Summary
TINF2 encodes the TINF2 protein, which is a subunit in the shelterin complex critical for telomere regulation. Three recent studies have associated six truncating germline variants in TINF2 that have previously been associated with a cancer predisposition syndrome (CPS) caused by elongation of the telomeres. This has added TINF2 to the long telomere syndrome genes, together with other telomere maintenance genes such as ACD, POT1, TERF2IP, and TERT.
We report a clinical study of 102 Danish patients with multiple primary melanoma (MPM) in which a germline truncating variant in TINF2 (p.(Arg265Ter)) was identified in four unrelated participants. The telomere lengths of three variant carriers were >90% percentile. In a routine diagnostic setting, the variant was identified in two more families, including an additional MPM patient and monozygotic twins with thyroid cancer and other cancer types. A total of 10 individuals from six independent families were confirmed carriers, all with cancer history, predominantly melanoma. Our findings suggest a major role of TINF2 in Danish patients with MPM.
In addition to melanoma, other cancers in the six families include thyroid, renal, breast, and sarcoma, supporting a CPS in which melanoma, thyroid cancer, and sarcoma predominate. Further studies are needed to establish the full spectrum of associated cancer types and characterize lifetime cancer risk in carriers.
Keywords: germline TINF2, long telomeres, multiple primary melanoma
We report a germline TINF2 variant associated with long telomeres, which we identified in 10 individuals from six independent families, all with cancer history, predominantly melanomas, supportive of long-telomere-associated cancer predisposition. Our findings suggest a major role of TINF2 in Danish patients with multiple primary melanoma.
Main text
High-risk melanoma susceptibility genes include CDKN2A (MIM: 600160), CDK4 (MIM: 123829), and BAP1 (MIM: 603089), all genes involved in cell cycle regulation; however, BAP1 is also involved in regulation of DNA damage response, cell senescence, and apoptosis, as well as the more recently discovered TERT (MIM: 187270), POT1 (MIM: 606478), ACD (MIM: 609377), and TERF2IP (MIM: 605061) genes involved in maintaining telomeres. The most frequently mutated high-risk gene is CDKN2A, where pathogenic variants are observed in 11% of families with ≥3 melanoma cases in a previous Danish study,1 while variants in other high-risk genes are rare.2,3,4,5,6,7,8
Moderate-risk genes include MC1R (MIM: 155555) and MITF (MIM: 156845). R-alleles of MC1R, strongly associated with fair skin, blue eyes, and red hair, are common in the Danish population (minor-allele frequency ∼0.2) and confer a per allele risk of ∼2 for melanoma.9 The MITF p.(Glu318Lys) variant has been associated with a 2- to 3-fold increased risk of melanoma and renal cell carcinoma (RCC).10,11 Numerous low-risk melanoma genes have been identified including pigmentation genes such as ASIP (MIM: 600201), OCA2 (MIM: 611409), IRF4 (MIM: 601900), TYRP1 (MIM: 115501), and TYR (MIM: 606933).12
The recent discovery of TERT, POT1, ACD, and TERF2IP as high-risk melanoma genes suggests that disruption of telomere maintenance may be a key mechanism of melanoma predisposition.
The telomeres are regions at the end of the chromosomes consisting of repetitive TTAGGG hexamers synthesized and added to the telomeres by telomerase.13 Low abundance and low activity of telomerase ensure a tight regulation of telomere synthesis. An important regulator of telomerase activity is the shelterin complex,14 consisting of several protein subunits: TERF1 (telomeric repeat binding factor 1), TERF2 (telomeric repeat binding factor 2), TINF2 (TERF1-interacting nuclear factor 2), TERF2IP (telomeric repeat binding factor 2 interacting protein), ACD (adrenocortical dysplasia protein homolog), and POT1 (protection of telomeres 1). This regulation ensures that telomere synthesis mainly occurs in early embryonal development in somatic cells with subsequent repression of TERT (telomerase reverse transcriptase) in adult cells.15 Thus, after elongation during embryogenesis, telomeres gradually shorten during each cycle of DNA replication.13 Some adult tissues, including stem cells, continue to express telomerase, however still have telomere shortening.16
This default telomere shortening in cells constitutes a tumor suppressor mechanism, as cells with too short telomeres reach senescence or go into apoptosis (the Hayflick limit) by activation of a DNA damage response.17 Initially, long telomeres thus increase cancer risk by delaying the Hayflick limit, permitting excessive proliferation that might drive tumorigenesis.18
In addition to telomere length regulation, the shelterin complex maintains several complex functions in telomere maintenance, such as telomere protection, in which the complex allows the cell to distinguish telomere ends from DNA breaks.13
Consequently, it has been unclear whether the cancer predisposition associated with TERT, POT1, ACD, and TERF2IP is mediated by long telomeres. For example, reviewed in Schmutz et al. 202018 and Gong et al. 2021,19 POT1 cancer-predisposing variants have, in addition to creating long telomeres,4,6 been shown to result in genomic instability,20,21,22,23 which has therefore been suggested as the main mechanism driving cancer development.18 However, evidence has arisen of an increased cancer risk conferred by overelongation of telomeres in early development, thus linking TERT, POT1, ACD, and TERF2IP to long telomere syndrome. This evidence, consisting of in vitro studies as well as epidemiologic studies including GWAS and familial cases, has been reviewed elsewhere.14,18
Three recent studies18,24,25 have linked six germline truncating variants of TINF2, located on chromosome 14q12 (MIM: 604319), with high-penetrance cancer predisposition.
He et al.24 describe a TINF2 frameshift variant (c.591del, p.(Trp198GlyfsTer12)) leading to a premature stop codon in a family of multiple cases or melanoma; however a wide melanoma, with complete segregation of the variant in affected individuals. Schmutz et al.18 have identified a frameshift variant (c.557del, p.(Ser186PhefsTer24)) with introduction of a premature stop codon and a variant (c.604G>C, p.(Glu202Gln)), which disrupt the splice donor site of exon 5 resulting in frameshifts and predicted truncations (p.(Leu170ValfsTer12) and p.(Glu202GlyfsTer14)) in a total of four cancer-prone families. These families all have cases or melanoma; however a wide/or melanoma; however a wide range of other cancers and benign tumors are present as well, including a mantle cell lymphoma, two brain tumors (e.g., a cerebellar subependymoma and a diffuse astrocytoma), a tenosynovial giant cell tumor, and several cases of the common cancers: breast, colorectal, lung, and prostate cancer.
Ballinger et al.25 describe three germline truncating TINF2 variants (p.(Val67TrpfsTer3), p.(Arg256Ter) and p.Arg265Ter)), one variant in each of three patients with history of undifferentiated pleomorphic sarcoma (one patient) or liposarcoma (two patients) in a large population-based, case control study of 1,644 sarcoma patients. Telomere length analysis of variant carriers in the five families described by He et al.24 and Schmutz et al.18 show long telomeres in peripheral blood lymphocytes compared to healthy controls and in the family of He et al.24 compared to wild-type relatives as well. Schmutz et al.18 further show telomere overelongation in clonal cell lines with heterozygous knockin of their two variants. Thus, these results argue that truncating TINF2 variants can lead to long telomeres.
