Abstract
PURPOSE.
Interstitial cystitis/painful bladder syndrome (IC) is a chronic syndrome of bladder-centric pain with unknown etiology that has an adverse impact on quality of life. We sought to analyze the urine and serum metabolomes of a cohort of IC patients and non-disease controls (NC) to identify possible diagnostic strategies for clinical stratification of patients.
MATERIALS AND METHODS.
Home collection of serum and urine samples was obtained from 19 IC and 20 NC females in the Veterans Affairs (VA) Health Care System. IC was diagnosed independently by thorough review of medical records using established criteria. Integrated structural lipidomics and metabolomics analyses were conducted. Biostatistics and bioinformatics analyses, including univariate analysis, unsupervised clustering, random forest analysis, and metabolite set enrichment analysis, were utilized to identify potential IC biomarkers.
RESULTS.
Metabolomics and lipidomics profiling revealed distinct expression patterns between NC and IC. Random forest analysis of urine samples suggested discriminators specific to IC. which showed a strong potential clinical value as a diagnostic signature (AUC, 0.92). Lipidomics profiling showed a distinct difference in patterns between IC and NC; however, there were no identifiable statistically defined lipid biomarker candidates.
CONCLUSIONS.
Analysis of serum and urine revealed that women with IC have distinct metabolomes, highlighting key metabolic pathways that may provide insight into the pathophysiology of IC. The findings from this pilot study suggest that integrated analyses of urinary metabolites, purine, phenylalanine, 5-oxoproline, and 5-hydroxyindoleacetic acid, can lead to promising IC biomarkers. Validation of these results using a larger dataset is currently underway.
Keywords: Metabolomics, biomarker, interstitial cystitis, metabolites, VA
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC) is defined as the “an unpleasant sensation (pain, pressure, and discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes” by the Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction (SUFU).1 There is a significant overlap in symptoms between IC, overactive bladder syndrome (OAB), vulvodynia, and endometriosis in women, and chronic prostatitis and chronic orchalgia in men.2, 3 However, according to AUA guidelines, the major distinguishing feature of IC is the presence of bladder-centric pain.4, 5 Estimates of the prevalence and natural history of IC fluctuate widely because of different diagnostic standards, populations studied, and challenges inherent in following patients over time.5–9
Despite the pressing burden of IC on public health, making an accurate diagnosis remains challenging and is often subjective.10 There are many different diagnostic tests for IC; these include urinalysis, urine culture, potassium sensitivity testing, cystoscopy, bladder biopsy, and hydrodistension of the bladder.5 However, none of these tests can definitively diagnose IC and often IC is a diagnosis of exclusion. Therefore, there is an urgent need to identify objective diagnostic markers that can differentiate IC from other conditions and be used as a definitive diagnostic standard.
There is a paucity of information known about the mechanisms underlying IC.11–13 In particular, bladder pain is a key symptom of IC and, in a majority of patients, is accompanied by urinary frequency and urgency, which suggests an inflammatory component to the disease.14–17 In practice, urine, which contains thousands of proteins, metabolites, and other compounds, may be a rich source of clues regarding disease mechanisms and novel diagnostic biomarkers. Compared to cystoscopy, urine collection and testing is more easily performed in the clinical setting. Urine can be obtained non-invasively and shows increased stability over serum or blood, which allows for easy sampling at multiple time points. Because urine is in direct contact with the diseased bladder, urinary biomarker detection may provide a wealth of important information on bladder health. Since levels of blood-based biomarkers are less influenced by environmental changes, fluid intake, diet, and exercise, a screening blood test incorporating blood-based molecular biomarkers is an attractive option.
The ultimate goal of this study is to improve the methodology for diagnosing IC; thereby, leading to the development of new insights into this condition. To achieve this, our pilot study focused on analyzing and comparing blood and urine samples from Veterans Affairs (VA) patients with or without IC to develop an accurate urine and/or serum-based diagnostic assay. Multi-omics approaches have recently become the focus of analyses of molecular signatures for applications in precision medicine.18 Considering how the metabolome integrates whole-body metabolism, unbiased metabolomics profiling may be able to identify different patterns of metabolites and lipids in patients with IC compared to non-disease controls (NC). This can further lead to valuable information on the mechanisms underlying IC.
METHODS AND MATERIALS
Study Approval and Ethical Statement.
All volunteers in this study gave informed consent prior to participation. Research use of samples was conducted in accordance to the terms outlined and set forth in the Declaration of Helsinki. Diet and other physiological parameters were not controlled. After IRB approval (Pro00041326), the VA Informatics and Computing Infrastructure (VINCI) was used to identify all active users of the VA system, defined as patients who had had two visits within the past calendar year prior to study start. ICD 9/10 codes for IC/BPS (595.1/N30.10) were used to sort patients into groups; one of which were patients diagnosed using the IC codes and the others as NC. Patients underwent a detailed chart review to confirm IC diagnosis and study eligibility. Informed written consent was obtained from all participants.
