Abstract
Purpose
To characterize the secondary anterior cruciate ligament (ACL) injury rates after primary allograft anterior cruciate ligament reconstruction (ACLR) and to identify the age cut-score at which the risk of allograft failure decreases.
Methods
All patients who underwent primary ACLR within a single orthopaedic department between January 2005 and April 2020 were contacted at a minimum of 2 years post-ACLR to complete a survey regarding complications experienced post-surgery, activity level, and perceptions of knee health. Patients were excluded for incidence of previous ACLR (ipsilateral or contralateral) and/or age younger than 14 years. Relative proportions were calculated, binary regression analysis was performed, and receiver operating characteristic analysis was used to identify the threshold age for maximal sensitivity and specificity to predict high risk of allograft failure, defined as undergoing revision ACLR.
Results
Of the 939 surveys completed, 398 patients underwent primary allograft ACLR (mean age 39.5 years; range 16.0-66.1 years; 54.3% female). The secondary ACL injury rate was 11.6% (5.8% ipsilateral revision ACLR, 5.8% contralateral ACL injury). Male and female patients had similar revision (5.5% male, 6.0% female, P = .82) and contralateral ACL injury rates (6.6% male, 5.1% female, P = .52). Receiver operating characteristic analysis indicated that age ≤34 years was threshold for differentiating high risk of allograft failure (area under the curve 0.65, 95% confidence interval 0.55-0.76; P = .014). Patients aged ≤34 years had a greater secondary injury rate than patients >34 years (20.4% (10.2% revision ACLR, 10.2% contralateral ACL injury) versus 6.9% (3.5% revision ACLR, 3.5% contralateral ACL injury; P < .001). Binary regression analysis demonstrated that decreasing age was associated with increased risk of graft failure (χ2 = 7.9, P = .02.).
Conclusions
Allograft ACLR showed similar failure rates between sexes but displayed suboptimal graft failure outcomes in younger and active patients. By age 34 years, the increased revision risk for younger patients diminished.
Level of Evidence
Level IV, therapeutic case series.
Anterior cruciate ligament (ACL) tears are a common sport-related injury, particularly in activities that involve pivoting and cutting.1,2 For patients who sustain an ACL tear and want to return to their sport or activity, anterior cruciate ligament reconstruction (ACLR) is the recommended course of surgical intervention.3, 4, 5 The use of an allograft for ACLR can be advantageous due to reduced surgical time, postoperative pain, donor-site morbidity, and quadriceps strength loss compared with autografts.6, 7, 8, 9 Another added benefit of allograft is improved ability to control the size of the graft, ensuring patients have adequately sized grafts for successful reconstruction.10, 11, 12 In relatively older patients who may be slower to rehabilitate after autograft ACLR, the benefit of reduced donor-site morbidity and faster initial recovery is particularly appealing, and thus allografts are a preferred graft choice for many surgeons when treating this patient population.13
An important indicator of ACLR outcomes is the incidence of secondary ACL injury, particularly graft failure. There are many variables that can contribute to graft failure, including graft type, age, sex, and activity level.14,15 A multicenter cohort study used multivariate regression analysis to identify predictors of graft failure, and they found that graft type and age were associated with graft failure.15
Although reduced surgical time and donor-site morbidity make allografts an appealing option, age-dependent variations in allograft failure rates contribute to the debate regarding allograft use in younger, more active populations.16, 17, 18 There has been a reluctance to use them in younger athletic populations due to evidence of greater risk of graft failure.19 Although autografts have been consistently shown to have lower failure rates than allografts in young, athletic populations, these differences diminish as patient age increases.15 For patients at an age or activity level at which the greater failure risk associated with allograft ACLR becomes negligible, the absence of donor-site morbidity and potentially easier rehabilitation make allografts a desirable graft choice. However, there is limited epidemiologic evidence that suggests at what age allografts can be safely used for ACLR.