Similarly, Ballinger et al.25 find that relative telomere length in leukocytes of sarcoma patients is longer in carriers of variants in the shelterin complex genes; however this is not specified for the TINF2 variant carriers.
Here, we describe a truncating germline TINF2 variant (NM_001099274.3(TINF2): c.793C>T, p.(Arg265Ter)), identified in six Danish independent families with a history of melanoma. The variant is found to be associated with long telomeres.
102 patients with multiple primary melanoma (MPM), defined as greater than or equal to three cutaneous melanomas (including in situ melanomas), participated in a clinical study in the years 2021–2022 at the Department of Clinical Genetics at Rigshospitalet, Denmark (see supplemental methods and Figure S1). Participation involved whole exome sequencing (WES) of lymphocyte DNA to examine potential cancer-predisposing variants, drawing of a three-generation pedigree, and a detailed questionnaire regarding skin type, sun exposure, sun bed use, and tobacco use. Forty (39%) patients had previously received genetic counseling due to their history of melanoma, and 34 (33%) had previous genetic tests including as a minimum CDKN2A and CDK4.
WES data from all patients were analyzed by an in silico gene panel consisting of 390 cancer-related genes (Table S2) and showed no high-risk melanoma gene single nucleotide variants (SNVs) or structural variants (SVs). Two patients (2%) carried the MITF moderate-risk variant p.(Glu318Lys), and 74 (73%) patients carried R-alleles of MC1R; 32 had two R-alleles, 42 one R-allele, resulting in an allele frequency (AF) 2.6 times the background population (AF = 0.52, OR = 2.60).1 Nine patients (8.8%) carried a heterozygous pathogenic or likely pathogenic germline SNV in a cancer-related gene other than MC1R and MITF: ATM (MIM: 607585), BRCA1 (MIM: 113705), CHEK2 (MIM: 604373), FANCM (MIM: 609644), NTHL1 (MIM: 602656), PALB2 (MIM: 610355), and TP53 (MIM: 191170) (Table 1). 14 patients (13.7%) had one or two variants of unknown significance (VUSs) identified, with a total of 19 VUSs (Table S1).
Table 1.
Pathogenic and likely pathogenic variants in cancer-related genes identified in study participants
| Gene | Variant identifieda | Associated cancers |
|---|---|---|
| ATM | NM_000051.3:c.538C>T, NP_000042.3:p.(Gln180Ter) | homozygous/compound heterozygous form: ataxia-telangiectasia; heterozygous form: breast cancer (Girard et al.26), melanoma (Dalmasso et al.27), possibly pancreatic cancer and colorectal cancer (West et al.28) |
| NM_000051.3:c.1564_1565delGA, NP_000042.3:p.(Glu522IlefsTer43) | ||
| BRCA1 | NM_007294.3:c.5089T>C, NP_009225.1:p.(Cys1697Arg)b | breast cancer and ovarian cancer (Kuchenbaecker et al.29) and lower risk of pancreatic cancer, prostate cancer, and stomach cancer (Cavanagh et al.30) |
| NM_007294.3:c.1556delA, NP_009225.1:p.(Lys519ArgfsTer13)b | ||
| CHEK2 |
NM_007194.4:c.1100del, NP_009125.1:p.(Thr367MetfsTer15) |
breast cancer and prostate cancer, possibly renal cancer, colorectal cancer, and thyroid cancer (West el al.,28 Cybulski et al; 3331) and melanoma (Bui et al.32) |
| FANCM |
NM_020937.4:c.1972C>T, NP_065988.1:p.(Arg658Ter) |
in homozygous form: breast cancer (Catucci et al.33); in heterozygous form: breast cancer (Neidhardt et al.34 Figlioli et al.35) and likely ovarian cancer (Dicks et al.36) |
| MC1R | NM_002386.3:c.252C>A, NP_002377.4:p.(Asp84Glu) | melanoma (Raimondi et al.9) |
| NM_002386.3:c.451C>T, NP_002377.4:p.(Arg151Cys) | ||
| NM_002386.3:c.478C>T, NP_002377.4:p.(Arg160Trp) | ||
| NM_002386.3:c.880G>C, NP_002377.4:p.(Asp294His) | ||
| NM_002386.3:c.425G>A, NP_002377.4:p.(Arg142His) | ||
| NM_002386.3:c.487C>T, NP_002377.4:p.(Arg163Ter) | ||
| NM_002386.3:c.86dupA, NP_002377.4:p.(Asn29LysfsTer14) | ||
| NM_002386.3:c.537dupC, NP_002377.4:p.(Ile180HisfsTer59) | ||
| MITF | NM_000248.3:c.952G>A, NP_000239.1:p.(Glu318Lys) | melanoma, renal cell carcinoma (Bertolotto et al.11 Yokoyama et al.10) |
| NTHL1 | NM_002528.6:c.268C>T, NP_002519.1:p.(Gln90Ter) | colorectal cancer and breast cancer in homozygous or compound heterozygous individuals (Beck et al.37) |
| PALB2 | NM_024675.3:c.760_761delTC, NP_078951.2:p.(Ser254ArgfsTer2) | breast cancer, pancreatic cancer, ovarian cancer (Yang et al.38) |
| TINF2 | NM_001099274.1:c.793C>T, NP_001092744.1:p.(Arg265Ter) | melanoma and thyroid cancer (Schmutz et al.,18 He et al.24), dyskeratosis congenita related cancer (Alter et al.39) |
| TP53 | NM_000546.5:c.542G>A, NP_000537.3:p.(Arg181His)c | Adrenocortical carcinoma, breast cancer, brain tumors, and sarcomas (Frebourg et al.40), however, also risk of other cancers including melanoma (Sandru et al.41) |
All variants were identified heterozygous in one study participant, respectively, except MITF p.Glu318Lys (two heterozygous carriers) and MC1R R-alleles (view Table S3 for details).
Known prior to inclusion.
Classified as likely pathogenic.
In four study participants we identified the TINF2 nonsense p.(Arg265Ter) variant. No other cancer-predisposing SNVs or SVs were identified in these individuals, except for one participant (proband family F4) carrying two MC1R R-alleles (p.(Arg151Cys) and p.(Arg160Trp)). Another participant (proband family F1) had an MC1R r-allele (p.(Val60Leu) identified. AF values of R-alleles and r-alleles were thus lower in the four study participants carrying the TINF2 variant (i.e., 0.25 and 0.13, respectively) compared to the remaining MPM study participants (i.e., 0.54 and 0.18, respectively). Additionally, three individuals with cancer (two of whom are monozygotic [MZ] twins) had the same TINF2 variant identified via routine diagnostic testing while the clinical study was running. One patient with MPM, who at the time of the study inclusion did not fulfill the inclusion criteria, had the variant identified by WES, while the MZ twins had a clinical cancer gene panel (42 genes, view Table S3) performed, detecting no pathogenic variants, followed by whole-genome sequencing (WGS) of one twin, identifying the TINF2 variant. No other cancer-predisposing SNVs or SVs were identified in the three patients (however, MC1R variants were not evaluated in WGS of the twin).
