Abstract
Patients with one or more developmentally absent teeth are routinely encountered in dental practice. Tooth agenesis can be associated with significant functional, aesthetic and psycho-social problems. The present article provides an overview of the prevalence and aetiology of tooth agenesis, as well as the condition’s clinical characteristics and management options with reference to the evidence base. A timely diagnosis can facilitate the appropriate planning and management which might not be straightforward, and patient care will likely require multi- and inter-disciplinary input. It is critical that dental care practitioners are aware of the clinical characteristics and management options for tooth agenesis.
Keywords: Aetiology of hypodontia, Dental agenesis, Hypodontia, Management of hypodontia, Tooth agenesis
1. Introduction
Tooth agenesis occurs when there is a developmental absence of one or more of the ‘normal’ complement of 20 teeth in the primary dentition and/or 32 teeth in the permanent dentition [1]. It is a relatively common dental aberration which can range from the absence of a single tooth to the failure of development of an entire dentition [2]. The definition of agenesis type is based on the number of teeth developmentally absent which usually excludes consideration of the permanent third molars [3]. Hypodontia is defined as the developmental absence of one to five teeth, whereas oligodontia is the agenesis of six or more teeth [4]. Anodontia occurs when all teeth fail to develop [5]. The terms ‘agenesis’ and ‘hypodontia’ are commonly used interchangeably in the literature and both terms are used in this review.
Tooth agenesis is associated with a variety of genetic and environmental factors [6]. It may occur in isolation or be associated with other dental anomalies and/or general medical conditions [2]. It can be associated with significant functional, aesthetic and psychological problems, and often requires multi- and interdisciplinary management [3], [4], [7], [8].
Evidence-based practice in dentistry requires the application of pertinent high-quality research integrated with a consideration of patient needs and preferences and the experience and expertise of the oral healthcare professional [9]. Although some relevant high-quality research has been published, the clinical management of agenesis is largely reliant on retrospective and opinion-based reports.
The aims of the present article are to:
-
•
Review the diagnosis, prevalence, and aetiology of tooth agenesis and
-
•
Describe, with reference to current evidence, the associated clinical characteristics and management options.
The article will conclude with a brief overview of the management considerations of the two most commonly agenic permanent teeth which are the mandibular second premolar and the maxillary lateral incisor.
2. Diagnosis
2.1. Clinical presentation
A tooth is suspected of being developmentally absent if it has not erupted into the mouth and is not evident on a radiograph at an expected timepoint [10]. Reference to the ‘expected’ timing of eruption of the teeth is helpful in assessing whether the tooth is present. Table 1 shows the mean ages of eruption of the permanent teeth of patients attending the Outpatient Clinic of the Department of Pediatric Dentistry of Tokyo Dental College [11]. Table 1 also shows that the corresponding data from Australia and the U.K. are broadly similar [12], [13].
Table 1.
Approximate ages at which permanent teeth erupt.
| Dentition | Tooth | Age of eruption |
|||||
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Japan [11] |
Australia [12] |
United Kingdom [13] |
|||||
| Mean (SD) | Median | Median | |||||
| Male | Female | Male | Female | Male | Female | ||
| Maxilla | Central incisor | 7.29 (0.8) | 7.38 (0.65) | 7.4 | 7.2 | 7.3 | 7.42 |
| Lateral incisor | 8.29 (0.77) | 8.2 (0.72) | 8.6 | 8.2 | 8.44 | 8.66 | |
| Canines | 10.74 (0.96) | 10.6 (1.05) | 11.8 | 11.2 | 11.74 | 11.98 | |
| First premolar | 10.78 (0.99) | 10.6 (1.09) | 11.3 | 10.8 | 11.06 | 11.25 | |
| Second premolar | 11.68 (1.09) | 11.68 (1.49) | 12.1 | 11.7 | 12.01 | 12.25 | |
| First molar | 6.69 (0.89) | 6.92 (0.99) | 6.7 | 6.6 | 6.66 | 6.77 | |
| Second molar | 12.89 (1.03) | 12.68 (1.68 | 12.7 | 12.3 | 12.67 | 12.8 | |
| Mandible | Central incisor | 6.92 (0.59) | 6.38 (0.56) | 6.6 | 6.4 | 6.47 | 6.6 |
| Lateral incisor | 7.26 (0.74) | 7.17 (0.68) | 7.8 | 7.5 | 7.61 | 7.76 | |
| Canines | 10.25 (0.95) | 9.67 (0.93) | 11.0 | 10.1 | 10.57 | 11.0 | |
| First premolar | 10.6 (1.01) | 10.44 (0.97) | 11.2 | 10.6 | 10.94 | 11.19 | |
| Second premolar | 11.54 (1.05) | 11.41 (1.18) | 12.1 | 11.7 | 12.1 | 12.21 | |
| First molar | 6.49 (0.58) | 6.53 (0.60) | 6.6 | 6.4 | 6.59 | 6.76 | |
| Second molar | 12.15 (0.96) | 11.94 (1.23) | 12.2 | 11.8 | 12.15 | 12.26 | |
Furthermore, some clinical presentations suggest agenesis. For example, the failure of the contralateral lateral incisor or second premolar to erupt within four-to-six months of its antimere indicates likely absence [11], [12]. Primary tooth infra-occlusion occurs when its eruptive mechanism fails to keep the tooth aligned with the occlusal plane of the adjacent teeth [14]. Up to two thirds of individuals with missing premolars have infra-occlusion of the corresponding primary molars [15].
2.2. Definitive diagnosis
A definitive diagnosis is usually determined by a radiographic evaluation but care in determining a tooth's absence is essential as there can be a wide variation in chronological development. Second premolars, for example, can commence development as late as 9 or 10 years of age [16]. Nevertheless, all primary teeth should have erupted by 3 years of age and all permanent teeth (apart from the third permanent molars) should have erupted by the age of 13–14 years.
3. Prevalence
Caution is advised in the determination of the prevalence of tooth agenesis. Variation in the ages of subjects and methodologies used to identify agenic teeth in evaluated populations may preclude accurate reporting of the condition.
3.1. Primary dentition
The prevalence of developmentally absent teeth in the primary dentition ranges from 0.4% to 2.4% in European and Japanese populations [17], [18], [19]. Males and females appear to be equally affected with one or two missing incisor teeth as the most common presentation. The upper primary lateral incisor is most commonly absent in European populations while the lower primary incisor fails to develop most commonly in Japanese individuals [17], [19]. The absence of a primary predecessor indicates the likely absence of a permanent tooth.
3.2. Permanent dentition
A recent meta-analysis calculated an overall hypodontia prevalence of 6.4% (95% CI: 5.7–7.2) [20]. The prevalence of developmentally absent teeth in the permanent dentition, however, varies between populations. Prevalence rates of up to 36.4% have been reported within some cohorts [5]. In Africa, a rate of 13.4% has been recorded whereas in Europe, 7% of the population have developmentally absent teeth [20], [21]. The prevalence of agenic teeth in Australia is 6.3% of the Caucasian population [22], [23]. This is greater than the recorded rates of 5.0% in North America and 4.4% in Latin America [20]. However, it is less than the 8.5% reported among 3358 orthodontic patients treated in Nippon University Hospital and 8.7–10.8% found in a 2008 study of 2072 paediatric patients in Matsudo, Japan [24], [25]. A 2016 study of 9584 high school students showed a prevalence rate of agenesis of 3.8% which suggested that agenesis in Japan may not be as high as previously thought [26].
In contrast to the circumstances regarding supernumerary teeth, the prevalence of missing permanent teeth was reported to be higher in females with a combined female: male ratio of 1.22:1 (95%CI: 1.14, 1.3) reported in a systematic review by Khalaf et al. [27], [20]. However, this gender difference was not noted in a recent large epidemiological survey in Japan [26]. In addition, the evidence indicates that there is a higher prevalence among those presenting with a class III malocclusion compared with other malocclusion types [28], [29].
Table 2 shows the breakdown of the developmentally absent teeth by type in a descending order of prevalence. The information was derived from an evaluation of 145,848 subjects in 22 studies [20]. Of those with missing teeth, 41.9% were missing only one, 39.7% were missing two, 7.2% were missing three, 5.4% were missing four, 1.7% were missing five and 3.1% were missing six teeth or more [20]. Many of the findings were similar to the results from a survey carried out by Endo et al. of Japanese orthodontic patients in 2006, in which 76.3% participants were missing one or two teeth and the lower second premolar was the most commonly developmentally absent permanent tooth [24]. Of note, however, the most commonly agenic tooth in the more recent Hagiwara et al. survey was the upper second premolar [26].
Table 2.
