Abstract
Synchronously Amplified Photoacoustic Image Recovery (SAPhIRe) offers improved background suppression using non-linear properties of modulatable contrast agents. Using SAPhIRe, multiple contrast agents in the same absorption window can be detected independently based on their unique triplet-state lifetimes. Here, we have demonstrated the unmixing of rose bengal and eosin Y signals from solution based on triplet-state lifetime mapping using both fluorescence and photoacoustics. Varying the pump-probe delay enables resolution and recovery of fast-decaying rose bengal and of slowly decaying eosin Y modulated photoacoustic signals, resulting from optically gated triplet state residence. Distinct images were reconstructed within tissue-mimicking phantom using the fitting coefficients of triplet-state lifetimes. Fluorescence was used to screen for modulation prior to photoacoustic imaging. The results suggest that lifetime unmixing can be utilized to simultaneously detect multiple pathologies with overlapping spectra using photoacoustic imaging.
Keywords: Triplet-state, Lifetime, Modulation, OADF, Imaging, Fluorescence, Photoacoustics
Graphical Abstract
1. Introduction
Photoacoustics (PA) can be used for noninvasive imaging by detecting optically generated acoustic signals from deep tissues. As acoustic waves are scattered and attenuated 100–1000-fold less compared to optical waves, photoacoustic signals are readily recoverable from deep within tissue [1], [2], [3], [4], making PA a much more practical in vivo imaging modality than all-optical methods such as fluorescence (FL) [5], [6]. As with all medical imaging modalities, PA sensitivity can also be limited as endogenous background in tissue is very high [1], even in the optical window of 700 nm–1000 nm [7], [8], [9], [10], [11], [12]. Ultrasound (US) imaging provides excellent quality anatomical information, however standard US imaging does not provide functional or molecular contrast. PA imaging combines optical excitation and acoustic detection to achieve both molecular contrast and extended penetration depth [13], [14], [15], [16].
Photoacoustic signals are generated by rapid optical excitation and decay of chromophores resulting in locally induced pressure wave generation. The acoustic signal detected using an ultrasound transducer permits the signal source to be spatially-resolved [2], [3], [17]. Because endogenous light-absorbers such as hemoglobin (Hb) produce strong PA signals, transferring fluorescence modulation-based background reduction methods to PA imaging can drastically improve sensitivity by rejecting endogenous PA background signals.
Synchronously Amplified Fluorescence Image Recovery (SAFIRe) modifies ground and intermediate state populations using pump and probe excitations to suppress background in FL imaging and selectively recover signals of interest [18], [19]. By optically modulating chromophore state populations, such dual-laser background suppression techniques hold promise for improving other optical contrast methods which can benefit from multimodal detection in high background levels. Optical modulation works through a pump laser populating an excited state and a probe laser selectively exciting molecules in this pre-prepared level. Thus, unique signals are only observed when both lasers illuminate the sample, either simultaneously, or (in the case of a long-lived triplet-state) after a many microsecond delay. This sequential nonlinearity also enables mapping of the triplet-state lifetime to provide an additional dimension on which one can distinguish chromophores. Whether FL or PA, optical contrast is generated by modulating populations of absorbing chromophore states. Long-lived triplet states facilitate this sequential two-photon (nonlinear) absorption and corresponding signal recovery as Hb, for example, rapidly decays and shows only signals that are separately linear with pump and probe excitation, thereby enabling its complete subtraction. As the photoacoustic analog of SAFIRe, Synchronously Amplified Photoacoustic Image Recovery (SAPhIRe) is able to eliminate all non-gated PA signals of endogenous chromophores and provide background-free PA images using 532 nm pump and 1064 nm probe excitation [20].
Recently, dye-doped silica nanoparticles [20] and polymer-embedded photo-switchable dyes [21] have enabled selective signal recovery from high background both in vivo and ex vivo. Using an oxygen-sensitive chromophore, recent gated PA studies even investigated oxygen distribution in biological tissues and tumors [22], [23], [24], [25]. Because fluorescence is easier to use as a screen for modulatable PA dyes, demonstrating the correspondence between the two modalities for optical modulation opens new directions in ultrasensitive PA imaging. It is this correspondence and connection between fluorescence modulation and PA modulation that is demonstrated here. Mapping of the triplet-state lifetimes for simultaneously distinguishing multiple emitters provides a direct connection to advances made in fluorescence modulation while offering multiple “color” probes in PA imaging based on triplet-state lifetime discrimination. Based on the different triplet state lifetimes of the contrast agents, SAPhIRe/SAFIRe algorithms can simultaneously distinguish several contrast agents that exhibit strongly overlapped absorption spectra. This makes it possible to independently quantify and localize multiple exogenous contrast agents present in the imaging tissue region simultaneously via PA lifetime imaging using each dye’s unique triplet-state lifetime. This extra dimension in SAPhIRe imaging will allow for the simultaneous differentiation of several targeted pathologies with real-time biological marker monitoring.
