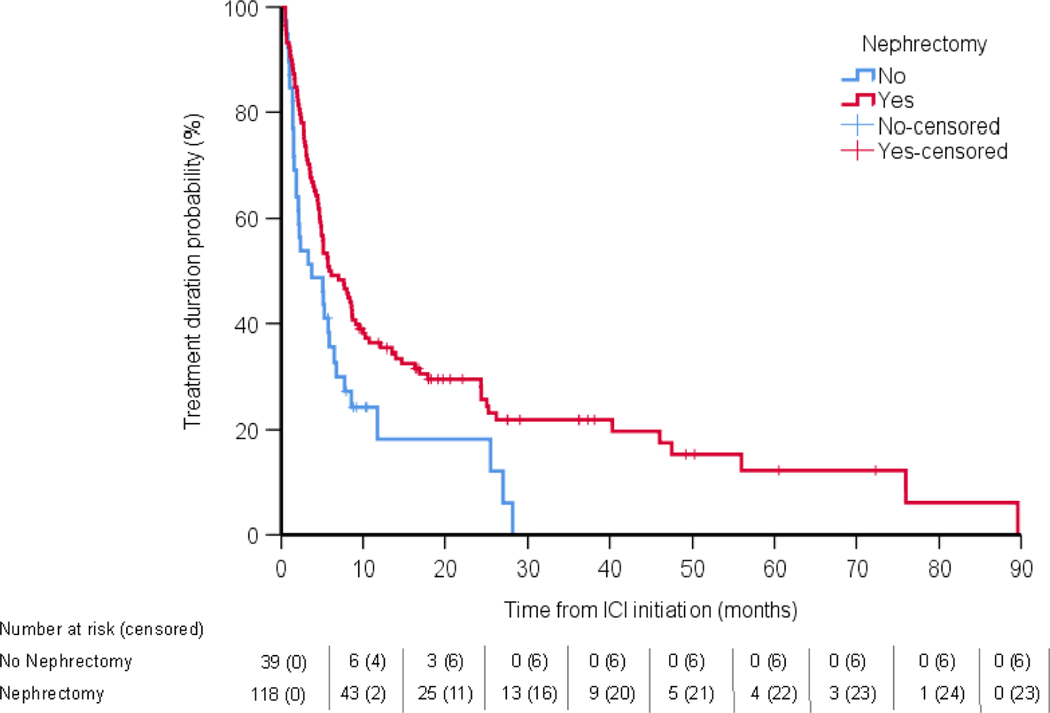
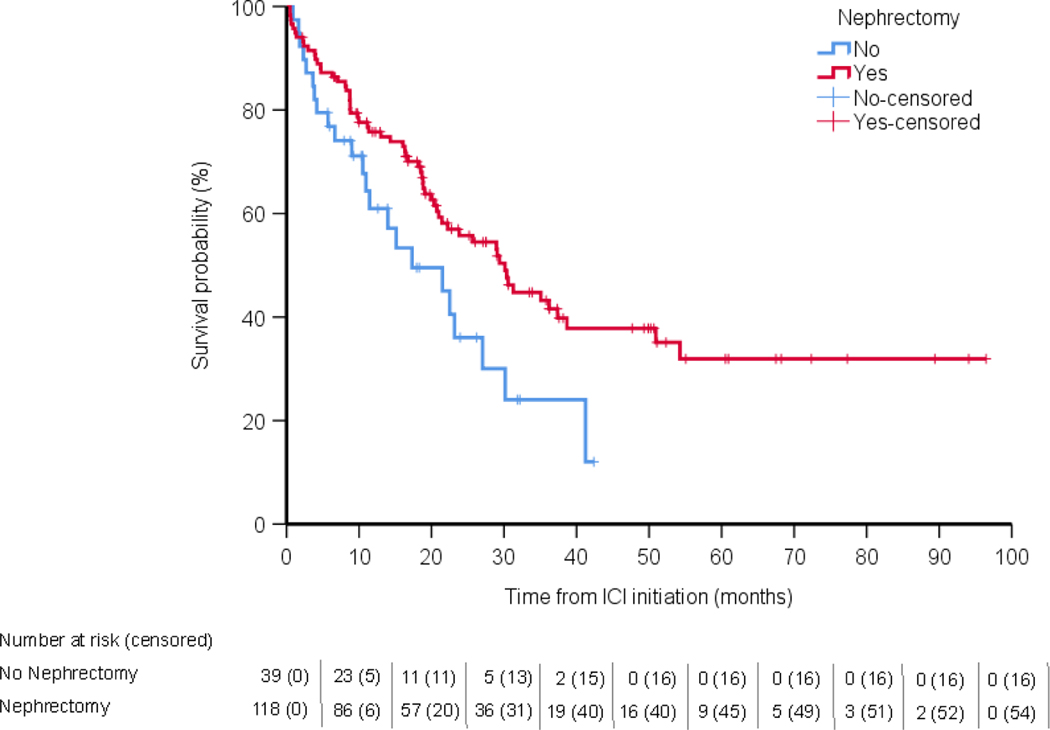
The median follow-up for the cohort was 18.3 mo, and 84 of 157 patients had died at the time of analysis. After controlling for the immortal time bias, receipt of CN was not significantly associated with TD on ICT (5.9 vs 3.7 mo, HR 0.98, 95% CI 0.65–1.47, p = 0.94; Fig. 1) or OS from ICT initiation (30.1 vs 17.3 mo, HR 0.79, 95% CI 0.47–1.33, p = 0.37; Fig. 2). In a secondary analytic approach to account for the immortal time bias, we evaluated the association between upfront CN and treatment outcomes with ICT, excluding the 29 patients who underwent deferred CN and were at a risk of an immortal time bias. Upfront CN was not associated with ICT TD compared with no CN (HR 0.71, 95% CI 0.45–1.14, p = 0.16; Supplementary Fig. 2), nor was it associated with OS (HR 0.61, 95% CI 0.35–1.06, p = 0.08; Supplementary Fig. 3). Notably, when we did not account for the immortal time bias, CN was associated with a significant improvement in TD (HR 0.64, 95% CI 0.42–0.99) and OS (HR 0.55, 95% CI 0.33–0.91).
Fig. 1 –
Analysis of the association between treatment duration and ICT. ICI = immune checkpoint inhibitor; ICT = immune checkpoint therapy.
Fig. 2 –
Analysis of the association between survival and ICT. ICI = immune checkpoint inhibitor; ICT = immune checkpoint therapy.
