Abstract
Tularemia is a severe zoonotic disease caused by gram-negative bacillus Francisella tularensis. F. tularensis species account for most cases in the United States of America (USA). Apart from the six classical clinical presentations that include glandular, ulceroglandular, oculoglandular, pharyngeal, typhoidal, and pneumonic, skeletal disease is uncommon. Rare clinical manifestations include primary and secondary skin rashes, erythema nodosum, and erythema multiforme. Infrequent skeletal manifestations have presented as osteomyelitis and prosthetic joint infections. Prosthetic joint infection by F. tularensis is a rarity. PubMed literature review revealed a total of five prosthetic joint infection cases. Here we report the sixth and the third case in the USA in a 73-year-old white male with an acute left knee prosthetic joint infection (occurring after a recent episode of left lower extremity cellulitis with septic shock) successfully treated with 14 days of doxycycline.
Keywords: Francisella tularensis, Tularemia, Prosthetic joint infection, Doxycycline
Introduction
Prosthetic joint infection (PJI) depends on the patient's multifactorial exposure in daily life. The risk factors and the etiological agents vary from an early PJI to a late PJI. The overall incidence of PJI in total knee arthroplasty (TKA) ten years after surgery is 1.4 % [1]. PJI is the leading cause of TKA failure due to increased stress from weight-bearing and minimal structural support [2]. Native and prosthetic joint infections by Tularemia are highly uncommon. We report a case of F. tularensis Left TKA PJI after a recent left lower extremity (LLE) cellulitis episode with septic shock.
Case presentation
A 73-year-old male with a medical history significant for hypertension, hyperlipidemia, benign prostate hypertrophy, gastroesophageal reflux disorder, depression, sarcomatoid lung cancer on pembrolizumab, pulmonary embolism on rivaroxaban, iron deficiency anemia, bilateral shoulder osteoarthritis (on intraarticular corticosteroid injections every three months), left TKA 2005 presented to his primary care physician for acute left knee worsening painful swelling with no systemic symptoms for last four weeks. The patient denied recent gardening, camping, insect bite, or trauma to the left knee or LLE. The patient had a recent hospitalization five weeks prior for acute LLE cellulitis with septic shock, acute toxic encephalopathy, acute kidney injury treated with intravenous fluids, broad-spectrum antibiotics vancomycin, and piperacillin-tazobactam. The patient had a large circular area of violaceous erythema with warmth above the left ankle joint, with pustules and blisters on the anterior aspect of the shin. Blood cultures were negative. LLE cellulitis improved with antibiotics, and he was discharged on a 10-day course of oral clindamycin. Left knee pain started after antibiotic completion and was initially of low-grade intensity that gradually worsened. The left knee pain was located posteriorly, grade 8/10, worse with flexion and weight-bearing. The pain improved after walking six to seven steps. Vitals were stable and physical examination was benign with elevated inflammatory markers (Table 1a) and a normal left knee x-ray.
Table 1a.
Laboratory results at Primary care physicians' office.
| 1) White cell count (3600 – 11,200/mL) | 5900 |
| 2) Hemoglobin (13.1 – 16.8 gm/dL) | 9.6 |
| 3) Hematocrit (38.2–48.4 %) | 30.3 % |
| 4) Platelet count (150,000 – 400,000/mL) | 458,000 |
| 5) Uric acid level (3.5 – 7.2 mg/dL) | 3.4 |
| 6) C-Reactive protein (0 – 0.5 mg/dL) | 7.39 |
| 7) Erythrocyte sedimentation rate (0 – 20 mm/hr) | 82 |
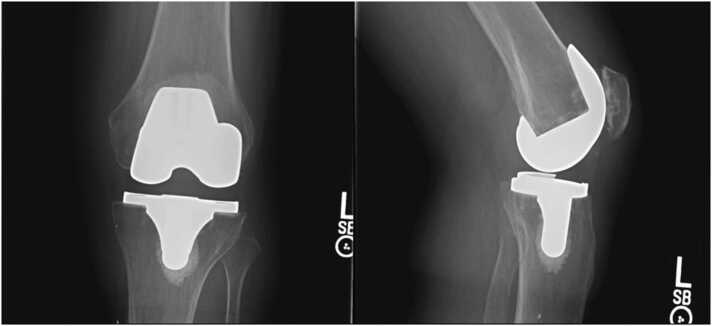
He was then referred to an orthopedic clinic for a left knee PJI concern, where examination revealed a non-erythematous left knee incision line without drainage but mild effusion and left hamstring muscle pain. Left knee range of motion was 0–90 degrees, and the sensation was intact to light touch. Left knee x-ray, two views revealed primary cemented components with good alignment and no evidence of loosening (Fig. 1). Left knee joint aspiration revealed 16 mL of clear straw-colored synovial fluid. Synovial fluid analysis (Table 1b) indicated acute infection, and culture revealed F.tularensis on the Vitek-2 system. The infectious disease team started him on doxycycline 100 mg twice daily for two weeks. The specimen was then subcultured on modified Thayer-Martin agar media for confirmation (laboratory personnel were notified), and a sample was sent to the state laboratory for confirmation. The state laboratory confirmed F. tularensis growth, and a convalescent antibody titer two weeks later was high at 1:640. After the antimicrobial therapy completion, his symptoms had subsided at the primary care follow-up. He had no pain with the left knee range of motion and weight-bearing. He has been symptom-free for the last twelve months and follows up with his primary care physician.
