Abstract
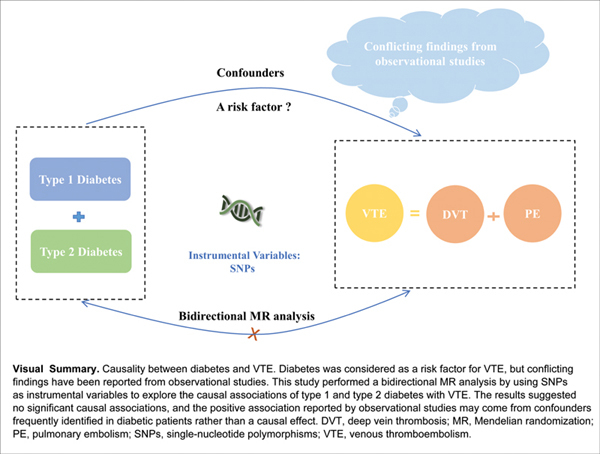
Background Diabetes was considered as a risk factor for venous thromboembolism (VTE), but conflicting findings have been reported from observational studies. This study aimed at investigating the causal associations of type 1 and type 2 diabetes with VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE).
Methods We designed a bidirectional two-sample Mendelian randomization (MR) analysis by using summary-level data from large genome-wide association studies performed in European individuals. Inverse variance weighting with multiplicative random effect method was used to obtain the primary causal estimates, and weighted median, weighted mode, and MR egger regression were replenished as sensitivity analyses to test the robustness of the results.
Results We found no significant causal effects of type 1 diabetes on VTE (odds ratio [OR]: 0.98, 95% confidence interval [CI]: 0.96–1.00, p = 0.043), DVT (OR: 0.98, 95% CI: 0.95–1.00, p = 0.102), and PE (OR: 0.98, 95% CI: 0.96–1.01, p = 0.160). Similarly, no significant associations of type 2 diabetes with VTE (OR: 0.97, 95% CI: 0.91–1.03, p = 0.291), DVT (OR: 0.96, 95% CI: 0.89–1.03, p = 0.255), and PE (OR: 0.97, 95% CI: 0.90–1.04, p = 0.358) were also observed. Results from multivariable MR analysis were consistent with the findings in univariable analysis. In the other direction, the results showed no significant causal effects of VTE on type 1 and type 2 diabetes.
Conclusion This MR analysis demonstrated no significant causal associations of type 1 and type 2 diabetes with VTE in both directions, in conflict with previous observational studies reporting positive association, which provided clues for understanding the underlying pathogenesis of diabetes and VTE.
Keywords: diabetes, venous thromboembolism, deep vein thrombosis, pulmonary embolism, Mendelian randomization
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major global burden of disease. As the third leading vascular disease after acute myocardial infarction and stroke, VTE affects nearly 10 million people worldwide per year. 1 With the enlarging aging population, improving economic conditions, and increasing prevalence of obesity and metabolic diseases, the incidence of VTE is expected to rise. 2 3
It was proposed that VTE and atherosclerotic disease have shared risk factors. 4 Many risk factors, including obesity, smoking, dyslipidemia, metabolic syndrome, and diabetes, were found to increase the risk of both VTE and atherosclerotic disease. 5 As a common risk factor of atherosclerotic disease, diabetes was considered as a risk factor for VTE as well. 6 Laboratory evidence supported that insulin resistance and acute and chronic hyperglycemia could induce coagulation activation and fibrinolysis impairment, resulting in a hypercoagulable state in patients with diabetes. 7 8 9 Lots of observational studies have also investigated the association between diabetes and VTE, whereas the results were inconsistent. 10 11 12 13 14 15 Two previous meta-analyses estimated a 1.4-fold increased risk of VTE for patients with diabetes compared with patients without, 10 11 while after adjusting the potential confounders, another large meta-analysis suggested that diabetes was unlikely to play a major role in VTE development. 12 Furthermore, a recent study evaluated the association of type 1 and type 2 diabetes with VTE, and they found that type 2 diabetes was related to the increased risk of VTE, but type 1 diabetes was not in the unadjusted model; however, in the fully adjusted model, type 1 diabetes was associated with a greater risk for VTE but type 2 diabetes was not. 13 Due to the limitations in terms of potential confounders and reversal causality bias, it is difficult to infer causality between diabetes and VTE from these observational studies. Mendelian randomization (MR) is a method that uses genetic variants as instrumental variables (IVs) to test the causal relation between a modifiable exposure and a disease. 16 17 As an individual's alleles were randomly allocated and fixed at conception, the MR method can circumvent reversal causality and environmental confounders that were inherent in traditional epidemiologic approaches. 18 19 20
To infer the causality between diabetes and VTE, we perform a bidirectional MR analysis to investigate the causal associations of type 1 and type 2 diabetes with VTE (including DVT and PE), and the multivariable MR (MVMR) method was also conducted to reduce the potential pleiotropic effects.
