Abstract
INTRODUCTION:
Cognitive resilience (CR) can be defined as the continuum of better- through worse-than-expected cognition, given the degree of neuropathology. The relation of healthy diet patterns to CR remains to be elucidated.
METHODS:
Using longitudinal cognitive data and postmortem neuropathology from 578 deceased older adults, we examined associations between the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) diet at baseline and two standardized CR measures reflecting higher cognitive levels over time (CR), and slower decline (CRSlope), than expected given neuropathology.
RESULTS:
Compared with individuals in the lowest tertile of MIND score, those in the top tertile had higher CR (mean difference [MD]=0.34;95%CI=0.14;0.55) and CRSlope (MD=0.27;95%CI=0.05;0.48), after multivariable adjustment. Overall MIND score was more strongly related to CR than the individual food components.
DISCUSSION:
The MIND diet is associated with both higher cognition and slower rates of cognitive decline, after controlling for neuropathology, indicating the MIND diet may be important to cognitive resilience.
Keywords: Alzheimer’s dementia, cognitive resilience, longitudinal study, MIND diet, neuropathology, nutrition
1. BACKGROUND
Cognitive resilience (CR) can refer to the ability to maintain better (or worse) cognition than expected given a certain level of Alzheimer’s disease (AD) and other neuropathologies.1,2 Clinical-pathologic studies indicate that approximately one-third of older adults without clinical dementia meet the neuropathologic criteria for AD,3–5 suggesting that cognitive resilience to pathology may be common in older people. Since there is currently no treatment for dementia pathology, observations of the disconnect between cognition and the degree of pathology suggest the value of identifying modifiable risk factors for CR as a path toward improving cognitive health without treating the ubiquitous neuropathologies in aging.6–8
There is a large body of epidemiological evidence suggesting that healthy dietary patterns, such as the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, are associated with slower cognitive decline and reduced risk of dementia,9,10 although the underlying mechanisms remain to be elucidated. One possible pathway is that diet may influence neural resources and enhance CR.11,12 Healthier diets have been associated with the formation of neurons in the adult hippocampus, a brain structure that is particularly vulnerable in the early stages of AD.13 Thus, by promoting and preserving CR over time, diet may partly explain the individual differences commonly observed in late-life cognitive trajectories.14–16
In our own work, we recently reported in participants of the Rush Memory and Aging Project, that greater MIND diet scores were associated with better cognitive function proximate to death and slower cognitive decline before death, independently of common neuropathologies, initial evidence that diet is a promising candidate to enhance CR.17 Here, we extend this work by examining not only the MIND diet but also its dietary components, and by considering two complementary measures of cognitive resilience which separately examine: (i) consistently higher levels of cognition as well as (ii) a slower rate of cognitive decline over time.18 We leveraged dietary assessments, longitudinal cognitive assessments, and postmortem neuropathology from older, deceased participants with autopsies in the Memory and Aging Project.
2. METHODS
2.1. Study participants
The Rush Memory and Aging Project (MAP) is an ongoing longitudinal clinical-pathologic study of aging and dementia, which started in 1997 and enrolls women and men from more than 40 retirement communities and housing facilities in the metropolitan Chicago area.19 Eligibility requires the absence of known dementia and agreement to annual clinical evaluations and brain donation at death. To date, the follow-up rate exceeds 90%. All participants signed an informed consent and an Anatomical Gift Act for organ donation and the study protocol was approved by an Institutional Review Board of Rush University Medical Center.
2.2. Dietary assessment
In MAP, collection of information on dietary intake began in February 2004, and has continued annually thereafter, through a 144-item semiquantitative food frequency questionnaire (FFQ), modified from the Willett FFQ for use in older individuals; validity and reliability of this FFQ has been established.20 For each food item, participants were asked to report the usual frequency of intake during the past year. Nutrient levels and total energy were based either on natural portion sizes (e.g., one apple) or according to age- and sex-specific portion sizes from national dietary surveys.20 Food frequency questionnaires with more than 55 items missing or with calories/day out of sex-specific ranges (women: [500–3,800]; men: [700–4,000]) were excluded.
2.3. MIND diet score
In this study, we focused on the first FFQ administered in assessing the MIND diet, which combines the Mediterranean diet and Dietary Approaches to Stop Hypertension diet, and targets foods associated with cognitive function in later life.21 The MIND diet score is based on greater intake of 10 healthy components (green leafy vegetables, other vegetables, berries, fish, poultry, beans, whole grains, nuts, olive oil, and wine) and less intake of 5 unhealthy components (red and processed meat, pastries and sweets, fast and fried foods, full-fat cheese, and butter/margarine). For each component, values of 0, 0.5, or 1 point are assigned based on a predefined number of servings,21 with higher scores representing healthier levels of intake; exceptions were olive oil consumption, which was scored 1 if identified by the participant as the primary oil usually used at home and 0 otherwise, and wine, where a value of 1 was assigned to those consuming one glass of wine per day, 0.5 to those consuming wine once per month to 6 times per week, and 0 to those who reported no intake or more than once per day. Thus, the possible MIND score ranged from 0 to 15 (with 15 as highest alignment to all components).