Deferred CN was utilized for 29 patients with S/R dedifferentiation with a similar distribution across dedifferentiation types. The dedifferentiation status was known prior to nephrectomy in nine patients (31%). Most of these patients had greater than one organ site of metastasis upon initiation of ICT (one organ site, seven patients; two to three organ sites, 16 patients; and four or more organ sites, six patients). The median time from the initiation of ICT to CN was 138 d (range 52–656 d). Not accounting for the immortal time bias, the median TD from ICT initiation was 10.7 mo and the median OS was 30.6 mo for patients who underwent deferred CN.
This cohort is noteworthy for the number of patients with mRCC and rhabdoid dedifferentiation (n = 49) or sarcomatoid plus rhabdoid dedifferentiation (n = 30). In contrast to sarcomatoid dedifferentiation, rhabdoid dedifferentiation occurred almost exclusively in patients with clear cell histology (98%), with one patient having unclassified RCC (2%). Patients with rhabdoid dedifferentiation were more likely to have three or fewer metastases at the time of ICT initiation than sarcomatoid dedifferentiation (95.9% vs 53.8%). Patients with rhabdoid dedifferentiation received a similar profile of ICT compared with those with sarcomatoid dedifferentiation (Table 1). A multivariable analysis did not contradict the supposition that mRCC with rhabdoid dedifferentiation as compared with sarcomatoid dedifferentiation had similar TD with ICT (5.1 vs 5.3 mo, HR 0.82, 95% CI 0.54–1.26, p = 0.40) and OS (30.4 vs 22.5 mo, HR 0.79, 95% CI 0.47–1.34, p = 0.38).
