Abstract
Key Clinical Message
Young patients with persistent rash and fevers despite antibiotic treatment should be evaluated for non‐infectious etiologies. In our patient's case, these findings led to a diagnosis of MAS, which ultimately affected how she was managed.
Abstract
Adult‐onset Still's disease (AOSD) is a rare, often difficult to diagnose autoimmune disease that typically presents as a rash, unresolving fevers and joint pains capable of mimicking a number of autoimmune diseases. Here, we present the case of a young postpartum woman whose clinical presentation, which included a pruritic maculopapular rash that evolved to include a flagellate component, and serological studies, chief among them cytopenias and a Ferritin >15,000 nm/mL) allowed us to make an early diagnosis of AOSD complicated by macrophage activation syndrome. We discuss the treatment for AOSD complicated by MAS with Hydrocortisone and Anakinra, the final discharge regimen prescribed for our patient, and report on her state 3 months post‐hospitalization, which was favorable. Our case is unique because we ultimately believe that pregnancy itself triggered her ASOD, because of how the quality of the flagellate component of her rash allowed us to narrow the differential diagnosis, and because of how the significant cytopenias and significant liver dysfunction alerted us to the possibility of MAS.
Keywords: adult Still's disease, adult‐onset Still's disease, anakinra, macrophage activating syndrome
The rashes of Adult‐Onset Still's Disease.
1. INTRODUCTION
The etiology of febrile maculopapular rashes can be challenging to diagnose given the diverse clinical presentations in which they can occur. In this case, we report on how misdiagnoses and the evolution of a previously healthy 32‐year‐old 13‐week postpartum female's maculopapular rash helped us make an early diagnosis of adult‐onset Still's disease (AOSD) complicated by macrophage activating syndrome (MAS).
AOSD is the adult form of systemic juvenile arthritis, a rare condition characterized by systemic inflammatory effects, quotidian fevers, and episodic maculopapular rash. 1 There is usually a trigger, most commonly AOSD itself (idiopathic) or an infection. 1 A fraction of AOSD can be complicated by MAS with most cases being diagnosed in young adults before 35 years of age. 2 It is often misdiagnosed initially as other autoimmune conditions or infections due to its rarity and similarity in presentation with a long list of medical conditions. We hope that this case will help providers appreciate the serological studies that should heighten their concern for AOS complicated by MAS as well as the subtle signs on presentation that may suggest it.
2. CASE HISTORY/EXAMINATION
The patient is a 32‐year‐old, 13‐week postpartum female with no other medical history. She came to our emergency department with a worsening itchy rash, joint pains in her wrists and fingers, and daily recurring high‐grade fevers for the past 6 weeks (as shown in Figure 1). Four weeks prior to presentation, she had been diagnosed with group A Streptococcus pharyngitis, prescribed, and completed a course of amoxicillin without response prompting an outside hospital admission where she was diagnosed with acute rheumatic fever and sepsis from Moraxella bacteremia that required brief ICU‐level care. She received 11 days' worth of IV Ceftriaxone with achievement of hemodynamic stability. However because of symptomatic worsening, she elected to leave against medical advice.
FIGURE 1.
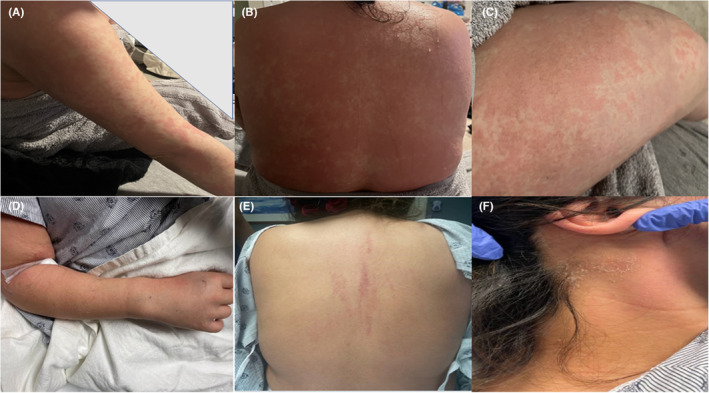
(A)–(C) are pictures of the maculopapular rash on the right arm, back, and right thigh, respectively early in the course of her disease while (D) shows mildly swollen right wrists and digits while (E and F) are images of the back and the right neck, respectively with residual desquamating rash at presentation to our facility.