Distribution of developmentally absent teeth by % tooth type of all developmentally absent teeth (excluding third permanent molars).
| Order of frequency | Tooth type* | % occurrence |
|---|---|---|
| 1 | 35, 45 | 29.9 |
| 2 | 12, 22 | 24.3 |
| 3 | 15,25 | 13.7 |
| 4 | 31,41 | 6.1 |
| 5 | 32, 42 | 4.3 |
| 6 | 14, 24 | 3.6 |
| 7 | 34, 44 | 2.7 |
| 8 | 13, 23 | 2.5 |
| 9 | 37, 47 | 1.8 |
| 10 | 17,27 | 1.5 |
| 11 | 33, 43 | 1.3 |
| 12 | 16, 26 | 1.1 |
| 13 | 36, 46 | 1 |
| 14 | 11, 21 | 1 |
KEY: %: Percentage. * Notation as per FDI World Dental Federation notation
4. Aetiology
The aetiology of hypodontia is not completely understood. Several theories have been proposed but it is commonly accepted that a multifactorial aetiology comprising genetic, epigenetic and environmental factors is involved [2], [6], [19]. The theories have been broadly considered as either evolutional or anatomical [30].
Evolutional theories are based on the rationale that the result of evolutionary changes to the craniofacial complex and/or dentition is a reduction in tooth number. In the 1940s, Dahlberg suggested that the more mesial tooth of each of the four types (incisor, canine, premolar and molar) was comparatively more stable than its more distal counterparts [31]. Clayton later suggested that the more distal teeth were superfluous to needs and became redundant during evolution [32]. Some propose that jaw size and tooth number are reducing as humans evolve [33]. However, the evidence to support this proposal is lacking [34], [35].
Anatomical theories originate from the concept that particular regions of a tooth’s dental lamina are vulnerable to environmental insult during dental development [30]. It has been proposed that the developmental absence of the maxillary lateral incisors and the second premolars and central incisors in the mandible occur because these teeth develop at sites of early fusion of the jaw [36]. Alternative thinking has suggested that agenesis was more likely in those areas where innervation was last to take place [37]. It is now accepted, however, that the developmental absence of a tooth or teeth is due to a complex interplay of genetic and environmental factors [1], [38].
4.1. Environmental factors
Developmental cascades are common in the formation of craniofacial structures and teeth [39]. This can be observed when syndromes involving tooth agenesis display dysplasias and clefts [5]. An environmental cause has been implicated in many craniofacial anomalies but evidence regarding environmental involvement in hypodontia is not robust. Investigations have indicated that medications such as the use of thalidomide while the mother is pregnant may result in hypodontia in her child [38], [40]. Rubella infection during pregnancy has also been proposed as a causative factor of hypodontia in the newborn [41].
Maternal smoking and/or the consumption of alcohol during pregnancy have been associated with craniofacial anomalies such as cleft lip and palate [42]. In particular, smoking can deleteriously impact upon the development of neural crest cells resulting in craniofacial anomalies [43]. As hypodontia and some craniofacial anomalies share specific signalling pathways, it has been speculated that a correlation exists between environmental factors and hypodontia.
Radiation and chemotherapy treatments for childhood cancers have been shown to have a detrimental impact on dental development, including tooth agenesis [1]. Krasuska-Slawinska et al. showed that with increased doses of chemotherapeutic agents such as vincristine, cyclophosphamide and doxorubicin over a long treatment period was associated with increased tooth agenesis [44]. In addition, the exposure to therapeutic radiation doses of 2000–4000 centigray during treatment for childhood cancers is known to result in dental anomalies often involving agenesis [45].
However, the evidence is weak regarding the link between trauma induced by inferior dental nerve blocks and/or trauma to the alveolar process containing the developing tooth germ and hypodontia [5].
4.2. Genetic factors
Current research suggests that the ‘inheritance’ of tooth agenesis can occur by different mechanisms [5]. X-linked (sex linked) autosomal recessive and autosomal dominant mechanisms have been implicated. Non-syndromic hypodontia can occur ‘randomly’ within an individual or be inherited with variations in expressivity and penetrance [2]. An association with additional dental and occlusal anomalies provides evidence of a genetic aetiology [6]. A genetic basis is further suggested by:
-
•
Sexual dimorphism. Greater prevalence of tooth agenesis has been reported in females.
-
•
Ethnic variation. Developmentally absent teeth appear to occur more frequently in individuals in North America than in Europe and Australia.
-
•
Occurrence in families. Tooth agenesis appears to occur more frequently in monozygotic twins compared with dizygotic twins and is more common among ‘blood’ relatives than in the general population and ‘non-blood’ relatives [2], [20], [33], [46], [47].
Non-syndromic hypodontia is the most common form of tooth agenesis [20]. Localised incisor–premolar hypodontia, in which only one or a few teeth are developmentally absent, is the presentation that occurs most frequently [48].
As tooth development is under genetic control, it is logical to presume that hypodontia is genetically linked and murine studies have provided information pointing to the identity of genes associated with agenesis in humans [2]. Agenesis may be due to the impairment of molecules which facilitate cell adhesion, the malfunctioning of extracellular matrix molecules and defective signalling pathways [49]. Although there are over 300 genes involved in tooth development, there appears to be a few key genes implicated in non-syndromic hypodontia [5].
Mutations of the human homeobox MSX1 (muscle segment homeobox 1) gene is linked with familial oligodontia [50]. An alteration of the PAX9 (paired box gene 9) gene has been associated with developmentally absent molars [51]. Both genes are involved in coding for transcription factor proteins essential in tooth development [2]. PAX9, for instance, codes for factors in tooth mesenchyme during tooth development with disturbances in the gene being involved in aborting development at the bud stage [52].
A third gene, AXIN 2 (axis inhibition protein 2), is responsible for regulating the Wnt signalling pathway [53]. Wnt proteins play an extensive part during embryonic development which includes dental development [2]. If the Wnt pathway is disrupted as a result of mutations of AXIN 2, tooth development is unlikely to occur [5]. AXIN 2 has been associated with the developmental absence of a lower incisor and has been observed in some forms of oligodontia [54]. Interestingly, the ‘malfunctioning’ of AXIN 2 is also associated with an increased risk of colo-rectal cancer which indicates the importance of the gene in a wide variety of molecular pathways [55]. Investigations have shown that mutations in EDA (ectodysplasin A) and EDA-receptor genes have resulted in the absence of maxillary incisors as well as other forms of sporadic tooth agenesis [56].
Most tooth agenesis is non-syndromic. However, there are several dozen syndromic conditions in which tooth agenesis constitutes a phenotypic spectrum of genetic abnormalities (Table 3) [2], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68].
Table 3.
Syndromes and medical disorders frequently associated with tooth agenesis.
| Syndrome/medical disorder | Responsible gene | Author (year) |
|---|---|---|
| Anhidrotic ectodermal dysplasia | EDA | Anbouba et al. (2020) [58] |
| Cleft lip and palate | TGFB3, MSX 1 | Tannure et al. (2012) [59] |
| Down syndrome | Trisomy 21 | Cobourne (2007) [2] |
| Ehlers Danlos syndrome | ADAMTS2 | Colige et al. (1999) [61] |
| Fraser Syndrome | FRAS1, FREM2, and GRIP1 | Kunz et al. (2020) [65] |
| Hemifacial Microsomia | OTX2, PLCD3, and MYT1 | Chen et al. (2018) [64] |
| Incontentia pigmenti | NEMO | Smahi et al. (2000) [62] |
| Limb mammary syndrome | TP63 | Van Bokhoven et al. (2001) [63] |
| Popliteal Petrygium Syndrome | Irf6 | Wu Chou et al. (2013) [67] |
| Rieger syndrome (Type 1) | Pitx2 | Semina et al. (1996) [60] |
| Van der Woude syndrome | Irf6 | Wang et al. (2003) [68] |
| Wiktop syndrome | Msx 1 | Jumlongras et al. (2001) [66] |
KEY: EDA: ectodysplasin A. TGFB3: Transforming growth factor beta 3. MSX1: Msh homeobox 1. ADAM; A disintegrin and metalloproteinase with thrombospondin motifs 2. FRAS 1: Fraser extracellular matrix complex subunit 1. FREM 2: FRAS1 related extracellular matrix 2. GRIP 1: Glutamate receptor interacting protein OTX 2: Orthodenticle homeobox 2. PLCD3; phospholipase C delta 3. MYT1: Myelin transcription factor 1. Nemo: NF-kappa-B essential modulator. TP63: Tumour protein 63. IRF6: Interferon regulatory factor 6. PITX2: Paired like homeodomain 2.
5. Assessment of patients with tooth agenesis
It is essential that patients presenting with tooth agenesis undergo a comprehensive dental assessment. Factors such as patient age, patient and family concerns and expectations, general medical and dental health and the number, quality and position of teeth present are important aspects of the assessment.