Creation of a VA IC Cohort and Cohort Assembly.
We developed a nationally representative longitudinal cohort of IC patients. To accomplish this, we took advantage of the largest nationally integrated healthcare system in the country, the VA (www.va.gov/health). To perform metabolomics and lipidomics analyses, blood and urine samples were collected from 19 women with chart-proven diagnosis of IC and 20 NC. Home collection of samples was achieved using a mobile phlebotomy company that was able to collect samples from patients across the country. Patients were instructed in clean catch procedures; they were not asked to fast or provide a first void specimen.
Diagnosis of IC.
Clinical symptoms consistent with recommendations by the American Urological Association (AUA) IC Guidelines were used to diagnose IC patients. Participants must have met all of the inclusion criteria and none of the exclusion criteria listed in Table 1.
Table 1.
Criteria for this study
| CRITERIA | |
|---|---|
| Inclusion Criteria for IC Diagnosis |
|
| Exclusion Criteria for IC Diagnosis |
|
Selection of Non-disease Controls.
NCs were selected using the VINCI query to identify all patients who did not have one of the ICD-9/10 codes for IC. The lack of IC diagnosis was confirmed via chart review. A random number generator was then used to randomly select 100 participants from this list to be enrolled. More specifically, a list of 500 randomly-selected healthy controls (250 men and 250 women) was created, and the first 20 women were contacted for this study. We chose to focus on women in this initial pilot study for several reasons: 1) women have higher rates of IC, 2) potential noise that can arise from gender differences is eliminated, and 3) given that the VA population is 90% male, we wanted to prove that women could be adequately sampled for this study. For this pilot study, if a participant declined participation, the next patient on the list was contacted until the enrollment quota of 20 was met.
Sampling of Blood and Urine (Home Collection).
Blood samples were collected by a mobile phlebotomy company (Phlebotek, Oakland Park, FL) that goes to the participants’ homes, draws blood, spins the samples to separate the serum, and ships the serum on ice to our laboratory at the Durham VA. Participants provided a urine sample at home using standard urine sample collection procedures (e.g. wipe beforehand, midstream clean catch). Patients sent the urine specimen back to the Durham VA overnight along with a cold pack. Once urine samples were received, they were processed by quick centrifugation at 3500 × g for 10 min, allowing for the separation of supernatants and pellets. The supernatants and pellets were stored in a −80°C freezer until shipping to Berg LLC (Framingham, MA) by an overnight carrier. These samples were used to identify metabolomic and lipidomic differences between IC patients and NCs.
Serum and Urine Sample Preparation and Unbiased Metabolomics.
The provided study workflow presents how metabolomics analyses were conducted in this study (Supplementary Figure 1).
All metabolomic analyses were conducted by Berg LLC. Serum and urine samples for the integrated HILIC-LC-MS/MS and GC-MS analyses were prepared as previously described.19–21 Metabolite extraction from serum was achieved using a mixture of isopropanol, acetonitrile, and water at a ratio of 3:3:2 v/v. Urine was mixed with an equal amount of acetonitrile, vortexed, and further centrifuged to remove sediments. Extract analyses were performed using GC-MS, RPLC-MS, and HILIC-LC-MS/MS protocols as previously described.14 We used the NEXERA XR UPLC system (Shimadzu, Columbia, MD, USA), coupled with the Triple Quad 5500 System (AB Sciex, Framingham, MA, USA) to perform hydrophilic interaction liquid chromatography analysis, the NEXERA XR UPLC system (Shimadzu, Columbia, MD, USA), coupled with the Triple TOF 6600 System (AB Sciex, Framingham, MA, USA) to perform reversed-phase liquid chromatography analysis, and an Agilent 7890B Gas Chromatograph (Agilent, Palo Alto, CA, USA) interfaced to a Time-of-Flight Pegasus HT Mass Spectrometer (Leco, St. Joseph, MI, USA). The GC system was fitted with a Gerstel Temperature-Programmed Injector, which is a cooled injection system (model CIS 4). An automated liner exchange (ALEX) (Gerstel, Muhlheim an der Ruhr, Germany) was used to eliminate cross-contamination from the sample matrix that was occurring between sample runs.
Quality control was performed using metabolite standard mixtures and pooled samples. A standard quality control sample containing a mixture of amino and organic acids was injected daily to monitor mass spectrometer response. Pooled quality control samples were obtained by taking aliquots of the same volume from all samples in the study and injecting it daily with a batch of analyzed samples to determine the optimal dilution and validate metabolite identification and peak integration. Collected raw data was manually inspected, merged, imputed, and normalized by the sample median.
