Abstract
The Semaphorin family is a group of proteins studied broadly for their functions in nervous systems. They consist of eight subfamilies ubiquitously expressed in vertebrates, invertebrates, and viruses and exist in membrane-bound or secreted forms. Emerging evidence indicates the relevance of semaphorins outside the nervous system, including angiogenesis, cardiogenesis, osteoclastogenesis, tumour progression, and, more recently, the immune system. This review provides a broad overview of current knowledge on the role of semaphorins in the immune system, particularly its involvement in inflammatory and infectious diseases, including chlamydial infections.
Keywords: Semaphorin, Sema3E, Immunity, Inflammation, Infection, Chlamydia
Graphical abstract
Highlights
-
•
Semaphorins were described initially as guidance molecules that were required to direct neuronal axons to their respective targets.
-
•
Recent studies indicate the relevance of semaphorins outside the nervous system, including angiogenesis, cardiogenesis, tumor progression, osteoclastogenesis, and immune cell regulation.
-
•
This review highlights the functions of semaphorins in immune cell function, inflammation and host defense.
1. Semaphorins
The first member of semaphorins was discovered as a new axonal glycoprotein in the grasshopper named “fasciclin IV” (Kolodkin et al., 1992). Later studies identified another protein called “Collapsin-1” in the chick brain, which shares homology with fasciclin IV (Luo et al., 1993). A group of molecules showing similar structural features, such as a “Sema” domain, were then identified and categorized into the semaphorin family (Roth et al., 2009). Although semaphorins were initially described as guidance molecules required to direct neuronal axons to their respective targets, recent studies indicate the relevance of semaphorins outside the nervous system, including angiogenesis, cardiogenesis, tumour progression, osteoclastogenesis, and immune cell homeostasis (Suzuki et al., 2008).
Semaphorins exist in secreted and membrane-associated forms and share a conserved ‘Sema’ domain with approximately 500 amino acid residues (Roth et al., 2009). They are divided into eight subclasses based on their structural elements and similarities in the amino acid sequences. Invertebrate semaphorins fall into classes I and II, whereas vertebrate semaphorins are grouped into classes III-VII (Suzuki et al., 2008; Janssen et al., 2010; Goodman et al., 1999). In addition, there are reports of semaphorin molecules encoded within viral genomes and are classified as class VIII semaphorins (Suzuki et al., 2008; Spriggs, 1999). Out of these, classes II, III, and VIII semaphorins exist in secreted form (Suzuki et al., 2008). Structurally, semaphorins are characterized by a sema domain at N terminals followed by a cysteine-rich plexin semaphorin integrin (PSI) domain (Janssen et al., 2010).
All semaphorins consist of an N-terminal 500 residue sema domain with a seven-blade β-propeller fold conformation (Worzfeld and Offermanns, 2014). The sema domain is tightly coupled to an adjacent cysteine-rich domain named the “PSI domain”(plexin–semaphorin–integrin) (Worzfeld and Offermanns, 2014). Except for class VI semaphorins, all other semaphorins contain “immunoglobulin (Ig)-like domains” or “thrombospondin type 1 repeats” (Worzfeld and Offermanns, 2014). The sema domain of semaphorins undergoes homodimerization and interacts with receptors, such as neuropilins and plexins.
1.1. Semaphorin receptors and co-receptors
Plexins and neuropilins are the two high-affinity receptors of semaphorins. Plexins (previously referred to as B2) was initially identified in Xenopus as a neuronal cell surface molecule (Takagi et al., 1987). Later sequence analysis revealed that plexins are single type 1 transmembrane receptors involved in the neuronal cell adhesion (Janssen et al., 2010; Ohta et al., 1995). They are divided into four subclasses: plexinA, plexinB, plexinC, and plexinD (Janssen et al., 2010). Plexins consist of an N-terminal Sema domain, a combination of three-PSI domains, and six IPT (Ig domain shared by plexins and transcription factors) domains in their extracellular regions (Takamatsu and Kumanogoh, 2012). The N-terminal Sema-PSI domain of plexin mediates the binding of plexins to semaphorins. Plexins also have an R-Ras and M-Ras GTPase-activating protein (GAP) domain in their cytoplasmic region. The cytoplasmic tail of plexins is involved in signal transmission upon ligand binding (Kumanogoh and Kikutani, 2013). Following the binding of semaphorins to plexins, GAP domain activation results in downstream signalling through molecules such as GTPase, protein kinase and associated molecules (Pasterkamp, 2012).
In addition to plexins, classes III semaphorins need neuropilin as a co-receptor for their activity. Neuropilins are divided into Nrp-1 and Nrp-2. They are single-pass transmembrane glycoproteins with large extracellular domains, a short transmembrane domain, and a long cytoplasmic sequence (Geretti and Klagsbrun, 2007). The extracellular portion of neuropilin contains two a1 and a2 domains, b1 and b2 domains and a MAM (meprin, A-5 protein, and receptor protein-tyrosine phosphatase mu) domain (Worzfeld and Offermanns, 2014). The a1 and a2 domains bind to class III semaphorins, b1 and b2 domains bind to ligands such as vascular endothelial growth factor (Worzfeld and Offermanns, 2014). The MAM domain of neuropilins is involved in the dimerization of neuropilins (Worzfeld and Offermanns, 2014).
1.2. Semaphorins and the immune system
In recent years, a growing body of evidence indicates the roles of different classes of semaphorins in various phases of immune response, including immune cell trafficking, migration, development and cell-cell interaction (Takamatsu and Kumanogoh, 2012).