Prudent clinical and radiographic monitoring is crucial to ensure a timely and accurate diagnosis of developmentally absent teeth (Fig. 1) [10], [69]. When considering management strategies, clinicians must be certain that the tooth is absent and not merely delayed in its development. Treatment options range from no intervention to comprehensive multi- and inter-disciplinary input from general dental practitioners, medical practitioners, orthodontists, prosthodontists, periodontists, oral surgeons, genetic counsellors and psychologists amongst others [3], [4], [69], [70], [71]. Additionally, and particularly in the more severe cases of hypodontia, the clinician may need to advise the patient/family that medical investigation is required to determine the presence of a previously undiagnosed syndrome [5].
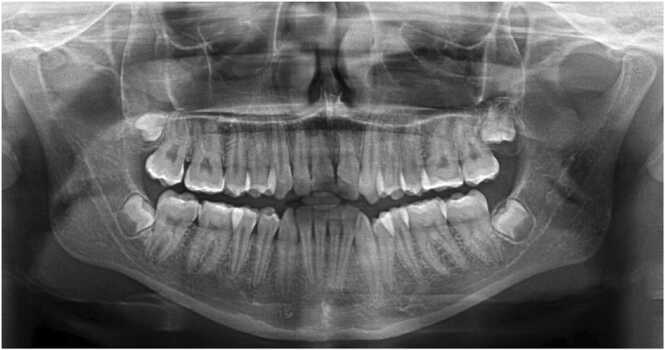
Fig. 1.
A dental pantomogram of a 15-year-old female illustrating agenesis of the upper left lateral incisor.
5.1. Psycho-social impact of tooth agenesis
Clinicians must also be aware of the potential psychosocial impact that agenesis might have on the individual. Although spacing is a common patient complaint, the literature has not always been equivocal in this regard [7], [8], [72], [73], [74], [75]. Laing et al., for example, suggested that there was no difference in the psychosocial effects between individuals presenting with hypodontia and individuals with equivalent ‘non-hypodontia’ orthodontic treatment needs [8]. However, the same study found that there were negative functional impacts on a participant’s chewing capacity when primary teeth without permanent successors had exfoliated [8].
A recent prospective cross-sectional study incorporated a hypodontia-specific quality of life tool as part of its methodology. The study found that the presentation and planned treatment of hypodontia adversely impacted on the social and emotional well-being of the 97 teenage participants, with more severe hypodontia associated with greater adverse effects [7]. The study also concluded that early and effective engagement between the affected individual and the interdisciplinary team is essential to minimise further negative impacts associated with the agenesis.
5.2. Occlusal and dental anomalies associated with tooth agenesis
It is also advisable for the clinician to be alert to the occlusal disturbances associated with hypodontia (Table 4, Fig. 2) [15], [76], [77], [78], [79], [80], [81], [82]. However, care is advised in the interpretation of the findings of studies investigating the prevalence of anomalies associated with hypodontia due to the wide range of methodologies adopted in the studies.
Table 4.
Reported % cases of occlusal and dental anomalies associated with tooth agenesis.
| Anomaly | Prevalence | Author |
|---|---|---|
| Conical incisors | 8.9% | Lai and Seow (1989) [15] |
| Hypoplastic enamel | 11% | Baccetti (1998) [79] |
| Impaction of teeth (notably maxillary canines) | 5.2–16% | Garib et al. (2009) Baccetti (1998) Garib et al. (2010) Al-Abdallah et al. (2015) [76], [79], [80], [81] |
| Infra-occlusion of primary teeth (agenesis of permanent premolars) | 15–65.7% | Baccetti (1998) [79] |
| Peg-shaped lateral incisors | 18–46.7% | Baccetti (1998) Garib et al. (2010) Al-Abdallah et al. (2015) [79], [80], [81] |
| Retained primary teeth | Up to 60% | Al-Abdallah et al. [81] |
| Smaller crown and root size (microdontia) | 20.6% | Garib et al. (2009) [76] |
| Transposition | 4.7% | Al-Abdallah et al. (2015) [81] |
| Taurodontism | Up to 38% | Kim and Lai [82] |
| KEY: %: Percentage | ||
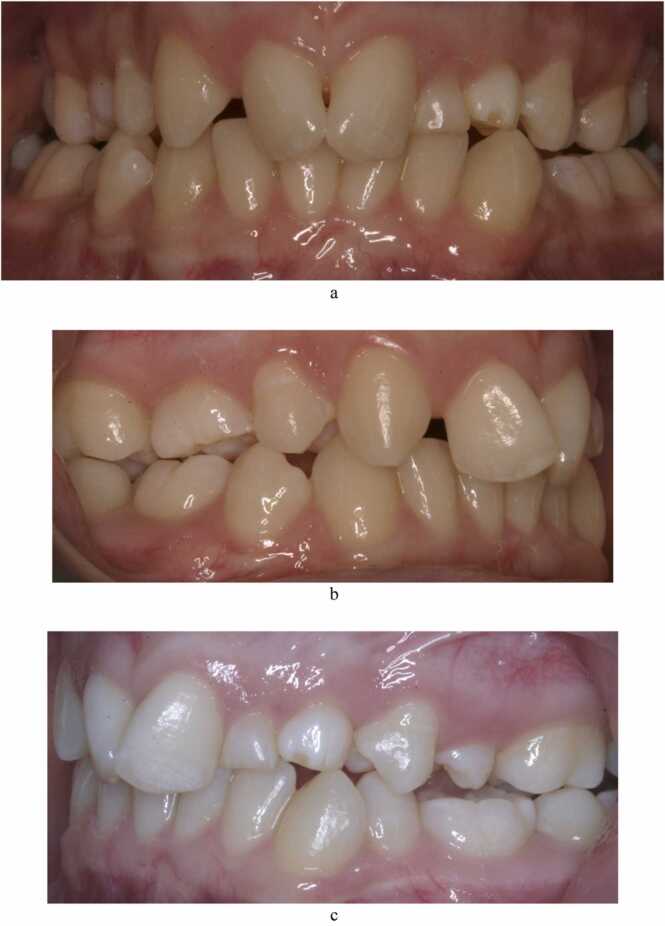
Fig. 2.
(a-c): Photographic images of a 16-year-old female with agenesis of both upper permanent lateral incisors, the upper right second premolar and both lower second premolars. She also presented with an upper retained primary lateral incisor and an upper left primary canine associated with the palatally ectopic permanent successor.
Additional characteristics associated with hypodontia include over-eruption of the tooth opposing the agenesis, an increased overbite with increasing tooth agenesis, delayed tooth eruption and deficient alveolar bone development [71], [83]. In addition, cephalometric studies have indicated an increased tendency for reduced mandibular plane angles, concave profiles, retrusion and overeruption of incisors and more retrusive upper lips associated with agenesis of greater severity [84], [85]. Ota and Arai showed that agenesis was twice as common within Japanese orthodontic patients with an Angle Class II Division 2 malocclusion compared to orthodontic patients in other groups [86]. More severe hypodontia has also been found to be associated with a class III skeletal tendency [85], [87].
6. Management of patients with tooth agenesis
The available evidence regarding management is principally comprised of case reports, case series, retrospective studies and clinician opinion. The stage of the patient’s dental development, however, may offer guidance on how to manage patients with tooth agenesis.
6.1. Primary and (early) mixed dentition
Clinicians must be aware that delayed dental development is a frequent finding in those with agenesis [78]. In severe oligodontia and anodontia, associated aesthetic, functional and psycho-social issues may require intervention in young children [3], [88]. An assessment of oro-facial functions of speech and mastication should be part of the management strategy [4], [70]. Treatment options include the build-up of malformed primary and permanent teeth with composite resin (CR), removable partial dentures and acrylic teeth attached to palatal arches can help alleviate aesthetic and functional concerns [3]. The patient, however, is committed to routine checks and adjustment of any fitted appliances during the patient’s remaining growth and development.
In milder forms of agenesis, ‘routine’ interceptive orthodontic interventions, such as the correction of an anterior crossbite, can be adopted to encourage favourable dental development. The extraction of deciduous teeth in the primary/early mixed dentition has been suggested as a strategy to promote favourable movement of adjacent permanent teeth but the literature suggests that additional later intervention is usually preferable to complete space closure [89], [90].
6.2. Late mixed and permanent dentition
By the age of 10 years, a provisional plan involving any necessary orthodontic and prosthodontic treatment is recommended. Because many agenic cases require orthodontic treatment to facilitate tooth positioning for management, early input from an orthodontist is commonly recommended [91], [92].