4. Discussion
The role for CN in the ICT era is unclear, and given the benefit patients with sarcomatoid dedifferentiation experience with ICT, we sought to evaluate clinical outcomes with ICT by CN status. In this bi-institutional cohort of mRCC with S/R dedifferentiation, CN was not significantly associated with improved ICT TD or superior OS when accounting for the immortal time bias. The group of patients at risk of an immortal time bias included those who underwent deferred CN because they were immortal from ICT initiation until the time of deferred CN, and we addressed this pernicious bias using two approaches: the primary time-dependent Cox regression model and a secondary analysis excluding the at-risk patients who underwent deferred CN. The immortal time bias would have significantly impacted our results as evidenced by the fact that CN was significantly associated with treatment outcomes when we did not control for this bias. While our analysis did not demonstrate a significant improvement with CN, it also did not show inferior survival for patients who underwent CN, which is a theoretical concern for upfront CN because it delays the initiation of ICT. The survival curves demonstrate a trend toward improved survival with CN, and individual patients had prolonged responses to systemic ICT after CN. Our findings suggest that there is a role for CN in select patients with S/R dedifferentiation in the ICT era. Of note, we focused only on testing specific hypotheses in this study, guided by DAGs, and accordingly intentionally omitted showing the HRs and CIs from the confounding variables to prevent the “table 2 fallacy,” whereby presenting adjusted effect estimates from several confounders derived from multivariable models invites misinterpretation [23].
The overarching rationale for CN in patients with asymptomatic, synchronous mRCC is to reduce tumor burden, particularly in those with low-volume metastases, and to decrease the production of clones resistant to systemic therapy. After the publication of the CARMENA and SURTIME trials, the European Association of Urology recommended against upfront CN in patients with MSKCC intermediate- or poor-risk mRCC requiring angiogenesis targeted therapy and recommended considering deferred CN in intermediate-risk patients who derive long-term benefit [24]. Our cohort deviates from these contemporary recommendations because 75% of our patients underwent upfront CN. Multiple retrospective studies from the targeted therapy era suggested that patients with sarcomatoid dedifferentiation benefit from CN [4,18,19]. In a study from the IMDC group in 2014, patients with mRCC and sarcomatoid dedifferentiation who underwent CN had longer OS than those who did not (10.2 vs 5.5 mo) [19]. Similarly, in a population-based study of 472 patients with mRCC and sarcomatoid dedifferentiation, those who underwent CN had longer 1-, 3-, and 5-yr disease-specific survival than those who did not [4]. To our knowledge, this report constitutes the largest experience of CN for patients with S/R dedifferentiation who were treated with ICT. It is noteworthy that we did not observe a significant benefit with CN, and the trend favoring CN strengthened when patients who underwent deferred CN were excluded from the analysis. It is possible that a potential benefit of CN across cohorts becomes diluted as ICT becomes the standard of care backbone for first-line treatment of mRCC.
Broadly speaking, improved patient selection for CN may strengthen the benefit seen as systemic therapies improve, and this may be accomplished by a thorough evaluation of clinical covariates and an assessment of the response to upfront systemic therapy for those considering deferred CN. The MDACC group previously identified preoperative variables associated with an increased risk of death in patients with any mRCC who underwent CN [25]. These factors included lactate dehydrogenase, albumin, symptomatic metastatic disease, metastatic site, and clinical T stage. If patients had four or more of these factors, they were unlikely to benefit from CN. In a population-based study from the same group, sarcomatoid dedifferentiation was also associated with an increased risk of death in any mRCC patient who underwent CN [25]. However, sarcomatoid dedifferentiation was not included in subsequent scores due to the difficulty of identifying heterogeneous dedifferentiation on preoperative biopsies, which we also observed [17]. Furthermore, the impact that the degree of sarcomatoid dedifferentiation has on survival with ICT or CN remains unknown. Most recently, the MDACC group showed that patients with metastatic ccRCC who undergo deferred CN have improved OS if they experience at least 10% radiographic shrinkage with systemic therapy [26]. Patient selection for this dedifferentiation cohort is challenging, but future studies may consider evaluating how these patient variables and radiographic response influence outcomes specifically in S/R dedifferentiation, particularly against those who did not undergo CN. Furthermore, there is a need to understand how distinct patterns of genomic, clonal evolution occur in patients with dedifferentiation because prospective identification of clonal patterns may biologically refine patient selection for CN.