2.4. Covariates
All covariates used here were defined according to reports at the annual evaluation concurrent with the first dietary assessment, except sex, smoking (never smoked, former smoker, or current smoker) and years of formal education, which were reported at enrollment. Depressive symptoms were assessed using a modified, 10-item version of the Center for Epidemiologic Studies Depression (CES-D) scale, with one point assigned for each reported depressive symptom.22 A composite measure of the total number of medical comorbidities was determined based on 7 self-reported medical conditions: diabetes, hypertension, cancer, hypothyroidism, stroke, heart disease, and head trauma; with one point assigned for each reported condition. Physical activity, expressed in hours per week, was self-reported using 5 items from a modified version of the 1985 National Health Interview Survey (walking, gardening, bicycling, swimming, and general exercise). The frequency of participation in cognitively stimulating activities was calculated based on reported time spent in 7 activities, such as reading, writing letters, or playing games like chess23; possible scores range from 1 to 5, with higher scores indicating more cognitive activities. All these covariates have been demonstrated to be important potential confounding factors in the study of dietary habits and cognitive health in older adults.17,21,24,25
2.5. Cognitive resilience outcomes
We examined two continuous measures of CR recently developed by our group and published in detail elsewhere.18 These two measures are calculated using longitudinal data on global cognition, and 9 measures of postmortem neuropathology.18 Briefly, we focused on a composite score of global cognition based on 17 psychometric tests administered annually until death.19 Raw test scores were converted into z-scores, using the baseline mean and standard deviation (SD); at each assessment, the z-scores were averaged together, with higher scores indicating better function. Global cognition was assessed at annual cognitive evaluations.
Approximately 70% of participants received their first dietary assessment after their first cognitive evaluation at cohort enrollment (mean of 1.9 [SD=2] years from enrollment to first FFQ). However, dietary patterns remained largely stable over time in our study sample; for example, the mean MIND diet score was 7.6 [SD=1.6] and 7.4 [SD=1.7] points on the first and second FFQ assessments, respectively. Thus, in estimating CR, we chose to use all available repeated cognitive measures, from enrollment through death.
To determine CR, we considered nine neuropathologies systematically examined at autopsy: global AD pathology, hippocampal sclerosis, neocortical Lewy bodies, TAR DNA-binding protein 43 (TDP-43), chronic macroscopic and microinfarcts, arteriolosclerosis, atherosclerosis, and cerebral amyloid angiopathy.26,27
We used two complementary approaches to estimate cognitive resilience. In one measure of CR, we considered longitudinal measures of global cognition, adjusting for neuropathologies, where CR was defined as the average of the differences between estimated person-specific and marginal (expected) levels of cognition at each follow-up time. Our second CR measure focused on slopes of cognitive decline rather than levels of cognition, such that CRSlope was defined as the estimated residual slope of cognitive change, given a specific profile of neuropathologies. These two versions (see Wagner and colleagues18 for more details) capture different dimensions of CR; in particular, CR is strongly related with baseline cognitive level, and thus may reflect the accumulation of cognitive resources through the participant’s life, whereas CRSlope focuses on residual cognitive change during follow-up, likely reflecting more immediate resilience to neuropathology.
2.6. Eligible participants for analysis
Of the 1,143 decedents from MAP who had completed the baseline clinical evaluation at the time of these analyses, we first identified the 964 individuals with a complete brain autopsy (Supplementary Figure A). We then excluded 59 participants who did not have data on all nine neuropathologies of interest, 45 who died before the start of the dietary sub-study, 196 with FFQ data but at least half of items missing or calories per day out of range, 18 with FFQ data processing still underway, and 57 participants with a clinical diagnosis of dementia as of the first dietary assessment. Lastly, we excluded 11 participants without at least two complete cognitive evaluations from enrollment through death, resulting in a study sample of 578 participants.
To evaluate whether the selected MAP decedents adequately represented all MAP decedents, we compared key characteristics between our analytic sample (n=578) to the full MAP sample (n=1,143), and we found that they were generally similar: for example, 28% were male in the analytic sample and 27% in all decedents; mean education was 15 years in the analytic sample and in all decedents, and age at study entry in the analytic sample (mean=82 years) was similar to that in the overall group (mean=81 years). Thus, our analytic sample well represents overall MAP decedents.
2.7. Statistical analyses
In the primary analyses, for each CR measure, we applied multivariable linear regression models to test the hypothesis that a greater MIND diet score was associated with higher CR. The demographic-adjusted models included terms for the MIND diet score and sex, education (continuous, years), age (continuous, years), and total caloric intake (continuous, kcal/day) at the first dietary assessment. The fully-adjusted models included additional terms for smoking status (never vs. former or current), number of depressive symptoms (continuous), number of medical conditions (continuous), physical activity (continuous, hours/week), and frequency of participation in cognitively stimulating activities (continuous) at the first dietary assessment. We first considered the MIND diet score in tertile categories, and then as a continuous variable using the original score.
We also examined associations between each of the MIND dietary components and the two CR outcomes to evaluate whether certain components may drive any observed associations between the MIND diet pattern and CR. For green leafy vegetables, poultry, beans, and olive oil, we created categories identically to the MIND scoring system, as described above; for the remaining components, we combined two of the standard MIND categories since sample sizes within categories were modest.21,24 For other vegetables, there were few participants in the two lowest standard categories and we considered tertiles of the distribution (see Supplementary Table A for each category used for each component). When examining the MIND diet components, to control for overall diet quality and the possible relations of components, in each model we added a covariate for the MIND diet score re-calculated after excluding the one component of interest in that model.
Finally, in sensitivity analyses, we examined whether defining the two CR measures based on cognitive data collected starting with the first dietary assessment instead of cohort enrollment altered findings; these analyses also excluded 25 participants who did not have at least 2 cognitive assessments as of the first FFQ. Additionally, we assessed whether mild cognitive impairment at FFQ may impact our findings by conducting analyses excluding the 163 participants diagnosed with mild cognitive impairment (MCI) at the time of FFQ. Lastly, since we were concerned that older persons with diabetes or vascular diseases at the time of FFQ assessment may have changed their diet to treat these diseases we conducted three additional sensitivity analyses excluding the 81, 77, and 66 participants with a history of type 2 diabetes, stroke, and heart disease at analytical baseline, respectively.