The orthodontic assessment should incorporate the general features of the malocclusion and the specific issues associated with the reduced tooth number [71]. The orthodontist will provide advice on whether the absent tooth or teeth is helpful in addressing any of the features of an underlying malocclusion [4]. Growth modification may be considered to address skeletal discrepancies during the late mixed/permanent dentition while the use of orthodontic fixed appliances can help enable the planned positioning of the teeth to fulfil the objectives of the treatment plan [4]. Anchorage requirements can be especially challenging when the number of the absent teeth is large [5]. The use of temporary anchorage devices has helped overcome many of the problems associated with the controlled tooth movement in this regard [83].
6.3. Management of agenic second permanent premolars
Clinicians may first be alerted to agenic second premolars due to infra-occlusion of the primary second molar [93]. Infra-occlusion occurs when the eruptive mechanism fails to keep a tooth aligned with the occlusal plane of adjacent teeth (Fig. 3) [94]. Up to 65.7% of individuals with missing second premolars have infra-occlusion of the corresponding primary molar [15], [76]. Management of the infra-occluded primary molar, without a permanent successor, depends on the:
-
•
Degree of infra-occlusion
-
•
Age of the patient
-
•
Likely progression of the infra-occlusion
-
•
Prognosis of the infra-occluded tooth and
-
•
Whether adverse effects of the infra-occlusion are present, such as a centreline shift to the site of the infra-occlusion and tipping of adjacent teeth toward site of the infra-occluded teeth [95].
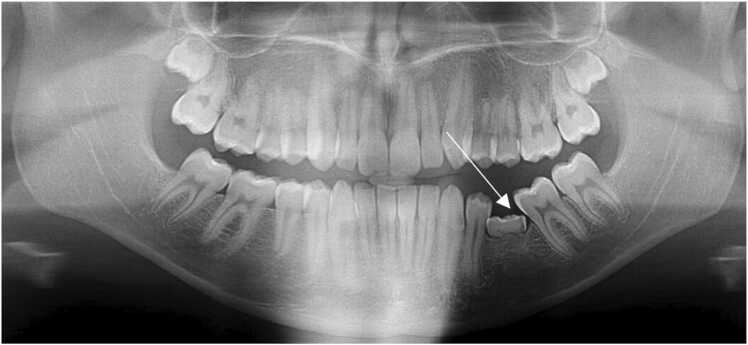
Fig. 3.
A dental pantomogram illustrating infra-occlusion of the lower left primary molar associated with an agenic second premolar. Note the tipping of the adjacent to the infra-occluded tooth and the associated small root sizes of the upper buccal segment teeth.
A mildly infra-occluded tooth is considered to be below the occlusal plane of the adjacent teeth but not below the contact points. It must be noted that the small step present between an infra-occluded primary molar and adjacent teeth may be due to differences in crown heights rather than the infra-occlusion. Moderate infra-occlusion occurs when the occlusal plane of the infra-occluded tooth is between the contact points and the alveolar crest, and the severely infra-occluded tooth is below the alveolar crest [95].
Early extraction may be indicated when the infra-occlusion is severe and adverse effects on the occlusion are observed [96]. This may result in some spontaneous space closure and potentially avoid further worsening of bony defects. The timely extraction of upper primary second molars without permanent successors may be helpful as permanent molars are likely to drift in a mesial direction to reduce the created space. Space closure subsequent to the extraction of lower primary second molars is more challenging as the permanent molars do not tend to drift in a mesial direction as easily. A 2008 study found that the long term survival of over 90% of retained primary molars associated with agenic second premolars was observed in 99 subjects, indicating that non-extraction of the primary second molar is an acceptable treatment option for many [97].
If the primary second molar is to be retained, a process called ‘slenderisation’ may be considered [98]. This involves the removal of up to 2.5 mm of enamel so that the mesiodistal width of the primary second molar is equivalent to that of a second premolar [99]. This can facilitate a Class I buccal occlusion and help ensure any future restoration is the appropriate size in the event of future loss the primary molar. However, this is not without risk. Removal of enamel beyond the enamel/dentine border will have negatively impact the tooth’s prognosis. Furthermore, the primary molar’s comparatively divergent roots risk being resorbed if space closure subsequent to ‘slenderisation’ results in contact with the roots of the adjacent teeth [98]. In addition, CR build-up of the occlusal surface of the primary molar can be considered if the tooth is mildly infraoccluded [98].
In general, however, the optimal time for orthodontic treatment for agenesis of second premolars, particularly in the mandible, is early adolescence (Fig. 4, Fig. 5, Fig. 6, Fig. 7) [3], [71], [100]. This is to ensure that treatment coincides with remaining facial growth and the eruption of the remaining permanent teeth.
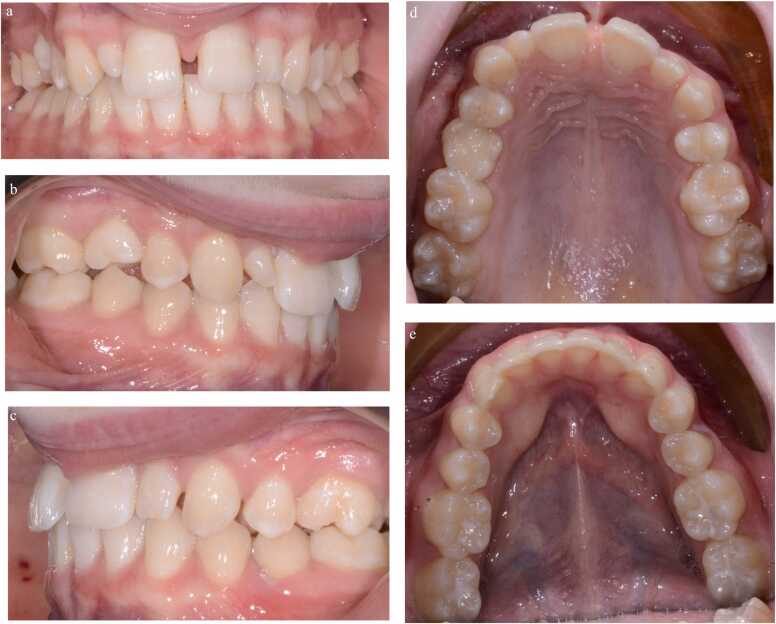
Fig. 4.
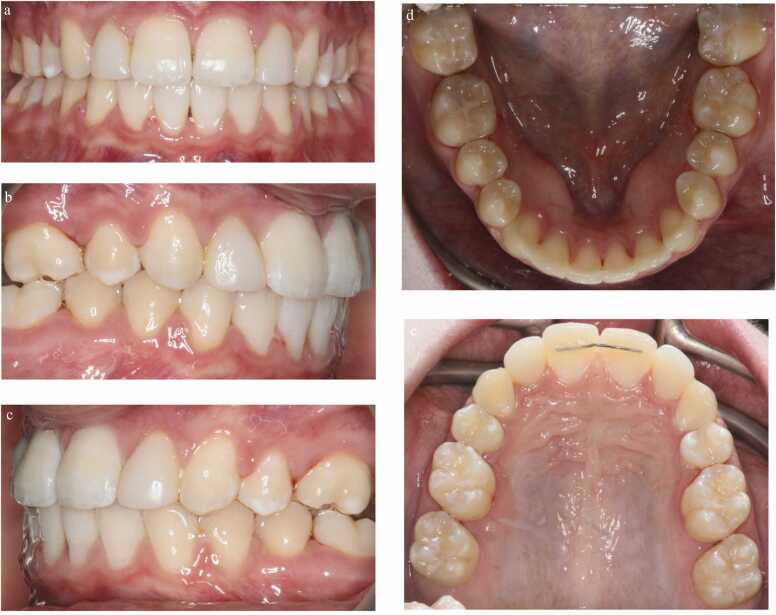
(a-e): Pre-treatment photographic images of a 14-year-old female (AB) with bilateral agenic upper second premolars, a retained and infra-occluded upper right primary second molar, peg-shaped upper right lateral incisor, microdont upper left lateral incisor and an upper midline diastema associated with a pronounced labial frenum.
Management required extraction of the retained primary molar, orthodontic treatment to enable space creation for the restorative build-up of the lateral incisors with composite resin and a frenectomy to reduce the risk of the diastema reopening.