Limited data are available about outcomes for mRCC with rhabdoid dedifferentiation, and our cohort of 49 patients with rhabdoid dedifferentiation is among the largest to date. Rhabdoid dedifferentiation has a unique histopathological appearance compared with sarcomatoid dedifferentiation. Both are considered WHO/ISUP grade 4 disease; yet, among grade 4 RCC patients, only sarcomatoid dedifferentiation is associated with inferior survival when considering all stages of disease [6]. Two studies have evaluated the biology underlying rhabdoid dedifferentiation. In a study from the MDACC group, ccRCC with rhabdoid dedifferentiation was transcriptomically distinct and had unique somatic DNA alterations compared with high-grade ccRCC without dedifferentiation [27]. A subsequent study from the Dana-Farber Cancer Institute performed multiomic analyses of S/R dedifferentiation, and it found that the transcriptomic and immune microenvironment changes were consistent across sarcomatoid, rhabdoid, and sarcomatoid plus rhabdoid dedifferentiation [28]. The same study also reported improved outcomes with ICT for the combined S/R cohorts, but it did not report the outcomes by individual types of dedifferentiation [28]. In our study, we found that rhabdoid dedifferentiation occurred almost exclusively in the setting of ccRCC, whereas sarcomatoid dedifferentiation occurred in variant histologies. Other baseline characteristics were similar between rhabdoid and sarcomatoid dedifferentiation (Table 1). Prior to this study, it was unknown how patients with mRCC and only rhabdoid dedifferentiation responded to ICT. We found that TD on ICT was similar between rhabdoid and sarcomatoid dedifferentiation. OS from ICT initiation did not differ significantly by type of dedifferentiation; yet OS was numerically longer in the rhabdoid cohort. Our study is limited by its retrospective design and the unique practice patterns of two high-volume cancer centers. There is a risk for selection bias when retrospectively evaluating CN, and we attempted to account for this bias by reporting Charlson comorbidity index and IMDC risk score across groups. Diagnosis of sarcomatoid and rhabdoid dedifferentiation was performed at each individual site by expert Genitourinary pathologists. There is a potential bias in the degree of dedifferentiation present between the CN and no CN cohorts because patients who did not undergo CN are more likely to have a higher percentage of tumor with dedifferentiation present than patients who underwent CN and have a full nephrectomy specimen available for evaluation. Our analysis is limited by the assumption that the dedifferentiation type does not change the effect of CN on ICT outcomes, but we accounted for the dedifferentiation type as a potential confounder in our multivariable model. Our cohort included patients who received any ICT given the improved responses with dedifferentiation and ICT combinations or monotherapy. It is possible that the magnitude of benefit from CN may differ between ICT combinations, and our analysis was not designed to answer that question.
5. Conclusions
In this multi-institutional cohort of mRCC with S/R dedifferentiation treated with ICT, CN was not significantly associated with improved ICT TD or superior OS when accounting for an immortal time bias. Our results highlight the importance of accounting for the immortal time bias in studies of CN. Given the trend toward benefit observed with CN, there are likely a subset of patients who derive meaningful benefit from CN, so patient selection remains key and improved tools for stratification prior to CN are needed to optimize outcomes.