2. Materials and method
2.1. Materials
Rose bengal (RB) sodium salt, 3-aminopropyltriethoxysilane (APTES), Tetraethylorthosilicate (TEOS), Sodium hydroxide, Cetyl trimethyl ammonium bromide (CTAB), Cyclohexane, Polyvinyl alcohol (PVA) and IGEPAL CO-520 were purchased from Millipore Sigma. Eosin-5-isothiocyanate (EY) was bought from Biotium. Ammonium hydroxide (28%) was bought from Fisher Scientific. Anhydrous Ethanol was purchased from Decon Labs,Inc. All chemicals were used without further purification. Milli-Q water (18.2 MΩ) was used throughout the experiment.
2.1.1. Nanoparticle (NP) synthesis
Rose bengal nanoparticles (RBNPs) were synthesized according to methods in the literature [20]. Briefly, 200 mg of CTAB was dissolved in 95 mL of DI water. 0.8 mL RB (5 mg/ mL) and 0.7 mL of 2 M sodium hydroxide were added to the solution. The reaction was heated to 80 °C and allowed to stabilize for about 1 h, then 1 mL of TEOS was added and stirred continuously. After 2 h, another 1 mL of TEOS was added dropwise to limit formation of additional silica beads and was kept stirring for one more hour. Particles were centrifuged and washed three times with water to remove unreacted dye. Finally, particles were dispersed in water and stored at 4 °C.
Eosin Y silica nanoparticles (EYNPs) were synthesized using a reverse microemulsion procedure [26]. First, 0.01 mmol (0.0075 g) EY was dissolved in 2.5 mL anhydrous ethanol, followed by addition of 0.02 mmol APTES. The resulting solution was stirred overnight in the dark. Then a 0.1 mL aliquot of this solution of was added to the mixture of 0.64 mL IGEPAL CO-520 and 9.2 mL cyclohexane and stirred for 30 min. Finally, 0.11 mL of TEOS and 0.164 mL of ammonium hydroxide were added to the previous solution and stirred overnight in the dark. All the reactions were done at room temperature.
2.1.2. Sample preparation of modulatable contrast agent mixtures
For FL experiments, immobilized samples of RB and EY were made using 15 wt% PVA in water. Similarly absorbing stock solutions of RB and EY were prepared in water and ethanol, respectively. The necessary concentrations were then achieved by mixing various ratios of RB and EY stock solutions to PVA under continuous stirring. Finally, a conventional glass coverslip was coated with a 40 μL drop of PVA-dye solution and dried in an oven at 60 °C for 30 min.
Samples prepared for photoacoustic imaging had 2% silica added to the PVA / dye mixtures to provide ultrasound contrast. A clear PVA layer was subsequently applied on top of the dye/PVA layer once it dried, and a final overcoat of acrylic nail polish was used to prevent the samples from dissolving while submerged in water during imaging. NP mixtures were prepared in the same way as dye mixtures for both fluorescence and PA experiments.
2.1.3. Photostability
Photostability was measured by irradiating aqueous nanoparticle solutions for several different pulse counts (532 nm, 2 mJ/cm2 fluence, as used during PA imaging). Absorption spectra were taken before and after lasing using a Biotek Synergy HT plate-reader.
2.1.4. Tissue-mimicking phantom
A tissue-mimicking phantom with several inclusions was created in synthetic rubber (Hummic Medical #5 gel). The molten rubber was mixed with 2% silica powder to provide ultrasound scattering. Three small inclusions were created for RB, EY, and India ink (non-modulatable control). RB, EY, and India ink were mixed with 15 wt% PVA solution, and one droplet of each of the three solutions were placed on a solidified rubber phantom. The phantom with droplets was dried at room temperature. After several hours, molten rubber gel was poured on top and let cool, producing a cube-shaped phantom with three inclusions, each with one particular dye.