All statistical analyses were conducted using R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). We used the lcmm function of lcmm R package version 1.7.8. for mixed models28 and lm function of stats R package version 4.0.3. for linear regression models.
3. RESULTS
3.1. Characteristics of study participants
The analytic sample included 578 MAP participants (Table 1), predominantly women (72%), non-Hispanic white (98%), with a mean of 14.9 (SD=2.8) years of education. Mean age was 84.1 (SD=5.8) years at the first dietary assessment and 91.4 (SD=6.1) at death. The average MIND diet score was 7.6 (SD=1.6). Follow-up spanned an average of 9 (SD=4; range=[1–23]) years from cohort enrollment to death, with a mean of 8 (SD=4; range=[2–22]) annual cognitive assessments.
Table 1.
Characteristics of participants at the first dietary assessment, according to tertiles of the MIND diet score (n=578).
| Characteristic | All | MIND score | ||
|---|---|---|---|---|
|
| ||||
| Tertile 1 [3.5–7] (n=239) | Tertile 2 [7–8.5] (n=197) | Tertile 3 [8.5–12] (n=142) | ||
|
| ||||
| Female, No. (%) | 417 (72) | 164 (69) | 145 (74) | 108 (76) |
| Age at diet assessment, mean (SD), years | 84.1 (5.8) | 84.1 (6.3) | 84.5 (5.5) | 83.5 (5.2) |
| Age at death, mean (SD), years | 91.4 (6.1) | 90.8 (6.6) | 92.0 (6.0) | 91.5 (5.4) |
| Education, mean (SD), years | 14.9 (2.8) | 14.4 (3.2) | 15.1 (2.7) | 15.4 (2.3) |
| Non-Hispanic white, No. (%) | 568 (98) | 234 (98) | 194 (98) | 140 (99) |
| Smoking status, No. (%) | ||||
| Never | 352 (61) | 147 (62) | 125 (63) | 80 (56) |
| Former | 210 (36) | 80 (33) | 70 (36) | 60 (42) |
| Current | 16 (3) | 12 (5) | 2 (1) | 2 (1) |
| Depressive symptoms, median (IQR) | 1 (0;2) | 1 (0;2) | 1 (0;2) | 0 (0;1) |
| Co-morbidities, median (IQR) | 2 (1;2) | 2 (1;3) | 2 (1;2) | 2 (1;3) |
| Co-morbidities, No. (%) | ||||
| Diabetes | 81 (14) | 42 (18) | 21 (11) | 18 (13) |
| Hypertension | 348 (60) | 149 (62) | 113 (57) | 86 (61) |
| Cancer | 226 (39) | 100 (42) | 70 (36) | 56 (39) |
| Hypothyroidism | 145 (25) | 51 (21) | 54 (27) | 40 (28) |
| Stroke | 77 (13) | 42 (18) | 16 (8) | 19 (13) |
| Heart disease | 66 (11) | 30 (13) | 13 (7) | 23 (16) |
| Head injury with loss of consciousness | 31 (5) | 12 (5) | 9 (5) | 10 (7) |
| Physical activity, mean (SD), hours/week | 2.9 (3.1) | 2.3 (2.5) | 3.0 (3.0) | 3.8 (3.7) |
| Cognitive activity, mean (SD), score | 3.2 (0.7) | 3.1 (0.7) | 3.3 (0.7) | 3.4 (0.6) |
| MIND diet, mean (SD), score | 7.6 (1.6) | 6.1 (0.8) | 8.0 (0.4) | 9.8 (0.8) |
| MIND diet score components | ||||
| Servings/week | ||||
| Green leafy vegetables | 4.4 (3.1) | 3.1 (2.7) | 4.5 (2.6) | 6.5 (3.0) |
| Other vegetables | 16.6 (8.1) | 13.6 (7.4) | 17.6 (7.5) | 20.3 (8.1) |
| Berries | 0.6 (0.6) | 0.4 (0.4) | 0.7 (0.6) | 0.9 (0.7) |
| Fish (not fried) | 1.8 (1.3) | 1.4 (1.2) | 1.9 (1.3) | 2.4 (1.3) |
| Poultry (not fried) | 1.6 (1.5) | 1.0 (1.1) | 1.8 (1.5) | 2.2 (1.5) |
| Beans | 1.4 (1.5) | 0.8 (1.2) | 1.5 (1.5) | 2.1 (1.8) |
| Whole grains | 5.4 (5.1) | 4.2 (3.8) | 5.2 (4.9) | 7.8 (6.4) |
| Nuts | 1.0 (1.3) | 0.7 (0.9) | 1.0 (1.3) | 1.4 (1.5) |
| Red meat and products | 3.7 (2.4) | 4.2 (2.6) | 3.7 (2.3) | 3.1 (2.1) |
| Pastries and sweets | 12.0 (8.6) | 13.6 (9.0) | 11.8 (8.0) | 9.5 (7.9) |
| Fast fried foods | 0.6 (0.8) | 0.9 (1.1) | 0.5 (0.6) | 0.3 (0.6) |
| Full fat cheese | 2.8 (2.9) | 2.9 (3.1) | 2.9 (3.0) | 2.4 (2.1) |
| Butter, margarine | 4.5 (6.9) | 6.3 (8.7) | 3.7 (5.4) | 2.7 (4.5) |
| Wine consumption, g/day | 2.4 (6.4) | 1.2 (5.2) | 2.8 (6.9) | 3.8 (7.4) |
| Frequent use of olive oil at home, % | 179 (31) | 32 (13) | 57 (31) | 90 (63) |
Abbreviations: BMI, body mass index; CR, cognitive resilience; IQR, inter-quartile range; MIND, Mediterranean-Dietary Approaches to Stop Hypertension diet intervention for neurodegenerative delay; SD, standard deviation.
