Abstract
Background and Aims
Prognostic models provide evidence‐based predictions and estimates of future outcomes, facilitating decision‐making, patient care, and research. A few of these models have been externally validated, leading to uncertain reliability and generalizability. This study aims to externally validate four models to assess their transferability and usefulness in clinical practice. The models include the respiratory index of severity in children (RISC)‐Malawi model and three other models by Lowlavaar et al.
Methods
The study used data from the Clinical Information Network (CIN) to validate the four models where the primary outcome was in‐hospital mortality. 163,329 patients met eligibility criteria. Missing data were imputed, and the logistic function was used to compute predicted risk of in‐hospital mortality. Models' discriminatory ability and calibration were determined using area under the curve (AUC), calibration slope, and intercept.
Results
The RISC‐Malawi model had 50,669 pneumonia patients who met the eligibility criteria, of which the case‐fatality ratio was 4406 (8.7%). Its AUC was 0.77 (95% CI: 0.77−0.78), whereas the calibration slope was 1.04 (95% CI: 1.00 −1.06), and calibration intercept was 0.81 (95% CI: 0.77−0.84). Regarding the external validation of Lowlavaar et al. models, 10,782 eligible patients were included, with an in‐hospital mortality rate of 5.3%. The primary model's AUC was 0.75 (95% CI: 0.72−0.77), the calibration slope was 0.78 (95% CI: 0.71−0.84), and the calibration intercept was 0.37 (95% CI: 0.28−0.46). All models markedly underestimated the risk of mortality.
Conclusion
All externally validated models exhibited either underestimation or overestimation of the risk as judged from calibration statistics. Hence, applying these models with confidence in settings other than their original development context may not be advisable. Our findings strongly suggest the need for recalibrating these model to enhance their generalizability.
Keywords: epidemiology, model external validation, pediatrics and adolescent medicine
1. INTRODUCTION
Childhood mortality remains high in low and middle‐income countries (LMICs) despite a significant reduction since 1990, but it's uncertain how much in‐hospital mortality has changed over this duration. 1 , 2 Mortality rates among hospitalized children in sub‐Saharan Africa remain high, and most deaths occur within the first few hours of admission. 3 An example of such a comparison can be found in Sharrow et al. 4 which reported a marked disparity in under‐five mortality rates between low‐income and high‐income settings, with rates of 67% and 5%, respectively. Emergency Triage Assessment and Treatment (ETAT) guidelines, produced by the World Health Organization (WHO), guide immediate care for children admitted to hospitals. ETAT guidelines provide guidance on triage and use syndrome‐based approach to the management of common childhood conditions, but they are still used inconsistently and implemented suboptimally in Africa despite having been in existence since 2005. 5
Regardless of an underlying condition, children at risk of death during hospitalization often present with similar danger signs and prompt triage and immediate supportive management are most important in reducing mortality and morbidity in admitted children. 6 Identification of children at risk of in‐hospital mortality is the first step in directing supportive treatments that have the potential to reduce deaths. Therefore, clinical prediction models that identify the sickest children immediately upon arrival at the hospital for immediate supportive care and targeted close monitoring may be useful. 7 However, many prognostic models do not meet methodological standards, reducing their utility and generalizability. 8 , 9 A common methodological weakness is the small sample size which makes a resultant model to have a low signal‐to‐noise ratio, limited number of events‐per‐variable (EPV), with some having EPV of less than 20, which is thought to lead to biased estimates. 10 , 11 , 12 , 13 Other weaknesses include poor handling of incomplete data, inappropriate statistical analyses, and optimistic interpretations of the model output. Furthermore, overreliance on fully automated statistical techniques, such as the stepwise model selection algorithm (backward or forward), which do not require expert or consensus input, can lead to overoptimistic or biased models that are not always relevant in routine practice. 11 , 14 , 15 , 16
Independent external validation of risk scores is recommended to assess their transferability and generalizability to other patient populations before their use in clinical practice. However, few models have been validated externally hence most models have uncertain reliability and generalizability. 15 , 17 , 18 In this study, we conduct external validation of four prognostic models predicting in‐hospital mortality for pediatric patients in LMICs using routine hospital data collected by the Clinical Information Network (CIN) in Kenya. These models were identified in the recent systematic review of pediatric prognostic models. 8 , 9
2. METHODS
2.1. Ethics and reporting
This external validation study followed recommendations for developing, reporting and validating prognostic studies stipulated in the TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis) statement. 12 , 13 Kenya Medical Research Institute's (KEMRI) scientific and ethical review committee approved the CIN study (#3459). The Kenya Ministry of Health (MoH) permitted this work using deidentified routine patient care data abstracted from medical records after discharge without individual patient/clinician consent.
2.2. Patient and public involvement
Patients were not involved in the study's design, so research questions were not informed by patients' priorities, experiences, or preferences.
2.3. Study design and setting
This validation study uses data collected from CIN, which comprises 20 public county referral (previously district) hospitals in Kenya whose locations are shown in Figure 1. These hospitals serve as first‐referral level hospitals but also serve patients who seek care directly from their homes. The CIN is a collaboration between researchers from the KEMRI‐Wellcome Trust Research Programme (KWTRP), the Kenya MoH, the Kenya Paediatric Association, and participating hospitals. The selection of these hospitals under CIN was purposeful to represent wide geographical variation in Kenya and targeted those with a high volume of pediatric admissions, at least 1000 children per year. More details about the selection of these hospitals and their locations have been given elsewhere. 3
Figure 1.
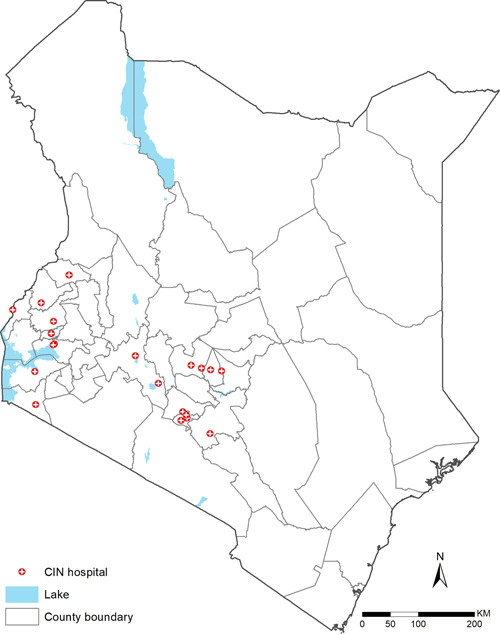
Locations of hospitals included in the validation cohort. CIN, Clinical Information Network.