3. Methods
3.1. Hardware/Instrumentation
3.1.1. Fluorescence study
Fluorescence experiments were conducted utilizing a 60X, 1.2 NA water immersion objective on an inverted optical microscope (Olympus IX71). FL signals were captured by a 100-μm multimode fiber (Thorlabs) directing light to a photon-counting avalanche photodiode (APD, PerkinElmer) linked to a PCI-6602 Counter (National Instruments) in a confocal setup. A 543 nm HeNe laser was used for primary excitation (Melles Griot), and a fiber-coupled CW 830 nm diode laser (Thorlabs) was used for secondary illumination. An acousto-optic modulator (NEOS-AOM) was employed for amplitude modulation of the 543 nm HeNe laser. Prior to entering the microscope, primary and secondary laser beams were combined on a dichroic mirror, and spatial beam overlap at the sample plane was verified using a charge-coupled device. Only the fluorescence signals were sent to the detector, with primary and secondary excitation light being filtered out by optical band-pass filters.
3.1.2. Photoacoustic study
Ultrasound and photoacoustic imaging were performed using a custom imaging system. Two Nd:YAG Q-switched lasers (Spectra-Physics Quanta-Ray) were used for the pump and probe optical excitations. The fundamental 1064 nm output from one unit generated the probe pulse, while the second unit’s output was frequency doubled to 532 nm using a second harmonic generator for the pump pulse. A programmable ultrasound imaging system (Vantage-256, Verasonics) was used in receive-only mode to acquire photoacoustic transients in addition to providing co-registered US imaging of the samples. The geometry used for imaging consisted of two coincident, co-located beams (pump and probe) passing through a diffuser and impinging the sample roughly normal to the imaging plane. A diffuser spatially homogenized the beam.
Timing coordination of the two lasers and ultrasound acquisition hardware was accomplished using a PXIe-based controller equipped with two counter/timer boards (PXIe-1095, PXIe-8880, and 2 PXIe-6612 boards, National Instruments). Photoacoustic experiments described in this paper consist of a b-mode US acquisition followed by a series of PA acquisitions at various pump-probe delays. For a given PA acquisition, trigger times for pump and probe pulses can be set arbitrarily, each with 5 ns precision. For lifetime measurements or contrast agent unmixing experiments, the sequence of PA acquisitions consists of monotonically increasing delays between the time the pump and probe lasers fire.
Similar to US image reconstruction, PA imaging requires beamforming. The background-free signal of interest is temporally synchronized with the probe laser excitation. Thus, prior to any data processing (e.g. SAPhIRe signal computation, contrast agent unmixing, etc.), the RF acquisitions are beamformed with respect to the probe laser firing time. Given that depth information for US and PA imaging is inherently based on acoustic wave propagation speed, and therefore time, acquisitions with low (<40 microseconds) pump-probe delays will inherently suffer from pump-induced photoacoustic transients overlapping those generated by the probe excitation. Normally, these pump-induced ‘ghost’ signals would need to be subtracted. Mitigation via signal subtraction of these unwanted pump-induced signals is directly influenced by jitter between the pump and probe firing times. Given the strong 532 nm absorptions of RB and EY, as well as most other endogenous materials, eliminating pump-induced signals is paramount. The results herein avoid this potential hazard by only employing pump-probe delays sufficiently long enough that potential temporal overlap is avoided given the axial size of the phantom (sample). Reverberations were eliminated using two methods: 1) using a large water tank (tank dimensions > 10-times phantom (sample) size, and 2) using a ‘perfect matching layer’ at the tank boundaries. The ‘perfect matching layer’ eliminated reverberations by continuously increasing both the attenuation and scattering coefficients through the depth of the layer.
4. Results and discussion
4.1. Temporal unmixing of RB and EY from different ratio solutions
RB and EY are chromophores exhibiting strong green-yellow absorption, high triplet yields, and modest fluorescence quantum yields. While potentially disadvantageous from a fluorescence brightness perspective, the high triplet yields of both RB and EY can actually improve sensitivity in high background biological imaging by drastically suppressing background in optically modulated fluorescence detection schemes. Optical excitation to produce the long-lived triplet enables one to selectively excite this pre-prepared triplet state at a long wavelength, many microseconds after the initial excitation pulse initially excites the molecule. Thus, delayed, long-wavelength probe excitation can regenerate the emissive S1 state and generate delayed fluorescence on zero background. The decay of this optically gated, delayed fluorescence signal reports on the triplet lifetime. Although RB and EY have similar spectral characteristics, their triplet lifetimes differ by an order of magnitude, enabling separation based on their delayed fluorescence decays [27]. This temporal separation is demonstrated here, and the same optical contrast is used to distinguish RB and EY with a new pump-probe PA imaging system.