Structural Lipid Analysis of Serum and Urine.
All lipidomic analyses were conducted by Berg LLC. All lipid standards were purchased from Avanti Polar Lipids, Nu-Chek Prep, Sigma-Aldrich, Cambridge Isotope Laboratory, Cayman Chemical Company, Avanti Polar Lipids, or Santa Cruz Biotechnology, Inc. All solvents were of HPLC or LC/MS grade and were acquired from Sigma-Aldrich, Fisher Scientific, or VWR International. Blinded lipidomic analyses of serum and urine samples were performed at BERG. A cocktail of deuterium-labeled and odd chain phospholipid standards from diverse lipid classes was added to 25 μl of serum and 500 μl of urine. Standards were chosen so that they represented each lipid class and were at designated concentrations chosen to provide the most accurate quantitation and dynamic range for each lipid species. Lastly, lipid extraction was performed as previously described.22–24 Samples were flushed with nitrogen and stored at −20 °C. Samples were then diluted 50 times using a solution of isopropanol:methanol:acetonitrile:water (3:3:3:1, by vol.) with 2 mM ammonium acetate to optimize ionization efficiency in both the positive and negative modes.
Electrospray ionization-mass spectrometry (EIS-MS) was performed on a TripleTOF® 5600+ (SCIEX, Framingham, MA), coupled to a customized direct injection loop on an EkspertmicroLC200 system (SCIEX). Lipids were analyzed using a customized data independent analysis strategy on TripleTOF® 5600+, which allows for MS/MSALL high resolution and high mass accuracy analysis, as previously described.24 Quantification was performed using an in-house library on MultiQuant™ (SCIEX).
Metabolite Pathway Analysis.
Metabolomics data were analyzed as previously described by Tolstikov et al.25 Identified metabolites were subjected to pathway analysis using MetaboAnalyst 4.0 with a metabolite set enrichment analysis (MSEA) module that consists of enrichment analyses relying on measured levels of metabolites and pathway topology and provides visualization of the identified metabolic pathways.26 The accession numbers of the detected metabolites (HMDB, PubChem, and KEGG Identifiers) were generated, manually inspected, and utilized to map the canonical pathways. MSEA was used to interrogate functional relation, which describes the correlation between compound concentration profiles and clinical outcomes.
Data Processing and Statistical Analysis.
Statistical analysis of metabolites was performed using one-way analysis of variance (ANOVA) and random forest. Temporal and two-factor data analysis, including data overview, two-way ANOVA, and empirical Bayes time-series analysis for detecting distinctive temporal profiles, was conducted when applicable. Analysis of accuracy of biomarkers to separate IC from NC was determined using area under the receiver operator characteristics curve (AUC). Statistical significance was assessed using R 3.0.2. Statistical significance for all analyses was determined at p<0.05. P-values for all pairwise comparisons were calculated using the Tukey-Kramer honest significant difference test.
RESULTS
VA IC cohort used for this pilot study.
As described in Table 2 , there were no differences in age, race, or BMI between the IC and NC groups.
Table 2.
Basic participant characteristics
| NC (N=20) | IC (N=19) | P-value | |
|---|---|---|---|
| Age, years | 0.616 1 | ||
| Mean (SD) | 50.5 (12.6) | 52.5 (12.4) | |
| Race | 0.175 2 | ||
| White | 10 (50%) | 15 (79%) | |
| Black | 7 (35%) | 4 (21%) | |
| Asian or Pacific Islander | 2 (10%) | 0 (0%) | |
| Unknown | 1 (5%) | 0 (0%) | |
| BMI | 0.684 3 | ||
| Median (IQR) | 29.2 (24.1, 38.2) | 27.1 (22.6, 38.8) |
T-Test
Fisher Exact
Wilcoxon
NC, non-disease controls
IC, interstitial cystitis
Metabolomics Analyses in Serum
To identify potential IC biomarker candidates in serum samples, metabolomics analyses were conducted. The heatmap indicates unsupervised clustering of the top 25 serum-based metabolites that could distinguish IC from NC from more than 500 detected in the initial profiling (Supplementary Figure 2A). The differentially expressed metabolites (DEMs) in the serum of IC patients include arachidonic acid (fold-change (FC) of 1.5359, p-value of 0.0092181), guanine (FC of 1.8883, p-value of 0.017327), oxo-octadecanoic acid (FC of 1.549, p-value of 0.021275), homocysteine (FC of 0.56719, p-value of 0.010477), beta-n-acetylglucosamine (FC of 0.44663, p-value of 0.010495), and phosphate (FC of 0.60255, p-value of 0.020363) (Figure 1A, Supplementary Table 1). Additional random forest analysis revealed the top 15 discriminators of IC, shown in Supplementary Figure 2B.