2.4. Outcome
In our study, the outcome of interest was all‐cause in‐hospital pediatric mortality. To minimize bias in outcome assessment, the hospital data clerks who abstracted data from medical records were kept unaware of the study, ensuring their inability to influence the outcome assessment and maintaining blindness to predictors. We aimed to comprehensively represent the target population by considering all pediatric patients admitted to the hospital during the study period. By including all eligible patients, we sought to avoid systematic selection bias related to the outcome of interest.
2.5. Prognostic models included for external validation
Models included in this external validation were obtained from a recent systematic review of prognostic models, 9 which identified 11 models predicting in‐hospital mortality for children admitted in LMICs hospitals. However, we excluded nine models 19 , 20 , 21 , 22 , 23 , 24 since they did not publish the relative weights of the risk factors and model intercept for the logistic regression models or the baseline hazard function for the survival models as required for the external validation. Before we decided to exclude these models, we contacted the corresponding authors of those studies by email, asking for the complete model formula without success. The following are the models included in the current external validation study.
2.5.1. Respiratory index of severity in children (RISC)‐Malawi prognostic model
RISC‐Malawi is a predictive model developed using prospectively collected clinical data from a cohort of 14,665 hospitalized children aged 2−59 months with pneumonia diagnosis in Malawi between 2011 and 2014. The total number of deaths in the model development cohort was 465, and the case‐fatality rate was 3.2% across the seven hospitals under study. 25 The authors utilized logistic regression to develop a prognostic model whose intercept and odds ratios for the seven prognostic factors are provided in Table 1. The author reported an area under the receiver operator characteristic curve (ROC) of 0.79 (95% CI: 0.76−0.82), demonstrating a fair ability to discriminate children's mortality risk.
Table 1.
Models to be externally validated.
| Study | Models | Inclusion criteria | Predictors | Model equation with intercept and odds ratios | Model derivation AUC (95% CI) | CIN sample size eligible for validation (% with mortality outcome) | |
|---|---|---|---|---|---|---|---|
| Hooli et al. 25 | RISC‐Malawi model | Age ≥2 and ≤59 months, pneumonia by danger signs (cough or difficult breathing and at least one danger sign (central cyanosis, grunting, chest wall indrawing, stridor, inability to drink, AVPU<A, or convulsion)) | Moderate hypoxemia, severe hypoxemia, moderately malnourished, severely malnourished, child‐sex (female), wheezing |
|
0.79 (95% CI: 0.76−0.82) | N = 50,669, mortality = 4406 (8.7%) | |
| Lowlaavar et al. 26 | Primary model | Age ≥6 and ≤60 months with proven or suspected infection | Abnormal Blantyre coma score, positive HIV, weight for age z‐score |
|
0.85 (95% CI: 0.80−0.89) |
N = 86,784, mortality = 4045 (4.7%) |
|
| Model 2 | Age ≥6 and ≤60 months with proven or suspected infection | Abnormal Blantyre coma score, positive HIV, middle upper arm circumference (MUAC) |
|
0.84 (95% CI: 0.79−0.89) |
N = 86,784, mortality = 4045 (4.7%) |
||
| Model 3 | Age ≥6 and ≤60 months with proven or suspected infection | Abnormal Blantyre coma score, MUAC |
|
0.82 (0.72‐0.91) |
N = 86,784, mortality = 4045 (4.7%) |
Abbreviations: AUC, area under the curve; CIN, Clinical Information Network; RISC, respiratory index of severity in children; WAZ, weight for age z‐score.
2.5.2. Lowlaavar et al. prognostic models
Lowlaavar et al. 26 developed three models utilizing a two‐site prospective observational study in Uganda which enrolled children between 6 months and 5 years admitted with a proven or suspected infection. In their study, 1307 children were enrolled consecutively, and 65 (5%) of participants died during their hospital stay. The study was conducted between March 2012 and December 2013. The primary model included three predictors namely weight‐for‐age z‐score (WAZ), Blantyre coma scale, and HIV status. Based on the derivation dataset, the area under the curve (AUC) was 0.85 (95% CI: 0.80−0.89). The second model included MUAC (mid‐upper arm circumference), Blantyre coma scale, and HIV status. The area under the ROC curve of this model was 0.84 (95% CI: 0.79−0.89). The third model included two variables MUAC and Blantyre coma scale, with an AUC of 0.82 (0.72−0.91). The equations of these three models are provided in Table 1.
2.6. Data collection
Patients admitted to CIN hospitals have the following data routinely collected and documented: biodata (e.g., age, gender), history of illness (e.g., length of illness, history of fever, diarrhea, vomiting, convulsions, vaccinations), examination findings (for instance vital signs, presence of thrush, edema, and visible wasting), investigations done during admission (e.g., malaria, hematology, glucose, HIV, and lumber puncture tests results), admission and discharge diagnoses, treatment received at admission (e.g., antibiotics, anti‐malarial, anti‐tuberculous medications), supportive care (oxygen support, blood transfusion, fluids, bolus fluids treatment), vital signs measurements for the initial 48 h of admission, and outcome at discharge. These patient details are systematically documented by clinicians and nurses using a standardized medical record form called Pediatric Admission Record (PAR), 27 adopted for use by hospitals participating in CIN. Upon discharge or death of a patient, a trained data clerk abstracts these data to a customized data capture tool designed using a nonproprietary Research Electronic Data Capture (REDCap) platform. 28 As part of quality assurance protocols, data quality checks (data completeness and transcription errors) are run locally using a script written in R programming language before data are synchronized to a central database. If any inconsistencies, data omissions or transcription errors occur, the data clerk corrects these after verification from the patient record. The data clerk does not make corrections to documentation errors made by the clinical or nursing teams.
2.7. Patient inclusion criteria
Patients hospitalized in pediatric wards across 20 CIN hospitals were eligible for inclusion from September 2013 to December 2021. Surgical cases of burns patients, healthy children accompanying sick babies, children admitted with poisonings such as organophosphate ingestion, or any other form of poisoning, and traumatic & road traffic cases were all excluded from the validation cohort. We also excluded patients admitted during healthcare workers (nurses and doctors) strikes. 29 These exclusions were done to make the validation dataset as similar as possible to the derivation cohort of the models whose performance is assessed in this study. The following eligibility criteria were applied to obtain a model‐specific cohort for external validation.