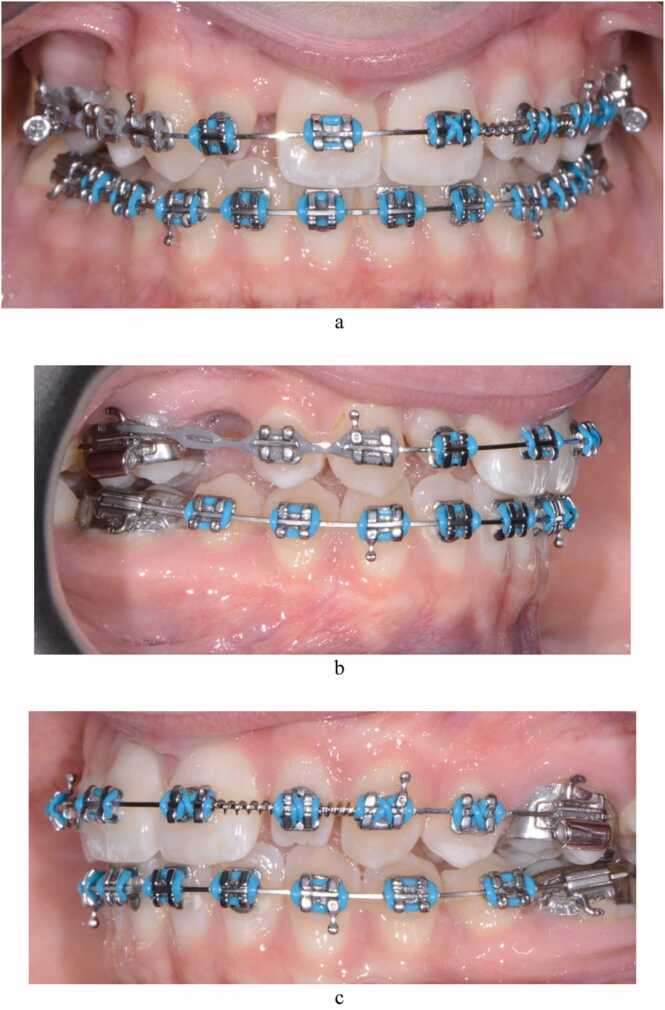
Fig. 5.
(a-c) AB 7 months after the extraction of the upper right primary molar and placement of fixed orthodontic appliances. The photographic images show the creation of space mesial and distal to the upper lateral incisors to enable their restorative build-up with composite resin, and closure of the space associated with the agenic upper right second premolar.
Fig. 6.
(a-c) AB 17 months after the extraction of the upper right primary molar and placement of fixed orthodontic appliances. The photographic image shows the upper lateral incisors which have been built up with composite resin and continued closure of the space associated with the agenic upper right second premolar.
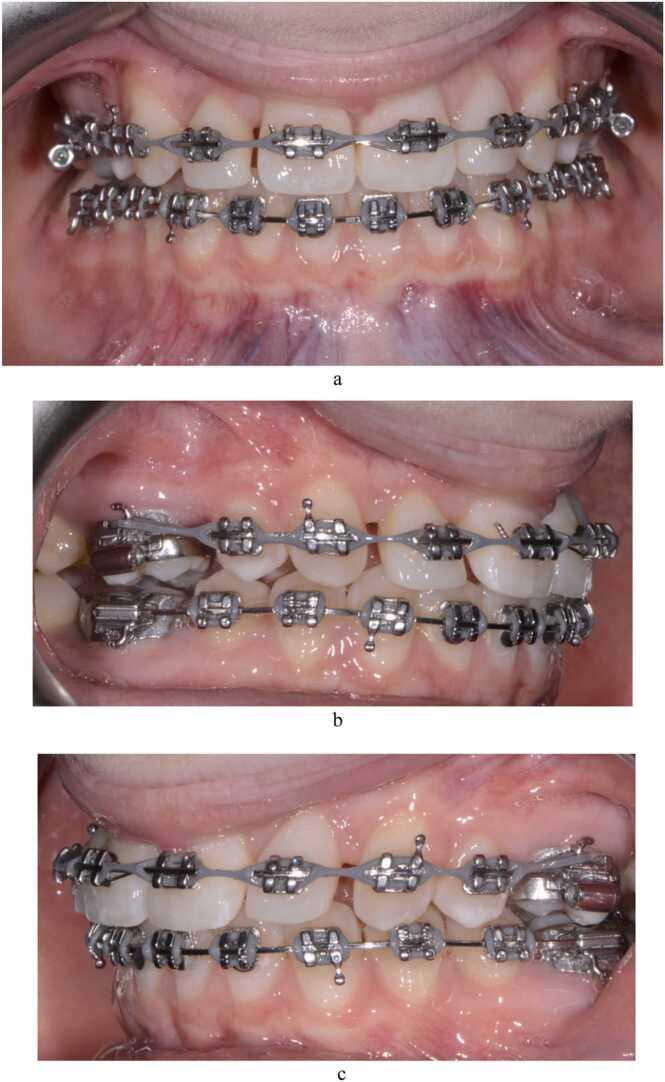
Fig. 7.
(a-e) Post-treatment photographic images of AB 24 months after the extraction of the upper right second primary molar and placement of fixed orthodontic appliances. Treatment included restorative build-up of the upper lateral incisors with composite resin and frenectomy of the labial frenum. The retention regimen included the bonding of a fixed lingual retainer to the upper central incisors to minimise reopening of the pre-treatment diastema.
6.4. Management of the agenic maxillary lateral incisor(s)
In a consideration of treatment duration, patient satisfaction, the effect on the temporomandibular joint and periodontal outcomes, the retrospective nature of current relevant evidence makes it unclear as to the greater effectiveness of either space closure or space opening (with prosthodontic replacement of the absent incisor(s)) in the management of agenic maxillary lateral incisors [101], [102], [103], [104]. According to Barber et al., the available research is focused on clinician and investigator outcomes rather than what is important to patients [105].
The initial malocclusion, however, can provide guidance on the management of agenic maxillary lateral incisors. The closer the presenting malocclusion is to a Class I incisor, canine and molar relationship with a ‘normal’ overbite, the more prosthodontic replacement of the lateral incisor may be indicated [91]. A Class III malocclusion may also benefit from this approach as prosthodontic replacement of the lateral incisor(s) will help to compensate for the position of the incisors in an associated relative maxillary deficiency. Prosthodontic replacement of the agenic lateral incisor may be via a resin-bonded fixed partial denture (FPD), a conventional full-coverage FPD, a cantilevered FPD, a removable partial denture or an implant [3], [91], [98], [106]. In addition, associated minor periodontal and surgical procedures such as alveolar ridge augmentation may be required to enable successful biological, functional and aesthetic treatment outcomes.
However, space closure can be the preferred option, particularly in those with a Class II malocclusion. This may involve extracting the primary lateral incisor and primary canine teeth to encourage eruption of the permanent canines next to the central incisors. The ‘excess’ space can be closed by the reduction of an increased overjet and/or protraction of the maxillary posterior teeth. A retrospective study showed that after 7.1 years, patients who had received ‘space-closure’ treatment for their agenic maxillary lateral incisor were more satisfied with their treatment and had superior periodontal health than those who had ‘space-opening’ and a prosthodontic replacement [104].
An important consideration in closing the space of an absent upper lateral incisor is the aesthetic outcome as a result of treatment [91], [107]. An assessment of the morphology, colour and gingival contour of the upper central incisor, upper canine, and upper premolar in addition to the smile line is essential [108]. This will help inform the clinician and patient whether closing the resulting space is a feasible aesthetic option, particularly as the latter two teeth will be required to mimic the characteristics of the lateral incisor and canine, respectively [106]. This often requires reshaping and/or the prosthodontic management of the upper six ‘anterior’ teeth and may also necessitate the reduction of the palatal cusp of the first premolar to prevent its interference with the occlusion in excursive movements. In addition, the premolar may need to be rotated mesio-palatally to replicate the wider canine (which can also help in using up excess space) [98]. Careful consideration must be given to the management of those cases in which the benefits to the occlusion of space closure conflict with a resulting sub-optimal aesthetic outcome.
6.5. Management of agenic molars
More severe forms of agenesis can result in the absence of several posterior teeth [24]. This may manifest itself as an increased overbite and a facial appearance that replicates that of an edentulous individual with lip eversion on closure and mandibular protrusion [109]. The reduced tooth number, the underdeveloped alveolar bone and the relatively small size of any present teeth herald considerable treatment planning difficulties which are better managed in a multi-disciplinary environment [75], [83].
7. Conclusions
Tooth agenesis is a relatively common condition. The present narrative overview has described the aetiology, prevalence, assessment, and management of affected patients with reference to the available evidence. Although the condition’s management is lacking in high-quality prospective research, it is clear that the presence of tooth agenesis can have significant negative aesthetic, functional, psychosocial and financial impacts.