Figure 1. Metabolic Biomarker Analysis of Serum Samples.
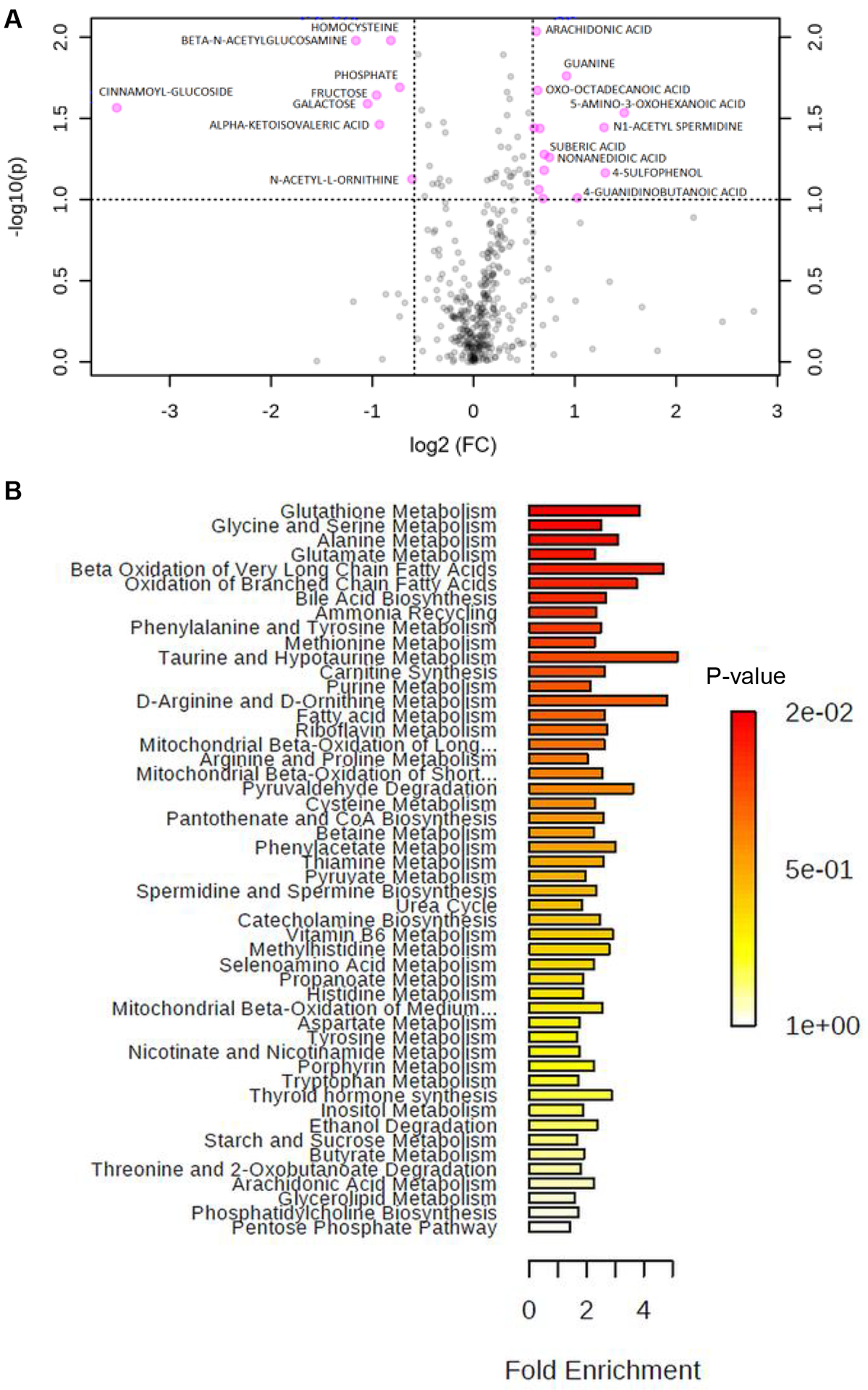
(A) A volcano plot shows differentially expressed metabolites (DEMs) between IC and NC samples. Univariate analysis was performed. (C) Top 15 discriminators by random forest analysis. (B) Enrichment overview of the top 50 DEMs in IC samples compared to NC, and the metabolic pathways associated with the metabolite sets.
Subsequent bioinformatics analysis found the top 50 differentially expressed metabolites in IC compared to NC; these included glutathione metabolism, glycine and serine metabolism, and alanine metabolism (Figure 1B). Genome-scale network modeling of human metabolism suggested glyceraldehyde-3-phosphate dehydrogenase, aldehyde dehydrogenase, and glycine hydroxymethyltransferase as being perturbed in IC compared to NC (Supplementary Figure 2C). While each of the top 4 ranked serum metabolites individually had a modest AUC for identifying IC vs. NC (0.77–0.78), in this pilot study, these results were not significantly different than an AUC of 0.5 (i.e. no value in identifying IC) (Supplementary Table 2).