When examining characteristics of participants according to tertiles of MIND score (Table 1), those with greater MIND diet scores were largely similar to those with lower scores. However, participants with higher MIND scores had a greater proportion of women, lower proportions of smokers and individuals with diabetes or stroke, and greater levels of education and physical activity.
3.2. Associations between the MIND diet and CR
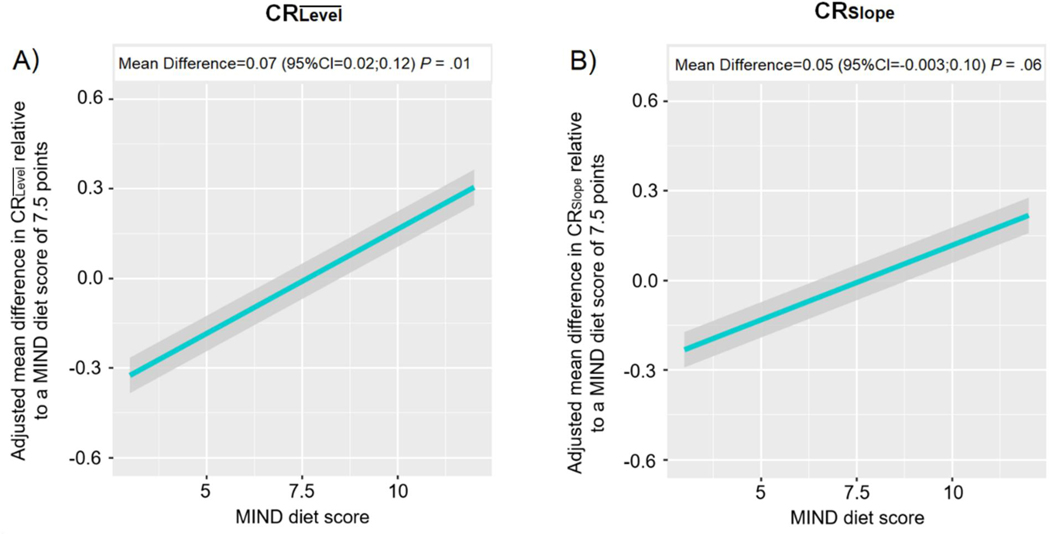
In the demographic-adjusted model (Table 2, Model A), individuals in the second and top tertiles of the MIND diet had significantly higher CR than those in the lowest tertile (tertile 2 versus tertile 1: mean difference [MD]=0.23 standard unit, 95%CI=0.05;0.42, P=.01; tertile 3 versus tertile 1: MD=0.39, 95%CI=0.18;0.59, P=.0002). These associations remained relatively consistent after additional adjustment for smoking status, depressive symptoms, medical conditions, physical activity, and cognitive activity (tertile 2 versus tertile 1: MD=0.23 standard unit, 95%CI=0.04;0.41, P=.04; tertile 3 versus tertile 1: MD=0.32, 95%CI=0.11;0.53, P=.003; Table 2, Model B). When we repeated the model replacing the tertiles with a continuous MIND score, we also found a trend of increasingly higher MIND diet score with increasingly higher CR, after full adjustment (for each one-unit increment in the MIND score: MD=0.07, 95%CI=0.02;0.12, P-trend=.01; Figure 1A).
Table 2.
Multivariable-adjusted mean difference in Cognitive Resilience (CR)a according to tertiles of the MIND diet score, Memory and Aging Project (n=578).
| Tertile 1 | Tertile 2 | Tertile 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean difference (95% CI) | P | Mean difference (95% CI) | P | |||
|
| ||||||
| CR | Model Ab | Ref. | 0.23 (0.05;0.42) | .01 | 0.39 (0.18;0.59) | .0002 |
| Model Bc | Ref. | 0.23 (0.04;0.41) | .02 | 0.34 (0.14;0.55) | .001 | |
|
|
||||||
| CRSlope | Model Cb | Ref. | 0.20 (0.01;0.39) | .04 | 0.32 (0.11;0.53) | .003 |
| Model Dc | Ref. | 0.20 (0.01;0.39) | .04 | 0.27 (0.05;0.48) | .01 | |
Abbreviations: CI, confidence interval; MIND, Mediterranean-Dietary Approaches to the Stop Hypertension (DASH) Diet Intervention for Neurodegenerative Delay.
After generating each CR variable, we standardized them to enable comparison of findings across the two CR measures.
Linear regression models adjusted for sex, education (continuous, years), age at first diet assessment (continuous, years), and total energy intakes (continuous, kcal/day).
Additionally adjusted for smoking status (never vs. former or current), number of depressive symptoms (continuous), number of medical conditions (continuous), physical activity (continuous, hours/week), and frequency of participation in cognitively stimulating activities (continuous) at the first dietary assessment.
Figure 1.
Estimated associations of MIND diet score with Cognitive Resiliencea (CR), Memory and Aging Project (n=578).
a After generating each CR variable, we standardized them to enable comparison of findings across the two CR measures. Plotted values denote the multivariable-adjusted mean difference in CRs for the range of MIND diet scores relative to the mean score of 7.5 points, adjusting for sex, education (continuous, years), age at first diet assessment (continuous, years), total energy intakes (continuous, kcal/day), smoking status (never vs. former or current), number of depressive symptoms (continuous), number of medical conditions (continuous), physical activity (continuous, hours/week), and frequency of participation in cognitively stimulating activities (continuous) at the first dietary assessment.