2.7.1. Eligibility criteria for RISC‐Malawi model's external validation cohort
As defined in the study that derived RISC‐Malawi model, the external validation cohort included patients aged 2−59 months with admission diagnoses of pneumonia that were defined as follows; history of cough or difficulty breathing and at least one of the danger signs, which included central cyanosis, grunting, chest‐wall indrawing, stridor, inability to drink/breastfeed, and or painful responsive (P) or unresponsiveness (U) based on the disability scale of AVPU (Alert, Verbal, Painful responsive, unresponsive) see Figure 2.
Figure 2.
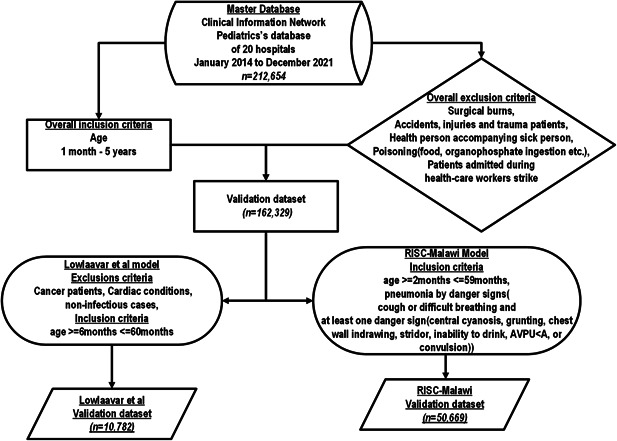
Flow chart of patients included in a model external validation cohort.
RISC‐Malawi model predictors were defined in the validation cohort as follows; moderate malnourished was defined as MUAC between 11.5 cm and 13.5 cm. Severe malnourished was defined as MUAC < 11.5 cm. Unconsciousness was assessed using the disability scale of AVPU (Alert, Verbal, Pain, Unresponsive). Thus, patients were assumed to be unconscious if they responded to pain only or were unresponsive altogether (P or U). Moderate hypoxemia was defined as oxygen saturation ranging from ≥90% to ≤92%, and severe hypoxemia was defined as oxygen saturation <90%.
To understand the performance of the RISC‐Malawi model in a scenario where the definition of predictors varied from the original study, we performed sensitivity analyses where pneumonia diagnosis was defined based on the clinical diagnosis instead of danger signs.
2.7.2. Eligibility criteria for Lowlavaar et al. models' external validation cohort
To match the clinical characteristics of the external validation cohort to that of the model derivation, we included children aged 6 to ≤60 months and excluded patients with the following features: malnourished cases (defined as clinical diagnoses of malnutrition), readmission cases, those with a cancer diagnosis, those with a heart condition, and patients with any parasitological confirmed or clinically suspected noninfectious illness see Figure 2.
Model predictors were defined as follows; weight‐for‐age z‐score was computed based on the reference materials in the WHO website (for patients <24 months) 30 , 31 and the National Centre for Health Statistics (for patients >24 months), 32 and abnormal Blantyre coma score (BCS).
Since BCS data was only collected in 6 out of 20 CIN hospitals, we limited the validation data to include patients from the 6 hospitals whose locations are shown in Figure 3. The collection of BCS data was introduced in September 2019 in the 6 hospitals participating in the WHO‐led study evaluating the subnational pilot introduction of the RTS,S/AS01 malaria vaccine in western Kenya—a region with high malaria transmission throughout the year. 33 Therefore, as defined in the model derivation study, a patient with a BCS of less than five was considered abnormal.
Figure 3.
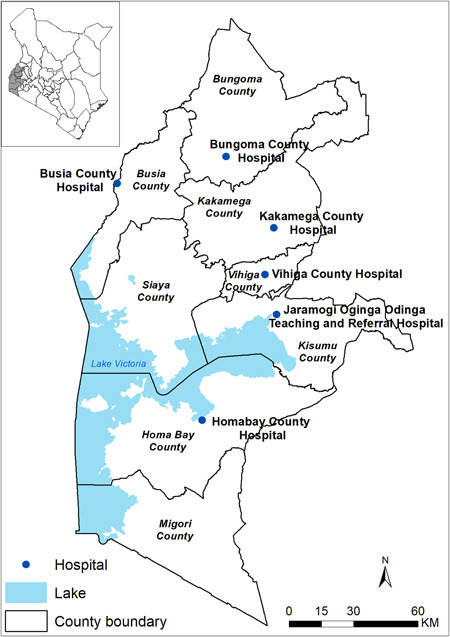
Locations of the six hospitals whose patients were included in the validation cohort of Lowlaavar et al. models.
2.8. Assessing performance of the prognostic model in the external validation
Model regression coefficients were used to determine predictions of the risk of mortality in the validation dataset as follows; for each patient in the validation cohort, the presence of the model predictor was assigned a value of 1, while its absence was assigned a value of 0. This was then multiplied by the corresponding regression coefficients and added together with the model intercept to get a linear predictor. The patient's predicted risk of in‐hospital mortality was then computed on the resultant linear predictor using the logistic function , where is the model intercept and is the regression coefficient for a given predictor X.