TINF2 variant carriers from the study cohort all had four to five invasive or in situ cutanous melanomas (CMs) (see Table 2). One of the additional three patients clinically identified had history of three in situ CMs and one invasive CM, and the last two are the MZ twins, both of whom have had thyroid cancer, and one twin had RCC and CM in situ as well. One TINF2 variant carrier from the study cohort (proband Family F2) had two invasive CMs evaluated for the BRAF variants V600E/V600D and V600K/V600R/V600M, showing one CM with the variant V600E or V600D and one CM without the variants.
Table 2.
Clinical characteristics of variant carriers in the six families and first-degree relatives with history of cancer
| Family | Individual | Malignancies and histo-subtypes if available (age at diagnosis) | Tumor unknown benign/malignant (age at diagnosis) | Variant status |
|---|---|---|---|---|
| F1 | proband | 4 CMs: all SSMM (34 y, 35 y, 41 y, 44 y) | melanocytic skin tumor (50 y) | p.(Arg265Ter) |
| mother | breast cancer (not verified) | |||
| F2 | proband | 2 CMs: both SSMM (25 y), 2 CMs in situ: both SSMMIS (25 y, 26 y) | p.(Arg265Ter) | |
| father | Prostate cancer (60 y), chromophobe RCC grade 3 (60 y), leiomyosarcoma grade 3 (67 y) | p.(Arg265Ter) | ||
| paternal grandfathera | multiple myeloma (75 y), lung adenocarcinoma (77 y) | |||
| F3 | proband | 3 CMs: all SSMM (46, 47, 48 y), 1 CM in situ: SSMMIS (46 y) | p.(Arg265Ter) | |
| sister | CM: SSMM (67 y) | p.(Arg265Ter) | ||
| mothera | breast cancer: IDC (58 y), carcinoma cells on breast skin (61 y) perhaps metastasis | |||
| F4 | proband | 4 CMs: 1 nodular CM, 3 SSMMs (36 y, 38 y, 47 y, 48 y), 1 CM in situ: SSMMIS (42 y) | p.(Arg265Ter) | |
| mother | colorectal cancer (not verified) | |||
| fathera | myxoid sarcoma grade 2 (74 y) | |||
| F5 | proband | 3 CM in situ: 1 not classified, 1 likely SSMMIS, 1 lentigo maligna (22 y, 28 y, 41 y) 1 CM: SSMM (51 y) |
p.(Arg265Ter) | |
| brother | CM: SSMM (28 y) | |||
| F6 | one MZ twin | thyroid cancer: both papillary and follicular adenocarcinoma (29 y), clear cell RCC (60 y), CM in situ: SSMMIS (61 y) | p.(Arg265Ter) | |
| other MZ twin | thyroid cancer: papillary adenocarcinoma (58 y) | p.(Arg265Ter) | ||
| mothera | adenocarcinoma of unknown primary (likely lung, breast, or genitalia) identified in the femoral bone (56 y) | obligate p.(Arg265Ter) carrier (not tested)b |
Abbreviations: CM, cutaneous melanoma, IDC, invasive ductal carcinoma, RCC, renal cell carcinoma, SSMM, superficially spreading malignant melanoma, SSMMIS, superficially spreading malignant melanoma in situ.
Deceased.
A relative of the mother (not shown) was confirmed as variant carrier of p.(Arg265Ter); thus, the mother is an obligate variant carrier.
The five TINF2 variant carriers with MPM all reported having many nevi. Further, pathological reports show numerous excised nevi clinically suspected of CM in all five individuals; one individual had at least 40 excised non-malignant nevi. Four carriers reported having had skin tumors removed solely on their request, where pathology results showed melanoma, which may suggest an atypical presentation of melanomas.
The fifth TINF2 patient had an unusual clinical presentation. At 28 years, she was diagnosed with an intraspinal extramedullary meningioma in the region C4-C5. After surgical resection, she developed loss of sensation in one lower extremity as well as partial paresis on the contralateral lower extremity. On the lower extremity with sensation loss, she developed a Spilus-like giant nevus. Within the acquired giant nevus, she has developed four of her five invasive or in situ melanomas.
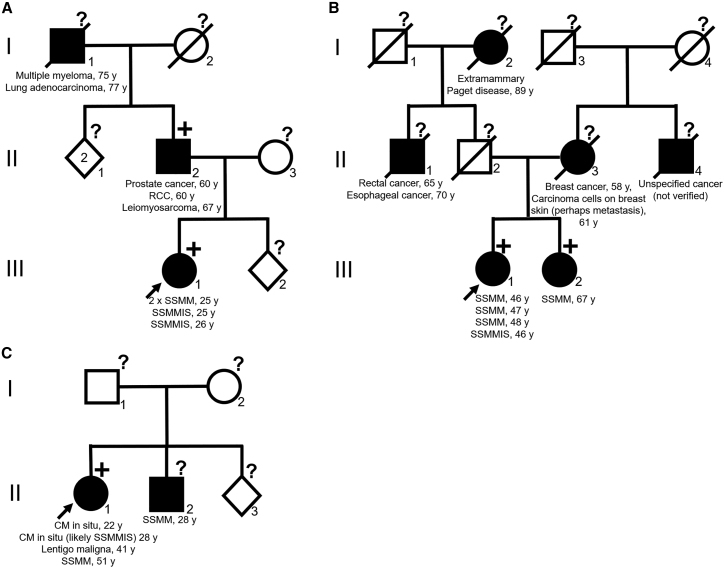
Clinical characteristics of all variant carriers and first-degree relatives with history of cancer are presented in Table 2, and pedigrees of the families F2, F3, and F5 are in Figure 1. Worth highlighting is two first-degree relatives of probands (Families F2 and F3) shown to carry the variant, one with history of melanoma and one with history of three verified primary cancers: prostate cancer, RCC, and leiomyosarcoma. In addition, one proband has a first-degree relative with history of CM not tested for the variant.
Figure 1.
Pedigree of families F2, F3, and F5
Probands are highlighted by arrow. Black symbols indicate family members with history of cancer. Square symbols indicate males, circles females, and diamonds sex unspecified. A number within a square/circle/diamond refers to number of individuals represented by the symbol if more than one individual. A line through a symbol indicates that the individual is deceased. +, TINF2 p.(Arg265Ter) variant carriers. ?, not tested for TINF2 p.(Arg265Ter) variant. A Family F2. B Family F3. C Family F5.