Supplementary Material
Financial disclosures:
Pavlos Msaouel certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Andrew W. Hahn reports advisory board consultation for Janssen and travel support from Dava Oncology. Ritesh R. Kotecha reports advisory board consultation for Eisai, and receiving institutional research funding from Pfizer, Novartis, Takeda, and Allogene Therapeutics. Chung-Han Lee reports advisory board consultation to Amgen, Aveo, Bristol-Myers Squibb (BMS), Exelixis, Eisai, Merck, Pfizer, EMD Serono, and Cardinal Health; institutional research funds from AstraZeneca, BMS, Calithera, Eisai, Eli Lilly, Exelixis, Merck, and Pfizer; and honoraria from AiCME, IDEOlogy Health, Intellisphere, Medscape, and Research to Practice. Amado J. Zurita reports advisory board consultation for Amedco, CancerNet LLC, AstraZeneca, Incyte, Bayer, Pfizer, and Biocept; institutional research funding from Infinity Pharma, Pfizer, ABX, Merck, and Curium; and honoraria from Amedco, Janssen-Cliag, AstraZeneca, Mckesson Specialty Health, CancerNet LLC, and Pfizer. Amishi Y. Shah reports advisory board consultation for BMS, Exelixis, and Pfizer; institutional research funds from BMS, Eisai, EMD Serono, and 4D Pharma; and travel support from Dava Oncology. Padmanee Sharma reports advisory board consultation for Achelois, Affini-T, Apricity, Asher Bio, BioAtla LLC, Candel Therapeutics, Catalio, Carisma, Codiak Biosciences, C-Reveal Therapeutics, Dragonfly Therapeutics, Earli Inc, Enable Medicine, Glympse, Henlius/Hengenix, Hummingbird, ImaginAb, Infinity Pharma, InterVenn Biosciences, LAVA Therapeutics, Lytix Biopharma, Marker Therapeutics, Oncolytics, PBM Capital, Phenomic AI, Polaris Pharma, Trained Therapeutix Discovery, Two Bear Capital, and Xilis, Inc; and private investment in Adaptive Biotechnologies, BioNTech, JSL Health, Sporos, and Time Bioventures. Robert J. Motzer reports institutional research funding from Pfizer, Eisai, Aveo Pharmaceuticals, Exelixis, Merck, Genentech, Roche, and BMS; and personal fees from Pfizer, Eisai, Aveo Pharmaceuticals, Exelixis, Merck, Genentech, Incyte, Roche, AstraZeneca, and EMD Serome. Nizar M. Tannir reports advisory consultation for BMS, Oncorena, Merck, Eli Lilly, Eisai Medical Research, and Nektar Therapeutics; institutional research funding from BMS, Nektar Therapeutics, Arrowhead Pharmaceuticals, Novartis, Calithera Biosciences, and Exelixis; and honoraria from Eisai Medical Research, BMS, Intellisphere, Oncorena, Merck, Neoleukin, Exelixis, and AstraZeneca. Martin H. Voss reports advisory board consultation for Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Merck, Natera, Onquality Pharmaceuticals, Novartis, and Pfizer; institutional research funding from BMS, Pfizer, and Genentech/Roche; honoraria from Novartis and BMS; and travel support from AstraZeneca, Eisai, Novartis, and Takeda. A. Ari Hakimi reports advisory board consultation for Merck. Pavlos Msaouel has received honoraria for service on a scientific advisory board for Mirati Therapeutics, Bristol Myers Squibb, and Exelixis; consulting for Axiom Healthcare Strategies; nonbranded educational programs supported by Exelixis and Pfizer; and research funding for clinical trials from Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center.
Funding/Support and role of the sponsor:
This work was supported in part by MD Anderson’s Prometheus informatics system. This study was supported in part by the Cancer Center Support Grant to MD Anderson Cancer Center (grant P30 CA016672) from the National Cancer Institute. Andrew W. Hahn is supported by the Rob Heyvaert and Paul Heynen Prostate Cancer Foundation Young Investigator Award, an Early Investigator Research Award by the Department of Defense, and philanthropic donations from Michael and Patricia Berns. Ritesh R. Kotecha is supported (in part) by the Academy of Kidney Cancer Investigators of the CDMRP/DOD (KC200127). Pavlos Msaouel was supported by the MD Anderson Khalifa Scholar Award, the Andrew Sabin Family Foundation Fellowship, a Translational Research Partnership Award (KC200096P1) by the United States Department of Defense, an Advanced Discovery Award by the Kidney Cancer Association, a Translational Research Award by the V Foundation, the MD Anderson Physician-Scientist Award, and philanthropic donations by the family of Mike and Mary Allen.
Footnotes
Paul V. Viscuse, Alberto C. Pieretti, Andrew J. Wiele, and Jianjun Gao report no conflicts of interest to disclose.