Methods
Study Design
This study was a bidirectional two-sample MR analysis, using single-nucleotide polymorphisms (SNPs) as IVs to investigate the causal associations of type 1 and type 2 diabetes with VTE (including DVT and PE). The causal effects assessed by MR method based on three assumptions: (1) IVs are associated with the exposure; (2) IVs are not associated with the outcomes except through the exposure; (3) IVs are not related to measured or unmeasured confounders.
At first, we used type 1 or type 2-associated SNPs to assess their causal effects on VTE. Then, to exclude the reversal causality, the VTE-associated SNPs were used as IVs for examining the causal associations in the other direction. Summary-level data for exposures (sample 1) and outcomes (sample 2) were extracted from the recent large genome-wide association studies (GWAS) of European descendants, which can be retrieved on MRC integrative Epidemiology Unit OpenGWAS data. 21
Data Source and Selection of Instrumental Variables
An overview of GWAS used for exposures and outcomes is shown in Supplementary Table S1 (available in the online version) . Summary-level data of type 1 diabetes were acquired from the GWAS conducted by Forgetta et al in 2020. 22 This study included 9,358 type 1 diabetes cases and 15,705 controls covering 12,783,129 SNPs from 12 European cohorts, with the objective to discover novel rare loci with large effects on risk of type 1 diabetes. For type 2 diabetes, summary-level data were extracted from a meta-analysis of GWAS with 62,892 type 2 diabetes cases and 596,424 controls, containing 5,030,727 SNPs. 23 This study meta-analyzed three GWAS datasets of European descendants: DIAbetes Genetics Replication and Meta-analysis (DIAGRAM, N = 149,821, participants from more than 10 countries), Genetic Epidemiology Research on Aging (GERA, N = 53,888, all White European American participants), and UK Biobank (UKB, N = 455,607, all UK participants), aiming at identifying functional genes and inferring possible mechanisms for type 2 diabetes. Moreover, GWAS datasets used for VTE (9,176 cases and 209,616 controls), PE (4,185 cases and 214,228 controls), and DVT (4,576 cases and 190,028 controls) were derived from FinnGen biobank ( www.finngen.fi ) that released in 2021. The FinnGen research project was launched in 2017, by combining genome information with digital health care data from Finnish participants. The project aims at improving human health through genetic research. The FinnGen study plans to utilize 500,000 unique samples collected from a nationwide network of Finnish biobanks. Once completing the study, the database will cover approximately 10% of the Finnish population. The study was conducted according to the guidelines of the Declaration of Helsinki. The informed consent was obtained from the participants. The study protocol had been approved by the institutional review committees of the original research studies.
The selection of SNPs used as IVs was according to the criteria as follows: (1) SNPs must meet genome-wide significance criteria ( p < 5 × 10 −8 ); (2) SNPs were not in linkage disequilibrium (defined as r 2 < 0.001 within 10,000 kbp window based on the European 1000 Genomes panel); (3) F-statistic [F-statistic = (β/SE) 2 ] of SNPs >10 to avoid weak instrument bias; (4) removing instruments with reverse causality by using Steiger filtering; (5) removing potential pleiotropic SNPs by the MR pleiotropy residual sum and outlier (MR-PRESSO) method. 24 25
Statistical Analysis
The inverse variance weighting with multiplicative random effect (IVW-MRE) method was used to obtain the primary causal estimates. Odds ratios (ORs) with 95% confidence intervals (CIs) are presented for the causal effects. The statistical significance was considered as p < 0.05/6 (2 exposures × 3 outcomes) = 0.008; findings with p- value between 0.008 and 0.05 were regarded as suggestive evidence of association.