Metabolomics Analyses in Urine
In urine samples, metabolomics profiling resulted in unsupervised clustering of the top urine IC-specific metabolites. As shown in the heatmap, IC had a distinct pattern in its metabolomics profile (Supplementary Figure 3A). Random forest analysis determined the top 15 discriminators (Supplementary Figure 3B). They include 1-palmitoylglycerophosphocholine, arachidonic acid, acetylcarnitine, and glycerate. DEMs in IC samples are shown in a separate volcano plot (Figure 2A). Levels of purine (FC of 2.1538, p-value of 4.29E-05), phenylalanine (FC of 2.1515, p-value of 0.00050223), 5-oxoproline (FC of 3.0523, p-value of 0.0011701), and tetradecadienylcarnitine (FC of 0.45607, p-value of 0.00099791) were significantly different in IC vs. NC (Figure 2A and Supplementary Table 3).
Figure 2. Metabolomic Biomarker Analysis of Urine Samples.
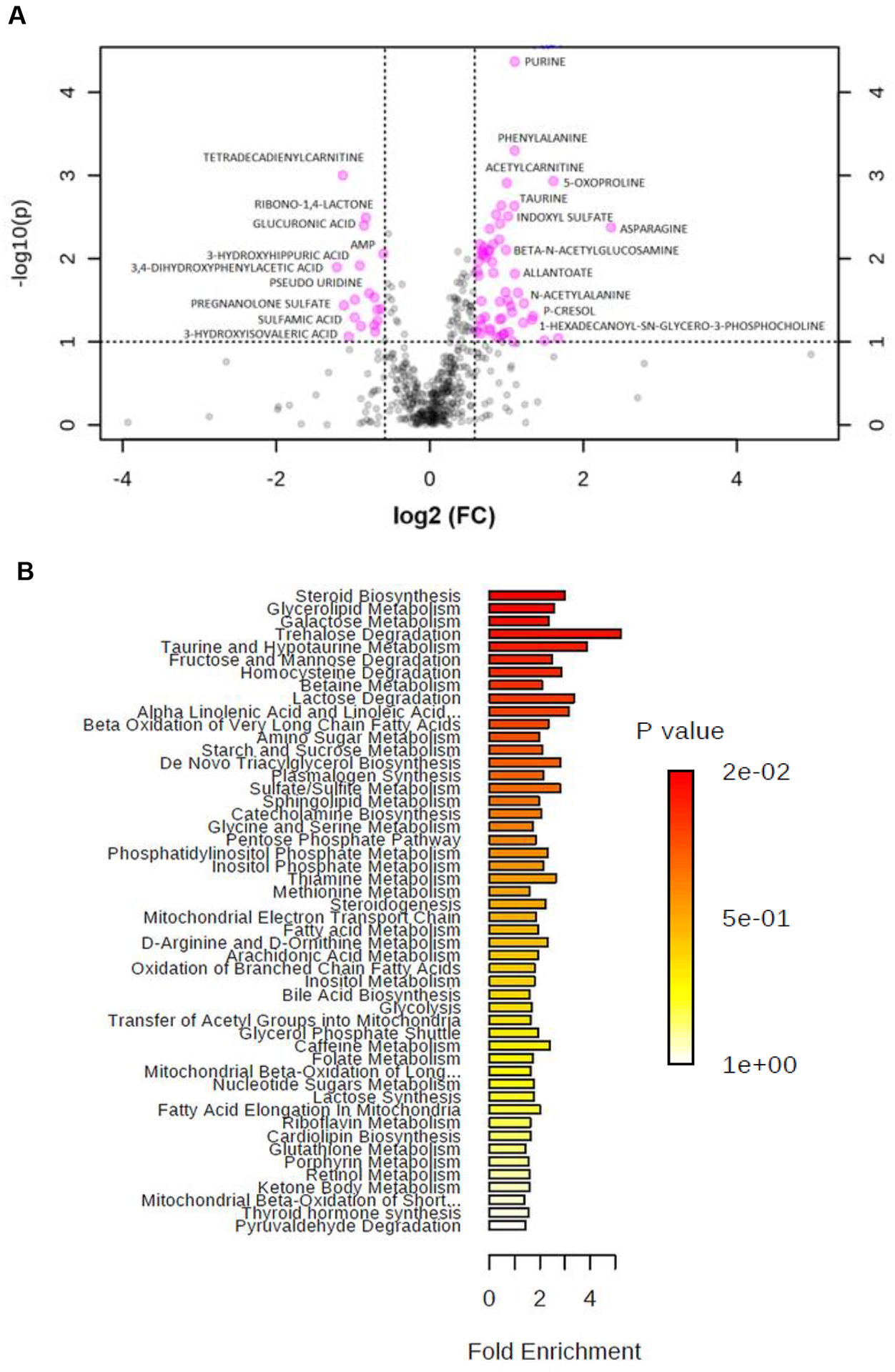
(A) A volcano plot showing DEMs between IC and NC samples. Univariate analysis was performed. (B) Enrichment overview of the top 50 DEMs in IC compared to NC, and the metabolic pathways associated with the metabolite sets.