1.3. Class 3 semaphorins in immune cell function, inflammatory and infectious diseases
Class 3 semaphorins generally bind to class A plexins and require neuropilins as co-receptor for their function (Nishide and Kumanogoh, 2018). However, semaphorin 3E (Sema3E) can bind to plexinD1 independent of neuropilin-1 (Nishide and Kumanogoh, 2018). Recently, Sema3A has been found to promote the migration of DC to lymphatics by activating myosin II (Takamatsu et al., 2010a). Sema3A also influences human T cell proliferation and cytokine production (Catalano et al., 2006). Later studies identified the requirement of plexinA4–Sema3A signaling for inflammatory cytokine production induced by sepsis (Wen et al., 2010). Furthermore, treatment with neutralizing anti-Sema3A monoclonal antibodies improved the survival of LPS-treated mice, possibly by reducing inflammatory cytokine production (Yamashita et al., 2015). On the other hand, intranasal treatment with Sema3A alleviates allergic symptoms by inhibiting the Th2/Th17 response and enhancing the Th1/Treg response. Exogenous Sema3A inhibits growth factor-induced proliferation of human airway smooth muscle cells, thereby minimizing the airway remodeling (Movassagh et al., 2016a). In systemic lupus erythematosus (SLE) patients, activated B cells expressed lower levels of CD72 than that of normal controls and this lower expression of CD72 was associated with lupus nephritis and SLE disease activity (Vadasz et al., 2014). Sema3A treatment enhances CD72 expression on B cells, and CD72 can be used as a biomarker to be followed during the treatment of SLE (Vadasz et al., 2014). Mechanistically, Sema3A binding with CD72 on B cells inhibits the phosphorylation of STAT-4 and HDAC-1 but induces the phosphorylation of p38-MAPK in B cells (Eiza et al., 2023). In vivo studies in mice revealed that Sema3A reduces lupus nephritis in NZB/W mice by preventing the deposition of immune complexes in the glomeruli (Bejar et al., 2018). These studies suggest that Sema3A administration restores self-tolerance and thereby reduces the pathogenesis of SLE (Eiza et al., 2023). Moreover, recent studies established the role of class 3 semaphorins like Sema3A, Sema3C, and Sema3F in promoting human DC migration by inducing the F-actin organization (Curreli et al., 2016) (Table 1).
Table 1.
Semaphorin functions and diseases.
| Semaphorins | Functions | Associated diseases |
|---|---|---|
| Sema3A | Migration of DC to lymphatics | Sepsis |
| Human T cell proliferation | Allergy | |
| SLE | ||
| Sema3C and Sema3F | Promote human DC migration | |
| Sema3E | Migration of maturing thymocytes | Sepsis |
| Chemoattractant for macrophages | Colitis | |
| NK-cell migration | Allergic asthma | |
| Reduce eosinophilic inflammation, serum IgE, IgG1, and Th2/Th17 cytokines in allergic asthma | Chlamyial infection | |
| Enhance Th1/Th17 response and reduce Treg response to chlamydial infection | Leishmania major infection | |
| Induce co-stimulatory molecules and production of IL-12 on DC | ||
| Sema4A | T cell differentiation and T cell priming | Asthma |
| Treg stability | Cancer | |
| Co-stimulatory molecule for T-cell activation | Rheumatoid arthritis | |
| Promotes macrophage migration | EAE | |
| MS | ||
| Sema4B | Inhibits IL-4 and IL-6 production in basophils | Lung adenocarcinoma |
| Increase infiltration of myeloid-derived suppressor cells (MDSCs) and T-regs | ||
| Sema4C | B cell polarization and function | Allergic asthma |
| Inhibits IgE, eosinophils, and Th2 cytokine | ||
| Sema4D | Promotes B cell activation by shutting off CD72 inhibitory signals | HIV |
| Induce expression of co-stimulatory molecules and IL-12 cytokine production of DC | Endodontic infection | |
| Negative regulator of the neutrophil activation | Asthma | |
| EAE | ||
| MS | ||
| Sema5A | Increase endothelial cell proliferation | Gastric cancer |
| Inhibits cell migration and invasion of glioma cells | Pancreatic cancer | |
| Stimulates T cell and NK cell proliferation Promotes the production of Th1/Th17 cytokines | Rheumatoid Arthritis | |
| Primary immune thrombocytopenia | ||
| Chronic spontaneous urticaria | ||
| Sema6D | Induce IL-12 production and upregulate MHC class II expression in dendritic cells | EAE |
| Regulates T cell activation | Allergic lung inflammation | |
| Maintain IL-10 producing ILC2s | Colitis | |
| Polarization of macrophage into an anti-inflammatory state | ||
| Migration of DC into lymphatics | ||
| Sema7A | Promotes differentiation of CD4+ T cells into Th1 and Th17 subclasses | LPS-induced lung injury |
| Stimulator of monocytes | Rheumatoid Arthritis | |
| Induces neutrophil migration | Idiopathic pulmonary fibrosis | |
| Promotes dendrite formation and migration of DC | West Nile virus (WNV) infection | |
| Breast cancer |
Sema3E, named initially as M-SemaH in mice, was discovered as a novel semaphorin family member shown to correlate with tumour progression positively (Eiza et al., 2023). The Sema3E transcripts encode 775 amino acids with the features of a secreted glycoprotein (Christensen et al., 1998). Initial studies by Christensen et al. suggest the involvement of the M-SemaH gene in embryonic development, which is found to be expressed in developing lungs, skeletal elements, and neural tube (Christensen et al., 1998). In humans, Sema3E is located on chromosome 7 and shares 87.4% similarity with mouse Sema3E (Nagase et al., 1997). Sema3E is highly expressed on DCs, Th2 cells, and thymic epithelial cells (Holl et al., 2012; Choi et al., 2008). Recent studies strongly suggest the importance of Sema3E in directing the migration of maturing thymocytes to the medulla (Choi et al., 2008). In the activated CD4+CD8+ double positive (DP) thymocytes, Sema3E-plexinD1 signaling inhibits CCR9/CCL25 signaling in the cortex (Choi et al., 2008). As a result, the activated DP thymocytes migrate to the medulla in response to CCL19/21 signaling (Choi et al., 2008). Later studies identified that Sema3E-plexinD1 axis control β1 integrin adhesion to regulate thymocyte migration (Choi et al., 2014). Recent studies by Ueda et al. identified the relevance of Sema3E in regulating thymocyte trafficking. Sema3E directly inhibits Rap1 activation through plexinD1 leading to impaired immunological synapse formation in thymocytes (Ueda et al., 2016).