To validate assumption 1, the IVs selected were strongly associated with the exposure of interest at genome-wide significance level. And also, SNP F-statistics, which assess the instrument strength, were all greater than 10 ( Supplementary Table S2, available in the online version ). 26 The assumptions 2 and 3 are independent from horizontal pleiotropy. 27 Several methods were conducted to reduce the potential pleiotropy. First, the potential pleiotropic SNPs were removed by the MR-PRESSO method. 24 25 Second, other MR analysis methods, including MR egger regression, weighted median, and weighted mode, were performed as additional sensitivity analyses. The potential pleiotropy was tested by MR egger regression, and an intercept did not statistically differ from zero ( p > 0.05) that suggested that there might be no horizontal pleiotropy. 27 28 Third, given that both smoking and body mass index (BMI) were related to diabetes and VTE, 29 30 31 it indicated that these factors may confound the diabetes–VTE association. Therefore, to further reduce the potential pleiotropy, MVMR with smoking and BMI as covariates was also implemented. An overview of GWAS summary statistics for covariates is listed in Supplementary Table S3 (available in the online version) .
Despite GWAS used for VTE (including DVT and PE) derived from FinnGen biobank, which only enrolled Finnish participants, GWAS used for type 1 and type 2 diabetes partially included Finnish individuals. A limited overlapping might present between the cohorts used in the exposures and outcomes GWAS, which was unavoidable due to the use of summary-level data. To avoid the weak instrument bias in MR analyses brought by potential overlapped datasets, IVs with F-statistic <10 were removed as mentioned above. The distribution (range and mean) of F-statistics is shown in Supplementary Table S2 (available in the online version) , which indicated that F-statistics were large enough to avoid the weak instrumental bias.
The analyses were performed using the R software (R Foundation for Statistical Computing, Austria, version 4.1.3), and the R package of “TwoSampleMR” and “MR-PRESSO” was used to perform MR analyses.
Results
A bidirectional MR analysis was performed to assess the association between diabetes and VTE. The numbers of eligible SNPs associated with exposures (diabetes or VTE) are shown in Figs. 1 and 2 , respectively. The causal estimates were calculated by both univariable and MVMR analyses.
Fig. 1.
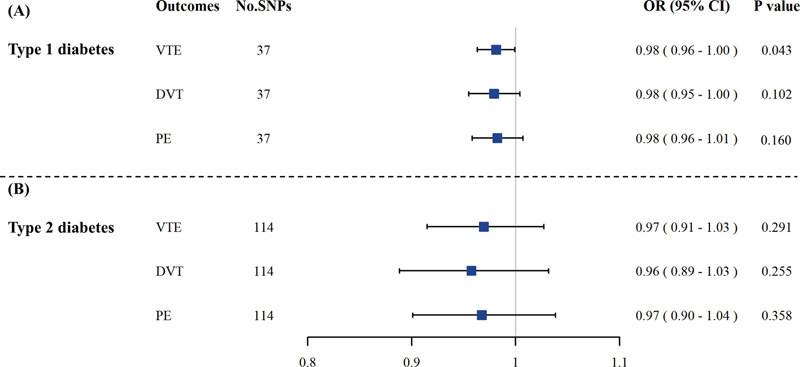
The causal association of diabetes with VTE. In univariable MR analysis, results of IVW-MRE suggested that there were no significant causal effects of type 1 ( A ) and type 2 ( B ) diabetes on VTE. CI, confidence interval; DVT, deep vein thrombosis; IVW-MRE, inverse variance weighting with multiplicative random effect method; MR, Mendelian randomization; No. SNPs, number of single-nucleotide polymorphisms; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
Fig. 2.