Fig. 1.
Anteroposterior and lateral view of left knee (at Orthopedic clinic) revealed primary cemented components with good alignment and no evidence of loosening.
Table 1b.
Left knee synovial fluid analysis at orthopedic clinic.
| 1) Total cells per cubic millimeter | 9299 |
| 2) Total White blood cells per cubic millimeter | 3299 |
| 3) Total Red blood cells per cubic millimeter | 6000 |
| 4) Neutrophils | 41 % |
| 5) Lymphocytes | 46 % |
| 6) Others | 15 % |
Discussion
Tularemia is an uncommon zoonosis with an incidence of 0.05 per 100,000 population in the USA caused by F. tularensis [3]. Multiple strains exist with variable virulence and severeness of clinical presentation, often during spring to early fall. Transmission vectors include ticks and deer fly in contact with an infected cottontail rabbit or a muskrat. The ticks frequently involved are the lone star (Amblyomma americanum), wood (Dermacentor andersoni), and dog tick (Dermacentor variabilis) in the USA [3]. None of the six classical presentations involve the skeletal system, which is rare and a complication. Cellular immunity develops within a few weeks and confers potent lifelong protection after infection in a healthy patient [3]. Recurrent infections are rare, with an ulceroglandular presentation and no systemic symptoms [3]. PJI occurs via hematogenous seeding from tularemia, inhalation of contaminated aerosols directly via tick bite, and lymphohematogenous spread. Hematogenous seeding is frequent in prosthetic joints due to the smaller inoculum needed, and prosthesis-induced acquired local granulocyte defect [4]. F. tularensis virulent attributes include type IV pili (adherence), polysaccharide capsule (inhibits IgM and C3 binding), capsule-like complex, or high molecular weight carbohydrate (stops antibody binding and complement deposition) [3], [5], and acid phosphatases (aid survival in macrophages). Biofilm formation does help its survival in the environment, and its role in PJI is unclear [5]. Except for a single case of osteomyelitis due to a cat bite, no native joint infections have been reported [6]. F. tularensis probably lacks synovial tropism due to a lack of fibronectin-binding ability or adhesins.
PubMed literature review revealed 5 cases of F. tularensis PJI (Table 2) [7], [8], [9], [10]. Including our patient, two were from Europe, and four were from North America. All patients were over 50 years old at presentation, predominantly males, with the knee joint being commonly affected. The frequent infection source was suspected tick bite to a lower extremity. Two patients underwent surgical revision, and the rest were treated with antimicrobials plus diagnostic or therapeutic aspiration. Two patients were on immunosuppression [7]. F. tularensis was isolated in culture from the synovial fluid in five patients and periprosthetic tissue in one patient. Serological assays were done in four patients, and 16 S rRNA (ribosomal Ribonucleic acid) analysis in three patients. The recommended antimicrobials are streptomycin/gentamicin, doxycycline, or quinolones, and the duration is unclear for PJI [3]. Currently, no guidelines exist to address F. tularensis PJI. It is unclear whether a dual antimicrobial combination is better than a single agent. One patient showed an excellent response to a combination of ciprofloxacin and rifampin than ciprofloxacin alone, possibly due to F. tularensis biovar type B PJI [7]. The fact that only one patient is on chronic antimicrobial suppressive therapy (delayed diagnosis and treatment) than others treated for a specific duration may suggest a lack of or ineffective biofilm formation, protective effect of cellular immunity, or a timely detection and treatment with a better response [10]. Unfortunately, no serological test was done to confirm acquired immunity in this patient. A fraction of culture-negative PJI due to F. tularensis could have been treated inadvertently with empirical regimens, including quinolones. Suppressive therapy may have a role if symptoms persist.