The role of Sema3E is shown in inflammatory diseases as well. In the mouse model of dietary obesity, the expression of Sema3E and its receptor plexinD1 was shown to be upregulated (Shimizu et al., 2013). In adipose tissue, Sema3E acts as a chemoattractant for macrophages, and p53-induced upregulation of Sema3E leads to tissue inflammation (Shimizu et al., 2013). Sema3E is also expressed in macrophages of atherosclerotic plaques and inhibits macrophage migration to chemokine by disrupting the re-organization of the actin cytoskeleton (Wanschel et al., 2013). The influence of Sema3E on macrophage function was also analyzed during the LPS-induced acute inflammatory response. Both Sema3E-deficient mice and mice with specific deletion of plexinD1in macrophages exhibit reduced inflammation and better clinical scores than wild-type controls in LPS-induced sepsis (Mohammed et al., 2020). The early inflammatory response to LPS in Sema3E KO mice was associated with reduced phosphorylation of ERK1/2, AKT, STAT3, and NF-κB in the macrophages (Mohammed et al., 2020). Mouse NK cells also express the Sema3E receptor, plexinD1, on their surface (Alamri et al., 2018). Activated NK cells showed higher migration towards the conditioned medium of immature Sema3E−/− DCs than Sema3E+/+ DCs, suggesting a suppressive effect of Sema3E produced by immature DCs in NK-cell migration (Alamri et al., 2018). In Sema3E deficient colitic mice, there was an exacerbated disease severity and was ameliorated by recombinant Sema3E treatment (Kermarrec et al., 2019). Sema3E modulates the pro‐inflammatory activity of CD11c+ cells via the NF‐κB‐dependent pathway to reduce colitis (Kermarrec et al., 2019). Furthermore, recombinant Sema3E treatment reduced apoptosis and p53-associated genes in intestinal epithelial cells (Eissa et al., 2019). Together, these findings suggest Sema3E as a novel inhibitor of intestinal inflammation.
There are numerous studies on the functions of Sema3E in allergic asthma. Sema3E inhibited human airway smooth muscle cell proliferation and migration, one of the critical events in the development of asthma (Movassagh et al., 2014). This effect was mediated by inducing F-actin depolymerization, suppressing Rac1 GTPase activity, and inhibiting phosphorylation of Akt and ERK ½ (Movassagh et al., 2014). The expression of Sema3E was significantly reduced in the airways of severe asthmatic patients and the mice with allergen sensitization and challenge (Movassagh et al., 2017a, 2017b). Sema3E deficiency in mice leads to exaggerated allergic airway inflammation, remodeling and airway hyperresponsiveness, while intranasal recombinant Sema3E treatment reduced house dust mite-induced allergic asthma (Movassagh et al., 2017b, 2017c). Sema3E−/− mice showed higher numbers of CD11b+ pulmonary DCs than WT controls after sensitization with allergen (Movassagh et al., 2017c). The adoptive transfer of these DCs to WT recipient mice enhanced house dust mite-induced Th2/Th17 inflammation (Movassagh et al., 2017c). Intranasal exogenous Sema3E protects mice from allergic asthma by reducing eosinophilic inflammation, serum IgE, IgG1, and Th2/Th17 cytokines (Movassagh et al., 2017b). Also, upon co-culture with T cells, CD103+ cDCs from Sema3E-treated, HDM-exposed mice promoted IFN-γ production by T cells (Movassagh et al., 2017b).
Apart from pulmonary DCs, Sema3E is also involved in regulating pulmonary neutrophil recruitment in a mouse model of allergic asthma. An enhanced accumulation of neutrophils is observed in the lungs of Sema3E KO mice after the allergen challenge, whereas exogenous Sema3E treatment reduced the allergen-induced neutrophil recruitment (Movassagh et al., 2016b). Moreover, human neutrophils exhibited a constitutive expression of Sema3E high-affinity receptor, plexinD1; and Sema3E inhibited CXCL8/IL-8–induced neutrophil migration via suppression of Ras-related C3 botulinum toxin substrate 1 GTPase activity and F-actin assembly (Movassagh et al., 2016b). Recently Sema3E is also reported to influence angiogenesis, an important feature of asthmatic airway remodeling. Sema3E inhibits the formation of new blood vessels in the allergic asthmatic airway by modulating pro-and anti-angiogenic factors such as vascular endothelial growth factor (VEGF), VEGF receptor 2 protein, and soluble VEGF receptor 1 (Tatari et al., 2019).
Recent studies evidenced the function of Sema3E in infectious diseases as well. We recently reported that Sema3E plays a significant role in protection against bacterial infection. Compared to WT mice, Sema3E-deficient mice showed more severe disease and higher bacterial growth following Chlamydia muridarum lung infection (Thomas et al., 2021). The disease severity of Sema3E KO mice was associated with lower Th1/Th17 cytokine responses, higher Th2 response, altered Ab response, and an increased number of regulatory CD4 T cells (Thomas et al., 2021). Moreover, DCs isolated from Sema3E KO mice showed lower expression of co-stimulatory molecules and the production of IL-12 but higher surface expression of inhibitory molecules and IL-10 production. Also, DCs from infected Sema3E KO mice failed to induce Th1 and Th17 cell responses compared to DCs from infected WT mice. After adoptive transfer, mice receiving DCs from Sema3E KO mice, unlike those receiving DCs from WT mice, were unprotected against challenge infection (Thomas et al., 2021).
Later studies revealed that treatment of WT and Sema3E KO mice with recombinant Sema3E-Fc protected mice from chlamydial infection with lower bacterial burden and inflammation in the lung (Thomas et al., 2022). The protective effect was correlated with enhanced Th1/Th17 response, reduced Treg response, higher co-stimulatory molecules, and lower immune-inhibitory marker PD-L1 on the surface of DCs. In addition, the recruitment of DCs, including the CD103+ subset, to the site of infection was also increased after the Sema3E treatment (Thomas et al., 2022) (Fig. 1). On the other hand, Sema3E deficient Leishmania major infected mice exhibited enhanced resistance to infection compared to WT mice (Ikeogu et al., 2021). Sema3E promotes susceptibility to Leishmania infection in mice by supressing Th1 immune response (Ikeogu et al., 2021). Enhanced resistance to L.major in Sema3E deficient mice was also associated with increased co-stimulatory molecules and 1L-12 cytokine production by CD11c + cells (Ikeogu et al., 2021). The reason for the discrepancy between the role of Sema3E in Chlamydia and Leishmania infection remains unclear. However, it suggests that the function of the Sema3 in different types of infectious diseases may be different due to the nature of infections. Notably, the study on L. major was done using C57BL/6 mice instead of BALB/c; the mouse difference should not be ignored, considering the dramatic difference of mouse strains in susceptibility and disease severity to L. major infection (Sacks and Noben-Trauth, 2002; Locksley et al., 1987; Biedermann et al., 2001). Further studies are required to understand the function of the Sema3E in specific infectious disease conditions.