In patients with metastatic renal cell carcinoma with sarcomatoid and/or rhabdoid dedifferentiation treated with immune checkpoint therapy, cytoreductive nephrectomy (CN) was not significantly associated with improved treatment duration or overall survival. Yet, there appears to be a subset of patients who derive benefit from CN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Blum KA, Gupta S, Tickoo SK, et al. Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol 2020;17:659–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gökden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol 2000;24:1329–38. [DOI] [PubMed] [Google Scholar]
- [3].Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol 2002;167:65–70. [PubMed] [Google Scholar]
- [4].Alevizakos M, Gaitanidis A, Nasioudis D, Msaouel P, Appleman LJ. Sarcomatoid renal cell carcinoma: population-based study of 879 patients. Clin Genitourin Cancer 2019;17:e447–53. [DOI] [PubMed] [Google Scholar]
- [5].Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013;37:1490–504. [DOI] [PubMed] [Google Scholar]
- [6].Zhang BY, Cheville JC, Thompson RH, et al. Impact of rhabdoid differentiation on prognosis for patients with grade 4 renal cell carcinoma. Eur Urol 2015;68:5–7. [DOI] [PubMed] [Google Scholar]
- [7].Kara O, Maurice MJ, Zargar H, et al. Prognostic implications of sarcomatoid and rhabdoid differentiation in patients with grade 4 renal cell carcinoma. Int Urol Nephrol 2016;48:1253–60. [DOI] [PubMed] [Google Scholar]
- [8].Kyriakopoulos CE, Chittoria N, Choueiri TK, et al. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer 2015;13:e79–85. [DOI] [PubMed] [Google Scholar]
- [9].Keskin SK, Msaouel P, Hess KR, et al. Outcomes of patients with renal cell carcinoma and sarcomatoid dedifferentiation treated with nephrectomy and systemic therapies: comparison between the cytokine and targeted therapy eras. J Urol 2017;198:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tannir NM, Signoretti S, Choueiri TK, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res 2021;27:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol 2021;39:308.33356420 [Google Scholar]
- [12].Choueiri TK, Eto M, Kopyltsov E, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Ann Oncol 2021;32(suppl_5):S678–724. [Google Scholar]
- [13].Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med 2018;379:417–27. [DOI] [PubMed] [Google Scholar]
- [14].Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol 2019;5:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Motzer RJ, Russo P. Cytoreductive nephrectomy—patient selection is key. N Engl J Med 2018;379:481–2. [DOI] [PubMed] [Google Scholar]
- [16].Shuch B, Said J, La Rochelle JC, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology—is up-front resection indicated and, if not, is it avoidable? J Urol 2009;182:2164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abel EJ, Carrasco A, Culp SH, et al. Limitations of preoperative biopsy in patients with metastatic renal cell carcinoma: comparison to surgical pathology in 405 cases. BJU Int 2012;110:1742–6. [DOI] [PubMed] [Google Scholar]
- [18].Silagy AW, Mano R, Blum KA, et al. The role of cytoreductive nephrectomy for sarcomatoid renal cell carcinoma: a 29-year institutional experience. Urology 2020;136:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704–10. [DOI] [PubMed] [Google Scholar]
- [20].Cho IS, Chae YR, Kim JH, et al. Statistical methods for elimination of guarantee-time bias in cohort studies: a simulation study. BMC Med Res Methodol 2017;17:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31:2963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol 2008;19:841–3. [DOI] [PubMed] [Google Scholar]
- [23].Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bex A, Albiges L, Ljungberg B, et al. Updated European Association of Urology guidelines for cytoreductive nephrectomy in patients with synchronous metastatic clear-cell renal cell carcinoma. Eur Urol 2018;74:805–9. [DOI] [PubMed] [Google Scholar]
- [25].Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer 2010;116:3378–88. [DOI] [PubMed] [Google Scholar]
- [26].Pieretti AC, Shapiro DD, Westerman ME, et al. Tumor diameter response in patients with metastatic clear cell renal cell carcinoma is associated with overall survival. Urol Oncol 2021;39:837.e9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh RR, Murugan P, Patel LR, et al. Intratumoral morphologic and molecular heterogeneity of rhabdoid renal cell carcinoma: challenges for personalized therapy. Mod Pathol 2015;28:1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bakouny Z, Braun DA, Shukla SA, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun 2021;12:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.