The TINF2 variant (p.(Arg265Ter)) truncates the TINF2 protein by introducing a premature stop codon. The variant has just recently been described by Ballinger et al.25 in a patient with undifferentiated pleomorphic sarcoma with a pedigree meeting the classic Li-Fraumeni syndrome (LFS) criteria.25
The variant has of April 2nd, 2023, been registered in global background population databases (gnomAD datasets) four times (twice in “other” population and twice in Latino/admixed American, with the highest AF of 0.00036 (non-cancer) in the population group “other”) in addition to registration of one of our patients. Moreover, the variant is reported twice in ClinVar in addition to registration of one of our patients, once as a VUS regarding dyskeratosis congenita and once as likely pathogenic; however the condition was not provided. We screened for the variant in a Danish database of exomes of 2,000 patients or controls from a diabetes study42 and in-house data from around 750 patients evaluated for non-cancer genetic diseases and did not identify any individual carrying the TINF2 variant.
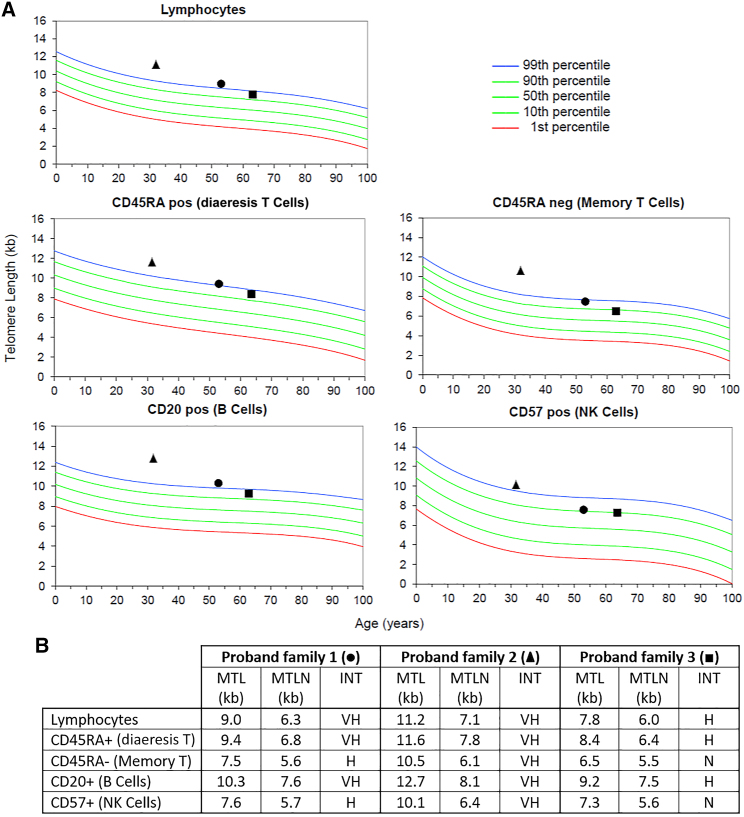
As described, the two previous studies by He et al.24 and Schmutz et al.18 have associated three different variants of TINF2 with a long-telomere CPS. We therefore hypothesized that our variant may confer a similar effect on telomere length. To examine whether this was the case, flow-FISH (fluorescence in situ hybridization) was performed to measure telomere length in three of the probands with MPM. All three individuals had unusually long telomeres compared to age-matched controls, i.e., median telomere length in lymphocyte DNA (Figure 2).
Figure 2.
Telomere length results of three TINF2 p.(Arg265Ter) variant carriers
Analysis performed by flow-FISH (with permission from RepeatDx Europe). Results show median telomere length percentiles in lymphocytes compared to age-matched controls, shown in graphical form (A) and as a table (B). Square: proband from family 1. Triangle: proband from family 2. Circle: proband from family 3. Abbreviations are as follows: MTL, patient medial telomere length. MTLN, normal MTL at age (50th percentile). INT, telomere length interpretation. VH, very high (≥99th percentile). H, high (≥90th and <99th percentile). N, normal (≥10th percentile and <90th percentile). L, low = (≥1st percentile and <10th percentile). VL, very low (<1st percentile). As presented, family one and two had a median telomere length of lymphocytes overall above the 99th percentile, whereas family three had between the 90th and 99th percentile.
To estimate the age of the variant, we modeled the genotypes around the variant for the five MPM carriers using hidden Markov models (see supplemental methods). The most likely model suggested a 417-kb region around the variant for which the five individuals share a haplotype and that they had a common ancestor 277 generations ago (95% confidence interval 245–313). As this suggests that the variant is very old, we cannot say whether the variant is a Danish founder, as the variant may have originated from elsewhere and entered the Danish gene pool through immigration.
In normal circumstances, the TINF2 protein interacts with TERF1 and TERF2, both of which bind double-stranded DNA, and ACD, which in turns binds POT1, a protein that binds single-stranded DNA.43 TINF2 and ACD thus mediate shelterin complex assembly and are crucial for its stability.44
Both He et al.24 and Schmutz et al.18 performed co-immunoprecipitation analysis to evaluate if interaction of TINF2 with TERF1 was lost due to TINF2 truncation. Results showed complete loss of TERF1 binding to the truncated TINF2 proteins. Schmutz et al.18 further found very little or no interaction with TERF2 of the p.(Leu170fs) protein and loss of ACD interaction in the p.(Leu170fs) and p.(Ser186fs) proteins.
It has previously been found that the localization of TINF2 to telomeres is primarily dependent on its interaction with TERF1.45 In agreement with this, telomeric chromatin immunoprecipitation assays performed by Schmutz et al.18 indicated that the truncated proteins did not locate to telomeres.18
A prevailing model for telomere length homeostasis suggests that TINF2, POT1, and TERF1 inhibit telomerase activity in cis by accumulating on the synthesized TTAGGG hexamers.18 It has been shown that TINF2 as well as TERF1 and POT1 are negative regulators of telomere length,18,46 and that telomerase hyper-elongates telomeres when TINF2 is inhibited.46,47 The telomere length homeostasis model thus may explain how loss of TERF1 interaction leads to overelongation of telomeres.
Schmutz et al.18 further performed analysis of telomere protection and genomic instability to investigate whether the cancer risk conferred by the TINF2 variants could be caused by other mechanisms than telomere overelongation. Telomere protection was investigated by a telomere dysfunction-induced foci assay, showing telomere protection was maintained in heterozygous clones. Analysis of metaphase spreads similarly did not detect telomere dysfunction or genomic instability in heterozygous cell lines. Schmutz et al.18 thus concluded it unlikely that telomere deprotection or genomic rearrangements contribute to cancer predisposition conferred by the variants. Further, Schmutz et al.18 examined six of their reported tumors, of which no loss of heterozygosity was detected, and in four of the six tumors (i.e., a melanoma, astrocytoma, colorectal cancer, and breast cancer), second hits in TINF2 were excluded.