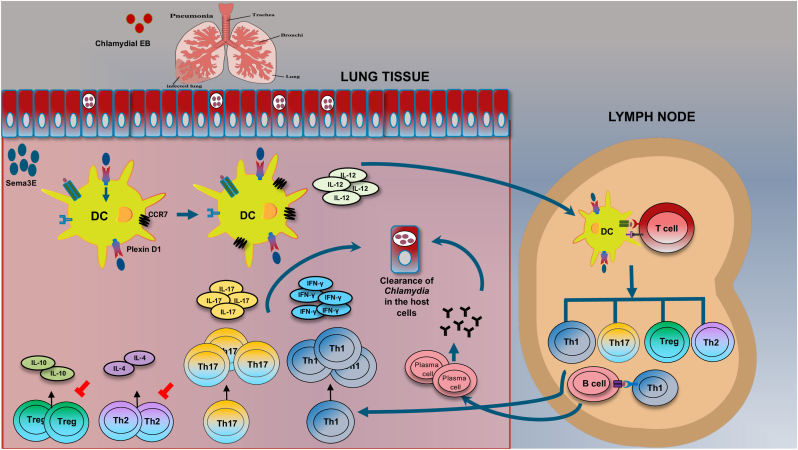
Fig. 1.
Schematic model showing the mechanism by which Sema3E protects against chlamydial infection. Chlamydial lung infection induces the production of Sema3E. Sema3E binds to its receptor, plexinD1, expressed on DCs, thus upregulating co-stimulatory molecules, chemokine receptor (CCR7), and IL-12 cytokine production. Sema3E modulated DC migrate to the draining lymph nodes and promotes Th1/Th17 response and inhibits Th2 and Treg response. The modulated DC can also promote IgG2a antibody production. These responses result in the clearance of Chlamydia (Thomas et al., 2021), (Thomas et al., 2022).
1.4. Class 4 semaphorins in immune cell function, inflammatory and infectious diseases
Class 4 semaphorins are membrane-bound and bind to class B plexins for their activity (Nishide and Kumanogoh, 2018). In addition, Sema4A bind to neuropilin-1 and plexinD1 (Nishide and Kumanogoh, 2018). The Sema4A molecule is a 761 aa long glycoprotein that exists in membrane-bound and soluble forms (Chapoval, 2018). Sema4A is highly expressed in Th1 cells and involved in their differentiation and T cell priming (Ito and Kumanogoh, 2016). Sema4A deficient mice showed reduced Th1 response and higher Th2 response (Kumanogoh et al., 2005). Sema4A deficiency also exacerbated Th2-like lung inflammation in the asthma (Mogie et al., 2013). Sema4A-Fc administration reduced lung inflammation and production of Th2-type cytokines in WT mice (Morihana et al., 2013). Later studies identified that Sema4A is required for Treg stability in tumours (Delgoffe et al., 2013). In contrast to observation in mice, human Sema4A induces Th2 immune responses (Lu et al., 2018). In addition to T cells, Sema4A is also expressed by dendritic cells (Kumanogoh et al., 2005). Sema4A-deficient DCs, compared to wild-type DCs, showed reduced stimulation of allogenic T cells (Kumanogoh et al., 2005). Thus, Sema4A acts as a co-stimulatory molecule for T-cell activation. Sema4A also acts as a chemoattractant/chemorepellent based on inflammatory conditions. Sema4A interaction with plexinD1 promotes macrophage migration but reduces endothelial cell migration (Meda et al., 2012; Toyofuku et al., 2007). Moreover, Sema4A can also be exploited as a biomarker for inflammatory diseases. Sema4A levels positively correlated with disease activity score and were involved in the rheumatoid arthritis pathogenesis (Wang et al., 2015a). Intravenous injection of an anti-Sema4A mAb improved the development of myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), in wild-type mice by decreasing the infiltration of mononuclear inflammatory cells into the spinal cord (Kumanogoh et al., 2002a). Sema4A expression is increased in patients with MS and cause pathogenesis by promoting Th17 skewing (Nakatsuji et al., 2012).
Sema4B is another semaphorin expressed by T cells, basophils, and B cells. In basophils, recombinant Sema4B inhibited IL-4 and IL-6 production (Nakagawa et al., 2011). Sema4B–KO mice had enhanced basophil-mediated IgE production, despite normal lymphocyte and dendritic cell functions (Nakagawa et al., 2011). Recent studies suggest that Sema4B expression was upregulated in lung adenocarcinoma tissues. Sema4B levels correlated with the increased infiltration of myeloid-derived suppressor cells (MDSCs) and T-regs leading to poor disease prognosis (Jiang et al., 2022). Sema4C, another class four semaphorin, is expressed in human memory B cells and binds to the receptor plexinB2 with high affinity (Xue et al., 2016; Maier et al., 2011). Sema4C is regarded as a factor needed for B cell polarization and function (Xue et al., 2016). In mouse models of allergic airway disease, Sema4C deficient mice exhibit higher IgE, eosinophils, and Th2 cytokines than WT mice (Xue et al., 2017). Sema4D (also known as CD100) was the first semaphorin discovered to have an immunoregulatory function and is highly expressed in dendritic cells, macrophages, activated B cells and T cells (Zhu et al., 2016). Several studies suggest that Sema4D promotes B cell activation by shutting off CD72 inhibitory signals (Kumanogoh et al., 2000). Sema4D promotes the viability of B cells and fine-tunes B-cell antigen receptor (BCR) signaling to generate proper immune response (Kumanogoh et al., 2000; Hall et al., 1996).