Enrichment overview of the top 50 DEMs in IC samples compared to NC suggested that steroid biosynthesis, glycerolipid metabolism, and galactose metabolism are significantly altered (Figure 2B). Genome-scale network modeling of human metabolism indicated potential enzymatic changes of fatty-acyl-CoA desaturase, which is responsible for fatty-acyl-CoA elongation (Supplementary Figure 3C).
It is notable that the top 4 differentially expressed analytes showed a robust AUC when analyzed as individual metabolites (Figure 3A). The AUC for purine, phenylalanine, 5-oxoproline, and 5-hydroxyindoleacetic acid (5-HIAA) were 0.873, 0.85, 0.817. and 0.938, respectively (Figure 3A, 3B). When combined, this biomarker panel had an AUC of 0.92 (Figure 3C). Additional specificity calculations showed positive predictive value (PPV), negative predictive value (NPV), and odds ratio (OR) (Figure 3D).
Figure 3. Statistical Consideration of Potential Urinary Biomarkers.
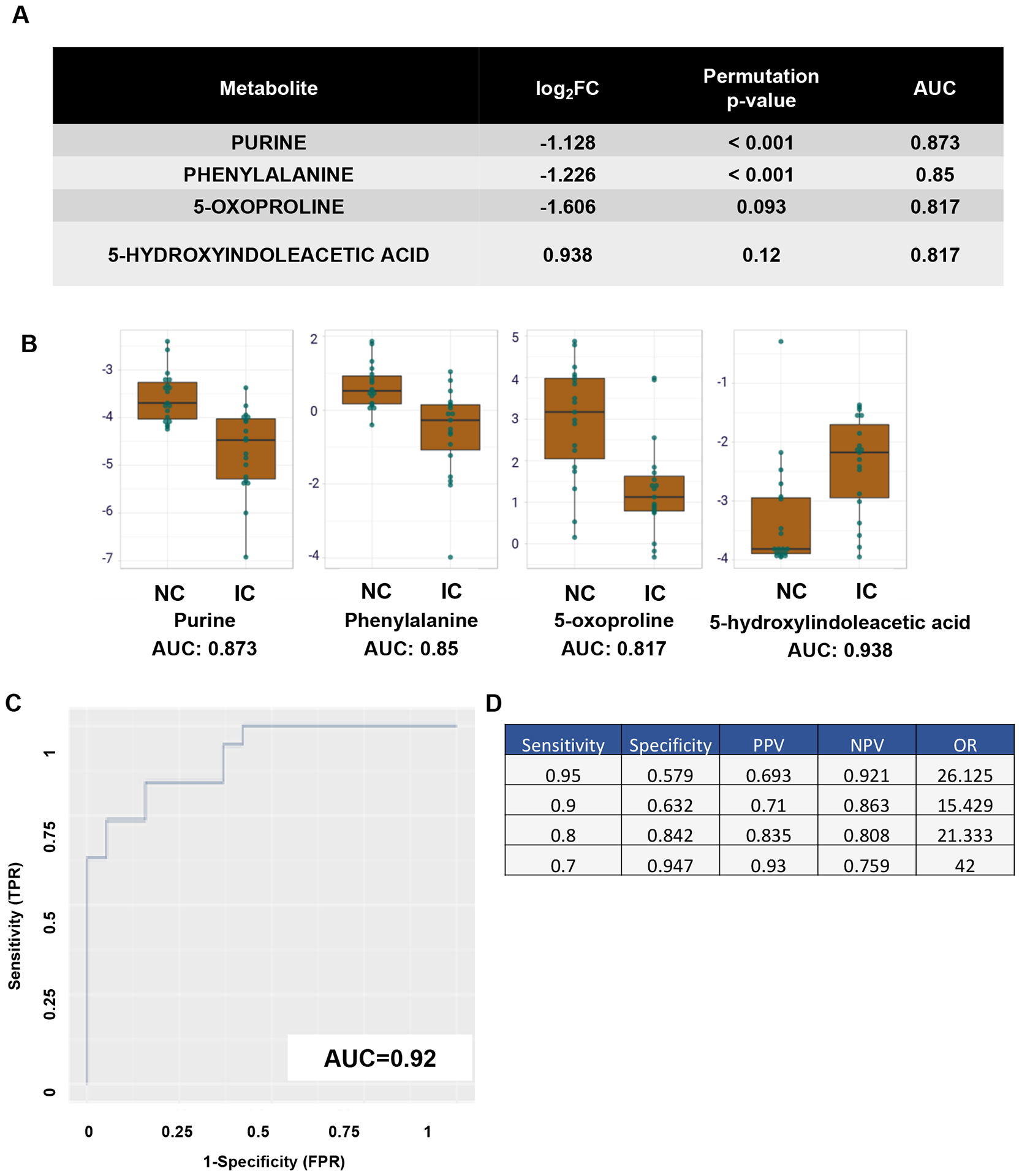
(A) Top 4 ranked differentially expressed analytes. (B) Area under the curve (AUC) analysis of the top 4 analytes. Y-axis shows AUC. X-axis illustrates either the NC or IC groups. (C) AUC of the potential biomarker panel, true positive rate (TPR) vs false positive rate (FPR). (D) AUC sensitivity and specificity calculations of the biomarker panel.