Different ratio solutions of RB and EY were excited from the S0 ground state to the S1 excited state by a pulsed primary laser (543 nm), resulting in both prompt (∼1 ns-lived) fluorescence and significant population of the triplet state, T1. A lower energy 830 nm CW secondary laser was used to excite T1 to Tn and partially repopulate the S1 state to produce optically activated delayed fluorescence (OADF). OADF is gated by the primary laser that indirectly populates the T1 level via S1, but OADF decays over many microseconds as the secondary laser induces reverse intersystem crossing to repopulate S1 by exciting the T1-Tn transition. The corresponding OADF decay results from the decay of the shelved T1 population and is linearly dependent on the secondary laser intensity. The triplet-state decay rate, , is
in which is the natural triplet-state decay rate, is the secondary laser intensity, is the secondary laser photon energy and is the action cross-section for reverse intersystem crossing to S1. The natural triplet-state lifetime (is obtained by plotting the estimated decay rate with correspond secondary laser intensity and extrapolating to zero secondary intensity. The secondary laser-induced fluorescence decays biexponentially for the various dye mixtures and monoexponentially for RB and EY separately with RB exhibiting a shorter triplet lifetime than EY (Fig. 1). The triplet-state lifetimes of RB and EY are 99 ± 3 μs and 334 ± 7 μs, respectively. Thus, probes with different triplet-state lifetime are readily separable.
Fig. 1.
OADF-measured triplet decays from a 1:1 mixture of RB: EY in PVA. A) the OADF decay at different secondary laser intensity after initial primary pulse (20 μs width, 400 Hz repetition rate). Higher secondary laser intensity resulted in faster OADF decay (inset). B) Secondary laser-induced fluorescence decay has passed through the sample is plotted vs secondary excitation intensity, with the y-intercept indicating the inverse of the natural triplet-state lifetime.
Both OADF and modulation in general rely on controlling ground and triplet-state populations in time. As a result, these same schemes are directly applicable to photoacoustic imaging. The PA imaging scheme used to discriminate RB and EY within various mixture ratios uses a 532 nm ( ~10 ns pulsed) pump laser to excite the S0 – S1 transition, indirectly populating the T1 state. Then, a 1064 nm ( ~10 ns pulse) probe laser excites molecules in the T1 state, resulting in rapid decay primarily from Tn back to T1. Thus, neither RISC nor OADF is required to generate SAPhIRe signals. Because T1 state populations are created by the pump laser, SAPhIRe signal only arises from successive excitation by both pump and probe lasers – the probe-only excitation does not produce any SAPhIRe signal alone. Since the T1 state naturally decays between pump and probe pulses, SAPhIRe signals are more prominent at short pump – probe intervals. By varying the pump-probe delay, different SAPhIRe signal amplitudes are observed. Triplet-state lifetimes were determined by mapping SAPhIRe signal amplitudes vs pump-probe delay (Fig. 2). Biexponential SAPhIRe decays were only observed when both RB and EY are present, enabling their separation based on their different triplet-state lifetimes (280 ± 50 μs and 2.40 ± 0.40 ms, respectively). Since the nonlinear features of chromophores produce the SAPhIRe signal, and the RB triplet state has a shorter lifetime than does the EY triplet state, their SAPhIRe signal amplitudes at various pump-probe decays alone enables independent quantification of each dye within mixtures, without the need to perform spectroscopic PA imaging.
Fig. 2.
SAPhIRe signals from 100% RB, 100% EY and 50:50 ratios of RB:EY in PVA at different pump-probe delays. 100 pump-probe delays were recorded for each sample.