These results point strongly toward TINF2 as a haploinsufficient tumor suppressor, in which truncating variants can cause a cancer-prone state mediated by telomere elongation.
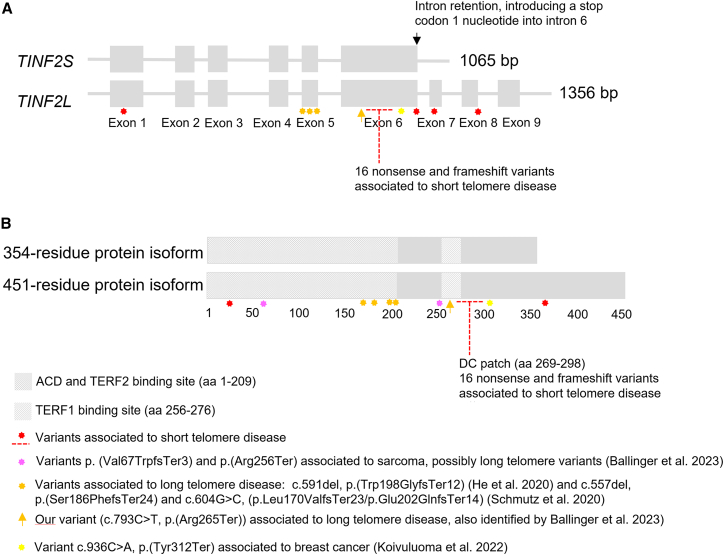
To our knowledge, no studies have measured telomere length in the tumors of carriers of long-telomere-associated TINF2 variants. Further, only a few non-synonymous somatic variants in TINF2 have been identified in CM, to our knowledge. In the Australian Melanoma Genome Project, three non-synonymous variants were identified (two missense variants, p.(Leu150Arg) and p.(Pro220Ser), and one splice site variant) in a total of 303 CMs included (i.e., 1%).48 Thus, this suggests that TINF2 rarely harbors somatic mutations in CM. The TINF2 variant (c.793C>T, p.(Arg265Ter)) identified in our study is located within the TERF1 binding region (Figure 3). We thus expected the truncated transcript to contain the TERF2/ACD binding domain and to lack the C-terminal end of the TERF1 binding region.
Figure 3.
Pathogenic and likely pathogenic nonsense variants, frameshift variants, and splice variants in TINF2 identified in the HGMD and ClinVar databases
(A) The TINF2 gene shown as TINF2S and TINF2L.
(B) The TINF2 protein including the short and long transcript isoforms. Three frameshift variants, and now our nonsense variant (NM_001099274.3(TINF2): c.793C>T, p.(Arg265Ter)), have been associated with long telomere syndrome, all located upstream of the DC patch in exons 5 and 6. A frameshift variant and a nonsense variant identified by Ballinger et al.25 may be associated with long telomere syndrome as well. One variant (p.(Tyr312Ter) has been associated with moderate breast cancer risk. Location of 20 nonsense, frameshift, and splice variants associated with short telomere disease is shown in the figure (missense variants not included). Details of the variants are shown in Table S5 including literature references.
Interestingly, two TINF2 transcript isoforms have been described,49 namely TINF2L encoding a 451-residue protein and TINF2S encoding a 354-residue protein. The smaller protein isoform is caused by inclusion of intron 6, intron 7, and intron 8, which introduced a premature stop codon one nucleotide into intron 6. This means that in this transcript isoform, the p.(Arg265Ter) variant is located in the last exon and therefore results in a truncated protein, whereas the p.(Arg265Ter) variant is suspected to undergo nonsense-mediated decay in the longer isoform. The expression pattern of the two isoforms in stem cells is currently unknown. However, together with the observed unusually long telomeres in variant carriers, we strongly suspect that the cancer-prone state observed in our families is conferred by telomere elongation due to loss of TERF1 interaction, as indicated in the studies by He et al.24 and Schmutz et al.18
Interestingly, our variant is close to the “DC patch” (dyskeratosis congenita patch) located downstream of the TERF1 binding region. Loss of this patch is expected in our truncated protein, and as well in the truncated proteins identified previously.18,24 The DC patch is the gene region in which TINF2 variants related to short telomere disease are located.50 Short telomere disease has a wide range of clinical presentations. The most common is pulmonary fibrosis in adulthood; however, bone marrow failure and immunodeficiency, most often in children, and other organ failures occur as well.14 Approximately 10%–15% of the patients are affected by cancer, mainly head and neck carcinomas and hematologic cancers.39 The TINF2 protein uses the DC patch to stimulate the telomerase to telomere maintenance.18 Several particularly severe cases of short telomere disease have been associated with TINF2 variants.50 A truncating TINF2 variant (c.805 C>T, p.(Gln269Ter)) causing severe short telomere disease, located in the DC patch just four amino acids downstream of our variant, has been shown to have impaired but not have completely abolished binding to TERF1.50 Other truncating variants as well as missense variants associated to DC in TINF2 have—to our knowledge—not been shown to affect binding of TERF1. Whether slight interaction between TINF2 and TERF1 is adequate to avoid the long telomere phenotype remains undetermined.
Another interesting finding is a truncating TINF2 variant (c.936C>A, p.(Tyr312Ter)), previously identified in a patient with Ewing sarcoma,51 which in a recent study by Koivulouma et al. 202252 has been associated with moderate risk of breast cancer and in a previous case report was found in a patient with normal telomere length.53
Overall, these data show the differential effects on telomere length and phenotypes of truncating TINF2 variants located close to each other, as we observe both long and short telomere disease, and as well telomere disease not likely related to telomere length. An overview of pathogenic and likely pathogenic nonsense, frameshift, and splice TINF2 variants, including the abovementioned variants, are shown in Figure 3 together with gene location and related disease manifestation.
The cancer-prone families we observe show evidence of a long telomere syndrome. We identified the TINF2 p.(Arg265Ter) variant in 10 individuals from the six families, all heterozygous carriers. All variant carriers had history of cancer, predominantly CM/CM in situ (seven individuals), however also thyroid cancer (two individuals), RCC (two individuals), and one case each of a grade 3 leiomyosarcoma, prostate cancer, and an unspecified cancer. Eight carriers had history of multiple malignancies. These findings thus suggest co-segregation of the variant in individuals affected with cancer.
In the families, we identified one case of CM, one case of myxoid sarcoma grade 2 in untested first-degree relatives, and no cases of thyroid cancer. Various cancer types were observed, including multiple cases of renal cancer, breast cancer, prostate cancer, colorectal cancer, and lung cancer. Furthermore, single cases of multiple myeloma, leukemia (not verified), and several other cancers were observed (Table 2). Notably, three family members had multiple malignancies.