Table 2.
Patient characteristics in Prosthetic joint infections (PJI) by F. tularensis[8].
| Reference | 1) Cooper CL [7] | 2) Chrdle A [8] | 3) Chrdle A [8] | 4) Rawal H, [9] | 5) Azua EN [10] | 6) Current case |
|---|---|---|---|---|---|---|
| Country | Ontario, Canada | Switzerland | Switzerland | Illinois, USA | Colorado, USA | Missouri, USA |
| Age/Gender | 68/Male | 84/Female | 84/Male | 77/Male | 58/Male | 73/Male |
| Infection source | Hunter (Tick bite six months prior to surgery) | Suspected rabbit dust inhalation | Suspected typhoidal tularemia | Hunter (suspected animal exposure) | Farmer (Suspected rabbit carcass exposure) | Suspected tick bite six weeks before |
| Immunosuppression | Methotrexate | None | None | None | None | Pembrolizumab, Intra-articular shoulder Corticosteroids |
| Diagnosis Year | 1998 | 2016 | 2016 | 2017 | 2020 | 2021 |
| Joint involved | Knee | Knee | Knee | Hip | Knee | Knee |
| Time interval to diagnosis after last surgery | 1 year | 12 years | 8 years | 1 week after revision of total hip arthroplasty (25 yrs) | 5 months after the revision | 16 years |
| Clinical Features | Painful swelling and copious incision line serous drainage with no systemic symptoms. | Joint redness and pain with no systemic symptoms. | Abdominal pain, encephalopathy, painful swelling with fever. | Right hip pain and fever. | Bilateral joint recurrent effusion with no systemic symptoms. | Painful joint swelling with effusion and no systemic symptoms. |
| Inflammatory markers | ESR 47 mm/hr CRP not done | ESR 69 mm/hr CRP 8.1 mg/dL | ESR not done CRP 9.8 mg/dL | ESR 96 mm/hr CRP 16.20 mg/dL | ESR not done CRP not done | ESR 82 mm/hr CRP 7.39 mg/dL |
| Diagnostic method | Culture (synovial fluid) | Culture and 16 S rRNA analysis (tissue culture) | Culture and 16 S rRNA analysis (synovial fluid) | Culture and MALDI-TOF MS (synovial fluid) | Culture and 16 S rRNA analysis (synovial fluid) | Vitek-2 and culture (synovial fluid) |
| Serology | Antibody titer of 1:320 | IgM 232.6 (<10 U/mL) IgG 126.4 (< 0 U/mL) | Antibody titer of 1:80 | Not done | Not done | Antibody titer of 1:640 |
| Surgical treatment | 2 stage revision | 2 stage revision | Therapeutic aspiration with retention | Retention after diagnostic aspiration | Therapeutic aspiration with retention | Retention after diagnostic aspiration |
| Antimicrobial Choice/duration | Ciprofloxacin and Rifampin for five to six months. | Doxycycline for six weeks | Doxycycline (20 days) and Gentamicin (10 days), followed by 20 days of ciprofloxacin | Doxycycline for one year | Doxycycline Chronic suppression | Doxycycline for 14 days |
ESR = Erythrocyte sedimentation rate, CRP = C-Reactive protein, RNA = Ribonucleic acid,
MALDI-TOF MS = matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Our patient resides in a Mid-Missouri county rural area farmhouse with no pets or animal exposure. He denied recent visits to parks or consuming contaminated agricultural products and water. Our patient presented after a suspected ulceroglandular lesion from a tick bite with a superimposed bacterial infection and septic shock. His symptoms started immediately after the completion of the clindamycin course. Clindamycin demonstrates an antibiofilm effect, excellent bone, and joint penetration, and is bactericidal against F. tularensis [11], [12], [13]. Our patient had an acute PJI, and symptoms resolved after arthrocentesis and antimicrobial therapy [14]. Clindamycin inadvertently might have contributed in treating our patient's acute PJI. Despite being on pembrolizumab and intraarticular steroids, he has remained symptom-free currently. It is unknown if the acquired robust immunity after F. tularensis infection has any protective role in preventing PJI recurrence or relapse.