Model performance was determined based on two metrics: discriminatory index and model calibration. To determine the discriminative ability of the model, we used the AUC, also known as c‐statistic which is a measure of the power of a model/score to distinguish between two classes. 11 We classified the model's discriminatory ability using the following cutoffs; (AUC) ≥ 0.90 was classified as “excellent discrimination,” AUC ranging from 0.80 to 0.89 was classified as “good discrimination,” AUC ranging from 0.70 to 0.79 as “fair discrimination,” and “poor discrimination” was for the model whose AUC was <0.70. 34 , 35
Model calibration was assessed by plotting the predicted probability of in‐hospital death against the observed proportion. The observed proportion is calculated using the proportion of events that occurred relative to the total number of observations in the datasets used for validation. The predictive model calculates the probability of an event occurring for each observation in the dataset to obtain the predicted probability. Two calibration metrics are computed, namely calibration slope and calibration intercept. 36 The calibration slope, which has a reference value of 1, examines the dispersion of the predicted risks such that a slope value of <1 suggests that estimated risks are too extreme, while a slope value of >1 indicates that the estimated risks are too low. On the other hand, the calibration intercept is a measure of calibration‐in‐the‐large and has a reference value of 0 such that a calibration intercept of <0 indicate overestimation while that of >0 indicates an underestimation of risk. 36 The confidence intervals for both c‐statistic, calibration slope and intercept were calculated through bootstrap resampling using the CalibrationCurves package in R. 37
2.9. Handling missing data
The two models selected for external validation in the current study used variables that had varying levels of documentation in the validation cohort. For instance, the data in MUAC, which was used to determine malnutrition status, were missing in 49.8% of the eligible population for the RISC‐Malawi model. Documentation of this variable was also not very well documented in the derivation dataset by Hooli et al. whereby the data were missing in 45.8% of the eligible population. See Table 4 for the data missingness of predictors in the RISC‐Malawi model and Table 5 for the Lowlavaal et al. models.
Table 4.
Comparing predictors used in RISC‐Malawi model in derivation and validation datasets.
| Predictor | Variable in model derivation dataset | Variable equivalent in external validation dataset | N in the derivation dataset | N in the validation dataset |
|---|---|---|---|---|
| Oxygen saturation | Normal | Oxygen saturation 93%−100% | 10,586 (64.3%) | 16,897 (33.3%) |
| Moderate hypoxemia (90%−92%) | Oxygen saturation 90%−92% | 1382 (8.4%) | 3875 (7.6%) | |
| Severe hypoxemia | Oxygen saturation <90% | 2094 (12.7%) | 8949 (17.7%) | |
| Missing oxygen saturation | 2413 (14.7%) | 20,947 (41.3%) | ||
| Malnutrition | Normal | MUAC >13.5 cm | 4557 (27.7%) | 15,234 (30.1%) |
| Moderately malnourished | MUAC (11.5−13.5 cm) | 3382 (20.5%) | 8699 (17.2%) | |
| Severely malnourished | MUAC <11.5 cm | 991 (6.0%) | 3042 (6.0%) | |
| Missing MUAC data | 7545 (45.8%) | 25,232 (49.8%) | ||
| Wheeze | Wheezing = yes | Wheezing = yes | 4117 (25.0%) | 6666 (13.2%) |
| Wheezing = no | Wheezing = no | 8767 (53.2%) | 42,701 (84.3%) | |
| Missing wheezing data | Missing data | 3591 (21.8%) | 1302 (2.6%) | |
| Unconsciousness | Unconscious = yes | Painful responsive or unresponsive in the disability scale of AVPU (Alert, Verbal, Painful responsive, unresponsive) | 608 (3.7%) | 3221 (6.4%) |
| Unconscious = no | Alert or verbal response based on the disability scale of AVPU | 12,529 (76.1%) | 45,915 (90.6) | |
| Missing data | 3338 (20.3%) | 1533 (3.0%) |
Abbreviations: MUAC, mid‐upper arm circumference; RISC, respiratory index of severity in children.
Table 5.
Comparing predictors used in Lowlavaal et al. model in derivation and validation datasets.
| Predictor | Variable in model derivation dataset | Variable equivalent in external validation dataset | N in the derivation dataset | N in the validation dataset |
|---|---|---|---|---|
| Blantyre coma score | Abnormal Blantyre coma score (score <5) | Verbal response based on the disability scale of AVPU | Not provided | 2023 (2.3%) |
| Missing data | Not provided | 103 (1.0%) | ||
| HIV diagnosis | Positive HIV diagnosis | Positive HIV diagnosis | 66 (5.1%) | 850 (1.0%) |
| Weight for age z‐score (WAZ) | Severely stunted (WAZ < −3) | Weight for age z‐score < −3 | 206 (15.9%) | 481 (4.5%) |
| Underweight (WAZ < −2) | Weight for age z‐score < −2 | 372 (28.6%) | 2649 (24.7%) | |
| Missing data | No provided | 45 (0.4%) | ||
| Mid‐upper arm circumference | MUAC < 125 mm | MUAC < 125 mm | 187 (14.5%) | 898 (8.8%) |
| MUAC < 115 mm | MUAC < 115 mm | 94 (7.3%) | 292 (2.8%) | |
| Missing data | No provided | 531 (4.9%) |
Abbreviation: MUAC, mid‐upper arm circumference.
To avoid bias that may have resulted from excluding observations with missing data, we undertook multiple imputations to account for the uncertainty caused by missing data. 38 , 39 To do this, we created 50 imputation datasets under the assumption of missing at‐random mechanism. Variables to be imputed were ordered based on their levels of data missingness from low to high. This was meant to fully benefit from the imputing algorithm's chained equations and boost convergence. The simulation error in the multiple imputations was minimized by using 100 iterations between imputations. Validation of the prognostic model was carried out on each of the imputed datasets. Rubin's rules 40 were used to pool estimates from the 50 multiply‐imputed datasets. As recommended, 41 a plot of densities of both the observed and imputed values suggested that the imputations generated from the imputing algorithm were plausible, as shown in Supporting Information: Figure 1.
All analyses were done using R 3.6.3 (R Foundation for Statistical Computing; http://www.cran.r-project.org).
3. RESULTS
3.1. Eligible population
The CIN's database had 212,654 patients admitted and 162,329 patients were eligible to be included in the validation cohort from all hospitals (n = 20). We further applied model‐specific exclusions, as shown in Figure 2 to obtain n = 50,669 and n = 10,782 patients eligible for the external validation of the RISC‐Malawi and Lowlaavar models, respectively. Model performance results are presented using multiply‐imputed datasets.
3.1.1. Characteristics of the cohort used in the external validation of the RISC‐Malawi prognostic model
We had n = 50,669 patients who met the eligibility criteria to be included in the validation dataset of the RISC‐Malawi model. Out of this cohort, the pneumonia case fatality ratio was 8.7%, ranging from 1.9% to 16.3% across hospitals. Upon examining the characteristics of this cohort, we observed that 3221/50,669 (6.4%) of the patients were unconscious, of which 1281/3221 (39.8%) died. 3042/50,669 (6%) of all patients were severely malnourished, and 604 (19.9%) of them died. In addition, the data also suggested 8949/50,669 (17.7%) patients experienced severe hypoxemia, of which 14% (1253/8949) died, as shown in Table 2.