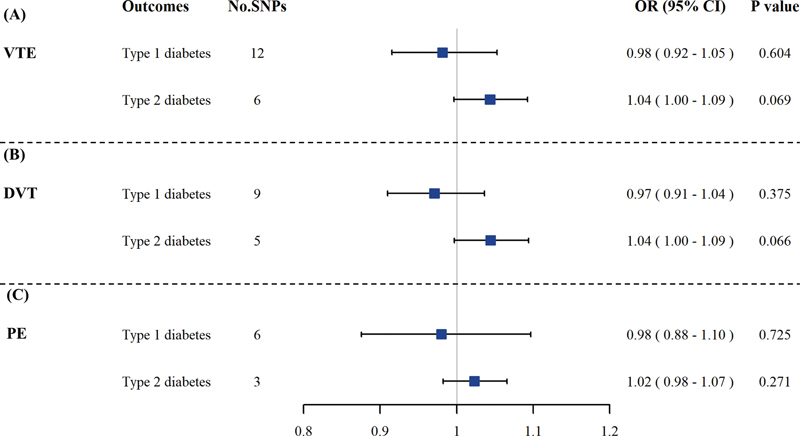
The causal associations of VTE with type 1 and type 2 diabetes. The results showed that there were no causal effects of VTE ( A ), DVT ( B ), and PE ( C ) on type 1 and type 2 diabetes by IVW-MRE in univariable MR analysis. CI, confidence interval; DVT, deep vein thrombosis; IVW-MRE, inverse variance weighting with multiplicative random effect method; MR, Mendelian randomization; No. SNPs, number of single-nucleotide polymorphisms; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
Causal Effects of Diabetes on VTE
The causal associations of type 1 diabetes and type 2 diabetes with VTE by univariable MR analysis are shown in Fig. 1 . The results of IVW-MRE suggested that there was a suggestive causal effect of type 1 diabetes on VTE (OR: 0.98, 95% CI: 0.96–1.00, p = 0.043). However, no significant effects were observed on DVT (OR: 0.98, 95% CI: 0.95–1.00, p = 0.102) and PE (OR: 0.98, 95% CI: 0.96–1.01, p = 0.160), respectively.
For type 2 diabetes, no significant association with VTE was found (OR: 0.97, 95% CI: 0.91–1.03, p = 0.291) ( Fig. 2 ). And also, there were no causal associations of type 2 diabetes with DVT (OR: 0.96, 95% CI: 0.89–1.03, p = 0.255) and PE (OR: 0.97, 95% CI: 0.90–1.04, p = 0.358), respectively.
In sensitivity analysis ( Supplementary Table S4, available in the online version ), for type 1 diabetes and type 2 diabetes–VTE associations, the estimated ORs calculated by MR egger regression, weighted median, and weighted mode showed similar directions with the results from the IVW-MRE method. The pleiotropy test using MR egger regression indicated that pleiotropy may exist for causal association between type 1 diabetes and DVT ( p < 0.05) ( Supplementary Table S5, available in the online version ), and the MR Egger regression also suggested a significant causal association of type 1 diabetes with DVT (OR: 0.95, 95% CI: 0.91–0.98, p = 0.004). Moreover, no significant pleiotropy biased the other causal estimations ( p > 0.05).
The diabetes and VTE risk were correlated with smoking and BMI. To further reduce the influence of potential pleiotropy from these factors, MVMR analysis was conducted. After adjusting for smoking and BMI, no significant associations of type 1 and type 2 diabetes with VTE were observed ( Table 1 ).
Table 1. The results of multivariable MR analysis for associations between diabetes and VTE.
| Exposures | Outcome | No. SNPs | OR (95% CI) | p -Value |
|---|---|---|---|---|
| Type 1 diabetes | VTE | 25 | 0.98 (0.96–1.00) | 0.080 |
| DVT | 25 | 0.98 (0.95–1.01) | 0.197 | |
| PE | 25 | 0.98 (0.95–1.01) | 0.166 | |
| Type 2 diabetes | VTE | 89 | 0.97 (0.91–1.03) | 0.277 |
| DVT | 89 | 0.96 (0.89–1.04) | 0.274 | |
| PE | 89 | 0.96 (0.89–1.04) | 0.310 |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; MR, Mendelian randomization; No. SNPs, number of single-nucleotide polymorphisms; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
Note: The table provided the results of multivariable MR after controlling smoking and body mass index.