Moreover, Sema4D and its receptor CD72 are expressed in large amounts in T cells and DC, respectively. Sema4D deficient DC exhibit reduced expression of co-stimulatory molecules and IL-12 cytokine production (Kumanogoh et al., 2002b). In addition to T cells and DC, Sema4D is also expressed in neutrophils. Sema4D-plexinB2 interaction decreased NET formation and acted as a negative regulator of the neutrophil activation (Nishide et al., 2017). More recent studies also showed a protective role for Sema4D in HIV infection (Eriksson et al., 2012). It was found that CD8+ T cells lacking Sema4D expression were functionally impaired and present in increased numbers in HIV-1–infected individuals. The functions of Sema4D were also examined in the context of endodontic infections. Enterococcus faecalis, a bacterial pathogen implicated in endodontic infections, specifically induces the expression of Sema4D in osteoclast precursor cells to inhibit bone formation (Wang et al., 2015b). In a mouse model of OVA-induced experimental asthma, Sema4D deficient mice, compared to WT mice, showed a significant decrease in eosinophilic airway infiltration and was associated with decreased IL-5, IL-13, TGF-β1, IL-6, and IL-17A levels in BAL (Shanks et al., 2013). In addition, reduced T cell proliferation and increased Treg cell numbers were observed in Sema4D deficient mice, compared to WT mice (Shanks et al., 2013). During EAE development, treatment with Sema4D blocking Abs significantly inhibited neuroinflammation (Okuno et al., 2010). In addition, Sema4D receptor (plexinB1)–deficient mice were resistant to the development of EAE after adoptive transfer of myelin oligodendrocyte glycoprotein (MOG)-specific T cells (Okuno et al., 2010). Similarly, anti-Sema4D treatment significantly attenuates EAE by preserving BBB integrity and protecting axonal myelination (Smith et al., 2015). Sema4D (CD100) expression was increased in the T cells of patients with MS and expression of Sema4D receptor, CD72, on B lymphocytes was reduced (Kuklina et al., 2014). These findings indicate the relevance of blocking Sema4D for the treatment of neuroinflammatory diseases.
1.5. Class 5 semaphorins in immune cell function, inflammatory and infectious diseases
Few studies identified the role of class 5 semaphorins in the functions of the immune system. Sema5A is reported to have immune functions among the class 5 semaphorins. The biological roles of Sema5A are exerted by its interactions with receptors plexinA1, and plexinB3 (Artigiani et al., 2004; Lyu et al., 2015). Initial studies described Sema5A as a factor that increase endothelial cell proliferation to promote angiogenesis (Sadanandam et al., 2010). Other studies suggest the association of Sema5A with metastatic ability in gastric and pancreatic cancers (Sadanandam et al., 2010; Pan et al., 2009). On the other hand, tumor suppressor functions of Sema5A is reported. Sema5A-plexin B3 activation significantly inhibits cell migration and invasion of glioma cells (Li and Lee, 2010). Sema5A act to maintain the epithelial phenotype of malignant pancreatic cancer cells and regulates tumor growth (Saxena et al., 2018).
Soluble Sema5A stimulates T cell and NK cell proliferation and promotes the production of Th1/Th17 cytokines (Gras et al., 2014). In addition, high serum levels of Sema5A are associated with Rheumatoid Arthritis (RA) and can act as a promising biomarker for diagnosis of RA (Gras et al., 2014). Increased levels of Sema5A in synovial fluids promote cytokine secretion and suppress apoptosis through PI3K/AKT/mTOR signaling pathway (Cheng et al., 2022). In addition, in patients with primary immune thrombocytopenia (ITP), an immune-mediated disorder, elevated plasma Sema5A levels correlated with Th1 polarization (Lyu et al., 2015). Sema5A was highly expressed in skin CD4+ T cells and mast cells of patients with chronic spontaneous urticaria (CSU) (Lobna et al., 2021). Sema5A and IL-17A co-expressed in the skin of CSU patients and Sema5A stimulation increased IL-17A-expressing CD4+ T cells in healthy controls to CSU levels (Lobna et al., 2021).
1.6. Class 6 semaphorins in immune cell function, inflammatory and infectious diseases
Class 6 semaphorins are membrane-bound semaphorins and bind to class A plexins. Among the class 6 semaphorins, Sema6D is expressed by T cells, B cells, and NK cells (Takegahara et al., 2006). Sema6D exists in both soluble and membrane-bound forms (Toyofuku et al., 2004). Sema6D receptor (plexinA1) deficient dendritic cells, compared with wild-type dendritic cells, poorly stimulated allogeneic T-cells (Takegahara et al., 2006). In addition, soluble Sema6D induced IL-12 production and upregulated of MHC class II expression in dendritic cells (Takegahara et al., 2006). These functions of Sema6D suggest that it can act as an indirect T cell costimulatory molecule. Sema6D induce DC activation through plexinA1–Trem-2–Dap12 receptor complex (Takegahara et al., 2006). PlexinA1 deficient mice were resistant to experimental autoimmune encephalomyelitis showing relevance of plexinA1–Trem-2–Dap12 signaling axis in DC activation (Takegahara et al., 2006). T cells express Sema6D upon TCR stimulation and regulates T cell activation at later stages of immune response (O'Connor et al., 2008). Upon targeting Sema6D with either Sema6D-Ig or anti-Sema6D antibody, there was a reduced T cell activation in vivo. Moreover, Sema6D act as a niche signal required for group 2 innate lymphoid cells (ILC2s) and helps to maintain IL-10 producing ILC2s (Naito et al., 2022). Sema6D deficient mice showed a reduction in ILC2s in peripheral tissues and are resistant to develop allergic lung inflammation (Naito et al., 2022). In addition to functional roles in DC and T cells, Sema6D is required for the polarization of macrophage into an anti-inflammatory state and in turn prevents the development of colitis (Kang et al., 2018). Furthermore, Sema6D receptor, plexinA1, plays a crucial role during the entry of DC into lymphatics (Takamatsu et al., 2010b).
1.7. Class 7 semaphorins in immune cell function, inflammatory and infectious diseases
Out of the class 7 semaphorins, Sema7A (also known as CD108) was reported to act as immune semaphorins. Human Sema7A consists of 666 amino acids (Xu et al., 1998) and exists in a soluble form in addition to the functional membrane-bound form (Althoff et al., 2001). Sema7A interacts with two main functional receptors; plexinC1 and integrin receptors containing the β1 subunit (β1 integrin) (Song et al., 2021). Sema7A is expressed by several immune cells, including T cells and dendritic cells. Soluble Sema7A promotes differentiation of CD4+ T cells into Th1 and Th17 subclasses by inducing T-bet and retinoic acid receptor–related orphan nuclear receptor γt (RORγt), respectively (Xie and Wang, 2017). Sema7A acts as a potent monocyte stimulator (Holmes et al., 2002) and initiates T cell mediated inflammatory response (Suzuki et al., 2007). Sema7A also functions as a factor which induces neutrophil migration into hypoxic tissue sites (Roth et al., 2016). During LPS-induced lung injury, Sema7A deficient mice showed a reduction in inflammatory cytokines and neutrophils (Roth et al., 2016).