The purposes of this study were to characterize the secondary ACL injury rates after primary allograft ACLR and to identify the age cut-score where risk of allograft failure decreases. We hypothesized that younger and more active patients who had an allograft ACLR would demonstrate increased risk of failure relative to older and less-active study participants, and that the increased risk would diminish by a patient’s mid-30’s.14
Methods
Patient Selection and Data Collection
Institutional review board approval was obtained (Emory University Institutional Review Board #:3512), and all patient participants gave electronic consent before completing the survey. All patients who underwent ACLR within a single orthopaedic department (11 sports medicine fellowship-trained orthopedic surgeons) between January 2005 and April 2020 were identified from electronic medical records using a custom query of Current Procedural Terminology code (29888). Nonprimary ACLRs were excluded from analysis by removing patients who indicated they had a previous ACLR (ipsilateral or contralateral), as previous ACL injury is an independent risk factor for subsequent ACL injury.20 In addition to nonprimary ACLRs, patients younger than the age of 14 years were excluded. All surgeries were performed by 1 of 11 orthopaedic surgeons. Graft type, tunnel placement, and fixation methods were based on surgeon preference. Patients were contacted via e-mail with a REDCap link to complete a follow-up survey regarding the outcomes of their primary ACLR at our institution. The survey asked patients to identify their preinjury activity level, graft type used for primary ACLR (quadriceps tendon, hamstring tendon, patellar tendon, allograft, or other), subsequent ACL complications, ability to return to sport, and incidence of ACL reconstruction at any institution before their primary ACLR at our institution. Postoperative Knee Osteoarthritis Outcomes Survey (KOOS) and Marx Activity Scale questionnaires also were collected, and scores were calculated for each respondent. Verification of data such as graft type and incidence of secondary injury was performed via operative report review before data analysis. Analysis of follow-up surveys was performed by the lead author (C.B.P.), a graduate (B.S.) research fellow, and the senior author (J.D.L.), a board-certified fellowship-trained sports medicine surgeon.
Subanalysis of Allograft Population
Patient data were sorted according to graft type, age at the time of primary surgery, and sex. Data analysis was conducted for 398 patients who underwent primary allograft ACLR. Secondary injury analysis included the incidence of revision ACLR and contralateral ACL injury. Revision ACLR was considered an indicator of graft failure.
Self-Reported Activity Level and Return-to-Sport
Patients self-classified their pre-ACL injury activity level in the following categories: competitive athlete, recreational athlete, heavy manual labor job, light manual labor job, active lifestyle, somewhat active, or sedentary. Patients also identified pre-ACL injury sport participation, level of competition, and ability to return to the same sport at any level post-ACLR. All patients who indicated no preinjury sport participation were excluded from return to sport rate calculation.
KOOS and Marx Activity Scale
Activity level was further characterized by the Marx Activity Scale, a measure that assesses the frequency of running, cutting, decelerating, and pivoting based on the subjects’ healthiest and most active state in the past year.21 The total score calculated from this scale ranges from 0 to 16, with a greater score representing a greater activity level. KOOS scores, ranging from 0 (worst) to 100 (best), were also used to evaluate the functional status and quality of life of patients post-ACLR.22
Statistical Analysis
Patient Demographics
Demographic distributions were calculated and reported. Overall secondary injury rate was also reported.
Receiver Operating Characteristic (ROC) Analysis
ROC analysis and Youden’s J calculation were used to identify the cut-score for age whereby risk of allograft failure increases.23 Youden’s J value was calculated for all points of the ROC curve, and the maximum value was used to select the optimum cut-score.23 Revision ACLR incidence and age at time of primary ACLR surgery were factored into the cut-score analysis.
Binary Logistic Regression
A binary logistic regression was performed to ascertain the effects of age and activity level (athlete vs nonathlete) on the likelihood that participants experienced graft failure. Incidence of revision ACLR was the dependent variable, and age and activity level (athlete vs nonathlete) were covariates. For the regression analysis, the activity level descriptive categories were further grouped into “athlete” and “nonathlete” categories. Competitive and recreational athletes were grouped as “athlete” and the remaining categories (active lifestyle, somewhat active, heavy manual labor job, light manual labor job, and sedentary) were grouped as “nonathlete.”
Subanalysis of Allograft Population
Pearson χ2 tests of independence were conducted to test the significance of the differences in revision and contralateral ACL injury rates between age groups (≤34 years and >34 years) and sex. The significance level was set to .05.
Self-Reported Activity Level and Return to Sport
The activity level percentage breakdown was reported for the population. Pearson χ2 tests of independence were conducted to test the significance of the differences between revision rates among activity levels (athletes vs non-athletes). The significance of the differences in return to sport rates between age groups (≤34 years and >34 years) was also calculated.
KOOS and Marx Activity Scale
Independent samples t-tests were conducted to test the significance of the differences in mean KOOS (as well as each KOOS subscale) and Marx scores between age groups (≤34 years and >34 years). The number of patients in each age group (≤34 years and >34 years) who met the minimum patient acceptable symptom state score for each KOOS subscale was also calculated.24,25 Independent Samples t-tests were also conducted to test the significance of the difference in mean KOOS and Marx scores for patients who underwent a revision ACLR versus those who did not.