Conclusion
Due to advances in joint prostheses, THA and TKA are being done in a younger population compared to a few decades back. The incidence of F. tularensis PJI might increase over the next few decades due to better diagnostic processes and activity resumption post-surgery. Obtaining occupational and environmental exposure is imperative before THA/TKA. Due to the lack of definite therapeutic guidelines, the F. tularensis PJI treatment approach is individualized based on patient risk factors. A high degree of clinical suspicion for F. tularensis infection in culture-negative PJI should be entertained as an earlier diagnosis can help in specific antimicrobial therapy, prevent surgical interventions, and retain the prosthesis.
Ethics approval
Care was taken to ensure that all patient identifiers were removed in the process of creating this case report, the patient was made aware of this case report.
Funding
There are no sources of funding to report for this case report.
CRediT authorship contribution statement
Javier Escovar - Principal author. Sachin M Patil - Co-author, Faculty advisor and contributor. William Roland - Faculty advisor and contributor.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
None.
Consent
Written and verbal informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- 1.Tsaras G., Osmon D.R., Mabry T., et al. Incidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, olmsted county, Minnesota, 1969-2007. Infect Control Hosp Epidemiol. 2012;33(12):1207–1212. doi: 10.1086/668421. PMID 23143357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beam E., Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32(4):843–859. doi: 10.1016/j.idc.2018.06.005. PMID 30241717. [DOI] [PubMed] [Google Scholar]
- 3.Paul G. Auwaerter, Robert L.Penn Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Francisella tularensis (Tularemia) 227 (9th ed.) Elsevier, Philadelphia (2020). p: 2759–2773.e4.
- 4.Zimmerli W., Trampuz A., Ochsner P.E. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. PMID 15483283. [DOI] [PubMed] [Google Scholar]
- 5.van Hoek M.L. Biofilms: an advancement in our understanding of Francisella species. Virulence. 2013;4(8):833–846. doi: 10.4161/viru.27023. PMID 24225421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen J.C., Malotky M.V. Francisella tularensis osteomyelitis of the hand following a cat bite: a case of clinical suspicion. Plast Reconstr Surg. 2011;128(1):37e–39e. doi: 10.1097/PRS.0b013e3182174626. PMID 21701312. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C.L., Van Caeseele P., Canvin J., Nicolle L.E. Chronic prosthetic device infection with Francisella tularensis. Clin Infect Dis. 1999;29(6):1589–1591. doi: 10.1086/313550. PMID 10585830. [DOI] [PubMed] [Google Scholar]
- 8.Chrdle A., Trnka T., Musil D., et al. Francisella tularensis periprosthetic joint infections diagnosed with growth in cultures. J Clin Microbiol. 2019;57(8):e00339–19. doi: 10.1128/JCM.00339-19. Published 2019 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawal H., Patel A., Moran M. Unusual case of prosthetic joint infection caused by Francisella Tularensis. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-221258. 2017:bcr2017221258. Published 2017 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azua E.N., Voss L.A. Tularemia in a prosthetic joint infection. Orthopedics. 2020;43(1):e54–e56. doi: 10.3928/01477447-20190627-01. PMID 31269216. [DOI] [PubMed] [Google Scholar]
- 11.Schilcher K., Andreoni F., Dengler Haunreiter V., Seidl K., Hasse B., Zinkernagel A.S. Modulation of Staphylococcus aureus Biofilm Matrix by Subinhibitory Concentrations of Clindamycin Antimicrob Agents Chemother. 2016;60(10):5957–5967. doi: 10.1128/AAC.00463-16. . Published 2016 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabit A.K., Fatani D.F., Bamakhrama M.S., Barnawi O.A., Basudan L.O., Alhejaili S.F. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136. doi: 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Madrid P.B., Chopra S., Manger I.D., et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parvizi J., Tan T.L., Goswami K., et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplast. 2018;33(5):1309–1314.e2. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]