It is essential that dental care practitioners are aware of the condition’s clinical characteristics and management options. Early diagnosis can facilitate appropriate planning and management of issues arising from developmentally absent teeth. It is crucial that dental care practitioners communicate with patients and their families that management may not be straightforward and patient care may require multi- and inter-disciplinary input.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Parkin N., Elcock C., Smith R.N., Griffin R.C., Brook A.H. The aetiology of hypodontia: the prevalence, severity and location of hypodontia within families. Arch Oral Biol. 2009;54:S52–S56. doi: 10.1016/j.archoralbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Cobourne M.T. Familial human hypodontia–is it all in the genes? Br Dent J. 2007;203:203–208. doi: 10.1038/bdj.2007.732. [DOI] [PubMed] [Google Scholar]
- 3.Jepson N.J., Nohl F.S., Carter N.E., Gillgrass T.J., Meechan J.G., Hobson R.S., Nunn J.H. The interdisciplinary management of hypodontia: restorative dentistry. Br Dent J. 2003;194:299–304. doi: 10.1038/sj.bdj.4809940. [DOI] [PubMed] [Google Scholar]
- 4.Gill D.S., Barker C.S. The multidisciplinary management of hypodontia: a team approach. Br Dent J. 2015;218:143–149. doi: 10.1038/sj.bdj.2015.52. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ani A.H., Antoun J.S., Thomson W.M., Merriman T.R., Farella M. Hypodontia: an update on its etiology, classification, and clinical management. BioMed Res Int. 2017;2017:9378325. doi: 10.1155/2017/9378325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook A.H. Multilevel complex interactions between genetic, epigenetic and environmental factors in the aetiology of anomalies of dental development. Arch Oral Biol. 2009;54:S3–S17. doi: 10.1016/j.archoralbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johal A., Huang Y., Toledano S. Hypodontia and its impact on a young person's quality of life, esthetics, and self-esteem. Am J Orthod Dentofac Orthop. 2022;161:220–227. doi: 10.1016/j.ajodo.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Laing E., Cunningham S.J., Jones S., Moles D., Gill D. Psychosocial impact of hypodontia in children. Am J Orthod Dentofac Orthop. 2010;137:35–41. doi: 10.1016/j.ajodo.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Chiappelli F. Evidence-based dentistry: two decades and beyond. J Evid Based Dent Pr. 2019;19:7–16. doi: 10.1016/j.jebdp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Fleming P.S., Johal A., DiBiase A.T. Managing malocclusion in the mixed dentition: six keys to success part 1. Dent Update. 2008;35:607–613. doi: 10.12968/denu.2008.35.9.607. [DOI] [PubMed] [Google Scholar]
- 11.Makino E., Tsujino K., Ishii T., Shintani S., Sueishi K. Difference in bilateral timing of eruption of permanent teeth. Bull Tokyo Dent Coll. 2018;59:277–284. doi: 10.2209/tdcpublication.2018-0009. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti J., Townsend G.C. New standards for permanent tooth emergence in Australian children. Aust Dent J. 2003;48:39–42. doi: 10.1111/j.1834-7819.2003.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 13.Elmes A., Dykes E., Cookson M.J. A cross-sectional survey to determine the ages of emergence of permanent teeth of Caucasian children of the Colchester area of the UK. Br Dent J. 2010;209 doi: 10.1038/sj.bdj.2010.672. [DOI] [PubMed] [Google Scholar]
- 14.Odeh R., Mihailidis S., Townsend G., Lähdesmäki R., Hughes T., Brook A. Prevalence of infraocclusion of primary molars determined using a new 2D image analysis methodology. Aust Dent J. 2016;61:183–189. doi: 10.1111/adj.12349. [DOI] [PubMed] [Google Scholar]
- 15.Lai P.Y., Seow W.K. A controlled study of the association of various dental anomalies with hypodontia of permanent teeth. Pedia Dent. 1989;11:291–296. [PubMed] [Google Scholar]
- 16.Wisth P.J., Thunold K., Boe O.E. The craniofacial morphology of individuals with hypodontia. Acta Odontol Scan. 1974;32:281–290. doi: 10.3109/00016357409026344. [DOI] [PubMed] [Google Scholar]
- 17.Yonezu T., Hayashi Y., Sasaki J., Machida Y.J. Prevalence of congenital dental anomalies of the deciduous dentition in Japanese children. Bull Tokyo Dent Coll. 1997;38:27–32. [PubMed] [Google Scholar]
- 18.Brook A.H. Dental anomalies of number, form and size: their prevalence in British school children. J Int Assoc Dent Child. 1974;5:37–53. [PubMed] [Google Scholar]
- 19.Nieminen P. Genetic basis of tooth agenesis. J Exp Zool B: Mol Dev Evol. 2009;15(312):320–342. doi: 10.1002/jez.b.21277. [DOI] [PubMed] [Google Scholar]
- 20.Khalaf K., Miskelly J., Voge E., Macfarlane T.V. Prevalence of hypodontia and associated factors: a systematic review and meta-analysis. J Orthod. 2014;41:299–316. doi: 10.1179/1465313314Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 21.Maatouk F., Baaziz A., Ghnima S., Masmoudi F., Ghedira H. Survey on hypodontia in Sayada, Tunisia. Quintessence Int. 2008;39:115–120. [PubMed] [Google Scholar]
- 22.Lynham A. Panoramic radiographic survey of hypodontia in Australian Defence Force recruits. Aust Dent J. 1990;35:19–22. doi: 10.1111/j.1834-7819.1990.tb03021.x. [DOI] [PubMed] [Google Scholar]
- 23.Davies P.L. Agenesis of teeth in the permanent dentition: a frequency study in Sydney schoolchildren. Aust Dent J. 1968;13:146–150. doi: 10.1111/j.1834-7819.1968.tb02254.x. [DOI] [PubMed] [Google Scholar]
- 24.Endo T., Ozoe R., Kubota M., Akiyama M., Shimooka S. A survey of hypodontia in Japanese orthodontic patients. Am J Orthod Dentofac Orthop. 2006;129:29–35. doi: 10.1016/j.ajodo.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Goya H.A., Tanaka S., Maeda T., Akimoto Y. An orthopantomographic study of hypodontia in permanent teeth of Japanese pediatric patients. J Oral Sci. 2008;50:143–150. doi: 10.2334/josnusd.50.143. [DOI] [PubMed] [Google Scholar]
- 26.Hagiwara Y., Uehara T., Narita T., Tsutsumi H., Nakabayashi S., Araki M. Prevalence and distribution of anomalies of permanent dentition in 9584 Japanese high school students. Odontology. 2016;104:380–389. doi: 10.1007/s10266-015-0225-2. [DOI] [PubMed] [Google Scholar]
- 27.Meade M.J. Supernumerary Teeth: An overview for the general dental practitioner. Dent Update. 2020;47:729–738. [Google Scholar]
- 28.Chung C.J., Han J.H., Kim K.H. The pattern and prevalence of hypodontia in Koreans. Oral Dis. 2008;14:620–625. doi: 10.1111/j.1601-0825.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 29.Uslu O., Akcam M.O., Evirgen S., Cebeci I. Prevalence of dental anomalies in various malocclusions. Am J Orthod Dentofac Orthop. 2009;135:328–335. doi: 10.1016/j.ajodo.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Galluccio G., Castellano M., La, Monaca C. Genetic basis of non-syndromic anomalies of human tooth number. Arch Oral Biol. 2012;57:918–930. doi: 10.1016/j.archoralbio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Dahlberg A.A. The changing dentition of man. J Am Dent Assoc. 1945;32:676–690. [Google Scholar]
- 32.Clayton J.M. Congenital dental anomalies occurring in 3,557 children. J Dent Child. 1956;23:206–208. [Google Scholar]
- 33.Vastardis H. The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofac Orthop. 2000;117:650–656. [PubMed] [Google Scholar]
- 34.Mattheeuws N., Dermaut L., Martens G. Has hypodontia increased in Caucasians during the 20th century? A meta-analysis. European. J Orthod. 2004;26:99–103. doi: 10.1093/ejo/26.1.99. [DOI] [PubMed] [Google Scholar]
- 35.Rakhshan V., Rakhshan H. Meta-analysis and systematic review of the number of non-syndromic congenitally missing permanent teeth per affected individual and its influencing factors. Eur J Orthod. 2016;38:170–177. doi: 10.1093/ejo/cjv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svinhufvud E., Myllärniemi S., Norio R. Dominant inheritance of tooth malpositions and their association to hypodontia. Clin Genet. 1988;34:373–381. doi: 10.1111/j.1399-0004.1988.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 37.Kjær I., Kocsis G., Nodal M., Christensen L.R. Aetiological aspects of mandibular tooth agenesis—focusing on the role of nerve, oral mucosa, and supporting tissues. Eur J Orthod. 1994;16:371–375. doi: 10.1093/ejo/16.5.371. [DOI] [PubMed] [Google Scholar]