3.3. Associations between the MIND diet and CRSlope
In the demographic-adjusted model (Table 2, Model C), individuals in the second and top tertiles of the MIND diet score had higher CRSlope than those in the lowest tertile (tertile 2 versus tertile 1: MD=0.20 standard unit, 95%CI=0.01;0.39, P=.04; tertile 3 versus tertile 1: MD=0.32, 95%CI=0.11;0.53, P=.003). These associations remained consistent in the fully-adjusted model (tertile 2 versus tertile 1: MD=0.20 standard unit, 95%CI=0.01;0.39, P=.04; tertile 3 versus tertile 1: MD=0.27, 95%CI=0.05;0.48, P=.01; Table 2, Model D). We found a trend of increasingly higher CRSlope with increasingly higher MIND diet score when examining the continuous MIND diet, although after full adjustment, this association did not reach formal statistical significance (for each one-unit increment in the MIND score: MD=0.05, 95%CI=−0.003;0.10, P-trend=.06; Figure 1B).
3.4. Associations between the MIND dietary components and Cognitive Resilience
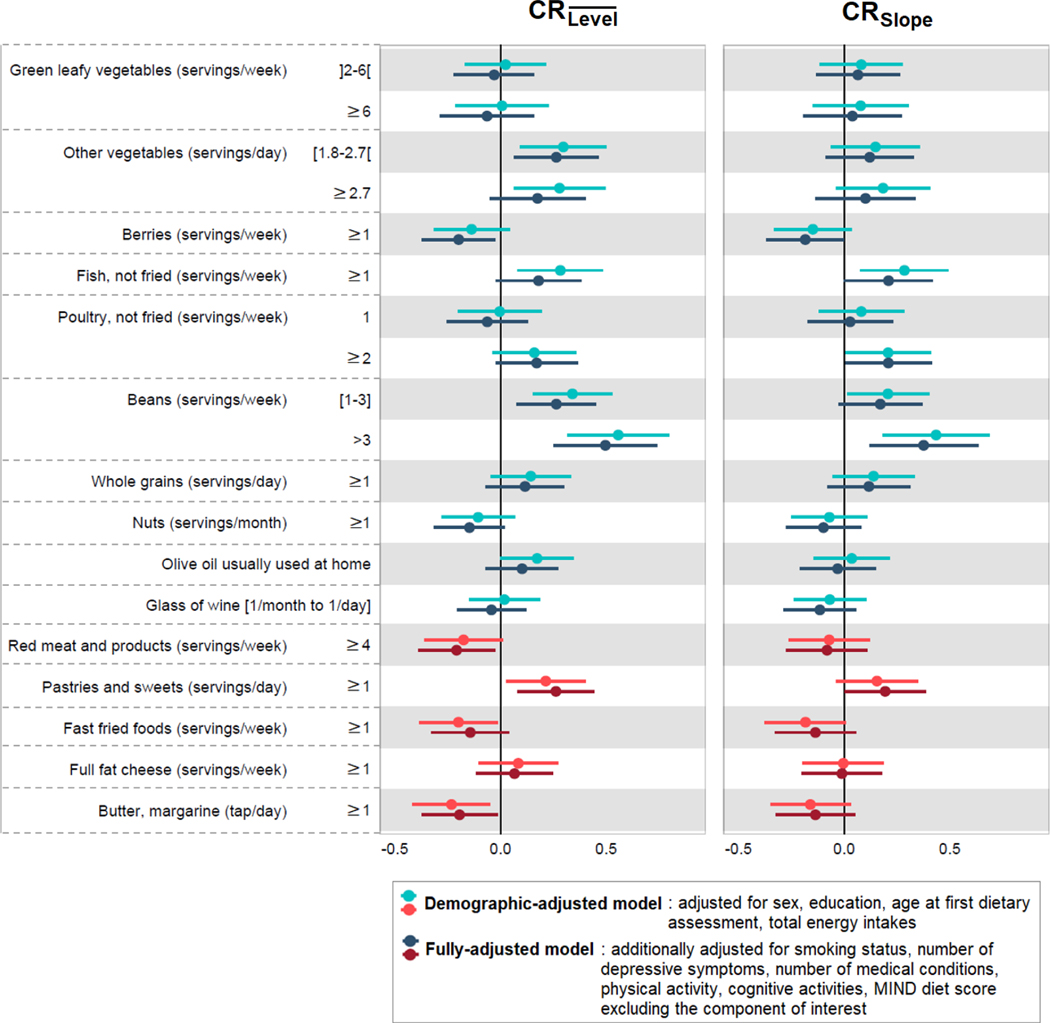
We also considered the relation of each component of the MIND diet with CR. For CR, we found that a higher intake of other vegetables (≥1.8 servings/day), fish (≥1 serving/week), and beans (≥1 serving/week) were associated with higher CR in the basic-adjusted models (all P<.01)(Figure 2, Models A). In contrast, higher intake of fast fried foods (≥1 serving/week) and butter/margarine (≥1 tap/day) were associated with lower CR in the basic-adjusted models (all P<.04)(Figure 2, Models A). When accounting for overall diet quality (i.e., the MIND diet score after removing the component of interest) in the fully-adjusted models, we found that higher intake of fish and beans were independently associated with higher CR and that greater intake of butter/margarine and red meat (≥4 servings/week) were associated with lower CR (all P<.04)(Figure 2, Models B). Unexpectedly, we found that higher intake of berries (≥1 serving/week) and nuts (≥1 serving/month) were associated with lower CR and that higher intake of pastries/sweets (≥1 serving/week) was associated with higher CR after full adjustment (all P<.04)(Figure 2, Models B). Because we were concerned about the possibility of reverse causation, such that those with diabetes had reduced their intake of sweets, we conducted analyses excluding those with prevalent diabetes at the FFQ; however, the associations remained similar (results not shown).