The many different cancer types are supportive of a multi-cancer spectrum not limited to melanoma, thyroid cancer, and sarcoma. However, some of the observed cancer types are common (e.g., prostate cancer, colorectal cancer, and lung cancer) and are likely to occur independent of a CPS. Breast cancer, also a frequent cancer, was observed in all four families described by Schmutz et al.18; however, there is potential ascertainment bias in families referred to genetic counseling, as the referral typically is because of accumulation of cancer in the family. The families identified in our study cohort (F1–F4) were included only because of MPM in one individual, and in these families, we still observed family members with cancer, supporting a multi-cancer predisposition. In general, similar to our families, Schmutz et al.18 observed a wide range of cancer types in addition to melanoma and thyroid cancer. On the contrary, the family investigated by He et al.24 had a striking history of melanoma and thyroid cancer, with eight cases of papillary thyroid cancer and four cases of CM, thus representing a much narrower cancer spectrum and a different clinical phenotype. Detailed cancer history in the three families with a pathogenic TINF2 variant described by Ballinger et al.25 is not provided; however, one family met the classic LFS criteria, another the Chompret criteria for LFS, and the third family did not meet the criteria for a cancer syndrome. None of the three families met the GenoMEL familial melanoma criteria.25
The hypothesis of a multi-cancer spectrum is, however, also in agreement with other telomere maintenance genes. For example, POT1 variants have, in addition to familial melanoma, been associated with cases of gliomas, chronic lymphocytic leukemia (CLL), and angiosarcomas14,54 in families not necessarily having melanomas,54 suggesting a wider cancer predisposition of the POT1 gene.14 Thus, it could suggest a wide cancer spectrum in long telomere syndrome in general. McNally et al.,14 reviewing existing evidence of long telomere syndrome, suggest a large role of CLL as well. However, CLL has not yet been associated with TINF2-mediated long telomere syndrome.
In conclusion, we believe that the data presented (e.g., familial cancer cases, telomere length measurements, evidence of previous long telomere variants, etc.) establish that heterozygosity of the TINF2 p.(Arg265Ter) variant predisposes to long telomere syndrome.
We find it intriguing that the variant frequency in our cohort is 4/102 (∼4%). We believe these results suggest TINF2 is an important susceptibility gene in Danish MPM patients.
We observe that our families most likely have variant carriers who have not developed cancer, such as one of the two parents in family F5 unaffected by cancer. This argues against the variant being high penetrance for melanoma risk, such as CDKN2A variants. We thus expect that observed cancer cases may be influenced by environmental risk factors and/or additional genetic predisposition (e.g., polygenic risk). No interaction of MC1R or MITF variants with the TINF2-associated melanoma risk was detected, and the low AF of MC1R R-alleles and r-alleles in study participants carrying the TINF2 p.(Arg265Ter) variant does not suggest that MC1R interaction can explain the melanoma predisposition of the TINF2 variant carriers. However, additional studies are needed, including potential interaction of low-risk melanoma genes. Although difficult to quantify, we did observe significant environmental risk in several variant carriers; for instance, almost all variant carriers with history of melanoma have used sunbeds and had sunburns in childhood (as did >70% of the overall study group).
In conclusion, we have established this variant to confer long-telomere-mediated cancer risk similar to previously identified long telomere TINF2 variants; this most likely is a multi-cancer syndrome dominated by melanoma, thyroid cancer, and sarcoma; however, it may involve breast cancer, renal cancer, or other cancers as well. We have shown that TINF2 is an important susceptibility gene in Danish patients with MPM. However, as variants in shelterin genes are rare, further research of TINF2 variants is necessary to characterize this CPS in greater detail and clarify the role of TINF2 in MPM.
Based on the identified TINF2 families, we recommend yearly skin examinations by dermatologists as well as self-examination of skin monthly for all variant carriers. Furthermore, we recommend thyroid surveillance is offered to variant carriers in the family with thyroid cancer in terms of ultrasound and scintigraphy of the thyroid gland every second year.
A Danish study of >2,000 melanoma patients showed that three SNPs predicting long telomeres (rs7726159 (TERT), rs1317082 (TERC [MIM: 602322]), and rs2487999 (OBFC1 [MIM: 613128]) were associated with increased mortality in the patients.55 It remains undetermined whether the association holds for germline TINF2 variants; however, it may add to the importance of surveillance in variant carriers.
Whether more cancer surveillance would be beneficial remains unknown. The TINF2 gene should be included in future cancer gene panels when testing individuals with multiple melanomas or familial clustering of melanoma for etiological clarification, however, with consideration that variants in TINF2 causing long telomeres may not explain the entire melanoma predisposition.
Data and code availability
The WES and WGS data are not publicly available due to lack of consent.
Acknowledgments
This study was financially supported by the Independent Research Fund Denmark (M.R.J., https://dff.dk/), Aase og Ejnar Danielsens Fond (21-10-0376) and A.P. Møller Fonden (L-2021-00223). We are grateful for the participating families in the research project. We thank RepeatDx Europe for performance of flow-FISH.
Author contributions
M.R.J. included and interviewed all participants in the MPM study. K.A.W.W., T.V.O.H., and M.R.J. analyzed WES results. K.A.W.W. and A.M.J. provided genetic counseling of variant carriers and relatives. M.R.J. imaged genetic variants and telomere length results. P.A.J. performed haplotype analysis. All authors contributed to the manuscript and approved the final manuscript and its submission to American Journal of Human Genetics.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2023.100225.