PA conversation efficiency is essential for separating each chromophore from various mixtures and predict the relative concentration. PA signals arise from the product of chromophore concentration and PA conversion efficiency. Thus, the PA signals from the two dyes in a given mixture can be described as
is the total photoacoustic signal amplitude, and offset is contributed by background PA and system noise. and are the independent contributions of each of the two dyes to the total photoacoustic signal. The PA signal amplitude contributed from each dye can be broken down further into conversion efficiencies, , and dye concentrations, and , of RB and EY present in the sample. Given the collection of acquisitions at various pump-probe delays, relative dye concentrations were obtained by fitting the conversion efficiencies to weighted exponential decay curves and fitting the collection of ‘offset’ values to a static constant. Least squares regression was used to calculate the conversion efficiency. Setting the higher efficiency dye initially to 1.0, the relative conversion efficiencies of RB and EY were calculated as 0.99 ± 0.06 and 0.78 ± 0.07 respectively. Fig. 3 shows that the PA conversion efficiency is independent of relative concentration in the mixed PA signal.
Fig. 3.
The portion of the PA signal that each dye contributed to the total signal plotted against the relative concentration in each sample. A line was fit to determine the conversion efficiency (R2 = 0.99 for EY and R2 = 0.98 for RB).
4.2. Temporal unmixing of RBNPs and EYNPs from different ratio mixtures
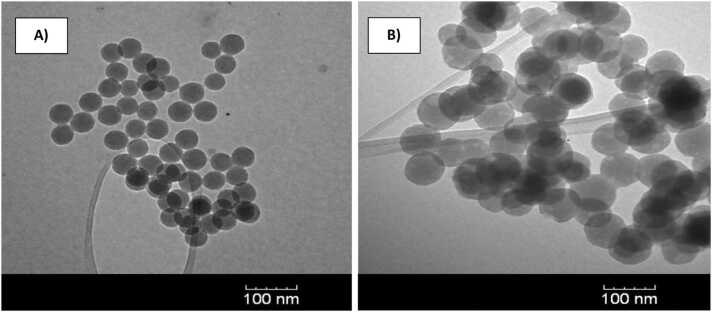
PA signals benefit from high dye concentrations to produce large acoustic waves at lower laser fluences. Resolution, however, is improved by having PA signals resulting from highly localized regions. Thus, to increase local dye concentration while maintaining small sizes, we synthesized silica nanoparticles embedded with either RB or EY at 5 mM and 3 mM local concentrations, respectively, confirmed by absorption spectroscopy and measured particle densities. Locally high intra-nanoparticle dye concentrations allow for high signals, but overall reasonable equivalent free dye concentrations (moles of dye/L solution), of 39 μM for RB and 16 μM for EY. SAPhIRe signals from mixtures of these nanoparticles were then studied and unmixed. The per-particle RB concentration was ∼5 mM and the EY concentration was ∼3 mM according to UV–vis studies of RB and EY absorption and estimated nanoparticle particle densities. Nanoparticles of both RB in silica and EY in silica appeared largely homogeneous and spherical, with particle sizes of 95 ± 10 nm and 43 ± 5 nm, respectively (Fig. 4). (TEM size distributions, absorption and emission spectra: Fig. S1, S2 and S3. Zeta potential Table S1). Photostability under 532 nm excitation shows retention of greater than ∼90% of nanoparticle signal even after 1000 pulses (Fig. S1).
Fig. 4.
TEM images of A) Eosin-Y doped silica nanoparticles (43 ± 5 nm) and B) Rose bengal doped silica nanoparticles (95 ± 10 nm).
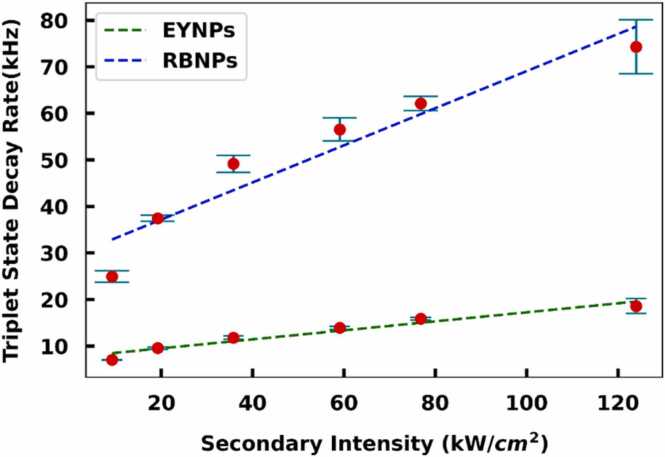
OADF was measured from mixtures of dye-doped nanoparticles based on the triplet-state lifetimes. The different RBNPs and EYNPs triplet-state lifetimes (34 ± 1 μs for RBNPs and 132 ± 2 μs for EYNPs) yield readily resolved decays within a nanoparticle mixture (Fig. 5). Triplet-state lifetimes depend on nanoenvironmental effects such as oxygen access and mobility within the nanoparticle that can alter triplet lifetimes. Although dyes are immobilized within nanoparticles, trapped oxygen and larger voids likely enable greater oxygen access in the mesoporous silica than in annealed PVA films.