Causal Effects of VTE on Diabetes
We further examined the causal effects of VTE on type 1 and type 2 diabetes. In both univariable ( Fig. 2 ) and MVMR analysis ( Table 2 ), no significant causal effects of VTE on type 1 and type 2 diabetes were observed. Moreover, the sensitivity analysis ( Supplementary Table S6, available in the online version ) supported the results from IVW-MRE in univariable analysis, and the pleiotropy test using MR egger regression indicated that no significant pleiotropy biased the causal estimations ( p > 0.05) ( Supplementary Table S7, available in the online version ).
Table 2. The results of multivariable MR analysis for associations between VTE and diabetes.
| Exposures | Outcome | No. SNPs | OR (95% CI) | p -Value |
|---|---|---|---|---|
| VTE | Type 1 diabetes | 9 | 1.04 (0.92–1.18) | 0.493 |
| Type 2 diabetes | 5 | 1.05 (0.89–1.24) | 0.525 | |
| DVT | Type 1 diabetes | 5 | 1.05 (0.95–1.16) | 0.321 |
| Type 2 diabetes | 3 | 1.07 (0.92–1.25) | 0.381 | |
| PE | Type 1 diabetes | 4 | 1.01 (0.88–1.16) | 0.866 |
| Type 2 diabetes | 3 | 1.05 (0.92–1.21) | 0.465 |
Abbreviations: CI, confidence interval; DVT, deep vein thrombosis; MR, Mendelian randomization; No. SNPs, number of single-nucleotide polymorphisms; OR, odds ratio; PE, pulmonary embolism; VTE, Venous thromboembolism.
Note: The table provided the results of multivariable MR after controlling smoking and body mass index.
Discussion
In comparison with some well-recognized risk factors of VTE, such as immobility, major trauma, older age, cancer, and thrombophilia, conflicting results were reported for diabetes. 10 11 12 The current study provided the first evidence from MR analysis on the causal associations of type 1 and type 2 diabetes with VTE, and no significant causal effects of type 1 and type 2 diabetes on VTE risk were observed in univariable analysis. After controlling for potential confounders, the causal associations were not changed.
Despite possible pleiotropy existed between type 1 diabetes and DVT, and the MR Egger test suggesting a significant causal association between type 1 diabetes and DVT, it should be noted that MR Egger is statistically less powerful than IVW and no causal association was indicated by MVMR analysis. Thus, we believe no causal effects of type 1 diabetes on DVT. In addition, a bidirectional design also excluded the reversal causality between VTE and diabetes.
Hyperglycemia and insulin resistance, the main metabolic abnormalities in diabetes, have been proposed to contribute to the diabetic prothrombotic status through a series of events, including endothelial dysfunction, platelet hyperactivity, impaired fibrinolysis, oxidative stress, and low-grade inflammation. 32 Not only atherothrombotic events, VTE was also seen to occur more frequently in patients with diabetes. 33 Moreover, according to the known evidence, current guidelines considered diabetes as a weak risk factor (OR < 2) for VTE. 6 Our null findings for these reasons came as surprising. Nevertheless, some observational studies were in line with our findings when confounders were meticulously taken into account. 12 15 34
The results of the current study contradicted the findings from two previous meta-analyses that suggested that diabetes increased the risk of VTE. 10 11 However, BMI, race, and other potential confounders that may affect the diabetes–VTE association were not considered in these previous studies, thus making their results difficult to interpret. After adjusting for BMI, Bell et al found the absence of statistical significance. 12 Similarly, in a case–control study, diabetes was univariately identified as a risk factor for VTE, while the association disappeared after controlling for confounders. 15 In several observational studies, 15 35 36 on account of the positive association of diabetes with VTE was attenuated after adjusting for BMI, it was suggested that some of the associations may be attributed to a higher prevalence of obesity among individuals with diabetes. In addition, the commonly used adjustment factors in observational studies, such as obesity, hypertension, dyslipidemia, and smoking, may contribute to the reported hypercoagulability and endothelial dysfunction among diabetic patients. 34 On the other hand, Heit et al have shown that patients with diabetes were more frequently hospitalized for surgery or for acute medical illness, or confined to a nursing home, which tended to support that these risk factors predisposed these patients to develop VTE. 15
Pleiotropy is an important issue to be considered in MR analysis. In many cases, multiple genetic variants were used in MR analysis, and it may be difficult to ensure each genetic variant solely associating with the risk factor of interest. In the current study, for example, if some diabetes variants had effects on VTE through other pathways instead of diabetes, IV assumptions would not hold and the causal estimates were biased. Given that smoking and BMI were both related to diabetes and VTE, 29 30 31 we used MVMR analysis with adjustment for smoking and BMI to assess the independent effects of diabetes on VTE. Consistent with some observational studies, 12 15 34 37 the results indicated that there were still no significant causal effects of type 1 and type 2 diabetes on VTE risk. Since the MR method is able to examine the causal association and without the influence of reversal causality and environmental confounding, we believe that diabetes is not causally related to the development of VTE.