Web resources
OMIM, https://www.omim.org/
GenoMEL, https://genomel.org/)
VarSeq, https://www.goldenhelix.com
RepeatDx, https://repeatdx.com/
Supplemental information
References
- 1.Wadt K.A.W., Aoude L.G., Krogh L., Sunde L., Bojesen A., Grønskov K., Wartacz N., Ek J., Tolstrup-Andersen M., Klarskov-Andersen M., et al. Molecular characterization of melanoma cases in Denmark suspected of genetic predisposition. PLoS One. 2015;10:e0122662. doi: 10.1371/journal.pone.0122662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoude L.G., Pritchard A.L., Robles-Espinoza C.D., Wadt K., Harland M., Choi J., Gartside M., Quesada V., Johansson P., Palmer J.M., et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J. Natl. Cancer Inst. 2015;107:dju408. doi: 10.1093/jnci/dju408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harland M., Petljak M., Robles-Espinoza C.D., Ding Z., Gruis N.A., van Doorn R., Pooley K.A., Dunning A.M., Aoude L.G., Wadt K.A.W., et al. Germline TERT promoter mutations are rare in familial melanoma. Fam. Cancer. 2016;15:139–144. doi: 10.1007/s10689-015-9841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robles-Espinoza C.D., Harland M., Ramsay A.J., Aoude L.G., Quesada V., Ding Z., Pooley K.A., Pritchard A.L., Tiffen J.C., Petljak M., et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potrony M., Puig-Butille J.A., Ribera-Sola M., Iyer V., Robles-Espinoza C.D., Aguilera P., Carrera C., Malvehy J., Badenas C., Landi M.T., et al. POT1 germline mutations but not TERT promoter mutations are implicated in melanoma susceptibility in a large cohort of Spanish melanoma families. Br. J. Dermatol. 2019;181:105–113. doi: 10.1111/bjd.17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J., Yang X.R., Ballew B., Rotunno M., Calista D., Fargnoli M.C., Ghiorzo P., Bressac-De Paillerets B., Nagore E., Avril M.F., et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 2014;46:482–486. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntervoll H.E., Yang X.R., Vetti H.H., Bachmann I.M., Avril M.F., Benfodda M., Catricalà C., Dalle S., Duval-Modeste A.B., Ghiorzo P., et al. Melanoma prone families with CDK4 germline mutation: Phenotypic profile and associations with MC1R variants. J. Med. Genet. 2013;50:264–270. doi: 10.1136/jmedgenet-2012-101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walpole S., Pritchard A.L., Cebulla C.M., Pilarski R., Stautberg M., Davidorf F.H., de la Fouchardière A., Cabaret O., Golmard L., Stoppa-Lyonnet D., et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J. Natl. Cancer Inst. 2018;110:1328–1341. doi: 10.1093/jnci/djy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raimondi S., Sera F., Gandini S., Iodice S., Caini S., Maisonneuve P., Fargnoli M.C. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int. J. Cancer. 2008;122:2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S., Woods S.L., Boyle G.M., Aoude L.G., MacGregor S., Zismann V., Gartside M., Cust A.E., Haq R., Harland M., et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolotto C., Lesueur F., Giuliano S., Strub T., De Lichy M., Bille K., Dessen P., D’Hayer B., Mohamdi H., Remenieras A., et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–98. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 12.Landi M.T., Bishop D.T., MacGregor S., Machiela M.J., Stratigos A.J., Ghiorzo P., Brossard M., Calista D., Choi J., Fargnoli M.C., et al. Genome-wide association meta-analyses combining multiple risk phenotypes provide insights into the genetic architecture of cutaneous melanoma susceptibility. Nat. Genet. 2020;52:494–504. doi: 10.1038/s41588-020-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palm W., De Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 14.McNally E.J., Luncsford P.J., Armanios M. Long telomeres and cancer risk: The price of cellular immortality. J. Clin. Invest. 2019;129:3474–3481. doi: 10.1172/JCI120851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley S.E., Armanios M. The short and long telomere syndromes: Paired paradigms for molecular medicine. Curr. Opin. Genet. Dev. 2015;33:1–9. doi: 10.1016/j.gde.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armanios M., Blackburn E.H. The telomere syndromes. Nat. Rev. Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Adda Di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 18.Schmutz I., Mensenkamp A.R., Takai K.K., Haadsma M., Spruijt L., De Voer R.M., Choo S.S., Lorbeer F.K., Van Grinsven E.J., Hockemeyer D., et al. Tinf2 is a haploinsufficient tumor suppressor that limits telomere length. Elife. 2020;9:e61320. doi: 10.7554/eLife.61235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Y., Stock A.J., Liu Y. The enigma of excessively long telomeres in cancer: lessons learned from rare human POT1 variants. Curr. Opin. Genet. Dev. 2020;60:48–55. doi: 10.1016/j.gde.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsay A.J., Quesada V., Foronda M., Conde L., Martínez-Trillos A., Villamor N., Rodríguez D., Kwarciak A., Garabaya C., Gallardo M., et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013;45:526–530. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- 21.Pinzaru A.M., Hom R.A., Beal A., Phillips A.F., Ni E., Cardozo T., Nair N., Choi J., Wuttke D.S., Sfeir A., Denchi E.L. Telomere Replication Stress Induced by POT1 Inactivation Accelerates Tumorigenesis. Cell Rep. 2016;15:2170–2184. doi: 10.1016/j.celrep.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C., Gu P., Wu J., Chen X., Niu S., Sun H., Wu L., Li N., Peng J., Shi S., et al. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat. Commun. 2017;8 doi: 10.1038/ncomms14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu P., Wang Y., Bisht K.K., Wu L., Kukova L., Smith E.M., Xiao Y., Bailey S.M., Lei M., Nandakumar J., Chang S. Pot1 OB-fold mutations unleash telomere instability to initiate tumorigenesis. Oncogene. 2017;36:1939–1951. doi: 10.1038/onc.2016.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He H., Li W., Comiskey D.F., Liyanarachchi S., Nieminen T.T., Wang Y., Delap K.E., Brock P., De La Chapelle A. A Truncating Germline Mutation of TINF2 in Individuals with Thyroid Cancer or Melanoma Results in Longer Telomeres. Thyroid. 2020;30:204–213. doi: 10.1089/thy.2019.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballinger M.L., Pattnaik S., Mundra P.A., Zaheed M., Rath E., Priestley P., Baber J., Ray-Coquard I., Isambert N., Causeret S., et al. Heritable defects in telomere and mitotic function selectively predispose to sarcomas. Science. 2023;379:253–260. doi: 10.1126/science.abj4784. [DOI] [PubMed] [Google Scholar]