Table 2.
Demographic and clinical characteristics of the cohort used to externally validate RISC‐Malawi model.
| All patients | Survived | Died | |
|---|---|---|---|
| Population | n = 50,669 | 46,263/50,669 (91.3%) | 4406/50,669 (8.7%) |
| Child‐sex (female) | 22,184/50,669 (43.8%) | 20,001/22,184 (90.2%) | 2183/22,184 (9.8%) |
|
Age (months) median (IQR) |
13 (7−24) | 14 (7−25) | 9 (6−16) |
| Moderate hypoxemia | 3875/50,669 (7.6%) | 3591/3875 (92.7%) | 284/3875 (7.3%) |
| Severe hypoxemia* | 8949/50,669 (17.7%) | 7696/8949 (86%) | 1253/8949 (14%) |
| Moderately malnourished* | 8699/50,669 (17.2%) | 7988/8699 (91.8%) | 711/8699 (8.2%) |
| Severely malnourished* | 3042/50,669 (6%) | 2438/3042 (80.1%) | 604/3042 (19.9%) |
| Wheeze present | 6666/50,669 (13.2%) | 6181/6666 (92.7%) | 485/6666 (7.3%) |
| Unconsciousness* | 3221/50,669 (6.4%) | 1940/3221 (60.2%) | 1281/3221 (39.8%) |
Note: Unconscious* defined as either painful responsive or unresponsive in the disability scale of AVPU (Alert, Verbal, Painful responsive, unresponsive). Severe hypoxemia* defined as oxygen saturation <90%. Severely malnourished* defined as mid‐upper arm circumference (MUAC) <11.5 cm. Moderately malnourished* defined as MUAC between 11.5 and 13.5 cm.
Abbreviation: RISC, respiratory index of severity in children.
3.1.2. Characteristics of the cohort used in the external validation of the Lowlaavar et al. models
Since the derivation study of the Lowlaavar models included the BCS as a model predictor, the eligibility criteria for the model validation cohort included patients from six hospitals where the BCS data was collected. In this dataset, 10,782 children met the eligibility criteria, of which 570/10,782 (5.3%) experienced in‐hospital mortality. As defined in the model's derivation study, patients with a BCS <5 were considered to have abnormal BCS that was present in 1199 patients, of whom 236 (19.7%) died in the hospital, as shown in Table 3.
Table 3.
Demographic and clinical characteristics patients from six hospitals who were included in the validation cohort of Lowlaavar et al. models.
| Indicator | All patients | Survived | Died |
|---|---|---|---|
| Population | N = 10,782 | 10,212/10,782 (94.7%) | 570/10,782 (5.3%) |
| Gender (female) | 4508/10,782 (41.8%) | 4245/4508 (94.2%) | 263/4508 (5.8%) |
| Age (months) median (IQR) | 24 (14−42) | 24 (14−42) | 22.5 (11−38) |
| HIV diagnosis | 75/10,782 (0.7%) | 63/75 (84%) | 12/75 (16%) |
| Abnormal BCS | 1199/10,782 (11.1%) | 963/1199 (80.3%) | 236/1199 (19.7%) |
| WAZ | −0.5 (−1.5 to 0) | −0.5 (−1.5 to 0) | −1 (−2 to 0) |
| MUAC | 14.3 (13.5−15) | 14.3 (13.6−15) | 14 (13.1−14.8) |
Note: Abnormal BCS defined as BSC of <5.
Abbreviations: BSC, Blantyre coma score; MUAC, mid‐upper arm circumference; WAZ, weight for age Z‐score.
3.1.3. Comparison of patients' profile between validation and derivation cohort
A comparative analysis of patients' case mix between the validation and derivation cohort of the RISC‐Malawi model indicates a satisfactory degree of similarity in their characteristics. However, it is noteworthy that the dataset employed to validate the RISC‐Malawi model exhibited a relatively higher pneumonia case fatality rate (8.7%) compared to the dataset used in its derivation (3.2%). Additionally, there was a significantly higher prevalence of unconsciousness in the validation dataset (6.4%) compared to the derivation cohort (3.7%), as shown in Table 4. Similarly, the dataset employed in developing the Lowlaavar models demonstrated a mortality rate of 5% which was comparable to the mortality rate of the dataset used for validation purposes (5.3%). However, the derivation cohort exhibited relatively higher levels of malnutrition, as evidenced by their MUAC values and stunting levels, as presented in Table 5.
3.1.4. Performance of RISC‐Malawi prognostic model in external validation dataset
The discriminatory ability (c‐statistic) of the RISC‐Malawi model was 0.77 (95% CI: 0.77−0.78), whereas the calibration slope was 1.04 (95% CI: 1.00−1.06). The calibration intercept was 0.81 (95% CI: 0.77−0.84), indicative of a poorly calibrated model since it underestimates the risk (intercept >0) see Figure 4.
Figure 4.
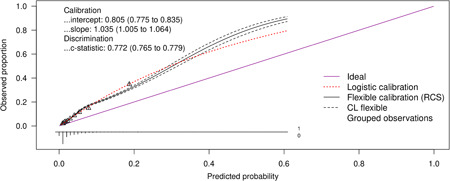
Performance of the RISC‐Malawi model in an external validation dataset. The figures show calibration curves and other model performance metrics. CL denotes confidence limits (95%); RCS denotes restricted cubic splines.
3.1.5. Performance of Lowlaavar et al. models in external validation dataset
We computed the performance statistics for the three models derived by Lowlaavar et al. and the findings were as follows; the primary model (model 1), which included three predictors (abnormal BCS, HIV+, weight for age z‐score) had a c‐statistic of 0.75 (95% CI: 0.72−0.77) while the calibration slope was 0.78 (95% CI: 0.71−0.84), and the calibration intercept was 0.37 (95% CI: 0.28−0.46). The second model (model 2) included the following three predictors: abnormal BCS, HIV+, and MUAC had a c‐statistic of 0.78 (95% CI: 0.77−0.80) while the calibration slope was 0.82 (95% CI: 0.76−0.89) and the calibration intercept was 0.92 (95% CI: 0.84−1.10). The third model with two predictors (abnormal BCS and MUAC) had a c‐statistic of 0.71 (95% CI: 0.68−0.73), while the calibration slope was 0.73 (95% CI: 0.67−0.80), and the calibration intercept was 0.39 (95% CI: 0.31−0.48) as shown in Figure 5.