Results
Patient Demographics
Of the 3,989 patients contacted, 1,200 patients completed the survey (30%) after up to 4 contact efforts were made. Of the 1,200 patients who completed the survey, 261 were excluded for incidence of previous ACLR and/or age younger 14 years (Fig 1). Of the 939 surveys included, 398 were from patients who underwent primary allograft ACLR (42.4%) (Fig 1). Female patients comprised 54.3% of the allograft population. The mean and median ages of patients undergoing allograft ACLR was 39.5 and 39.4 years, respectively (range 16.0-66.1 years). The mean time of follow-up was 8.4 ± 4.1 years post-primary allograft ACLR (range 2.0-17.2 years) (Table 1).
Fig 1.
Flowchart for patient inclusion/exclusion. The number of patients contacted and included/excluded based on survey completion and exclusion criteria is detailed. (ACLR, anterior cruciate ligament reconstruction; CPT, Current Procedural Terminology.)
Table 1.
Patient Demographics
| Factor | n, n (%), or mean (range) |
|---|---|
| Total allograft patients, n | 398 |
| Female, n (%) | 216 (54.3) |
| Male, n (%) | 182 (45.7) |
| Age, y, mean (range)∗ | 39.5 (16.0-66.1) |
| ≤34 y, n, mean (range) | 137, 28.0 (16.0-34.9) |
| >34 y, n, mean (range) | 261, 45.5 (35.0-66.1) |
| Follow-up, y, mean ± SD (range) | 8.4 ± 4.1 (2.0-17.2) |
ROC, receiver operating characteristic; SD, standard deviation.
Age group cohorts (≤34 years and >34 years) determined based on cut-score age (34 years) from ROC analysis.
Secondary ACL Injury
Among patients with allograft ACLR, there was a secondary ACL injury rate of 11.6% (5.8% ipsilateral revision ACLR, 5.8% contralateral ACL injury). Mean time from index ACLR to revision ACLR was 5.1 ± 4.0 years (range 1.2-15.1 years).
ROC and Logistic Regression Analysis
ROC analysis indicated that age older than 34 years was threshold for allograft failure to decrease (area under the curve 0.65, 95% confidence interval 0.55-0.76; P = .01) (Fig 2). The binary logistic regression analysis indicated that decreasing age was associated with an increased risk of graft failure. The model was statistically significant, χ2 = 7.9, P = .02. The model explained 5.5% (Nagelkerke R2) of the variance in graft failure and correctly classified 94.2% of cases.
Fig 2.
ROC curve. ROC curve used to determine the cut-score age with maximum sensitivity and specificity (maximum Youden’s J value). (ROC, receiver operating characteristic.)
Age and Sex Subanalysis According to Cut-Score Age
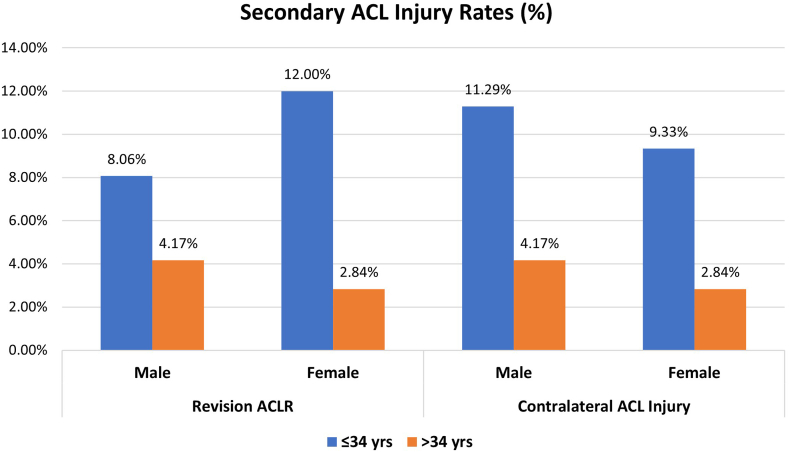
Patients aged ≤34 years (n = 137, median age 28.8 years) had a greater secondary ACL injury rate of 20.4% (10.2% revision ACLR, 10.2% contralateral ACL injury) compared with the secondary ACL injury rate of 6.9% (3.5% revision ACLR, 3.5% contralateral ACL injury) for patients older than 34 years (n = 261, median age 44.6 years) (P < .001). Patients aged ≤34 years had a greater revision rate compared with patients older than 34 years (10.2% vs 3.5%, P = .006). Patients aged ≤34 years also had greater contralateral injury rates than patients older than 34 years (10.2% vs 3.5%, P = .006). Overall, male and female patients had similar revision rates (male: 5.5%, female: 6.0%, P = .82) and contralateral ACL injury rates (males: 6.6%, females: 5.1%, P = .52).When we combined age and sex, female patients aged ≤34 years had greater revision and contralateral ACL injury rates than female patients aged older than 34 years (P = .007 and P = .039, respectively, Fig 3). Male patients did not have differences in revision or contralateral ACL injury rates between age groups (≤34 years and >34 years).