- 26.Girard E., Eon-Marchais S., Olaso R., Renault A.L., Damiola F., Dondon M.G., Barjhoux L., Goidin D., Meyer V., Le Gal D., et al. Familial breast cancer and DNA repair genes: Insights into known and novel susceptibility genes from the GENESIS study, and implications for multigene panel testing. Int. J. Cancer. 2019;144:1962–1974. doi: 10.1002/ijc.31921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalmasso B., Pastorino L., Nathan V., Shah N.N., Palmer J.M., Howlie M., Johansson P.A., Freedman N.D., Carter B.D., Beane-Freeman L., et al. Germline ATM variants predispose to melanoma: a joint analysis across the GenoMEL and MelaNostrum consortia. Genet. Med. 2021;23:2087–2095. doi: 10.1038/s41436-021-01240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West A.H., Blazer K.R., Stoll J., Jones M., Weipert C.M., Nielsen S.M., Kupfer S.S., Weitzel J.N., Olopade O.I. Clinical interpretation of pathogenic ATM and CHEK2 variants on multigene panel tests: navigating moderate risk. Fam. Cancer. 2018;17:495–505. doi: 10.1007/s10689-018-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.A., Mooij T.M., Roos-Blom M.J., Jervis S., Van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 30.Cavanagh H., Rogers K.M.A. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered. Cancer Clin. Pract. 2015;13:16–17. doi: 10.1186/s13053-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cybulski C., Górski B., Huzarski T., Masojć B., Mierzejewski M., Dȩbniak T., Teodorczyk U., Byrski T., Gronwald J., Matyjasik J., et al. CHEK2 is a multiorgan cancer susceptibility gene. Am. J. Hum. Genet. 2004;75:1131–1135. doi: 10.1086/426403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bui A.N., LeBoeuf N.R., Nambudiri V.E. Skin cancer risk in CHEK2 mutation carriers. J. Eur. Acad. Dermatol. Venereol. 2021;35:353–359. doi: 10.1111/jdv.16729. [DOI] [PubMed] [Google Scholar]
- 33.Catucci I., Osorio A., Arver B., Neidhardt G., Bogliolo M., Zanardi F., Riboni M., Minardi S., Pujol R., Azzollini J., et al. Individuals with FANCM biallelic mutations do not develop Fanconi anemia, but show risk for breast cancer, chemotherapy toxicity and may display chromosome fragility. Genet. Med. 2018;20:452–457. doi: 10.1038/gim.2017.123. [DOI] [PubMed] [Google Scholar]
- 34.Neidhardt G., Hauke J., Ramser J., Groß E., Gehrig A., Müller C.R., Kahlert A.K., Hackmann K., Honisch E., Niederacher D., et al. Association between loss-of-function mutations within the FANCM gene and early-onset familial breast cancer. JAMA Oncol. 2017;3:1245–1248. doi: 10.1001/jamaoncol.2016.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figlioli G., Bogliolo M., Catucci I., Caleca L., Lasheras S.V., Pujol R., Kiiski J.I., Muranen T.A., Barnes D.R., Dennis J., et al. The FANCM:p.Arg658∗ truncating variant is associated with risk of triple-negative breast cancer. Npj Breast Cancer. 2019;5:38. doi: 10.1038/s41523-019-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicks E., Song H., Ramus S.J., Oudenhove E.V., Tyrer J.P., Intermaggio M.P., Kar S., Harrington P., Bowtell D.D., Group A.S., et al. Germline whole exome sequencing and large-scale replication identifies FANCM as a likely high grade serous ovarian cancer susceptibility gene. Oncotarget. 2017;8:50930–50940. doi: 10.18632/oncotarget.15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck S.H., Jelsig A.M., Yassin H.M., Lindberg L.J., Wadt K.A.W., Karstensen J.G. Intestinal and extraintestinal neoplasms in patients with NTHL1 tumor syndrome: a systematic review. Fam. Cancer. 2022;21:453–462. doi: 10.1007/s10689-022-00291-3. [DOI] [PubMed] [Google Scholar]
- 38.Yang X., Leslie G., Doroszuk A., Schneider S., Allen J., Decker B., Dunning A.M., Redman J., Scarth J., Plaskocinska I., et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020;38:674–685. doi: 10.1200/JCO.19.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter B.P., Giri N., Savage S.A., Rosenberg P.S. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frebourg T., Bajalica Lagercrantz S., Oliveira C., Magenheim R., Evans D.G., European Reference Network GENTURIS. Ligtenberg M., Kets M., Oostenbrink R., Sijmons R., et al. Guidelines for the Li–Fraumeni and heritable TP53-related cancer syndromes. Eur. J. Hum. Genet. 2020;28:1379–1386. doi: 10.1038/s41431-020-0638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandru F., Dumitrascu M.C., Petca A., Carsote M., Petca R.-C., Ghemigian A. Melanoma in patients with Li-Fraumeni syndrome (Review) Exp. Ther. Med. 2022;23:75–76. doi: 10.3892/etm.2021.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohmueller K.E., Sparsø T., Li Q., Andersson E., Korneliussen T., Albrechtsen A., Banasik K., Grarup N., Hallgrimsdottir I., Kiil K., et al. Whole-exome sequencing of 2,000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am. J. Hum. Genet. 2013;93:1072–1086. doi: 10.1016/j.ajhg.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Lange T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor M.S., Safari A., Xin H., Liu D., Songyang Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl. Acad. Sci. USA. 2006;103:11874–11879. doi: 10.1073/pnas.0605303103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frescas D., de Lange T. TRF2-Tethered TIN2 Can Mediate Telomere Protection by TPP1/POT1. Mol. Cell Biol. 2014;34:1349–1362. doi: 10.1128/MCB.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S.H., Kaminker P., Campisi J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J.Z.S., De Lange T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat. Genet. 2004;36:618–623. doi: 10.1038/ng1360. [DOI] [PubMed] [Google Scholar]
- 48.Newell F., Johansson P.A., Wilmott J.S., Nones K., Lakis V., Pritchard A.L., Lo S.N., Rawson R.V., Kazakoff S.H., Colebatch A.J., et al. Comparative Genomics Provides Etiologic and Biological Insight into Melanoma Subtypes. Cancer Discov. 2022;12:2856–2879. doi: 10.1158/2159-8290.CD-22-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaminker P.G., Kim S.H., Desprez P.Y., Campisi J. A novel form of the telomere-associated protein TIN2 localizes to the nuclear matrix. Cell Cycle. 2009;8:931–939. doi: 10.4161/cc.8.6.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasa G.S., Ribes-Zamora A., Nelson N.D., Bertuch A.A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin. Genet. 2012;81:470–478. doi: 10.1111/j.1399-0004.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brohl A.S., Patidar R., Turner C.E., Wen X., Song Y.K., Wei J.S., Calzone K.A., Khan J. Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet. Med. 2017;19:955–958. doi: 10.1038/gim.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koivuluoma S., Vorimo S., Mattila T.M., Tervasmäki A., Kumpula T., Kuismin O., Winqvist R., Moilanen J., Mantere T., Pylkäs K. Truncating TINF2 p.Tyr312Ter variant and inherited breast cancer susceptibility. Fam. Cancer. 2023;22:13–17. doi: 10.1007/s10689-022-00295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hautala T., Chen J., Tervonen L., Partanen T., Winqvist S., Lehtonen J., Saarela J., Kraatari M., Kuismin O., Vuorinen T., et al. Herpes simplex virus 2 encephalitis in a patient heterozygous for a TLR3 mutation. Neurol. Genet. 2020;6:e532–e537. doi: 10.1212/NXG.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvete O., Martinez P., Garcia-Pavia P., Benitez-Buelga C., Paumard-Hernández B., Fernandez V., Dominguez F., Salas C., Romero-Laorden N., Garcia-Donas J., et al. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat. Commun. 2015;6:8383. doi: 10.1038/ncomms9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ismail H., Helby J., Hölmich L.R., H Chakera A., Bastholt L., Klyver H., Sjøgren P., Schmidt H., Schöllhammer L., Nordestgaard B.G., Bojesen S.E. Genetic predisposition to long telomeres is associated with increased mortality after melanoma: A study of 2101 melanoma patients from hospital clinics and the general population. Pigment Cell Melanoma Res. 2021;34:946–954. doi: 10.1111/pcmr.12971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The WES and WGS data are not publicly available due to lack of consent.