Upon arrival at our emergency department, the patient was found to be sinus tachycardic to 114 beats per minute but normotensive and afebrile. Broad spectrum antibiotics with vancomycin, aztreonam, and metronidazole were started after computerized tomography imaging raised concerns for acute cholecystitis. She was admitted to General Medicine where our examination identified the presence of a faint but diffuse maculopapular rash over most of the body except the palms and soles with associated desquamation of the back and face. More prominent erythematous linear plaques localized to her upper back (Figure 1) were also noted. Patient‐supplied pictures of the rash prior to presentation depicted an erythematous rash composed of slightly edematous papules coalescing into plaques on the upper extremities, thighs, and back (Figure 1). We also corroborated the description of this rash through outside hospital chart review. She had swollen and tender bilateral wrists and proximal interphalangeal joints. Her abdominal exam was benign and there was no Murphy sign.
Her white blood cell count was 11,000 10/μL (absolute neutrophil count of 6.3 × 10/μL); hemoglobin, 8.6 g/dL; platelets, 115 103/μL; AST, 535 units/L; ALT, 161 units/L; Total Bilirubin, 2.1 mg/dL; serum alkaline phosphatase, 986 units/L; C‐reactive protein, 101.9 mg/L; and erythrocyte sedimentation rate, 58 mm/h. The presence of cytopenias and elevated liver enzymes and bilirubin prompted additional serologic investigations, including a D‐dimer which was 57,547 ng/mL; INR, 1.4; aPTT 23.5 seconds; and Fibrinogen of 81 mg/dL. Her serum ferritin was elevated to greater than 15,000 ng/mL with soluble IL‐2 receptor and IL‐18 each greater than 4000 and 11,000 pg/mL, respectively. Antineutrophilic antibodies (ANA), antineutrophilic anticyclic antibodies (ANCA), and rheumatoid factor (RF) were all negative. A HIDA scan was negative for cholecystitis (Figure 2), an angiogram protocolled chest CT scan did not identify any infiltrate although noted enlarged hilar lymph nodes (Figure 2), while a TEE ruled out the presence of any valvular vegetations. She had a negative viral hepatitis panel, HIV, EBV, HSV, RSV, SARS‐CoV‐2, and QuantiFERON assays. Urine and blood cultures were also negative.
FIGURE 2.
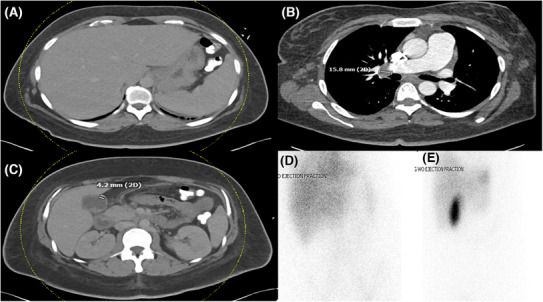
CT scan of (A) the abdomen depicting splenomegaly (B) of the chest showing an enlarged right hilar lymph node and (C) the abdomen showing a thickened gallbladder wall. (D and E) are HIDA scans showing the absence of acute cholecystitis.
To us, her constellation of symptoms suggested adult‐onset Still's disease complicated by MAS. Rheumatology was consulted, who agreed and recommended systemic treatment with intravenous Hydrocortisone and Anakinra. Her liver enzymes and bilirubin improved markedly by discharge (AST, 28 units/L; ALT, 38 units/L; serum alkaline phosphatase, 407 units/L; Total Bilirubin, 0.8 mg/gL; and Ferritin, 1334.2 ng/mL) and she was discharged on Day 8with oral prednisone and Anakinra. At her PCP visit 2 weeks post‐discharge, the patient's rash and fever had completely resolved and she was doing well. Her maintenance therapy included Anakinra and an ongoing month‐long steroid taper. During treatment, the patient's cytopenias also improved significantly and at her outpatient follow‐up appointment, her white blood cell and platelet counts had normalized while her hemoglobin level demonstrated significant improvement from 7.9 to 10.7 g/dL. Similarly, ferritin and CRP levels had normalized to 168.6 ng/mL and 3.8 mg/L, respectively (Figure 3).
FIGURE 3.