Fig 3.
Secondary ACL injury rates by sex and age. Secondary ACL injury rates calculated for each sex and age group by dividing the sum of the number of revision ACLR surgeries and CL ACL injuries by the total number of ACLR surgeries within each group. Patients who had a previous ipsilateral or contralateral ACL surgery were excluded. (ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; CL, contralateral.)
Activity Level
Overall, recreational athletes compromised 45.7% of the allograft patient population (Table 2). In both age groups (≤34 years and >34 years), recreational athletes compromised the greatest proportion of patients (43.8% and 46.7%, respectively) (Fig 4). The proportion of competitive athletes was greater among patients aged ≤34 years compared with patients older than 34 years (18.3% vs 5.8%, P < .001). Overall, athletes (competitive and recreational) had a trend toward greater revision than nonathletes, although it was not statistically significant (7.6% vs 3.4%, respectively, P = .075). In patients older than 34 years, athletes similarly had a trend toward greater revision than nonathletes, although it was also not statistically significant (5.1% vs 1.6%, P = .13).
Table 2.
Allograft Preinjury Activity Level
| Activity Level,∗ n (%) | Frequency |
Graft Failure |
|---|---|---|
| n = 398 | n = 23 | |
| Competitive athlete | 40 (10.1) | 4 (10.0) |
| Recreational athlete | 184 (46.2) | 13 (7.1) |
| Active lifestyle | 103 (25.9) | 5 (4.9) |
| Somewhat active | 54 (13.6) | 0 |
| Sedentary | 4 (1.0) | 0 |
| Light manual labor job | 8 (2.0) | 1 (12.5) |
| Heavy manual labor job | 5 (1.3) | 0 |
Activity level categories determined based on patients’ survey-selection.
Fig 4.
Allograft preinjury activity level breakdown. Proportions of patients who self-classified as competitive athlete, recreational athlete, heavy manual labor job, light manual labor job, active lifestyle, somewhat active, or sedentary were calculated for each age group.
Return to Sport
In patients aged ≤34 years, 67.7% returned to sport after primary ACLR compared with 81.9% in patients older than 34 years (P = .008).
KOOS and Marx Activity Scale Scores
There was no difference in Marx Activity Scale score at final follow-up when comparing patients aged ≤34 years and older than 34 years (6.0 vs 6.3, t[298] = –0.44, P = .66, Table 3).25 There were no differences in overall KOOS scores or individual KOOS subscale scores when comparing patients aged ≤34 years and older than 34 years (KOOS overall: 83.2 vs 85.5, t[369] = –1.4, P = .15, Table 3).25 The number of patients in each age group (≤34 years and >34 years) who met the minimum patient acceptable symptom state score for each KOOS subscale is listed in Table 3.24,25 The average overall KOOS score was lower for patients who experienced graft failure compared with patients who did not experience graft failure (76.1 vs 85.2, t[369] = 2.78, P = .006). The average KOOSQOL and KOOSSport/Rec subscale scores were also lower for patients who experienced graft failure compared with patients who did not experience graft failure (KOOSQOL: 57.4 vs 74.7, t[369] = 3.4, P < .001; KOOSSport/Rec: 71.9 vs 82.5, t[369] = 2.2, P = .027).
Table 3.
KOOS Subscale and Marx Activity Scores∗
| ≤34 Years | >34 Years | P Value† | |
|---|---|---|---|
| KOOS QOL | |||
| Score, mean | 70.8 | 75.3 | .073 |
| Met PASS score‡ (62.5), n | 96 | 187 | |
| KOOS Pain | |||
| Score, mean | 90.2 | 91.1 | .46 |
| Met PASS score‡ (88.9), n | 86 | 168 | |
| KOOS Sports, rec | |||
| Score, mean | 79.6 | 83.1 | .13 |
| Met PASS score‡ (75.0), n | 94 | 180 | |
| KOOS ADL | |||
| Score, mean | 94.8 | 94.3 | .65 |
| Met PASS score‡ (100), n | 64 | 117 | |
| KOOS Symptoms | |||
| Score, mean | 80.7 | 83.5 | .12 |
| Met PASS score‡ (57.1), n | 120 | 221 | |
| Marx Activity Scale | 6.0 | 6.3 | .66 |
ACLR, anterior cruciate ligament reconstruction; ADL, Activities of Daily Living; KOOS, Knee Osteoarthritis Outcomes Survey; PASS, patient acceptable symptom state; QOL, Quality of Life.