Figure 5.
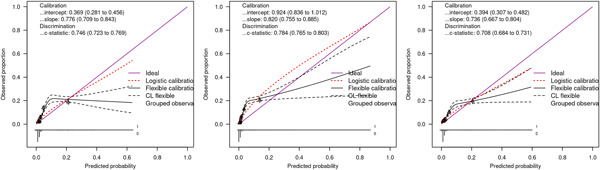
Performance of the Lowlaavar et al. (2016) models in an external validation dataset where abnormal Blantyre coma score (BCS) was defined as BCS < 5. The first panel to the left is the calibration curves of the primary model (model I), the panel in the middle are the calibration curves for model II, and the last panel to the right are the calibration curves of model III. CL denotes confidence limits (95%); RCS denotes restricted cubic splines.
3.2. Sensitivity analyses
As a sensitivity analysis, we varied the criteria of determining pneumonia diagnosis in the validation cohort of RISC‐Malawi such that instead of using danger signs (central cyanosis, grunting, indrawing, stridor, inability to drink, AVPU, and convulsion) as used in the original study to define pneumonia, we used clinical admission diagnosis of pneumonia. All other eligibility criteria remained unchanged, resulting in a sample size of 56,045 with a pneumonia case fatality rate of 7.6%. Evaluation of the RISC‐Malawi model performance in the sensitivity analyses dataset suggested a reduced performance compared to what was seen in the primary analyses, as shown in the Supporting Information: Figure 2.
We also undertook a sensitivity analysis of the Lowlavaar models using patients from all hospitals (n = 20) instead of the six hospitals as used in the main analyses. However, the abnormal BCS was defined using AVPU scores which is a disability scale such that patients who were at “V” during the clinical assessment by a physician were classified as having abnormal Blantyre scale. Those who met the eligibility criteria were 86,784 patients and in‐hospital mortality was 4.7% (n = 4045). Patients with abnormal BCS were 2023 (2.3%) out of which 268 (13.5%) died. Performance of the Lowlavaar models in the sensitivity analyses dataset were lower as compared to the main analyses as shown in the Supporting Information: Figure 3.
4. DISCUSSION
4.1. Summary of findings
The first step towards wider clinical application of clinical prediction rules is to validate existing prognostic models to identify children at risk of deterioration in diverse settings. In this study, we externally validated four predictive models initially developed by two studies to identify children at an increased risk of in‐hospital mortality in low‐resource settings. 25 , 26
Using a diverse population of children admitted to 20 hospitals from 2014 to December 2021, we performed an external validation to assess these four prognostic scores' discriminatory ability and calibration levels. All models had fair discriminatory values (AUC: 0.70−0.79). However, they all underestimated the mortality (calibration intercept >0), leading to the misclassification of patients at an increased risk of deterioration. These model performance measures were even lower when they were validated using the sensitivity analyses datasets where we varied the definitions of abnormal BCS and pneumonia from how it was defined in the original study by Lowlavaar et al. and Hooli et al. for RISC‐Malawi, respectively. This demonstrates the value addition of explaining predictors as used in the model derivation study.
The suboptimal performance of these models in the CIN data sets may be due to having more diverse patient populations and different case‐mix. Although we attempted to ensure that the patients' characteristics in the validation and derivation cohort were as similar as possible, we observed that the cohort we used to validate the RISC‐Malawi model had a higher pneumonia case fatality rate (8.7%) than the original patient group (3.2%). Despite this difference, the discriminatory ability of the RISC‐Malawi in the validation cohort had an AUC 0.77 (95% CI: 0.77−0.78), which was nearly similar to that observed in the model derivation cohort 0.79 (95% CI: 0.76−0.82).
While the calibration intercepts of all models we externally validated suggested underestimation of the risk of mortality in their predictions, calibration slopes of the same models illustrated that these predictions were too extreme, especially for the Lowlaavar et al. models whose calibration slopes were all <1. On the other hand, predictions of the risk of pneumonia‐related mortality by the RISC‐Malawi model were too low, as judged by the calibration slope of >1. The result may be partly explained by the inclusion of more physical examination variables as prognostic factors, which could make a model underperform in an external dataset because of the variations in interobserver agreement, which is more common in physical examination findings. 42 , 43 It is encouraged to include prognostic factors that do not have interobserver variations, such as blood lactate and other biomarkers, including C‐reactive protein, procalcitonin, and so forth, in the settings where these tests are available. However, while such biomarkers might have better prognostic values and are attractive to be included in the predictive models, they may not be readily available in limited‐resource settings and are costly to undertake.
In literature, the RISC‐Malawi model has been subjected to external validation in a diverse cohort of hospitalized children from the WHO's study group whose study patients were pooled from 10 studies on pediatric pneumonia from different countries. 44 In this cohort, there were 17,864 who met the eligibility criteria with a pneumonia case fatality ratio of 4.9%. The RISC‐Malawi score in that validation study had a fair discriminatory value (AUC = 0.75, 95% CI = 0.74−0.77), which was not significantly different from what was obtained in our validation study even though our validation cohort had a higher pneumonia case fatality ratio of 8.7% (Table 2). Furthermore, since calibration statistics were not reported, we could not determine how RISC‐Malawi's calibration measures in the validation study compares with what was obtained in the current study. To our knowledge, Lowlaavar et al. models have not been externally validated in any setting.
4.2. Limitations
While the CIN database is a rich source of data routinely collected from several hospitals over a long period and hence suitable for model development and validation, by design, these data were not meant for such purposes. Instead, the CIN was an essential initial step in efforts to understand and improve care in Kenyan hospitals. This led to missing data in variables of interest for many children, resulting in multiply imputing the data, a computationally prohibitive task. However, the CIN dataset had a substantial adequate sample size required for external validation studies. 45
Lastly, even though we attempted to make the validation population as similar as possible to that used in the derivation of the models we externally validated, we didn't exclude children who carried more than one diagnosis concomitantly, which could explain the reason why the validation case mortality rate is twice to that of the derivation cohort.