Sema7A could also be used as a therapeutic agent in rheumatoid arthritis as anti-Sema7A antibody treatment reduced arthritis scores in mice with collagen-induced arthritis (Xie and Wang, 2017). Sema7A is also expressed by fibrocytes, and Sema7A deficiency reduces TGF-β1–induced lung fibrosis as well as alveolar remodeling (Kang et al., 2007). Further studies identified an association of Sema7A expressing Treg cells with disease progression in people with idiopathic pulmonary fibrosis (IPF) (Reilkoff et al., 2013). Moreover, adoptive transfer of these cells induced fibrosis and airway remodeling in the TGF-β1–exposed murine lung (Reilkoff et al., 2013). Furthermore, West Nile virus (WNV) infection of Sema7A deficient mice exhibited lower viral load, reduced blood-brain barrier permeability, and increased survival (Sultana et al., 2012). Studies of Sema7A in DC showed that Sema7A is needed for dendrite formation and migration of DC (van Rijn et al., 2016). In murine model of breast cancer, Sema7A promote tumor growth by skewing monocytes into a pro-tumorigenic phenotype (Garcia-Areas et al., 2014).
2. Conclusion
Accumulating evidence suggests the relevance of semaphorins in cancer, autoimmunity, allergic disorders, and infectious diseases. Further study to address the question of how semaphorins influence the process of these diseases will provide new insight into the disease pathogenesis and host defence mechanisms. This molecule also has the potential to be a novel target in the treatment of these diseases. For example, the therapeutic ability of the uncleavable variant of Sema3E (Uncl-Sema3E) has been demonstrated in multiple tumor models where it acts as a novel inhibitor of tumor growth, metastasis, and angiogenesis (Casazza et al., 2010). Also, in vivo studies on mice indicate that Sema3E treatment enhances host defence against chlamydial infection (Thomas et al., 2022) and reduces allergic asthma (Movassagh et al., 2017b). Currently, several undergoing clinical trials targeting semaphorins in the context of cancer (clinicaltrials.gov, NCT03690986, NCT01313065), multiple sclerosis (clinicaltrials.gov, NCT01764737), Alzhimer's disease (clinicaltrials.gov, NCT04381468) and early sepsis (clinicaltrials.gov, NCT02692118) are available. However, the efficacy of semaphorins as a potential target in infectious/inflammatory conditions has remained to be evaluated in clinical settings. Further research on semaphorins will shed light on their functions in the immune system and provide new approaches to treating inflammatory and infectious diseases.
CRediT authorship contribution statement
Rony Thomas: Visualization, Writing – original draft, Writing – review & editing. Xi Yang: Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work is supported by a grant from Canadian Institutes of Health Research to X.Y. ((MOP130423).
Data availability
Data will be made available on request.
References
- Alamri A., et al. Semaphorin-3E produced by immature dendritic cells regulates activated natural killer cells migration. Front. Immunol. 2018;9:1005. doi: 10.3389/fimmu.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff K., et al. Recognition sequences and structural elements contribute to shedding susceptibility of membrane proteins. Biochem. J. 2001;353(Pt 3):663–672. doi: 10.1042/0264-6021:3530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S., et al. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5(7):710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar J., et al. Semaphorin3A: a potential therapeutic tool for lupus nephritis. Front. Immunol. 2018;9:634. doi: 10.3389/fimmu.2018.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann T., et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2001;2(11):1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- Casazza A., et al. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J. Clin. Invest. 2010;120(8):2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano A., et al. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107(8):3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- Chapoval S.P. Neuroimmune semaphorins as costimulatory molecules and beyond. Mol. Med. 2018;24(1):13. doi: 10.1186/s10020-018-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., et al. Semaphorin 5A suppresses ferroptosis through activation of PI3K-AKT-mTOR signaling in rheumatoid arthritis. Cell Death Dis. 2022;13(7):608. doi: 10.1038/s41419-022-05065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.I., et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29(6):888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.I., et al. Dynamic control of β1 integrin adhesion by the plexinD1-sema3E axis. Proc. Natl. Acad. Sci. U. S. A. 2014;111(1):379–384. doi: 10.1073/pnas.1314209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen C.R., et al. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58(6):1238–1244. [PubMed] [Google Scholar]
- Curreli S., et al. Class 3 semaphorins induce F-actin reorganization in human dendritic cells: role in cell migration. J. Leukoc. Biol. 2016 doi: 10.1189/jlb.2A1114-534R. jlb. 2A1114-534R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe G.M., et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501(7466):252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa N., et al. Semaphorin 3E regulates apoptosis in the intestinal epithelium during the development of colitis. Biochem. Pharmacol. 2019;166:264–273. doi: 10.1016/j.bcp.2019.05.029. [DOI] [PubMed] [Google Scholar]
- Eiza N., et al. CD72-semaphorin3A axis: a new regulatory pathway in systemic lupus erythematosus. J. Autoimmun. 2023;134 doi: 10.1016/j.jaut.2022.102960. [DOI] [PubMed] [Google Scholar]
- Eriksson E.M., et al. Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity. Blood. 2012;119(3):745–755. doi: 10.1182/blood-2010-12-324848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Areas R., et al. Semaphorin7A promotes tumor growth and exerts a pro-angiogenic effect in macrophages of mammary tumor-bearing mice. Front. Physiol. 2014;5:17. doi: 10.3389/fphys.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geretti E., Klagsbrun M. Neuropilins: novel targets for anti-angiogenesis therapies. Cell Adhes. Migrat. 2007;1(2):56–61. doi: 10.4161/cam.1.2.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C., et al. Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97(5):551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Gras C., et al. Secreted semaphorin 5A activates immune effector cells and is a biomarker for rheumatoid arthritis. Arthritis Rheumatol. 2014;66(6):1461–1471. doi: 10.1002/art.38425. [DOI] [PubMed] [Google Scholar]
- Hall K.T., et al. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc. Natl. Acad. Sci. U. S. A. 1996;93(21):11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl E.K., et al. Plexin-B2 and Plexin-D1 in dendritic cells: expression and IL-12/IL-23p40 production. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S., et al. Sema7A is a potent monocyte stimulator. Scand. J. Immunol. 2002;56(3):270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- Ikeogu N.M., et al. Semaphorin 3E promotes susceptibility to Leishmania major infection in mice by suppressing CD4+ Th1 cell response. J. Immunol. 2021;206(3):588–598. doi: 10.4049/jimmunol.2000516. [DOI] [PubMed] [Google Scholar]