KOOS (knee injury and osteoarthritis outcome score) and Marx Activity scores were captured post-ACLR at time of follow-up survey completion.
P values were not statistically significant for age group comparisons.
PASS score values are listed for each subscale.25 The number of patients who met each score is listed.
Discussion
The most important finding in this study was the age cut-score at which allograft ACL failure decreases: ROC analysis indicated that age older than 34 years at the time of primary allograft ACLR was the threshold age at which graft failure risk decreased. Our hypothesis was affirmed, as the failure rate was nearly 3-fold greater among patients aged 34 years or younger compared with older than 34 years. Although nonsignificant, the overall failure rate of athletes was more than double compared with nonathletes. These findings suggest that for patients undergoing ACLR who are ≤34 years old or who are athletes, there is an increased risk of allograft failure. Meanwhile, an allograft may be an acceptable graft choice for patients older than 34 years who do not participate in competitive or recreational athletics. Although it is likely that other patient-specific factors were not fully captured by this study that should be considered when determining the risk of allograft ACLR, these findings provide valuable information that may help guide clinical practice for ACLR. Namely, allografts appear to be an appropriate graft choice for nonathletes older than 34 years.
Overall, the allograft failure incidence in this study was 5.7%, which is lower than previous studies reporting revision ACLR rates after primary allograft ACLR between 10.3% and 26.5%.26, 27, 28 The failure rate was likely lower in the present study due to patient selection of low-failure-risk populations and potentially also in part due to retrospective survey bias. In addition, unlike a previous systematic review of allograft ACLR by Joyce et al.,28 which considered a composite cumulative failure risk as graft failure, grade 2+ or worse Lachman, grade 2+ or worse pivot shift, overall International Knee Documentation Committee grade C or D, or instrumented laxity with a side-to-side difference greater than 5 mm, graft failure in the present study was solely indicated by revision ACLR. The outcomes for allograft ACLR were particularly successful in the relatively older population in the present study, as patients older than 34 years had an average graft failure rate of only 3.5%. However, similar to reports from previous studies,12,15,29 allograft failure rate was markedly greater in younger patients.14,15,29, 30, 31 Although previous studies reported lower revision risk in a relatively older population, they did not indicate at what age this decreased risk was significant. As such, the present study introduced a novel cut-score analysis to determine the threshold age at which allograft failure decreases. When revision rates were calculated above and below the cut-score threshold, patients aged 34 years and younger had a nearly 3-fold greater revision rate compared with patients aged older than 34 years. However, a nearly 3-fold greater proportion of patients aged 34 years and younger were classified as competitive athletes (18.0%) compared with patients older than 34 years (6.6%). This cut-score does not suggest that every patient aged older than 34 years should have an allograft for ACLR. Although age older than 34 years is a good indicator of reduced allograft failure risk, activity level should be taken into consideration for graft selection on a case-by-case basis.32,33
In the present study, the graft failure rate in athletes was more than double compared with non-athletes, and greater than 3-fold for athletes older than 34 years old, although these differences did not meet statistical significance. The observed difference was likely not statistically significant due to an overall low number of athletes in this cohort, largely due to patient selection, and the study was likely underpowered to determine differences in failure rates when comparing athletes and nonathletes. In addition, activity level is likely already factored into the significance of the relationship between age and revision ACLR. Therefore, age can generally serve as a proxy for activity level, whereas age increases, activity level decreases.34 This is further demonstrated in our data where the proportion of competitive athletes decreases as patient age increases. Although significance was not found in this study, a previous study demonstrated increased odds of ACL graft failure for those with higher activity level compared with low activity level.32 Although the increased rate of graft failure among athletes was not statistically significant in the present study, it is consistent with previous study reports and warrants caution when considering allograft for the athletic population undergoing ACLR.32 Therefore, we are not recommending the use of allograft in all patients over the age of 34 years. Rather, consideration should be given as to which specific sporting activities the patient participates in and how frequently the patient participates or plans to participate in these activities postoperatively. In other words, the injury risk profile of each patient older than 34 years should be considered when deciding between autograft and allograft ACLR. Consistent with the findings of this study, the senior author (J.D.L.) generally uses autograft tissue in any cutting or pivoting athlete regardless of age, reserving the use of allograft for select patients older than 34 years of age who do not participate in cutting or pivoting activities and have failed a course of nonsurgical treatment before ACLR. Further investigation of patient-specific factors that may predict a satisfactory outcome following allograft ACLR may be warranted.