Lipidomics Analyses in Serum and Urine
Structural lipidomics analyses were performed as described in the Methods; however, there was no statistical difference between the NC and IC groups (data not shown).
DISCUSSION
In this study, we established that IC participants have distinct metabolic signatures in their urine compared to healthy controls. In contrast there were no significant differences in serum-based metabolomics.
This pilot study identified 4 urinary metabolic analytes capable of segregating IC patients from NCs; these metabolites are purine, phenylalanine, 5-oxoproline, and 5-HIAA. Each of these analytes had individual AUCs greater than 0.8. When combined into a single model, the AUC was 0.92. While the urinary levels of 5-HIAA were higher in IC compared to NC, urinary levels of purine, phenylalanine, and 5-oxoproline were significantly lower in IC participants compared to NC. Ultimately, while these results require validation in larger studies, if validated, the biomarker panel identified in this study may have a profound effect on facilitating IC diagnosis.
5-HIAA, the primary metabolite of serotonin, is known to be associated with aromatic L-amino acid decarboxylase deficiency, celiac disease, and sepiapterin reductase deficiency, which are all inborn metabolic errors (Source: Human Metabolome Database (HMDB) http://www.hmdb.ca/metabolites/HMDB0000763). In addition, elevated concentrations of 5-HIAA have been reported in individuals with metabolic abnormalities and chronic low-grade inflammation.26 This provides provocative evidence to suggest that IC may be a metabolic disorder. Phenylalanine was previously suggested as a possible IC biomarker assisting potassium sensitivity test (PST)-based diagnosis.27 That study conducted among 22 female volunteers showed that phenylalanine analysis using a Raman microspectroscopy, a non-destructive chemical analysis technique, was able to exactly certify the results of PST for diagnosis of IC. Our study found purine levels were decreased in urine samples from IC patients. There are no previous findings that suggest that purine is directly linked to IC symptoms; however, purinergic signaling has been widely studied in the bladder.11 Purinergic receptors, such as P2X7, have been suggested as therapeutic targets in IC28, and expression levels of P2X3 receptors were abnormally increased in bladder urothelial cells from patients with IC.29 There is no previous study about the role of 5-oxoproline in IC or any other bladder diseases.
Although there have been significant developments in metabolomics technology and databases, few studies have demonstrated the use of metabolomics or lipidomics to discover highly sensitive and specific biomarkers for IC diagnosis.28 The results from this pilot study strongly suggest that urine, but not serum, is a powerful potential resource for screening and monitoring IC in the clinical setting. However, because this is still an exploratory study, these results will need thorough validation.
There are several noted limitations in this study. First, home collection of samples was prepared by either the mobile phlebotomy company (blood) or participants (urine) and shipped to our laboratory at the Durham VA using a NanoCool shipping box kit. This shipping kit is designed to keep specimens between 2–8°C for 72 hours only. This collection methodology does not provide discrete data on the number of hours between collection and arrival at the lab. However, we are certain that collection methods and conditions were as similar as possible throughout and were non-differential by IC vs. NC, suggesting there is unlikely to be major bias in data output between groups. Second, there was no comparison done between home-based collection and hospital-based collection to determine if there were any effects of collection methods on the analyzed metabolomes. Third, this particular study did not capture some clinical characteristics of participants, such as menopausal status or molecular characteristics. Furthermore, due to limited sample size, there was no comparison between IC and other bladder diseases, such as overactive bladder, or no breakdown of participants based on IC subtypes (ulcer vs. non-ulcer, or flare vs. non-flare).