- Ito D., Kumanogoh A. Cell Adhesion & Migration; 2016. The Role of Sema4A in Angiogenesis, Immune Responses, Carcinogenesis, and Retinal Systems; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B.J., et al. Structural basis of semaphorin-plexin signalling. Nature. 2010;467(7319):1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., et al. Semaphorin 4B promotes tumor progression and associates with immune infiltrates in lung adenocarcinoma. BMC Cancer. 2022;22(1):632. doi: 10.1186/s12885-022-09696-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.R., et al. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J. Exp. Med. 2007;204(5):1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., et al. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat. Immunol. 2018;19(6):561–570. doi: 10.1038/s41590-018-0108-0. [DOI] [PubMed] [Google Scholar]
- Kermarrec L., et al. Semaphorin-3E attenuates intestinal inflammation through the regulation of the communication between splenic CD11C. Br. J. Pharmacol. 2019;176(9):1235–1250. doi: 10.1111/bph.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A.L., et al. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9(5):831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- Kuklina E.M., et al. Semaforin Sema4D in the immune system in multiple sclerosis. Bull. Exp. Biol. Med. 2014;157(2):234–237. doi: 10.1007/s10517-014-2533-x. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A., Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 2013;13(11):802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A., et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13(5):621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A., et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419(6907):629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A., et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J. Immunol. 2002;169(3):1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A., et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22(3):305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Li X., Lee A.Y. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. J. Biol. Chem. 2010;285(42):32436–32445. doi: 10.1074/jbc.M110.120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobna M., et al. The expression of IL-17, in chronic spontaneous urticaria is linked to Semaphorin5A. Biomolecules. 2021;11(3) doi: 10.3390/biom11030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R.M., et al. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur. Immunol. 1987;138(5):744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Lu N., et al. Human Semaphorin-4A drives Th2 responses by binding to receptor ILT-4. Nat. Commun. 2018;9(1):742. doi: 10.1038/s41467-018-03128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Raible D., Raper J.A. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Lyu M., et al. Elevated Semaphorin 5A correlated with Th1 polarization in patients with chronic immune thrombocytopenia. Thromb. Res. 2015;136(5):859–864. doi: 10.1016/j.thromres.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Maier V., et al. Semaphorin 4C and 4G are ligands of Plexin-B2 required in cerebellar development. Mol. Cell. Neurosci. 2011;46(2):419–431. doi: 10.1016/j.mcn.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda C., et al. Semaphorin 4A exerts a proangiogenic effect by enhancing vascular endothelial growth factor-A expression in macrophages. J. Immunol. 2012;188(8):4081–4092. doi: 10.4049/jimmunol.1101435. [DOI] [PubMed] [Google Scholar]
- Mogie G., et al. Neuroimmune semaphorin 4A as a drug and drug target for asthma. Int. Immunopharm. 2013;17(3):568–575. doi: 10.1016/j.intimp.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., et al. Semaphorin 3E regulates the response of macrophages to lipopolysaccharide-induced systemic inflammation. J. Immunol. 2020;204(1):128–136. doi: 10.4049/jimmunol.1801514. [DOI] [PubMed] [Google Scholar]
- Morihana T., et al. An inhibitory role for Sema4A in antigen-specific allergic asthma. J. Clin. Immunol. 2013;33(1):200–209. doi: 10.1007/s10875-012-9798-5. [DOI] [PubMed] [Google Scholar]
- Movassagh H., et al. Neuronal chemorepellent Semaphorin 3E inhibits human airway smooth muscle cell proliferation and migration. J. Allergy Clin. Immunol. 2014;133(2):560–567. doi: 10.1016/j.jaci.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Movassagh H., et al. Human airway smooth muscle cell proliferation from asthmatics is negatively regulated by semaphorin3A. Oncotarget. 2016;7(49):80238–80251. doi: 10.18632/oncotarget.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassagh H., et al. Chemorepellent semaphorin 3E negatively regulates neutrophil migration in vitro and in vivo. J. Immunol. 2016 doi: 10.4049/jimmunol.1601093. [DOI] [PubMed] [Google Scholar]
- Movassagh H., et al. Expression of semaphorin 3E is suppressed in severe asthma. J. Allergy Clin. Immunol. 2017;140(4):1176–1179. doi: 10.1016/j.jaci.2017.04.031. [DOI] [PubMed] [Google Scholar]
- Movassagh H., et al. Semaphorin 3E alleviates hallmarks of house dust mite-induced allergic airway disease. Am. J. Pathol. 2017;187(7):1566–1576. doi: 10.1016/j.ajpath.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Movassagh H., et al. Semaphorin 3E deficiency exacerbates airway inflammation, hyperresponsiveness, and remodeling in a mouse model of allergic asthma. J. Immunol. 2017;198(5):1805–1814. doi: 10.4049/jimmunol.1601514. [DOI] [PubMed] [Google Scholar]
- Nagase T., et al. Prediction of the coding sequences of unidentified human genes. VII. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 1997;4(2):141–150. doi: 10.1093/dnares/4.2.141. [DOI] [PubMed] [Google Scholar]
- Naito M., et al. Semaphorin 6D-expressing mesenchymal cells regulate IL-10 production by ILC2s in the lung. Life Sci. Alliance. 2022;5(11) doi: 10.26508/lsa.202201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., et al. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J. Immunol. 2011;186(5):2881–2888. doi: 10.4049/jimmunol.1003485. [DOI] [PubMed] [Google Scholar]
- Nakatsuji Y., et al. Elevation of Sema4A implicates Th cell skewing and the efficacy of IFN-β therapy in multiple sclerosis. J. Immunol. 2012;188(10):4858–4865. doi: 10.4049/jimmunol.1102023. [DOI] [PubMed] [Google Scholar]
- Nishide M., Kumanogoh A. The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2018;14(1):19–31. doi: 10.1038/nrrheum.2017.201. [DOI] [PubMed] [Google Scholar]
- Nishide M., et al. Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann. Rheum. Dis. 2017;76(8):1440–1448. doi: 10.1136/annrheumdis-2016-210706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor B.P., et al. Semaphorin 6D regulates the late phase of CD4+ T cell primary immune responses. Proc. Natl. Acad. Sci. U. S. A. 2008;105(35):13015–13020. doi: 10.1073/pnas.0803386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., et al. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14(6):1189–1199. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- Okuno T., et al. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2010;184(3):1499–1506. doi: 10.4049/jimmunol.0903302. [DOI] [PubMed] [Google Scholar]