In a multicenter cohort study comparing graft failure rates, patients with allografts were 4 times more likely to retear their graft relative to those with autograft.15 This same study also reported that the odds of re-rupture decrease with increasing age, but it did not specifically consider the activity level of older patients, which is likely an important consideration when deciding on graft choice for ACLR regardless of age. In another study of a young, athletic population, allografts were reported as 3 times more likely to retear than autografts.26 Although this study considered the activity level of patients, it did not report failure rates for an older population. In a systematic review of randomized controlled trials comparing hamstring autograft and bone–patellar tendon–bone autograft, the overall autograft re-rupture rate was reported as 3.6%.35 Other studies have reported autograft failure rates ranging from 3.0% to 5.8% and contralateral reinjury rates ranging from 3.0% to 11.8%.36,37 When considering graft choice for ACLR, autograft is considered the “gold standard,” and therefore allograft should generally only be considered if the failure rate is less than or equal to autograft failure rates. In the present study, the allograft failure rate in older nonathletes is lower than the autograft failure rates in the previously referenced studies. As such, allograft may be an appropriate graft choice in relatively older non-athletes (i.e., noncutting/pivoting athletes).
Although there were differences in revision ACLR rates when comparing the 2 age groups, the KOOS and Marx Activity scores at final follow-up were similar. These results were surprising, as previous literature has shown that Marx Activity score is inversely correlated with age. These results also suggest selection bias, as less active young patients may have been selected to receive allograft rather than autograft.21 It is also possible that younger patients may not have returned to their preinjury activity level after allograft ACLR, as Marx Activity score was only obtained at final follow-up. Previous studies have also reported that older age at the time of surgery is a significant predictor of lower KOOS and Marx scores 10 years after ACLR.38,39 Although no differences were found for KOOS or Marx Activity scores according to age group, we did find that the average overall KOOS score and the average KOOSQOL and KOOSSport/Rec subscale scores were lower for patients who experienced graft failure compared with patients who did not experience graft failure. Consistent with previous studies on allograft ACLR, there were no differences in revision or contralateral ACL injury rates according to sex.14,33
Limitations
The authors acknowledge that the study results should be considered relative to the potential limitations. The inclusion of patients undergoing autograft ACLR could have provided a meaningful comparison with the allograft ACLR cohort. Despite a relatively large number of patients included in the study, there was a small number of graft failures, therefore contributing to a relatively low AUC score and potentially less reliable analyses. In addition, due to a small number of graft failures, subgroup analyses including comparison of failure rates in athletes and nonathletes may have been underpowered to detect a significant difference. There was a wide range of follow-up time points postsurgery (2-17 years), and since this study was survey-based, there is a potential for nonresponse bias. Graft choice was nonrandom, as it was decided upon by a shared decision-making process between the surgeon and patient, thereby introducing selection bias. Factors considered included patient age, activity level, and donor-site morbidity related to autograft choices.40 In addition, due to the length of the study period and the number of surgeons included, there was likely an evolution and variety of surgical techniques and post-operative rehabilitation protocols that could influence ACLR outcomes. Data regarding surgical technique and fixation methods was not collected from operative reports. The relatively low Marx Activity scores in each age group indicate patient selection bias for allograft. Although this patient selection was performed to reduce graft failure risk, it contributes to the younger population in this study not necessarily being representative of “all comers.” Furthermore, activity level was self-reported by patients and thus subject to varied interpretations since no direct measurement of physical activity was available. Another limitation is that graft failure was indicated by revision ACLR and therefore did not include patients who may have torn their allograft but chose not to undergo revision surgery. Additionally, no preoperative patient-reported outcomes scores were available. Lastly, we did not collect data on allograft type, which could be another confounding variable.