In summary, while we found a 4-biomarker panel that could discriminate IC from NC with high accuracy, the findings from this study strongly suggest that additional larger multicenter studies with independent validation cohorts are needed. In the future, we seek to improve and validate this biomarker panel, which can lead to potential future clinical applications.
Supplementary Material
Acknowledgments.
The authors acknowledge support from Centers for Disease Controls and Prevention (1U01DP006079, PIs, Freedland, Anger, Kim) and BERG Health.
Footnotes
CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Conflicts of Interest.
Some of authors declare conflict of interest. B.G., N.N., V.B., V.T., and M.K. are employees of BERG Health. The other authors have nothing to disclose.
REFERENCES
- 1.Hanno PM, Erickson D, Moldwin R et al. : Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol, 193: 1545, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Bogart LM, Berry SH, Clemens JQ: Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol, 177: 450, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Suskind AM, Berry SH, Ewing BA et al. : The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol, 189: 141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buffington CA: Re: Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review: L. M. Bogart, S. H. Berry and J. Q. Clemens. J Urol 2007; 177: 450–456. J Urol, 178: 1121, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hanno PM, Burks DA, Clemens JQ et al. : AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol, 185: 2162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordling J, Anjum FH, Bade JJ et al. : Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol, 45: 662, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Simon LJ, Landis JR, Erickson DR et al. : The Interstitial Cystitis Data Base Study: concepts and preliminary baseline descriptive statistics. Urology, 49: 64, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Sant GR, Hanno PM: Interstitial cystitis: current issues and controversies in diagnosis. Urology, 57: 82, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Moutzouris DA, Falagas ME: Interstitial cystitis: an unsolved enigma. Clin J Am Soc Nephrol, 4: 1844, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Skove SL, Howard LE, Senechal J et al. : The misdiagnosis of interstitial cystitis/bladder pain syndrome in a VA population. Neurourol Urodyn, 38: 1966, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson KE: Purinergic signalling in the urinary bladder. Auton Neurosci, 191: 78, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Birder LA: Pathophysiology of interstitial cystitis. Int J Urol, 26 Suppl 1: 12, 2019 [DOI] [PubMed] [Google Scholar]
- 13.You S, Yang W, Anger JT et al. : ‘Omics’ approaches to understanding interstitial cystitis/painful bladder syndrome/bladder pain syndrome. Int Neurourol J, 16: 159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tudrej KB, Piecha T, Kozlowska-Wojciechowska M: Role of NLRP3 inflammasome in the development of bladder pain syndrome interstitial cystitis. Ther Adv Urol, 11: 1756287218818030, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy L, Caldwell A, Brierley SM: Mechanisms Underlying Overactive Bladder and Interstitial Cystitis/Painful Bladder Syndrome. Front Neurosci, 12: 931, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen JM, Klumpp DJ: Mechanisms of pain from urinary tract infection. Int J Urol, 21 Suppl 1: 26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura N, Oguchi T, Yokoyama H et al. : Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int J Urol, 21 Suppl 1: 18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier M, Asmis R, Hawkins GA et al. : The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int J Mol Sci, 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskin AS, Linderman JD, Brychta RJ et al. : Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a beta3-Adrenergic Receptor Agonist. Diabetes, 67: 2113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drolet J, Tolstikov V, Williams BA et al. : Integrated Metabolomics Assessment of Human Dried Blood Spots and Urine Strips. Metabolites, 7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gacias M, Gaspari S, Santos PM et al. : Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife, 5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao F, McDaniel J, Chen EY et al. : Monoacylglycerol Analysis Using MS/MS(ALL) Quadruple Time of Flight Mass Spectrometry. Metabolites, 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Gross RW: Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev, 24: 367, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Simons B, Kauhanen D, Sylvanne T et al. : Shotgun Lipidomics by Sequential Precursor Ion Fragmentation on a Hybrid Quadrupole Time-of-Flight Mass Spectrometer. Metabolites, 2: 195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolstikov V, Nikolayev A, Dong S et al. : Metabolomics analysis of metabolic effects of nicotinamide phosphoribosyltransferase (NAMPT) inhibition on human cancer cells. PLoS One, 9: e114019, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukui M, Tanaka M, Toda H et al. : High plasma 5-hydroxyindole-3-acetic acid concentrations in subjects with metabolic syndrome. Diabetes Care, 35: 163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh TF, Yu KJ, Lin SY: Possible application of Raman microspectroscopy to verify the interstitial cystitis diagnosis after potassium sensitivity test: phenylalanine or tryptophan as a biomarker. Dis Markers, 23: 147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taidi Z MK, Bates L, Sana-Ur-Rehman H, Liu L Purinergic P2X7 receptors as therapeutic targets in interstitial cystitis/bladder pain syndrome; key role of ATP signaling in inflammation Bladder, 6: e38, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Chai TC: Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol, 171: 448, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