Fig. 5.
Triplet-state decays of 1:1 ratio of RBNPs and EYNPs in PVA. Secondary laser-induced fluorescence decay after the initial primary pulse (10µs width, 500 Hz repetition rate) has passed through the sample is plotted vs secondary excitation intensity, with the y-intercept indicating the inverse of the natural triplet-state lifetime.
To demonstrate SAPhIRe signal unmixing for NPs, identical PA experiments were conducted. Since both NPs have shorter triplet-state lifetimes than their respective dyes in PVA, photoacoustic signals were collected for various pump-probe delays up to 2 ms. SAPhIRe signal amplitudes were mapped against pump-probe delay and used to determine the triplet-state lifetime. Both RBNPs (60 ± 8 μs) and EYNPs (500 ± 45 μs) were identified and discriminated from the mixtures based on their triplet-state lifetime from SAPhIRe signals (Fig. 6). Again, while the triplet-state lifetimes of both RBNPs and EYNPs using SAPhIRe are shorter than in their corresponding annealed PVA environments, RB triplet decays produce the fast component while EY produces the longer decaying component in any given sample, enabling separation of SAPhIRe signals.
Fig. 6.
SAPhIRe signals from 100% RBNPs, 100% EYNPs and 50:50 ratios of RBNPs:EYNPs in PVA at different pump-probe delays. The NP-doped PVA sample preparation protocol was identical to those of dye samples for PA experiments. 50 pump-probe delays were recorded for each sample.
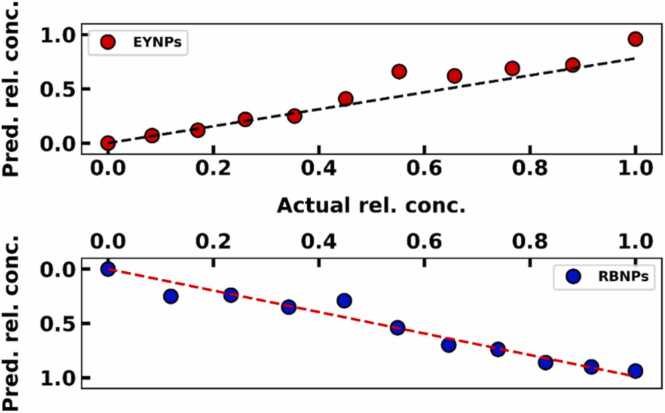
PA conversion efficiency of RB and EY was used to predict the relative concentration of RBNPs and EYNPs from different ratio mixtures. Fig. 7 represents the relationship between precited relative concentrations and actual relative concentrations. Relative amplitudes and decays from fits were combined with the relative PA efficiencies of RB and EY to determine relative nanoparticle and dye concentrations in each mixture. Fig. 7 shows that SAPhIRe signals can readily distinguish RBNPs and EYNPs signals, and accurately report their relative concentrations.
Fig. 7.
Relationship between predicted and actual relative concentration using PA conversion efficiencies of RB and EY (R2 = 0.89 for EYNPs and R2 = 0.95 for RBNPs).
4.2.1. SAPhIRe image reconstruction based on triplet-state lifetime mapping
Using the same collection as for Fig. 6, US and PA signals were captured from tissue-mimicking phantoms. Fig. 8(A) shows the US image of phantoms. The leftmost phantom is RB, the one in the middle is EY, and the one on the right is India ink (Ink) dye. Ink was used as a control because it can be excited with the same pump laser but is not modulatable. SAPhIRe signal intensities at each pixel vs time trace were individually normalized and fitted against pump-probe delay time with fixed RB and EY triplet-state lifetimes. Fitting coefficients of both RB and EY were used to construct background-free images. Imaging results for the phantom experiment are shown in Fig. 8(B). Reconstructed images were overlaid on the US image. It is evident from the figure that multiple contrast agents can be separated based on their unique triplet-state lifetimes. Since, Ink is not modulatable, no SAPhIRe signal decay was recoverable.