4.3. Fulfilled knowledge gaps and what to be done next
In the literature, it is more common to find model development studies than validation ones. Hence, many models risk not being utilized in clinical practice because they are yet to be externally validated using a diverse population as expected, thus becoming wasted research efforts. When evaluating a model for risk stratification, researchers should utilize preexisting knowledge and, if available, validate and update an existing model within a similar setting instead of building a new model from scratch with all the drawbacks of overfitting and lack of reproducibility. In this study, we have subjected four models to an external validation study to determine their clinical utility. In the ideal case of perfect validity where scores have AUC ≥ 0.8, calibration intercept = 0, and calibration slope = 1, the model could be recommended for clinical applications. However, if the model deviates from the ideal case, then there is evidence of miscalibration and model recalibration should be performed. 46 , 47 Our findings have suggested that the four models have significant miscalibration and hence underscores the necessity of recalibration as a next step. Recalibration is done by adjusting the intercept and slope of the logistic regression equation to match the observed proportion of outcomes in the external dataset. This involves fitting a new logistic regression model with the predicted probabilities from the original model and the observed outcomes in the external dataset. The intercept and slope are then adjusted to align the predicted probabilities with the observed outcomes.
4.4. Conclusions
Even though prognostic models underperform when subjected to different populations from the original population used in its derivation, none of the models externally validated in this current study displayed an outstanding discriminatory value of AUC ≥0.8 without under/overestimating the risk based on the calibration statistics. Consequently, these models may not be applied confidently in settings other than those in which they were developed. Based on our findings, recalibrating these models, or further developing prognostic models with greater sensitivity and specificity to identify children at risk of in‐hospital mortality may be warranted.
AUTHOR CONTRIBUTIONS
Morris Ogero: Formal analysis; methodology; visualization; writing—original draft; writing—review and editing. John Ndiritu: Methodology. Rachel Sarguta: Formal analysis; methodology; writing—original draft; writing—review and editing. Timothy Tuti: Formal analysis; methodology; visualization; writing—original draft; writing—review and editing. Samuel Akech: Formal analysis; funding acquisition; methodology; validation; visualization; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
KEMRI Scientific and Ethical Review Committee approved the CIN study (3459). The Kenya Ministry of Health gave permission for this work which entailed use of deidentified routine patient care data abstracted from medical records after discharge without need for individual patient/clinician consent.
TRANSPARENCY STATEMENT
The lead author Morris Ogero affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We would like to thank the Ministry of Health who gave permission for this work to be developed and have supported the implementation of the Clinical Information Network. The CIN team who contributed to the design of the data collection tools, collection of data and its management that form the basis of this research work include Mike English, Samuel Akech, Ambrose Agweyu, Grace Irimu, Michuki Maina, Jacquie Oliwa, Jalemba Aluvaala, Timothy Tuti, Paul Mwaniki, Henry Gathuri, George Mbevi, James Bukosia, Cynthia Asingwa, Sylvia Omoke, Livingstone Mumelo, John Wainana, Joyce Kigo, Kefa Wairoto, Henry Gathuri, and Dennis Muoki. Hospitals teams in CIN include Samuel N'garng'ar (Vihiga County Hospital), Ivan Muroki (Kakamega County Hospital), David Kimutai & Loice Mutai (Mbagathi County Hospital), Caren Emadau & Cecilia Mutiso (Mama Lucy Kibaki Hospital), Charles Nzioki (Machakos Level 5 Hospital), Francis Kanyingi & Agnes Mithamo (Nyeri County Hospital), Margaret Kuria (Kisumu East County Hospital), Samuel Otido (Embu County Hospital), Grace Wachira & Alice Kariuki (Karatina County Hospital), Peris Njiiri (Kerugoya County Hospital), Rachel Inginia & Melab Musabi (Kitale County Hospital), Hilda Odeny (Busia County Hospital), Grace Ochieng & Lydia Thuranira (Kiambu County Hospital); Priscilla Oweso (Vihiga County Hospital), Ernest Namayi (Mbale Rural Health and Demonstration Centre), Benard Wambani, Samuel Soita (Kakamega Provincial General Hospital), Joseph Nganga (Mbagathi District Hospital), Margaret Waweru, John Karanja (Kiambu County Hospital), Susan Owano (Mama Lucy Kibaki Hospital), Esther Muthiani (Machakos Level 5 Hospital), Alfred Wanjau (Nyeri Level 5 hospital), Larry Mwallo (Kisumu East District Hospital), Lydia Wanjiru (Embu Provincial General Hospital), Consolata Kinyua (Karatina District Hospital), Mary Nguri (Kerugoya District Hospital), and Dorothy Munjalu (Kitale District Hospital). Clinical information network (CIN) was supported by a Senior Wellcome Fellow award (number 207522 to Mike English) that provided funding for the study. S. A. was supported by the Initiative to Develop African Research Leaders Wellcome Trust (award number 107769). M. O. was supported by a scholarship from Kenya Higher Loans Education Board. The funders had no role the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Ogero M, Ndiritu J, Sarguta R, Tuti T, Akech S. Pediatric prognostic models predicting inhospital child mortality in resource‐limited settings: an external validation study. Health Sci Rep. 2023;6:e1433. 10.1002/hsr2.1433
DATA AVAILABILITY STATEMENT
Data for this report are under the primary jurisdiction of the Ministry of Health in Kenya. Enquiries about using the data can be made to the KEMRI‐Wellcome Trust Research Programme Data Governance Committee.