- 38.Brook A.H. A unifying aetiological explanation for anomalies of human tooth number and size. Arch Oral Biol. 1984;29:373–378. doi: 10.1016/0003-9969(84)90163-8. [DOI] [PubMed] [Google Scholar]
- 39.Matalova E., Fleischmannova J., Sharpe P.T., Tucker A.S. Tooth agenesis: from molecular genetics to molecular dentistry. J Dent Res. 2008;87:617–623. doi: 10.1177/154405910808700715. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert-Barness E. Teratogenic causes of malformations. Ann Clin Lab Sci. 2010;40:99–114. [PubMed] [Google Scholar]
- 41.Cameron J., Sampson W.J. Hypodontia of the permanent dentition. Case Rep Aust Dent J. 1996;41:1–5. doi: 10.1111/j.1834-7819.1996.tb05645.x. [DOI] [PubMed] [Google Scholar]
- 42.Little J., Cardy A., Munger R.G. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ. 2004;82:213–218. [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon M.J., Marazita M.L., Beaty T.H., Murray J.C. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasuska-Sławińska E., Brożyna A., Dembowska-Bagińska B., Olczak-Kowalczyk D. Antineoplastic chemotherapy and congenital tooth abnormalities in children and adolescents. Conte Oncol. 2016;20:394–401. doi: 10.5114/wo.2016.64602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonis A.L., Tarbell N., Valachovic R.W., Gelber R., Schwenn M., Sallan S. Dentofacial development in long‐term survivors of acute lymphoblastic leukemia: A comparison of three treatment modalities. Cancer. 1990;66:2645–2652. doi: 10.1002/1097-0142(19901215)66:12<2645::aid-cncr2820661230>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 46.Grahnén H. Hypodontia in the permanent dentition: a clinical and genetic investigation. Odontol Rev. 1956;7:1–100. [Google Scholar]
- 47.Polder B.J., Van’t Hof M.A., Van der Linden F.P., Kuijpers‐Jagtman A.M. A meta‐analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32:217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 48.Nieminen P., Arte S., Pirinen S., Peltonen L., Thesleff I. Gene defect in hypodontia: exclusion of MSX1 and MSX2 as candidate genes. Hum Genet. 1995;96:305–308. doi: 10.1007/BF00210412. [DOI] [PubMed] [Google Scholar]
- 49.Jernvall J., Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 50.Lidral A.C., Reising B.C. The role of MSX1 in human tooth agenesis. J Dent Res. 2002;81:274–278. doi: 10.1177/154405910208100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stockton D.W., Das P., Goldenberg M., D'Souza R.N., Patel P.I. Mutation of PAX9 is associated with oligodontia. Nat Genet. 2000;24:18–19. doi: 10.1038/71634. [DOI] [PubMed] [Google Scholar]
- 52.Mitsui S.N., Yasue A., Masuda K., Watanabe K., Horiuchi S., Imoto I., Tanaka E. Novel PAX9 mutations cause non-syndromic tooth agenesis. J Dent Res. 2014;93:245–249. doi: 10.1177/0022034513519801. [DOI] [PubMed] [Google Scholar]
- 53.Callahan N., Modesto A., Meira R., Seymen F., Patir A., Vieira A.R. Axis inhibition protein 2 (AXIN2) polymorphisms and tooth agenesis. Arch Oral Biol. 2009;54:45–49. doi: 10.1016/j.archoralbio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Küchler E.C., Lips A., Tannure P.N., Ho B., Costa M.C., Granjeiro J.M., Vieira A.R. Tooth agenesis association with self-reported family history of cancer. J Dent Res. 2013;92:149–155. doi: 10.1177/0022034512468750. [DOI] [PubMed] [Google Scholar]
- 55.Lammi L., Arte S., Somer M., Järvinen H., Lahermo P., Thesleff I., Pirinen S., Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alves-Ferreira M., Pinho T., Sousa A., Sequeiros J., Lemos C., Alonso I. Identification of genetic risk factors for maxillary lateral incisor agenesis. J Dent Res. 2014;93:452–458. doi: 10.1177/0022034514523986. [DOI] [PubMed] [Google Scholar]
- 57.Yin W., Bian Z. The gene network underlying hypodontia. J Dent Res. 2015;94:878–885. doi: 10.1177/0022034515583999. [DOI] [PubMed] [Google Scholar]
- 58.Anbouba G.M., Carmany E.P., Natoli J.L. The characterization of hypodontia, hypohidrosis, and hypotrichosis associated with X‐linked hypohidrotic ectodermal dysplasia: a systematic review. Am J Med Genet A. 2020;182:831–841. doi: 10.1002/ajmg.a.61493. [DOI] [PubMed] [Google Scholar]
- 59.Tannure P.N., Oliveira C.A., Maia L.C., Vieira A.R., Granjeiro J.M., de Castro Costa M. Prevalence of dental anomalies in nonsyndromic individuals with cleft lip and palate: a systematic review and meta-analysis. Cleft Palate Craniofac J. 2012;49:194–200. doi: 10.1597/10-043. [DOI] [PubMed] [Google Scholar]
- 60.Semina E.V., Reiter R., Leysens N.J., Alward W.L., Small K.W., Datson N.A., Siegel-Bartelt J., Bierke-Nelson D., Bitoun P., Zabel B.U., Carey J.C. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 61.Colige A., Sieron A.L., Li S.W., et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I Nproteinase gene. Am J Hum Genet. 1999;65:308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smahi A., Courtois G., Vabres P., et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 63.Van Bokhoven H., Hamel B.C., Bamshad M., Sangiorgi E., Gurrieri F., Duijf P.H., Vanmolkot K.R., van Beusekom E., van Beersum S.E., Celli J., Merkx G.F. p63 Gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand–split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–492. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q., Zhao Y., Shen G., Dai J. Etiology and pathogenesis of hemifacial microsomia. J Dent Res. 2018;97:1297–1305. doi: 10.1177/0022034518795609. [DOI] [PubMed] [Google Scholar]
- 65.Kunz F., Kayserili H., Midro A., de Silva D., Basnayake S., Güven Y., Borys J., Schanze D., Stellzig‐Eisenhauer A., Bloch‐Zupan A., Zenker M. Characteristic dental pattern with hypodontia and short roots in Fraser syndrome. Am J Med Genet A. 2020;182:1681–1689. doi: 10.1002/ajmg.a.61610. [DOI] [PubMed] [Google Scholar]
- 66.Jumlongras D., Bei M., Stimson J.M., Wang W.F., DePalma S.R., Seidman C.E., Felbor U., Maas R., Seidman J.G., Olsen B.R. A nonsense mutation in MSX1 causes Witkop syndrome. Am J Hum Genet. 2001;69:67–74. doi: 10.1086/321271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu-Chou Y.H., Lo L.J., Chen K.T., Chang C.S., Chen Y.R. A combined targeted mutation analysis of IRF6 gene would be useful in the first screening of oral facial clefts. BMC Med Genet. 2013;14:1–5. doi: 10.1186/1471-2350-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Liu J., Zhang H., Xiao M., Li J., Yang C., Lin X., Wu Z., Hu L., Kong X. Novel mutations in the IRF6 gene for Van der Woude syndrome. Hum Genet. 2003;113:382–386. doi: 10.1007/s00439-003-0989-2. [DOI] [PubMed] [Google Scholar]
- 69.Hobson R.S., Carter N.E., Gillgrass T.J., Jepson N.J., Meechan J.G., Nohl F., Nunn J.H. The interdisciplinary management of hypodontia: the relationship between an interdisciplinary team and the general dental practitioner. Br Dent J. 2003;194:479–482. doi: 10.1038/sj.bdj.4810184. [DOI] [PubMed] [Google Scholar]
- 70.Gill D.S., Jones S., Hobkirk J., Bassi S., Hemmings K., Goodman J. Counselling patients with hypodontia. Dent Update. 2008;35:344–352. doi: 10.12968/denu.2008.35.5.344. [DOI] [PubMed] [Google Scholar]
- 71.Carter N.E., Gillgrass T.J., Hobson R.S., Jepson N., Meechan J.G., Nohl F.S., Nunn J.H. The interdisciplinary management of hypodontia: orthodontics. Br Dent J. 2003;194:361–366. doi: 10.1038/sj.bdj.4809995. [DOI] [PubMed] [Google Scholar]
- 72.Wong A.T., McMillan A.S., McGrath C. Oral health-related quality of life and severe hypodontia. J Oral Rehabil. 2006;33:869–873. doi: 10.1111/j.1365-2842.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 73.Hashem A., Kelly A., O’Connell B., O'Sullivan M. Impact of moderate and severe hypodontia and amelogenesis imperfecta on quality of life and self-esteem of adult patients. J Dent. 2013;41:689–694. doi: 10.1016/j.jdent.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Anweigi L., Allen P.F., Ziada H. The use of the Oral Health Impact Profile to measure the impact of mild, moderate and severe hypodontia on oral health‐related quality of life in young adults. J Oral Rehab. 2013;40:603–608. doi: 10.1111/joor.12062. [DOI] [PubMed] [Google Scholar]