Figure 8.
(A) US signals from tissue-mimicking phantom with inclusions of RB, EY and nonmodulatable control India Ink. (B) Reconstructed images using the triplet-state lifetime fitting amplitudes indicating RB and EY only, overlaid on US images. The color bars (linear, from 0 to 1) indicate the amplitude of the fast (RB) or slow (EY) decay component as the optimal normalized decay vs. time for each pixel was fit. India Ink signals produce no pump-probe intensity decay, rendering it invisible in the reconstructed SAPhIRe image.
5. Conclusion
As optical modulation alters ground and triplet state populations in a sequential two-photon excitation scheme, fluorescence modulation is an excellent and facile screen for modulatable PA contrast agents. Using the different triplet state lifetimes of two spectrally similar dyes, rose bengal and eosin Y, amounts of each dye can be quantitatively recovered from mixtures. This two-“color” resolution scheme has been applied to normally monochromatic photoacoustic detection to resolve and detect the amount of each dye within mixtures in phantoms. Coupled with the background removal of SAPhIRe, the delayed two-photon PA excitation holds promise for multi-“color” imaging and resolution of multiple targeted features in PA imaging schemes. Using fluorescence as a screen opens the opportunity to more readily identify and use other modulatable PA contrast agents in more standard longer wavelength excitation regions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge financial support from National Institutes of Health R01EB028916.
Biographies

Md Shariful Islam is a PhD student in chemistry and biochemistry at the Georgia Institute of Technology working under the supervision of Dr. Robert Dickson. He received his undergraduate and Master’s degrees in chemistry in 2010 and 2012 from University of Dhaka, Bangladesh. Currently, his research focused on synthesis and characterization of modulable dye nanoparticles for non-invasive cancer/tumor detection using photoacoustic imaging.

Don VanderLaan is currently a research engineer in the department of Electrical & Computer Engineering at Georgia Institute of Technology, Atlanta GA. His interests include cardiovascular disease, ultrasound and photoacoustic imaging, medical devices, imaging system design, and experiment automation. Don earned his B.S. in Electrical Engineering at Michigan State University in 2009.

Josie Hickman is a PhD student in chemistry and biochemistry at the Georgia Institute of Technology working under the supervision of Dr. Robert Dickson. She received her undergraduate and Master's degrees in chemistry in 2018 and 2020 from East Carolina University. Currently, her research is focused on the characterization of modulatable dye to be used in photoacoustic imaging.

Dr. Stanislav Emelianov is a Joseph M. Pettit Endowed Chair, Georgia Research Alliance Eminent Scholar, and Professor of Electrical & Computer Engineering and Biomedical Engineering at the Georgia Institute of Technology and Emory University School of Medicine. Dr. Emelianov is also the Director of the Ultrasound Imaging and Therapeutics Research Laboratory. Projects in Dr. Emelianov's laboratory are focused on the discovery, development, and clinical translation of diagnostic imaging and therapeutic instrumentation, augmented with theranostic nanoagents.

Dr. Robert Dickson is the Vasser Woolley Professor of Chemistry at Georgia Tech. He earned undergraduate and graduate degrees from Haverford College and The University of Chicago, respectively. After postdoctoral work at UCSD, he joined Georgia Tech’s faculty in 1998, where he has pioneered the development and use of optically modulatable contrast agents to selectively recover weak signals buried within high background. Current efforts in his lab involve the development of chromophores and tailored application of modulation-based signal recovery to spy on biologically relevant processes in their native environments and statistical and optical methods for rapid antibiotic susceptibility testing from infected blood.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pacs.2023.100529.
Contributor Information
Stanislav Emelianov, Email: stas@gatech.edu.
Robert M. Dickson, Email: dickson@chemistry.gatech.edu.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on request.