Conclusions
Allograft ACLR showed similar failure rates between sexes but displayed suboptimal graft failure outcomes in younger and active patients. By age 34 years, the increased revision risk for younger patients diminished.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: S.G.K. reports consultant and payment for lectures including service on speakers’ bureaus, royalties for Smith & Nephew, outside the submitted work. K.E.H. reports educational speaking consultant for Arthrex, outside the submitted work. M.W.P. reports consultant for Zimmer Biomet Holdings and payment for lectures including service on speakers’ bureaus from Arthrex, outside the submitted work. S.A.L. reports editorial or governing board, Arthroscopy; and Eastern Orthopedic Association, board or committee member, outside the submitted work. G.D.M. consults with commercial entities to support commercialization strategies and applications to the U.S. Food and Drug Administration but has no direct financial interest in the products. His institution receives current and ongoing grant funding from National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants U01AR067997, R01 AR070474, R01AR055563, R01AR076153, and R01 AR077248 and industry sponsored research funding related to injury prevention and sport performance to his institution. He receives author royalties from Human Kinetics and Wolters Kluwer and is an inventor of biofeedback technologies (Patent No: US11350854B2, Augmented and Virtual reality for Sport Performance and Injury Prevention Application, Approval Date: 06/07/2022, Software Copyrighted) designed to enhance rehabilitation and prevent injuries that receives licensing royalties. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Csintalan R.P., Inacio M.C., Funahashi T.T. Incidence rate of anterior cruciate ligament reconstructions. Perm J. 2008;12:17–21. doi: 10.7812/tpp/07-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buller L.T., Best M.J., Baraga M.G., Kaplan L.D. Trends in anterior cruciate ligament reconstruction in the United States. Orthop J Sports Med. 2015;3 doi: 10.1177/2325967114563664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Failla M.J., Arundale A.J., Logerstedt D.S., Snyder-Mackler L. Controversies in knee rehabilitation: Anterior cruciate ligament injury. Clin Sports Med. 2015;34:301–312. doi: 10.1016/j.csm.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber-Westin S., Noyes F.R. One in 5 athletes sustain reinjury upon return to high-risk sports after ACL reconstruction: A systematic review in 1239 athletes younger than 20 years. Sports Health. 2020;12:587–597. doi: 10.1177/1941738120912846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahardja R., Zhu M., Love H., Clatworthy M.G., Monk A.P., Young S.W. Effect of graft choice on revision and contralateral anterior cruciate ligament reconstruction: Results from the New Zealand ACL Registry. Am J Sports Med. 2020;48:63–69. doi: 10.1177/0363546519885148. [DOI] [PubMed] [Google Scholar]
- 6.Marrale J., Morrissey M.C., Haddad F.S. A literature review of autograft and allograft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:690–704. doi: 10.1007/s00167-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 7.Hulet C., Sonnery-Cottet B., Stevenson C., et al. The use of allograft tendons in primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:1754–1770. doi: 10.1007/s00167-019-05440-3. [DOI] [PubMed] [Google Scholar]
- 8.Cristiani R., Mikkelsen C., Wange P., Olsson D., Stålman A., Engström B. Autograft type affects muscle strength and hop performance after ACL reconstruction. A randomised controlled trial comparing patellar tendon and hamstring tendon autografts with standard or accelerated rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2021;29:3025–3036. doi: 10.1007/s00167-020-06334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A.H., Capin J.J., Zarzycki R., Snyder-Mackler L. Athletes with bone-patellar tendon-bone autograft for anterior cruciate ligament reconstruction were slower to meet rehabilitation milestones and return-to-sport criteria than athletes with hamstring tendon autograft or soft tissue allograft: Secondary analysis from the ACL-SPORTS trial. J Orthop Sports Phys Ther. 2020;50:259–266. doi: 10.2519/jospt.2020.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiggins A.J., Grandhi R.K., Schneider D.K., Stanfield D., Webster K.E., Myer G.D. Risk of Secondary injury in younger athletes after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Am J Sports Med. 2016;44:1861–1876. doi: 10.1177/0363546515621554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett A.M., Craft J.A., Replogle W.H., Hydrick J.M., Barrett G.R. Anterior cruciate ligament graft failure: A comparison of graft type based on age and Tegner activity level. Am J Sports Med. 2011;39:2194–2198. doi: 10.1177/0363546511415655. [DOI] [PubMed] [Google Scholar]
- 12.van Eck C.F., Schkrohowsky J.G., Working Z.M., Irrgang J.J., Fu F.H. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40:800–807. doi: 10.1177/0363546511432545. [DOI] [PubMed] [Google Scholar]
- 13.Bowman E.N., Limpisvasti O., Cole B.J., ElAttrache N.S. Anterior cruciate ligament reconstruction graft preference most dependent on patient age: A survey of United States surgeons. Arthroscopy. 2021;37:1559–1566. doi: 10.1016/j.arthro.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Kaeding C.C., Pedroza A.D., Reinke E.K., Huston L.J., Spindler K.P. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: Prospective analysis of 2488 primary ACL reconstructions from the MOON Cohort. Am J Sports Med. 2015;43:1583–1590. doi: 10.1177/0363546515578836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeding C.C., Aros B., Pedroza A., et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber F.A., Cowden C.H., Sanders E.J. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30:483–491. doi: 10.1016/j.arthro.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Wasserstein D., Sheth U., Cabrera A., Spindler K.P. A Systematic review of failed anterior cruciate ligament reconstruction with autograft compared with allograft in young patients. Sports Health. 2015;7:207–216. doi: 10.1177/1941738115579030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabricant P.D., Kocher M.S. Management of ACL injuries in children and adolescents. J Bone Joint Surg Am. 2017;99:600–612. doi: 10.2106/JBJS.16.00953. [DOI] [PubMed] [Google Scholar]