REFERENCES
- 1. Emi Suzuki . Global Child Mortality Rate Dropped 49% Since 1990. World Bank; 2014. [Google Scholar]
- 2. UNICEF . Levels and trends in child mortality. 2021. https://data.unicef.org/resources/levels-and-trends-in-child-mortality/2022
- 3. Ayieko P, Ogero M, Makone B, et al. Characteristics of admissions and variations in the use of basic investigations, treatments and outcomes in Kenyan hospitals within a new Clinical Information Network. Arch Dis Child. 2016;101(3):223‐229. 10.1136/archdischild-2015-309269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharrow, D , Hug, L , You, D , et al. Global, regional, and national trends in under‐5 mortality between 1990 and 2019 with scenario‐based projections until 2030: a systematic analysis by the UN Inter‐agency Group for Child Mortality Estimation. The Lancet Global Health, 2022;10(2):e195‐e206. 10.1016/s2214-109x(21)00515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hands C, Hands S, Verriotis M, et al. Emergency triage assessment and treatment plus (ETAT+): adapting training to strengthen quality improvement and task‐sharing in emergency paediatric care in Sierra Leone. J Glob Health. 2021;11:04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayieko P, Ogero M, Makone B, et al. Characteristics of admissions and variations in the use of basic investigations, treatments and outcomes in Kenyan hospitals within a new Clinical Information Network. Arch Dis Child. 2016;101(3):223‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vogenberg FR. Predictive and prognostic models: implications for healthcare decision‐making in a modern recession. Am Health Drug Benefits. 2009;2(6):218‐222. [PMC free article] [PubMed] [Google Scholar]
- 8. Ogero M, Sarguta R, Malla L, Aluvaala J, Agweyu A, Akech S. Methodological rigor of prognostic models for predicting in‐hospital paediatric mortality in low‐and middle‐income countries: a systematic review protocol. Wellcome Open Res. 2020;5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogero M, Sarguta RJ, Malla L, et al. Prognostic models for predicting in‐hospital paediatric mortality in resource‐limited countries: a systematic review. BMJ Open. 2020;10(10):e035045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feinstein AR. Multivariable Analysis: an Introduction. Yale University Press; 1996:578‐582. [Google Scholar]
- 11. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins GS, Reitsma JB, Altman DG, Moons K. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med. 2015;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1‐W73. [DOI] [PubMed] [Google Scholar]
- 14. Collins GS, Altman DG. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open cohort study. BMJ. 2009;339:b2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453‐473. [DOI] [PubMed] [Google Scholar]
- 16. Sauerbrei W, Perperoglou A, Schmid M, et al. State of the art in selection of variables and functional forms in multivariable analysis—outstanding issues. Diagnostic and Prognostic Research. 2020;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- 18. Bleeker SE, Moll HA, Steyerberg EW, et al. External validation is necessary in prediction research. JCE. 2003;56(9):826‐832. [DOI] [PubMed] [Google Scholar]
- 19. Olson D, Davis NL, Milazi R, et al. Development of a severity of illness scoring system (inpatient triage, assessment and treatment) for resource‐constrained hospitals in developing countries. Tropical Medicine & International Health: TM & IH. 2013;18(7):871‐878. 10.1111/tmi.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reed C, Madhi SA, Klugman KP, et al. Development of the respiratory index of severity in children (RISC) score among young children with respiratory infections in South Africa. PLoS One. 2012;7(1):e27793. 10.1371/journal.pone.0027793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. George EC, Walker AS, Kiguli S, et al. Predicting mortality in sick African children: the FEAST paediatric emergency triage (PET) score. BMC Med. 2015;13(1):174. 10.1186/s12916-015-0407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helbok R, Kendjo E, Issifou S, et al. The Lambaréné organ dysfunction score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis. 2009;200(12):1834‐1841. 10.1086/648409 [DOI] [PubMed] [Google Scholar]
- 23. Conroy AL, Hawkes M, Hayford K, et al. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non‐malaria febrile illness. Crit Care. 2015;19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berkley JA. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ. 2003;326(7385):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hooli S, Colbourn T, Lufesi N, et al. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC‐Malawi) from Malawi. PLoS One. 2016;11(12):e0168126. 10.1371/journal.pone.0168126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowlaavar N, Larson CP, Kumbakumba E, et al. Pediatric in‐hospital death from infectious disease in Uganda: derivation of clinical prediction models. PLoS One. 2016;11(3):e0150683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ministry of Health (MOH) [Kenya] . Paediatric admitting record form. Kenya: Ministry of Health; 2015. http://www.idoc-africa.org/images/documents/Paeds%202_a_-%20PAR%20Paediatric%20Admitting%20Record%20Form.pdf2016
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377‐381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irimu G, Ogero M, Mbevi G, et al. Tackling health professionals' strikes: an essential part of health system strengthening in Kenya. BMJ Glob Health. 2018;3(6):e001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Boys weight for age z‐score, 2021.
- 31. World Health Organization . Girls weight for age z‐score, 2021.
- 32. Centers for Disease Control and Prevention . National Center for Health Statistics. In: Files Z‐sD, ed., 2021.
- 33. Akech S, Chepkirui M, Ogero M, et al. The clinical profile of severe pediatric malaria in an area targeted for routine RTS, S/AS01 malaria vaccination in Western Kenya. Clin Infect Dis. 2020;71(2):372‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bijlsma MW, Brouwer MC, Bossuyt PM, et al. Risk scores for outcome in bacterial meningitis: systematic review and external validation study. J Infect. 2016;73(5):393‐401. [DOI] [PubMed] [Google Scholar]
- 35. Muller MP, Tomlinson G, Marrie TJ, et al. Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community‐acquired pneumonia? Clin Infect Dis. 2005;40(8):1079‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Calster B, McLernon DJ, Van Smeden M, Wynants L, Steyerberg EW. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CalibrationCurves: Calibration performance [program] . R package version 0.1.2 version, 2016.
- 38. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377‐399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 40. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 41. Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1548‐7660. [Google Scholar]
- 42. Florin TA, Ambroggio L, Brokamp C, et al. Reliability of examination findings in suspected community‐acquired pneumonia. Pediatrics. 2017;140(3):e20170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sjoding MW, Cooke CR, Iwashyna TJ, Hofer TP. Acute respiratory distress syndrome measurement error. Potential effect on clinical study results. Ann Am Thorac Soc. 2016;13(7):1123‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rees CA, Hooli S, King C, et al. External validation of the RISC, RISC‐Malawi, and PERCH clinical prediction rules to identify risk of death in children hospitalized with pneumonia. J Glob Health. 2021;11:04062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vergouwe Y, Steyerberg EW, Eijkemans MJC, Habbema JDF. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. JCE. 2005;58(5):475‐483. [DOI] [PubMed] [Google Scholar]
- 46. Steyerberg EW, Borsboom GJJM, van Houwelingen HC, Eijkemans MJC, Habbema JDF. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med. 2004;23(16):2567‐2586. [DOI] [PubMed] [Google Scholar]
- 47. Steyerberg EW, Harrell FE Jr., Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models. JCE. 2001;54(8):774‐781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data for this report are under the primary jurisdiction of the Ministry of Health in Kenya. Enquiries about using the data can be made to the KEMRI‐Wellcome Trust Research Programme Data Governance Committee.