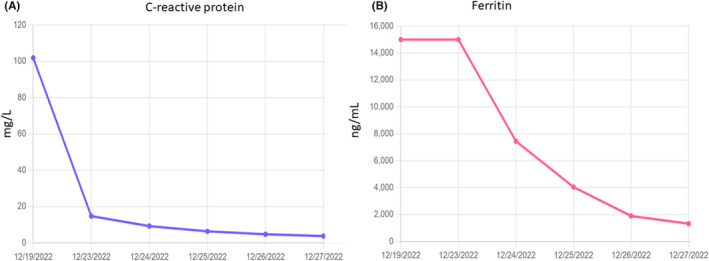
Ferritin (A) and C‐reactive protein levels (B) during admission and at follow‐up visits, demonstrating a decrease in these molecules following the start of anakinra and steroids. It should be noted that the slight increase in CRP at the end was thought to be caused by the patient's injection site reaction.
3. DISCUSSION
AOSD is a rare rheumatological disease that affects 1–6 per million people and is more common in women. 3 While classically characterized by quotidian fevers, pharyngitis, distal polyarthralgias, and a salmon‐pink colored rash, presentations can be atypical which makes it challenging to diagnose. It remains a diagnosis of exclusion and is based on the Yamaguchi criteria. 4 Although our patient had three major criteria—fever lasting 1 week or longer, arthralgias lasting 2 weeks or longer, suspicion for typical rash—and four minor criteria—sore throat, splenomegaly, abnormal liver function tests, and negative ANA and RF, we had to rule out other possibilities.
Atypical sepsis, acute rheumatic fever, endocarditis (due to recent Moraxella bacteremia), and drug reaction with eosinophilia and systemic symptoms (DRESS) were all considered. While she was previously diagnosed with acute rheumatic fever in the setting of having had GAS pharyngitis, our assessment found that she did not display other cardinal features like chorea, subcutaneous nodules, carditis symptoms, and erythema marginatum, which classically begins as enlarging macules that assume the shape of rings or crescent with central clearing. Contrastingly, our patient's rash was initially evanescent, maculopapular, and spared the palms and soles. 6 Furthermore, she had not improved despite appropriate antibiotics. 6 Similarly, while endocarditis may have explained her persistent fevers and systemic inflammation, there were no signs of it (i.e., cardiac murmur), a TEE was negative for vegetations, and we would have expected symptomatic improvement while on antibiotics. DRESS was also considered but given that her symptoms predated antibiotic use or use of any other culprit medication, we felt that this too was unlikely. In addition, this would not have accounted for all of her objective findings such as elevated liver function tests and lymphadenopathy. The patient's elevated inflammatory markers, ferritin levels, periodic fevers, joint pain, splenomegaly, lymphadenopathy, and recurrent rash lead us to suspect a primary rheumatologic process like AOSD, and while a skin biopsy could have helped with making the diagnosis, in the presence of fulfilled Yamaguchi criteria, it was not necessary and the patient declined it. 5 Importantly, it was the patient's rash, which had evolved to include a flagellate component that increased our suspicion even further for AOSD. As erythematous parallel linear or curvilinear pruritic plaques, the flagellate rash has a short diagnostic differential that includes shitake mushrooms, bleomycin‐intake, dermatomyositis, chikungunya, and AOSD. 7
The presence of bicytopenias (anemia and thrombocytopenia) should alert the provider to evaluate for complications of AOSD such as MAS which can complicate 12%–14% of AOSD and has a mortality rate of 10%–22%. 1 Prompt evaluation for coagulopathy and hyperferritinemia should ensue to help make this diagnosis. The lack of a definitive diagnostic test for AOSD complicated by MAS, its rarity, and its similar presentation to a long list of autoimmune, infectious (viral infections), neoplastic, and other rheumatologic diseases make it an easy diagnosis to miss. However, recent advances have highlighted a role for soluble Interleukin (IL)‐2 and IL‐18 in distinguishing AOSD with MAS from other forms of hemophagocytic lymphohistiocytosis in adults usually secondary to malignancies, infections (typically Epstein–Barr virus [EBV]), sepsis, chimeric antigen receptor T‐cell therapy. 8 , 9 Elevated soluble IL‐2r and IL‐8, as in our patient, helped to confirm the diagnosis of AOSD complicated by MAS. 8 , 9 After successfully ruling out other diagnoses, providers should make every effort to identify a trigger driving the episode of AOSD. In our patient's case, symptoms started well before her diagnosis of group A Streptococcal pharyngitis, acute rheumatic fever, or Moraxella bacteremia. We believe that pregnancy and the postpartum period triggered her episode. About 9.3% of AOSD occurs during the postpartum period and episodes occuring up to 6 months postpartum have been reported. 10 , 11
The approved treatment strategy of AOSD is based on the severity of the disease and whether there is MAS. In the case of the latter, combination therapy with a glucocorticoid and a disease‐modifying antirheumatic drug (DMARD) is recommended. As an IL‐1 inhibitor, Anakinra is an upstream inhibitor of the inflammatory cascade that drives AOSD and although evidence is limited studies have demonstrated rapid clinical improvement in cases complicated by MAS when used. 12 Maintenance therapy depends on the response to initial therapy, residual systemic or articular disease, and any side effects from the use of high‐dose steroids.