- 75.Hobkirk J.A., Goodman J.R., Jones S.P. Presenting complaints and findings in a group of patients attending a hypodontia clinic. Br Dent J. 1994;177:337–339. doi: 10.1038/sj.bdj.4808606. [DOI] [PubMed] [Google Scholar]
- 76.Garib D.G., Peck S., Gomes S.C. Increased occurrence of dental anomalies associated with second-premolar agenesis. Angle Orthod. 2009;79:436–441. doi: 10.2319/021308-87.1. [DOI] [PubMed] [Google Scholar]
- 77.Peck S., Peck L., Kataja M. Concomitant occurrence of canine malposition and tooth agenesis: evidence of orofacial genetic fields. Am J Orthod Dentofac Orthop. 2002;122:657–660. doi: 10.1067/mod.2002.129915. [DOI] [PubMed] [Google Scholar]
- 78.Dhamo B., Vucic S., Kuijpers M.A., Jaddoe V.W., Hofman A., Wolvius E.B., Ongkosuwito E.M. The association between hypodontia and dental development. Clin Oral Invest. 2016;20:1347–1354. doi: 10.1007/s00784-015-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baccetti T. A controlled study of associated dental anomalies. Angle Orthod. 1998;68:267–274. doi: 10.1043/0003-3219(1998)068<0267:ACSOAD>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 80.Garib D.G., Alencar B.M., Lauris J.R., Baccetti T. Agenesis of maxillary lateral incisors and associated dental anomalies. Am J Orthod Dentofac Orthop. 2010;137 doi: 10.1016/j.ajodo.2009.12.024. 732-e1. [DOI] [PubMed] [Google Scholar]
- 81.Al-Abdallah M., AlHadidi A., Hammad M., Al-Ahmad H. Prevalence and distribution of dental anomalies: a comparison between maxillary and mandibular tooth agenesis. Am J Orthod Dentofac Orthop. 2015;148:793–798. doi: 10.1016/j.ajodo.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Kim W., Lai P. Association of taurodontism with hypodontia: a controlled study. Pedia Dent. 1989;11:215. [PubMed] [Google Scholar]
- 83.Harrison J. Management of patients with hypodontia: what has changed? J Orthod. 2019;46(1_suppl):60–64. doi: 10.1177/1465312519840043. [DOI] [PubMed] [Google Scholar]
- 84.Gungor A.Y., Turkkahraman H. Effects of severity and location of nonsyndromic hypodontia on craniofacial morphology. Angle Orthod. 2013;83:584–590. doi: 10.2319/091012-722.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acharya P.N., Jones S.P., Moles D., Gill D., Hunt N.P. A cephalometric study to investigate the skeletal relationships in patients with increasing severity of hypodontia. Angle Orthod. 2010;80:699–706. doi: 10.2319/072309-411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ota K., Arai K. Prevalence and patterns of tooth agenesis in Angle Class II Division 2 malocclusion in Japan. Am J Orthod Dentofac Orthop. 2015;148:123–129. doi: 10.1016/j.ajodo.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Vucic S., Dhamo B., Kuijpers M.A., Jaddoe V.W., Hofman A., Wolvius E.B., Ongkosuwito E.M. Craniofacial characteristics of children with mild hypodontia. Am J Orthod Dentofac Orthop. 2016;150:611–619. doi: 10.1016/j.ajodo.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Nunn J.H., Carter N.E., Gillgrass T.J., Hobson R.S., Jepson N.J., Meechan J.G., Nohl F.S. The interdisciplinary management of hypodontia: background and role of paediatric dentistry. Br Dent J. 2003;194:245–251. doi: 10.1038/sj.bdj.4809925. [DOI] [PubMed] [Google Scholar]
- 89.Lindqvist B. Extraction of the deciduous second molar in hypodontia. Eur J Orthod. 1980;2:173–181. doi: 10.1093/ejo/2.3.173. [DOI] [PubMed] [Google Scholar]
- 90.Kokich V., Jr Early management of congenitally missing teeth. Sem Orthod. 2005;11:146–151. [Google Scholar]
- 91.Sabri R. Management of missing maxillary lateral incisors. J Am Dent Assoc. 1999;130:80–84. doi: 10.14219/jada.archive.1999.0032. [DOI] [PubMed] [Google Scholar]
- 92.Meade M.J., Dreyer C.W. Eruption disturbances in the mixed dentition: orthodontic considerations for primary dental care. Austr Dent J. 2022;67:S14–S23. doi: 10.1111/adj.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hua L., Thomas M., Bhatia S., Bowkett A., Merrett S. To extract or not to extract? Management of infraoccluded second primary molars without successors. Br Dent J. 2019;227:93–98. doi: 10.1038/s41415-019-0207-9. [DOI] [PubMed] [Google Scholar]
- 94.Kurol J., Thilander B. Infraocclusion of primary molars and the effect on occlusal development, a longitudinal study. Eur J Orthod. 1984;6:277–293. doi: 10.1093/ejo/6.4.277. [DOI] [PubMed] [Google Scholar]
- 95.Attwall R., Parker K., Gill D.S. Management of infra-occluded primary molars. Dent Update. 2018;45:625–633. [Google Scholar]
- 96.Kennedy D.B. Treatment strategies for ankylosed primary molars. Eur Arch Paediatr Dent. 2009;10:201–210. doi: 10.1007/BF03262683. [DOI] [PubMed] [Google Scholar]
- 97.Bjerklin K., Al-Najjar M., Kårestedt H., Andrén A. Agenesis of mandibular second premolars with retained primary molars. A longitudinal radiographic study of 99 subjects from 12 years of age to adulthood. Eur J Orthod. 2008;30:254–261. doi: 10.1093/ejo/cjn027. [DOI] [PubMed] [Google Scholar]
- 98.Lewis B.R., Gahan M.J., Hodge T.M., Moore D. The orthodontic-restorative interface: 2. Compensating for variations in tooth number and shape. Dent Update. 2010;37:138–152. doi: 10.12968/denu.2010.37.3.138. [DOI] [PubMed] [Google Scholar]
- 99.Kokick V.G., Kokich V.O. Congenitally missing mandibular second premolars: clinical options. Am J Orthod Dentofac Orthop. 2006;130:437–444. doi: 10.1016/j.ajodo.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 100.Mamopoulou A., Hägg U., Schröder U., Hansen K. Agenesis of mandibular second premolars. Spontaneous space closure after extraction therapy: a 4-year follow-up. Eur J Orthod. 1996;18:589–600. doi: 10.1093/ejo/18.6.589. [DOI] [PubMed] [Google Scholar]
- 101.Jamilian A., Perillo L., Rosa M. Missing upper incisors: a retrospective study of orthodontic space closure versus implant. Prog Orthod. 2015;16:2. doi: 10.1186/s40510-015-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seehra J., Al-Ali A., Pandis N., Cobourne M.T. Space closure versus space opening for bilateral absent upper lateral incisors: what is the duration of orthodontic treatment? Eur J Orthod. 2020;42:460–465. doi: 10.1093/ejo/cjz062. [DOI] [PubMed] [Google Scholar]
- 103.Josefsson E., Lindsten R. Treatment of missing maxillary lateral incisors: a clinical and aesthetic evaluation. Eur J Orthod. 2019;41:273–278. doi: 10.1093/ejo/cjy061. [DOI] [PubMed] [Google Scholar]
- 104.Robertsson S., Mohlin B. The congenitally missing upper lateral incisor. A retrospective study of orthodontic space closure versus restorative treatment. Eur J Orthod. 2000;22:697–710. doi: 10.1093/ejo/22.6.697. [DOI] [PubMed] [Google Scholar]
- 105.Barber S., Bekker H.L., Meads D., Pavitt S., Khambay B. Identification and appraisal of outcome measures used to evaluate hypodontia care: a systematic review. Am J Orthod Dentofac Orthop. 2018;153:184–194. doi: 10.1016/j.ajodo.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Zachrisson B.U., Rosa M., Toreskog S. Congenitally missing maxillary lateral incisors: canine substitution. Am J Orthod Dentofac Orthop. 2011;139:440. doi: 10.1016/j.ajodo.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 107.Rosa M., Olimpo A., Fastuca R., Caprioglio A. Perceptions of dental professionals and laypeople to altered dental esthetics in cases with congenitally missing maxillary lateral incisors. Prog Orthod. 2013;14:1–7. doi: 10.1186/2196-1042-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gill D.S., Naini F.B., Tredwin C.J. Smile aesthetics. Dent Update. 2007;34:152–158. doi: 10.12968/denu.2007.34.3.152. [DOI] [PubMed] [Google Scholar]
- 109.Hobkirk J.A., Brook A.H. The management of patients with severe hypodontia. J Oral Rehabil. 1980;7:289–298. doi: 10.1111/j.1365-2842.1980.tb00447.x. [DOI] [PubMed] [Google Scholar]