Figure 2.
Multivariable-adjusted mean difference in Cognitive Resilience (CR)a according to the individual MIND diet componentsb, Memory and Aging Project (n=578).
a After generating each CR variable, we standardized them to enable comparison of findings across the two strategies.
b For all food components, the reference group is not displayed but represents the lowest category; for example, for green leafy vegetables, the reference group is “≤2 servings/week” (see Supplementary Table A).
For CRSlope, we generally found associations with similar directions to those obtained for CR, although the magnitude of the effect estimates was smaller (Figure 2). In the basic-adjusted models, we found that greater intake of fish (≥1 serving/week) and beans (≥1 serving/week) were associated with higher CRSlope (all P<.04)(Figure 2, Models A). When accounting for overall diet quality in the fully-adjusted models, bean intake (>3 servings/week) remained independently associated with better CRSlope (P=.004)(Figure 2, Model B).
3.5. Sensitivity Analyses
When re-calculating CR and CRSlope using cognitive data from the first dietary assessment until death (Supplementary Table B), findings remained generally similar, supporting our use of all available cognitive data in primary analyses. In addition, when excluding 163 participants with MCI at first FFQ, findings remained largely consistent, suggesting that subtle cognitive disorders did not account for the association between the MIND diet and CR (Supplementary Table B). Similarly, findings remained unchanged after the exclusion of 81, 77, and 66 participants with a history of diabetes, stroke, and heart disease at the time of FFQ, respectively, suggesting that possible dietary changes due to these medical conditions did not alter the association between the MIND diet and CR (Supplementary Table B).
4. DISCUSSION
In this study of nearly 600 older adults followed annually for up to 23 years before death, we examined the relationship between the MIND diet score and two complementary measures of cognitive resilience, calculated using longitudinal cognitive data through death and 9 measures of postmortem neuropathology. We found clear associations of greater alignment with the MIND diet with both higher cognitive levels (CR) and slower residual slopes of cognitive decline (CRSlope), than would be expected marginally given the neuropathological profile. Further, our results suggested that the overall MIND diet may be more strongly related to CR than the individual food components. Overall, these striking findings indicate that the MIND diet may both maintain higher levels of cognition through older age (i.e., CR) and reduce cognitive decline in older individuals (i.e., CRSlope), regardless of accumulated neuropathology.
The relation between healthy dietary patterns and cognition have been reported in longitudinal studies over two decades. Diet has been associated with reduced Alzheimer’s dementia risk,10 better cognition,10,29–31 and slower cognitive decline,10,32,33 as well as less amyloid load,34,35 reduced infarcts,36 higher cortical thickness,37,38 and brain volumes.29,34,35,39,40 Our study suggests additional pathways by which the MIND diet may be associated with cognitive health via its association with resilience to pathology. Our results confirm and enhance previous work, including our own, which reported that the overall MIND diet was associated with higher cognitive scores proximate to death and flatter slopes of cognitive decline over time, independent of neuropathology17; here, we further directly quantified the association of 15 MIND dietary components to two complementary and longitudinal constructs of CR, and our findings generally support the ongoing belief in the nutrition field about the importance of a healthy dietary pattern, rather than one specific food group, to achieve optimal brain health.9 Dietary patterns may better capture the complex interactive and synergistic dynamic between nutrients and bioactives from various foods.
The mechanistic link between MIND diet and cognitive resilience is not fully understood. However, based on current research, we postulate several possible biologic pathways. For example, the MIND diet encourages a high intake of anti-inflammatory, antioxidant, and neuroprotective food compounds.41,42 The consumption of green leafy vegetables and berries, uniquely specified in the MIND diet, provides high amounts of antioxidants (vitamin E, carotenoids, and flavonoids),43,44 that appear to neutralize oxidative stress responsible for neurodegeneration.45–48 In animal models, antioxidant nutrients may downregulate overall neuroinflammation and prolonged activation of microglia; this may reduce brain cytokine production that may negatively impacts neurogenesis and may increase brain-derived neurotrophic factor (BDNF).43,44 Indeed, we previously reported that higher brain BDNF expression was associated with better CR (defined as slower cognitive decline independent of brain pathologies).49 In addition, MIND diet is also rich in other essential nutrients, such as long-chain omega-3 fatty acids from fish and nuts; B-vitamins from vegetables and whole grains; vitamin K and lutein from green leafy vegetables. These nutrients have been related to better neuronal plasticity, reduced gliosis and oxidative stress, and appear to maintain neurogenesis and blood-brain barrier integrity in in-vitro studies and animal models.50–52 Further, the MIND diet pattern also limits high saturated fat, high sugar foods, and red and processed meats. Saturated fats and sugars may induce neuroinflammation by mediating MAPK and NKκB signaling pathways, negatively impacting BDNF pathways and increasing blood-brain barrier permeability in the hippocampus causing synaptic dysfunction.53 Finally, a small randomized controlled trial of high versus low fat/high-glycemic-index diet found central nervous system changes in concentrations of lipoprotein, oxidative stress, and insulin.54 These metabolic disturbances can lead to worsened neuronal plasticity.55 Thus, there are an array of plausible mechanisms supporting our results here.
This study has important strengths including a validated FFQ, high follow-up rates, and two complementary measures of CR defined using long-term repeated cognitive measures before death, a comprehensive battery of 17 cognitive tests, and measures of 9 brain pathologies.