- Pan G.Q., et al. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World J. Gastroenterol. 2009;15(22):2800–2804. doi: 10.3748/wjg.15.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp R.J. Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 2012;13(9):605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Reilkoff R.A., et al. Semaphorin 7a+ regulatory T cells are associated with progressive idiopathic pulmonary fibrosis and are implicated in transforming growth factor-β1-induced pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013;187(2):180–188. doi: 10.1164/rccm.201206-1109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L., et al. The many faces of semaphorins: from development to pathology. Cell. Mol. Life Sci. 2009;66(4):649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J.M., et al. Semaphorin 7A aggravates pulmonary inflammation during lung injury. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D., Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2(11):845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Sadanandam A., et al. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc. Res. 2010;79(1):1–9. doi: 10.1016/j.mvr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., et al. Semaphorin-5A maintains epithelial phenotype of malignant pancreatic cancer cells. BMC Cancer. 2018;18(1):1283. doi: 10.1186/s12885-018-5204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks K., et al. Neuroimmune semaphorin 4D is necessary for optimal lung allergic inflammation. Mol. Immunol. 2013;56(4):480–487. doi: 10.1016/j.molimm.2013.05.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I., et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metabol. 2013;18(4):491–504. doi: 10.1016/j.cmet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Smith E.S., et al. SEMA4D compromises blood-brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol. Dis. 2015;73:254–268. doi: 10.1016/j.nbd.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Song Y., et al. The involvement of semaphorin 7A in tumorigenic and immunoinflammatory regulation. J. Cell. Physiol. 2021;236(9):6235–6248. doi: 10.1002/jcp.30340. [DOI] [PubMed] [Google Scholar]
- Spriggs M.K. Shared resources between the neural and immune systems: semaphorins join the ranks. Curr. Opin. Immunol. 1999;11(4):387–391. doi: 10.1016/S0952-7915(99)80065-X. [DOI] [PubMed] [Google Scholar]
- Sultana H., et al. Semaphorin 7A contributes to West Nile virus pathogenesis through TGF-β1/Smad6 signaling. J. Immunol. 2012;189(6):3150–3158. doi: 10.4049/jimmunol.1201140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446(7136):680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kumanogoh A., Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 2008;9(1):17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- Takagi S., et al. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev. Biol. 1987;122(1):90–100. doi: 10.1016/0012-1606(87)90335-6. [DOI] [PubMed] [Google Scholar]
- Takamatsu H., Kumanogoh A. Diverse roles for semaphorin− plexin signaling in the immune system. Trends Immunol. 2012;33(3):127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Takamatsu H., et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat. Immunol. 2010;11(7):594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H., et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat. Immunol. 2010;11(7):594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegahara N., et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 2006;8(6):615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- Tatari N., et al. Semaphorin 3E inhibits house dust mite-induced angiogenesis in a mouse model of allergic asthma. Am. J. Pathol. 2019;189(4):762–772. doi: 10.1016/j.ajpath.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Thomas R., et al. Semaphorin 3E protects against chlamydial infection by modulating dendritic cell functions. J. Immunol. 2021;206(6):1251–1265. doi: 10.4049/jimmunol.2001013. [DOI] [PubMed] [Google Scholar]
- Thomas R., et al. Exogenous Semaphorin 3E treatment protects against chlamydial lung infection in mice. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.882412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18(4):435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., et al. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26(5):1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y., et al. Sema3e/Plexin D1 Modulates Immunological Synapse and Migration of Thymocytes by Rap1 Inhibition. J. Immunol. 2016;196(7):3019–3031. doi: 10.4049/jimmunol.1502121. [DOI] [PubMed] [Google Scholar]
- Vadasz Z., et al. A regulatory role for CD72 expression on B cells in systemic lupus erythematosus. Semin. Arthritis Rheum. 2014;43(6):767–771. doi: 10.1016/j.semarthrit.2013.11.010. [DOI] [PubMed] [Google Scholar]
- van Rijn A., et al. Semaphorin 7A promotes chemokine-driven dendritic cell migration. J. Immunol. 2016;196(1):459–468. doi: 10.4049/jimmunol.1403096. [DOI] [PubMed] [Google Scholar]
- Wang L., et al. Expression of Semaphorin 4A and its potential role in rheumatoid arthritis. Arthritis Res. Ther. 2015;17:227. doi: 10.1186/s13075-015-0734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., et al. Enterococcus faecalis promotes osteoclastogenesis and semaphorin 4D expression. Innate Immun. 2015;21(7):726–735. doi: 10.1177/1753425915593162. [DOI] [PubMed] [Google Scholar]
- Wanschel A., et al. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler. Thromb. Vasc. Biol. 2013;33(5):886–893. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., et al. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J. Exp. Med. 2010;207(13):2943–2957. doi: 10.1084/jem.20101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T., Offermanns S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014;13(8):603–621. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang H. Semaphorin 7A as a potential immune regulator and promising therapeutic target in rheumatoid arthritis. Arthritis Res. Ther. 2017;19(1):10. doi: 10.1186/s13075-016-1217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., et al. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J. Biol. Chem. 1998;273(35):22428–22434. doi: 10.1074/jbc.273.35.22428. [DOI] [PubMed] [Google Scholar]
- Xue D., et al. Semaphorin 4C: a novel component of B-cell polarization in Th2-driven immune responses. Front. Immunol. 2016;7:558. doi: 10.3389/fimmu.2016.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue D., et al. Semaphorin 4C protects against allergic inflammation: requirement of regulatory CD138+ plasma cells. J. Immunol. 2017;198(1):71–81. doi: 10.4049/jimmunol.1600831. [DOI] [PubMed] [Google Scholar]
- Yamashita N., et al. Anti-Semaphorin 3A neutralization monoclonal antibody prevents sepsis development in lipopolysaccharide-treated mice. Int. Immunol. 2015;27(9):459–466. doi: 10.1093/intimm/dxv014. [DOI] [PubMed] [Google Scholar]
- Zhu Z., et al. Sema4D is required in both the adaptive and innate immune responses of contact hypersensitivity. Mol. Immunol. 2016;78:98–104. doi: 10.1016/j.molimm.2016.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.