- 19.Duchman K.R., Lynch T.S., Spindler K.P. Graft selection in anterior cruciate ligament surgery: Who gets what and why? Clin Sports Med. 2017;36:25–33. doi: 10.1016/j.csm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Smith H.C., Vacek P., Johnson R.J., et al. Risk factors for anterior cruciate ligament injury: A review of the literature-part 2: Hormonal, genetic, cognitive function, previous injury, and extrinsic risk factors. Sports Health. 2012;4:155–161. doi: 10.1177/1941738111428282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx R.G., Stump T.J., Jones E.C., Wickiewicz T.L., Warren R.F. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 22.Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., Beynnon B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 23.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Harris J.D., Brand J.C., Cote M.P., Faucett S.C., Dhawan A. Research Pearls: The significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy. 2017;33:1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 25.Muller B., Yabroudi M.A., Lynch A., et al. Defining thresholds for the patient acceptable symptom state for the IKDC subjective knee form and KOOS for patients who underwent ACL reconstruction. Am J Sports Med. 2016;44:2820–2826. doi: 10.1177/0363546516652888. [DOI] [PubMed] [Google Scholar]
- 26.Bottoni C.R., Smith E.L., Shaha J., et al. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43:2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 27.Singhal D., Kanodia N., Singh R., Singh S.K., Agrawal S. Predicting quadruple semitendinosus graft size for anterior cruciate ligament reconstruction by patient anthropometric variables: A cohort study of 280 cases. Malays Orthop J. 2021;15:71–77. doi: 10.5704/MOJ.2111.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyce C.D., Randall K.L., Mariscalco M.W., Magnussen R.A., Flanigan D.C. Bone–patellar tendon–bone versus soft-tissue allograft for anterior cruciate ligament reconstruction: A systematic review. Arthroscopy. 2016;32:394–402. doi: 10.1016/j.arthro.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Cruz A.I., Beck J.J., Ellington M.D., et al. Failure rates of autograft and allograft ACL reconstruction in patients 19 years of age and younger: A systematic review and meta-analysis. JB JS Open Access. 2020;5 doi: 10.2106/JBJS.OA.20.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maletis G.B., Chen J., Inacio M.C.S., Funahashi T.T. Age-related risk factors for revision anterior cruciate ligament reconstruction: A cohort study of 21,304 patients from the Kaiser Permanente Anterior Cruciate Ligament Registry. Am J Sports Med. 2016;44:331–336. doi: 10.1177/0363546515614813. [DOI] [PubMed] [Google Scholar]
- 31.Singhal M.C., Gardiner J.R., Johnson D.L. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23:469–475. doi: 10.1016/j.arthro.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Borchers J.R., Pedroza A., Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: A case-control study. Am J Sports Med. 2009;37:2362–2367. doi: 10.1177/0363546509340633. [DOI] [PubMed] [Google Scholar]
- 33.Salmon L., Russell V., Musgrove T., Pinczewski L., Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:948–957. doi: 10.1016/j.arthro.2005.04.110. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins M.S., Storti K.L., Richardson C.R., et al. Objectively measured physical activity of USA adults by sex, age, and racial/ethnic groups: A cross-sectional study. Int J Behav Nutr Phys Act. 2009;6:31. doi: 10.1186/1479-5868-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spindler K.P., Kuhn J.E., Freedman K.B., Matthews C.E., Dittus R.S., Harrell F.E. Anterior cruciate ligament reconstruction autograft choice: Bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32:1986–1995. doi: 10.1177/0363546504271211. [DOI] [PubMed] [Google Scholar]
- 36.Wright R.W., Dunn W.R., Amendola A., et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: A prospective MOON cohort study. Am J Sports Med. 2007;35:1131–1134. doi: 10.1177/0363546507301318. [DOI] [PubMed] [Google Scholar]
- 37.Wright R.W., Magnussen R.A., Dunn W.R., Spindler K.P. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: A systematic review. J Bone Joint Surg Am. 2011;93:1159–1165. doi: 10.2106/JBJS.J.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spindler K.P., Huston L.J., Chagin K.M., et al. Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: A MOON longitudinal prospective cohort study. Am J Sports Med. 2018;46:815–825. doi: 10.1177/0363546517749850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spindler K.P., Huston L.J., Wright R.W., et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39:348–359. doi: 10.1177/0363546510383481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouarbes D., Menetrey J., Marot V., Courtot L., Berard E., Cavaignac E. Anterior cruciate ligament reconstruction: A systematic review and meta-analysis of outcomes for quadriceps tendon autograft versus bone–patellar tendon–bone and hamstring–endon autografts. Am J Sports Med. 2019;47:3531–3540. doi: 10.1177/0363546518825340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.