However, there are some limitations to consider. First, self-reported dietary intake may not be perfectly accurate. To help reduce misclassification of diet, in primary analyses we modeled the MIND diet score in tertile categories to provide a more robust classification. Further, misclassification of diet would bias results towards the null, thus we may have underestimated the association of diet with CR. Second, we were also concerned about possible systematic misclassification if those with MCI may misreport diet (and have worse CR); however, our FFQ was validated in those with MCI, and in sensitivity analyses excluding participants with MCI at the dietary assessment, we found similar results. Third, although the presence of specific medical co-morbidities may lead to improvements in dietary habits, in our sensitivity analyses excluding participants with a history of diabetes, stroke or heart disease prior to the FFQ, we also found similar results. Further, we observed highly stable dietary patterns in our study sample, suggesting that focusing on the initial MIND diet score was valid as well as representative. Fourth, although we considered several important confounders in our analyses, many were self-reported (e.g., medical comorbidities), and residual confounding may still persist. Fourth, for analyses of MIND components, distributions of some individual dietary variables in MIND were skewed, making it harder to examine distinct categories of intake, and our sample size was modest, leading to imprecise results for some components. Lastly, these studies are primarily composed of non-Hispanic White older adults who consented to brain autopsy and donation; since both diet and cognition may differ in diverse populations, future longitudinal studies on diet and CR in diverse populations are needed.
Supplementary Material
RESEARCH IN CONTEXT.
SYSTEMATIC REVIEW:
Cognitive resilience can be considered as the continuum from better through worse than expected cognition, given the degree of neuropathology. Literature reviews via PubMed identify extensive research supporting a relation of healthier dietary habits to cognitive decline and dementia, whereas limited research examines whether dietary patterns may be associated with cognitive resilience, as a possible mechanism of brain health.
INTERPRETATION:
Using longitudinal cognitive data and postmortem neuropathology from 578 deceased, predominantly White older participants, we found that a higher score at baseline for the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) was associated with higher cognitive resilience – that is, higher cognitive levels and slower cognitive decline than would be expected given the participant’s neuropathologic profile.
FUTURE DIRECTIONS:
Future research should examine the relationship between healthy dietary patterns and CR in diverse populations.
ACKNOWLEDGMENTS
Dr. Maude Wagner is supported by a post-doctoral fellowship from the French Foundation for Alzheimer’s Research (alzheimer-recherche.org). We thank the participants of the Rush Memory and Aging Project and key staff members; Traci Colvin, MPH, for coordination of the clinical data collection; Karen Skish, MS, for coordination of the pathologic data collection; John Gibbons, MS, and Greg Klein, MS, for data management.
Funding
Study funded by NIH (R01AG17917, P30AG10161, R01AG15815, R01AG34374) and the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the writing of the report or the decision to submit it for publication.
Footnotes
Conflicts of interest
The authors have no conflicts to declare.
Data availability
All data in these analyses (and descriptions of the studies and variables) can be requested through the Rush Alzheimer’s Disease Center Research Resource Sharing Hub at www.radc.rush.edu. The R code to replicate the analyses of this study can be provided by the corresponding author upon reasonable request.
REFERENCES
- 1.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology. 2018;90(15):695–703. doi: 10.1212/WNL.0000000000005303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocancea DI, Loenhoud, Groot, Barkhof, Flier WM van der, Ossenkoppele R. Measuring Resilience and Resistance in Aging and Alzheimer Disease Using Residual Methods: A Systematic Review and Meta-analysis. Neurology. Published online 2021. doi: 10.1212/WNL.0000000000012499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58(4):376–388. doi: 10.1097/00005072-199904000-00008 [DOI] [PubMed] [Google Scholar]
- 4.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–376. doi: 10.1212/wnl.55.3.370 [DOI] [PubMed] [Google Scholar]
- 5.Ewbank DC, Arnold SE. Cool with plaques and tangles. N Engl J Med. 2009;360(22):2357–2359. doi: 10.1056/NEJMe0901965 [DOI] [PubMed] [Google Scholar]
- 6.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983–2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge HH, Zhu J, Woltjer R, et al. Risk of incident clinical diagnosis of Alzheimer’s disease-type dementia attributable to pathology-confirmed vascular disease. Alzheimers Dement. 2017;13(6):613–623. doi: 10.1016/j.jalz.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85(1):114–124. doi: 10.1002/ana.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. doi: 10.1016/S1474-4422(18)30338-7 [DOI] [PubMed] [Google Scholar]
- 10.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv Nutr. 2019;10(6):1040–1065. doi: 10.1093/advances/nmz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S, Stern Y, Gu Y. Modifiable lifestyle factors and cognitive reserve: A systematic review of current evidence. Ageing Res Rev. 2022;74:101551. doi: 10.1016/j.arr.2021.101551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zainuddin MSA, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull. 2012;103(1):89–114. doi: 10.1093/bmb/lds021 [DOI] [PubMed] [Google Scholar]
- 14.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age and Ageing. 2011;40(6):684–689. doi: 10.1093/ageing/afr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Boyle PA, Segawa E, et al. Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychology. 2015;29(3):335–343. doi: 10.1037/neu0000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA. Normative Cognitive Decline in Old Age. Ann Neurol. 2020;87(6):816–829. doi: 10.1002/ana.25711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhana K, James BD, Agarwal P, et al. MIND Diet, Common Brain Pathologies, and Cognition in Community-Dwelling Older Adults. Journal of Alzheimer’s Disease. 2021;83(2):683–692. doi: 10.3233/JAD-210107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner M, Wilson RS, Leurgans SE, et al. Quantifying longitudinal cognitive resilience to Alzheimer’s disease and other neuropathologies. Alzheimers Dement. Published online 2022. doi: 10.1002/alz.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213–1217. doi: 10.1093/aje/kwg290 [DOI] [PubMed] [Google Scholar]