Some limitations need to be addressed in our study. First, as stated above, although this study suggested no causal effects of type 1 and type 2 diabetes on VTE risk, the exact pathways that underlying high prevalence of VTE in these patients need further investigation. Second, to ensure the homogeneity of genetic background, we assess and compare the causal associations of type 1 and type 2 diabetes with VTE only in individuals of European descendants, thus limiting the generalizability of our results to populations with other races or ethnicities. Third, the summary-level data were used to performed MR analysis, thus the effects of diabetes on VTE in different subgroups, such as older versus younger or male versus female, cannot be answered. Fourth, a limited overlapping might exist between the cohorts used in the exposures and outcomes, but the F-statistics for IVs were large enough to avoid the weak instrumental bias. Nonetheless, it was the first bidirectional MR study to assess the causal association of diabetes with VTE, and both univariable and multivariable analyses obtained the consistent results.
Conclusion
In conclusion, the bidirectional MR analysis demonstrated that there were no significant causal associations of type 1 and type 2 diabetes with VTE. The positive association reported by observational studies may result from confounders frequently identified in diabetic patients rather than a causal effect of diabetes.
Funding Statement
Funding The study was supported by grants from CAMS Innovation Fund for Medical Sciences (CIFMS) (2022-I2M-C&T-B-040) and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (NCRC2020007).
Footnotes
Conflict of Interest None declared.
What is known about this topic?
Diabetes was considered as a risk factor for venous thromboembolism (VTE).
The association between diabetes and VTE was conflicting according to available research studies.
What does this paper add?
There were no significant causal effects of type 1 and type 2 diabetes on VTE.
No significant causal effects of VTE on type 1 and type 2 diabetes were also observed.
The exact mechanisms underlying high prevalence of VTE in these patients need further investigation.
Supplementary Material
References
- 1.Khan F, Tritschler T, Kahn S R, Rodger M A.Venous thromboembolism Lancet 2021398(10294):64–77. [DOI] [PubMed] [Google Scholar]
- 2.Liew N C, Alemany G V, Angchaisuksiri P et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36(01):1–20. doi: 10.23736/S0392-9590.16.03765-2. [DOI] [PubMed] [Google Scholar]
- 3.Venous Thromboembolism Collaboration . Siegal D M, Eikelboom J W, Lee S F et al. Variations in incidence of venous thromboembolism in low-, middle-, and high-income countries. Cardiovasc Res. 2021;117(02):576–584. doi: 10.1093/cvr/cvaa044. [DOI] [PubMed] [Google Scholar]
- 4.Duggirala M K, Cook D A, Mauck K F.An association between atherosclerosis and venous thrombosis N Engl J Med 200334904401–402., author reply 401–402 [DOI] [PubMed] [Google Scholar]
- 5.Piazza G, Goldhaber S Z. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121(19):2146–2150. doi: 10.1161/CIRCULATIONAHA.110.951236. [DOI] [PubMed] [Google Scholar]
- 6.ESC Scientific Document Group . Konstantinides S V, Meyer G, Becattini C et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(04):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 7.Lemkes B A, Hermanides J, Devries J H, Holleman F, Meijers J C, Hoekstra J B. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8(08):1663–1669. doi: 10.1111/j.1538-7836.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 8.Bryk A H, Prior S M, Plens K et al. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol. 2019;18(01):49. doi: 10.1186/s12933-019-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant P J. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262(02):157–172. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 10.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen P W. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(01):93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Ding X, Du X, Zhao X, Wang Z, Ma Z. Diabetes is associated with increased risk of venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2015;135(01):90–95. doi: 10.1016/j.thromres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Bell E J, Folsom A R, Lutsey P L et al. Diabetes mellitus and venous thromboembolism: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;111:10–18. doi: 10.1016/j.diabres.