4. CONCLUSION
We have presented the case of a young woman ultimately diagnosed with AOSD complicated by MAS after numerous interactions with the health‐care system failed to ameliorate her complaints. This case was unique because we believe the trigger for ASOD was pregnancy and the postpartum period and because the quality of the patient's rash evolved to include a flagellate component, allowing us to narrow the differential diagnosis considerably. We believe that there is value in stressing the importance of further investigation should cytopenias, especially anemia and thrombocytopenia, and significant liver dysfunction in the setting of persistent flagellate rash be identified. In our patient's case, these findings led to a diagnosis of MAS, which ultimately affected how she was managed.
We hope this case will encourage earlier consideration of non‐infectious causes of fever when it presents with a maculopapular or flagellate rash and provide the clinician with valuable insights into how to assess disease severity in AOSD.
AUTHOR CONTRIBUTIONS
Toluwalase Awoyemi: Conceptualization; resources; writing – original draft; writing – review and editing. Alexandra Conti: Resources; writing – original draft; writing – review and editing. Frank Aguilar: Supervision; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We would like to thank the patient for allowing us to write about her presentation.
Awoyemi T, Conti A, Aguilar FG. Adult‐onset Still's disease complicated by macrophage activation syndrome. Clin Case Rep. 2023;11:e7825. doi: 10.1002/ccr3.7825
DATA AVAILABILITY STATEMENT
All data necessary for this case report are available as part of this article and no additional source data are required.
REFERENCES
- 1. Efthimiou P, Kadavath S, Mehta B. Life‐threatening complications of adult‐onset Still's disease. Clin Rheumatol. 2014;33(3):305‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Efthimiou P, Kontzias A, Hur P, Rodha K, Ramakrishna GS, Nakasato P. Adult‐onset Still's disease in focus: clinical manifestations, diagnosis, treatment, and unmet needs in the era of targeted therapies. Semin Arthritis Rheum. 2021;51(4):858‐874. [DOI] [PubMed] [Google Scholar]
- 3. Rao S, Tsang LSL, Zhao M, Shi W, Lu Q. Adult‐onset still's disease: a disease at the crossroad of innate immunity and autoimmunity. Front Med. 2022;9:881431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19(3):424‐430. [PubMed] [Google Scholar]
- 5. Lee JYY, Hsu CK, Liu MF, Chao SC. Evanescent and persistent pruritic eruptions of adult‐onset still disease: a clinical and pathologic study of 36 patients. Semin Arthritis Rheum. 2012. Dec;42(3):317‐326. [DOI] [PubMed] [Google Scholar]
- 6. Sika‐Paotonu D, Beaton A, Raghu A, Steer A, Carapetis J. Acute rheumatic fever and rheumatic heart disease. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations [Internet]. University of Oklahoma Health Sciences Center; 2016. [cited 2023 Jun 30]. http://www.ncbi.nlm.nih.gov/books/NBK425394/ [Google Scholar]
- 7. Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol. 2014;80(2):149‐152. [DOI] [PubMed] [Google Scholar]
- 8. Shiga T, Nozaki Y, Tomita D, et al. Usefulness of interleukin‐18 as a diagnostic biomarker to differentiate adult‐onset still's disease with/without macrophage activation syndrome from other secondary hemophagocytic lymphohistiocytosis in adults. Front Immunol. 2021. Jan;1(12):750114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maruyama J, Inokuma S. Cytokine profiles of macrophage activation syndrome associated with rheumatic diseases. J Rheumatol. 2010;37(5):967‐973. [DOI] [PubMed] [Google Scholar]
- 10. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet Lond Engl. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult‐onset Still's disease. Nat Rev Rheumatol. 2018;14(10):603‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyseng‐Williamson KA. Anakinra in Still's disease: a profile of its use. Drugs Ther Perspect Ration Drug Sel Use. 2018;34(12):543‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for this case report are available as part of this article and no additional source data are required.