- 21.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LS R. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–407. [PubMed] [Google Scholar]
- 24.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussein YN, Samieri C, Livingston G, et al. Nutrition state of science and dementia prevention: recommendations of the Nutrition for Dementia Prevention Working Group. The Lancet Healthy Longevity. 2022;3(7):E501–E512. doi: 10.1016/S2666-7568(22)00120-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74–83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software. 2017;78(1):1–56. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 29.Melo van Lent D, O’Donnell A, Beiser AS, et al. Mind Diet Adherence and Cognitive Performance in the Framingham Heart Study. J Alzheimers Dis. 2021;82(2):827–839. doi: 10.3233/JAD-201238 [DOI] [PubMed] [Google Scholar]
- 30.Wesselman LMP, van Lent DM, Schröder A, et al. Dietary patterns are related to cognitive functioning in elderly enriched with individuals at increased risk for Alzheimer’s disease. Eur J Nutr. 2021;60(2):849–860. doi: 10.1007/s00394-020-02257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boumenna T, Scott TM, Lee JS, et al. MIND Diet and Cognitive Function in Puerto Rican Older Adults. J Gerontol A Biol Sci Med Sci. 2022;77(3):605–613. doi: 10.1093/gerona/glab261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherian L, Wang Y, Fakuda K, Leurgans S, Aggarwal N, Morris M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. J Prev Alzheimers Dis. 2019;6(4):267–273. doi: 10.14283/jpad.2019.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz-Garcia MI, Toledo E, Razquin C, et al. “A priori” Dietary Patterns and Cognitive Function in the SUN Project. Neuroepidemiology. 2020;54(1):45–57. doi: 10.1159/000502608 [DOI] [PubMed] [Google Scholar]
- 34.Rainey-Smith SR, Gu Y, Gardener SL, et al. Mediterranean diet adherence and rate of cerebral Aβ-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl Psychiatry. 2018;8(1):238. doi: 10.1038/s41398-018-0293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassilaki M, Aakre JA, Syrjanen JA, et al. Mediterranean diet, its components and amyloid imaging biomarkers. J Alzheimers Dis. 2018;64(1):281–290. doi: 10.3233/JAD-171121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarmeas N, Luchsinger JA, Stern Y, et al. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69(2):257–268. doi: 10.1002/ana.22317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosconi L, Murray J, Tsui WH, et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer’s Disease. J Prev Alzheimers Dis. 2014;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 38.Staubo SC, Aakre JA, Vemuri P, et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. 2017;13(2):168–177. doi: 10.1016/j.jalz.2016.06.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015;85(20):1744–1751. doi: 10.1212/WNL.0000000000002121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luciano M, Corley J, Cox SR, et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017;88(5):449–455. doi: 10.1212/WNL.0000000000003559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steele M, Stuchbury G, Münch G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp Gerontol. 2007;42(1–2):28–36. doi: 10.1016/j.exger.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 42.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367(1):31–37. doi: 10.1111/nyas.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey AN, Joseph JA. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br J Nutr. 2015;114(10):1542–1549. doi: 10.1017/S0007114515003451 [DOI] [PubMed] [Google Scholar]
- 44.Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology. 2018;90(3):e214–e222. doi: 10.1212/WNL.0000000000004815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Fata G, Weber P, Mohajeri MH. Effects of Vitamin E on Cognitive Performance during Ageing and in Alzheimer’s Disease. Nutrients. 2014;6(12):5453–5472. doi: 10.3390/nu6125453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida Y, Ito S, Ohtsuki S, et al. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem. 2009;284(48):33400–33408. doi: 10.1074/jbc.M109.054056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katayama S, Ogawa H, Nakamura S. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J Agric Food Chem. 2011;59(23):12691–12696. doi: 10.1021/jf203654c [DOI] [PubMed] [Google Scholar]
- 48.Chan A, Shea TB. Folate deprivation increases presenilin expression, gamma-secretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. J Neurochem. 2007;102(3):753–760. doi: 10.1111/j.1471-4159.2007.04589.x [DOI] [PubMed] [Google Scholar]
- 49.Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86(8):735–741. doi: 10.1212/WNL.0000000000002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stover PJ, Durga J, Field MS. Folate nutrition and blood-brain barrier dysfunction. Curr Opin Biotechnol. 2017;44:146–152. doi: 10.1016/j.copbio.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canovai A, Amato R, Melecchi A, et al. Preventive Efficacy of an Antioxidant Compound on Blood Retinal Barrier Breakdown and Visual Dysfunction in Streptozotocin-Induced Diabetic Rats. Front Pharmacol. 2021;12:811818. doi: 10.3389/fphar.2021.811818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen BY, Huang HS, Tsai KJ, et al. Protective Effect of a Water-Soluble Carotenoid-Rich Extract of Cordyceps militaris against Light-Evoked Functional Vision Deterioration in Mice. Nutrients. 2022;14(8):1675. doi: 10.3390/nu14081675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19(7):1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x [DOI] [PubMed] [Google Scholar]
- 54.Grisotto C, Taïlé J, Planesse C, et al. High-Fat Diet Aggravates Cerebral Infarct, Hemorrhagic Transformation and Neuroinflammation in a Mouse Stroke Model. Int J Mol Sci. 2021;22(9):4571. doi: 10.3390/ijms22094571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavaliere G, Trinchese G, Penna E, et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front Cell Neurosci. 2019;13:509. doi: 10.3389/fncel.2019.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in these analyses (and descriptions of the studies and variables) can be requested through the Rush Alzheimer’s Disease Center Research Resource Sharing Hub at www.radc.rush.edu. The R code to replicate the analyses of this study can be provided by the corresponding author upon reasonable request.