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton W, Nemeth B, de Lusignan S et al. Effect of type 1 diabetes and type 2 diabetes on the risk of venous thromboembolism. Diabet Med. 2021;38(05):e14452. doi: 10.1111/dme.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holst A G, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121(17):1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 15.Heit J A, Leibson C L, Ashrani A A, Petterson T M, Bailey K R, Melton L J., III Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arterioscler Thromb Vasc Biol. 2009;29(09):1399–1405. doi: 10.1161/ATVBAHA.109.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emdin C A, Khera A V, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 17.Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VA Million Veteran Program . Levin M G, Klarin D, Assimes T L et al. Genetics of smoking and risk of atherosclerotic cardiovascular diseases: a mendelian randomization study. JAMA Netw Open. 2021;4(01):e2034461. doi: 10.1001/jamanetworkopen.2020.34461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higbee D H, Granell R, Sanderson E, Davey Smith G, Dodd J W. Lung function and cardiovascular disease: a two-sample Mendelian randomisation study. Eur Respir J. 2021;58(03):2.003196E6. doi: 10.1183/13993003.03196-2020. [DOI] [PubMed] [Google Scholar]
- 20.Million Veteran Program . Larsson S C, Mason A M, Bäck M et al. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J. 2020;41(35):3304–3310. doi: 10.1093/eurheartj/ehaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsworth B, Lyon M, Alexander Tet al. The MRC IEU OpenGWAS data infrastructure.2020
- 22.DCCT/EDIC Research Group . Forgetta V, Manousaki D, Istomine R et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. 2020;69(04):784–795. doi: 10.2337/db19-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.eQTLGen Consortium . Xue A, Wu Y, Zhu Z et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(01):2941. doi: 10.1038/s41467-018-04951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Spiller W, Del Greco M F et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47(04):1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbanck M, Chen C Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(05):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi Y, Lee S J, Spiller W et al. Causal associations between serum bilirubin levels and decreased stroke risk: a two-sample Mendelian randomization study. Arterioscler Thromb Vasc Biol. 2020;40(02):437–445. doi: 10.1161/ATVBAHA.119.313055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(02):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwig F P, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(06):1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101–107. doi: 10.1016/j.trsl.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emerging Risk Factors Collaboration . Gregson J, Kaptoge S, Bolton T et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4(02):163–173. doi: 10.1001/jamacardio.2018.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan J S, Liu N N, Guo T T, Hu S, Hua L. Genetically predicted obesity and risk of deep vein thrombosis. Thromb Res. 2021;207:16–24. doi: 10.1016/j.thromres.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(01):121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson J W. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48(05):1017–1021. doi: 10.1007/s00125-005-1715-5. [DOI] [PubMed] [Google Scholar]
- 34.Gariani K, Mavrakanas T, Combescure C, Perrier A, Marti C. Is diabetes mellitus a risk factor for venous thromboembolism? A systematic review and meta-analysis of case-control and cohort studies. Eur J Intern Med. 2016;28:52–58. doi: 10.1016/j.ejim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Tsai A W, Cushman M, Rosamond W D, Heckbert S R, Polak J F, Folsom A R. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 36.Van Schouwenburg I M, Mahmoodi B K, Veeger N J et al. Insulin resistance and risk of venous thromboembolism: results of a population-based cohort study. J Thromb Haemost. 2012;10(06):1012–1018. doi: 10.1111/j.1538-7836.2012.04707.x. [DOI] [PubMed] [Google Scholar]
- 37.Glynn R J, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