References
- 1.Wang L.V., Wu H.-i. John Wiley & Sons; 2012. Biomedical Optics: Principles and Imaging. [Google Scholar]
- 2.Zhang H.F., et al. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006;24(7):848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 3.Xu M., Wang L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006;77(4) [Google Scholar]
- 4.Lao Y., et al. Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth. Phys. Med. Biol. 2008;53(15):4203. doi: 10.1088/0031-9155/53/15/013. [DOI] [PubMed] [Google Scholar]
- 5.Ntziachristos V. Fluorescence molecular imaging. Annu. Rev. Biomed. Eng. 2006;8(1):1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 6.Ale A., et al. FMT-XCT: in vivo animal studies with hybrid fluorescence molecular tomography–X-ray computed tomography. Nat. Methods. 2012;9(6):615–620. doi: 10.1038/nmeth.2014. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S., et al. Modulated fluorophore signal recovery buried within tissue mimicking phantoms. J. Phys. Chem. A. 2013;117(39):9501–9509. doi: 10.1021/jp312071n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgass K., et al. Application of FLIM-FIDSAM for the in vivo analysis of hormone competence of different cell types. Anal. Bioanal. Chem. 2010;398(5):1919–1925. doi: 10.1007/s00216-010-4127-4. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y., et al. Fluorescence lifetime imaging microscopy: in vivo application to diagnosis of oral carcinoma. Opt. Lett. 2009;34(13):2081–2083. doi: 10.1364/ol.34.002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marriott G., et al. Optical lock-in detection imaging microscopy for contrast-enhanced imaging in living cells. Proc. Natl. Acad. Sci. 2008;105(46):17789–17794. doi: 10.1073/pnas.0808882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng D., et al. Precise diagnosis in different scenarios using photoacoustic and fluorescence imaging with dual-modality nanoparticles. Nanoscale. 2016;8(30):14480–14488. doi: 10.1039/c6nr03809c. [DOI] [PubMed] [Google Scholar]
- 12.Anderson R.R., Parrish J.A. The optics of human skin. J. Invest. Dermatol. 1981;77(1):13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 13.Luke G.P., Yeager D., Emelianov S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012;40(2) doi: 10.1007/s10439-011-0449-4. 422-37. [DOI] [PubMed] [Google Scholar]
- 14.Maslov K., et al. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008;33(9):929–931. doi: 10.1364/ol.33.000929. [DOI] [PubMed] [Google Scholar]
- 15.Wang L.V. CRC press; 2017. Photoacoustic Imaging and Spectroscopy. [Google Scholar]
- 16.Wang L.V., Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosencwaig A., Gersho A. Theory of the photoacoustic effect with solids. J. Appl. Phys. 1976;47(1):64–69. [Google Scholar]
- 18.Richards C.I., Hsiang J.-C., Dickson R.M. Synchronously amplified fluorescence image recovery (SAFIRe) J. Phys. Chem. B. 2010;114(1):660–665. doi: 10.1021/jp909167j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan C., et al. All-optical fluorescence image recovery using modulated stimulated emission depletion. Chem. Sci. 2011;2(6):1080–1085. doi: 10.1039/C0SC00637H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demissie A.A., et al. Synchronously amplified photoacoustic image recovery (SAPhIRe) Photoacoustics. 2020;20 doi: 10.1016/j.pacs.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C., et al. Reversibly photoswitching upconversion nanoparticles for super‐sensitive photoacoustic molecular imaging. Angew. Chem. 2022;134(19) doi: 10.1002/anie.202116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashkenazi S., et al. Photoacoustic probing of fluorophore excited state lifetime with application to oxygen sensing. J. Biomed. Opt. 2008;13(3) doi: 10.1117/1.2927466. [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi S. Photoacoustic lifetime imaging of dissolved oxygen using methylene blue. J. Biomed. Opt. 2010;15(4) doi: 10.1117/1.3465548. [DOI] [PubMed] [Google Scholar]
- 24.Märk J., et al. Photons Plus Ultrasound: Imaging and Sensing 2014. International Society for Optics and Photonics; 2014. Photoacoustic imaging of a near-infrared fluorescent marker based on dual wavelength pump-probe excitation. [Google Scholar]
- 25.Jo J., et al. In vivo photoacoustic lifetime based oxygen imaging with tumor targeted G2 polyacrylamide nanosonophores. ACS nano. 2019;13(12):14024–14032. doi: 10.1021/acsnano.9b06326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabanov V., et al. Assessment of encapsulated dyes’ distribution in silica nanoparticles and their ability to release useful singlet oxygen. Chem. Commun. 2018;54(49):6320–6323. doi: 10.1039/c8cc03413c. [DOI] [PubMed] [Google Scholar]
- 27.Demissie A.A., Dickson R.M. Triplet shelving in fluorescein and its derivatives provides delayed, background-free fluorescence detection. J. Phys. Chem. A. 2020;124(7):1437–1443. doi: 10.1021/acs.jpca.9b11040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.