Abstract
Background
Vagal nerve stimulation (VNS) has been reported to reduce body weight and improve sympathovagal imbalance in both basic and clinical studies. Its effects on glycemic control were however unclear. The aims of this study were to investigate the effects of VNS with various parameters on blood glucose and its possible mechanisms in rats.
Methods
A hyperglycemic rodent model induced by glucagon was used initially to optimize the VNS parameters; then, a type 2 diabetic rodent model induced by high-fat diet combined with streptozotocin was used to validate the VNS method. The VNS electrodes were implanted at the dorsal subdiaphragmatic vagus; three subcutaneous electrodes were implanted at the chest area for recording electrocardiogram in rats induced by glucagon.
Results
(1) VNS with short pulse width of 0.3 ms but not 3 ms reduced blood glucose during an oral glucose tolerance test (OGTT), with a 38.4% reduction at 15 min and 26.9% at 30 min (P < 0.05, vs. sham-VNS respectively). (2) VNS at low frequency of 5 Hz but not 14 Hz or 40 Hz reduced blood glucose during the OGTT (P < 0.05, vs. sham-VNS). (3) Intermittent VNS was more potent than continuous VNS (P < 0.01). (4) No difference was found between unilateral VNS and bilateral VNS. (5) VNS enhanced vagal activity (P = 0.005). (6) The hypoglycemic effect of VNS was blocked by glucagon-like peptide-1 (GLP-1) antagonist exendin-4.
Conclusions
VNS at 5 Hz reduces blood glucose in diabetic rats by enhancing vagal efferent activity and the release of GLP-1.
Keywords: Vagal nerve stimulation, Diabetes, Blood glucose, Autonomic functions, Glucagon-like peptide-1
Introduction
Diabetes is a complex, chronic illness requiring continuous medical care with multifactorial risk-reduction strategies beyond glycemic control [1]. The prevalence diabetes continues to rise. It is estimated that as many as one in three US adults will have diabetes by 2050 if the current trend continues [2, 3]. Diabetes imposes a substantial burden on the economy of the USA with an annual cost of over $327 billion [2]. The majority (90%) of diabetes is type 2 diabetes mellitus (T2DM) [4]. T2DM is one of the leading epidemics in human history closely associated with obesity [5].
Pharmacologic therapy is the first-line standard of care approach for T2DM, including mono-therapy and combination therapy. The use of metformin as first-line therapy is the preferred initial pharmacologic agent for the treatment of T2DM; metformin has beneficial effects on A1C, weight, and cardiovascular mortality [1, 6]. However, metformin has side effects on gastrointestinal tract and it is contraindicated with renal insufficiency [6]; other medications such as sulfonylureas, thiazolidinedione, and GLP-1 agonist cause weight gain during the hypoglycemic therapy, which is an undesirable outcome with potential long-term adverse consequences on glycemic control itself [7]. Metabolic surgery has been considered for treating T2DM as well [8, 9]. Some studies reported that it achieved near or complete normalization of glycemia 2 years following surgery in 72% of patients, compared with 16% in a matched control group treated with lifestyle and pharmacological interventions [9, 10]. However, the surgical treatment is only recommended for patients with body mass index (BMI) ≥ 35 kg/m2. In addition, it is costly and associated with risks. Long-term concerns include dumping syndrome, vitamin and mineral deficiencies, anemia, osteoporosis, depression, and other major psychiatric disorders [1], which make it an unacceptable option for a large portion of diabetic patients. In words, there is still a need to develop novel therapies.
Vagal nerve stimulation (VNS) has been approved by the Food and Drug Administration (FDA) for treating epilepsy and depression [11–15]. It has also been shown to have an antiinflammatory effect due to activation of vagal activity [16]. VNS was reported to reduce food intake and body weight in both animals and humans [17–19] and lower blood glucose level in a few studies in animal models [20–22]. However, the parameters used for VNS differed substantially in these previous studies and no efforts have been made to optimize stimulation parameters. There is a need to explore the VNS method for glycemic control with possible parameter optimization.
We therefore aimed to optimize VNS parameters to reduce blood glucose; the parameter optimization was mainly targeting on the stimulation frequency, pulse width, locations and stimulation patterns; mechanisms related to vagal activity, insulin sensitivity, and glucagon-like peptide-1 (GLP-1) were investigated as well in rodent models of temporary hyperglycemia and T2DM.
Materials and Methods
Subjects
Twenty Sprague-Dawley (SD) rats (male, 200–250 g Charles River Laboratory, MD) were housed in the micro-isolator cage equipped with filter hoods under controlled temperature (20 °C), a 12-h/12-h light/dark cycle and free access to water and solid food. The experimental protocol was approved by the Animal Care and Use Committee of the Johns Hopkins University.
Animal Models
Acute Hyperglycemia
Acute hyperglycemia was induced in 10 rats by intraperitoneal injection of glucagon (0.5 mg/kg, Sigma-Aldrich, St. Louis, MO) during the oral glucose tolerance test (OGTT). It was reported that glucagon at this dose substantially increased blood glucose level during the 2-h OGTT in normal rats [23, 24].
Type 2 Diabetic Model
A type 2 diabetic model was established using a previous method [25]. The “ad libitum” diet was started at 7 weeks of age in 10 SD rats. The animals had free access to high-fat diet chow (fat 60% kcal, protein 20% kcal, carbohydrate 20% kcal, energy density 5.21 kcal/g, Research Diets Inc., D12492, New Brunswick, NJ) for a period of 4 weeks and were then injected intraperitoneally with a low dose of streptozotocin (STZ, 35 mg/kg). Rats with blood glucose ≥ 140 mg/dl 10 days after the STZ injection were selected for the experiments. This model was considered as T2DM [26, 27].
Surgical Procedure
In 10 rats fed with the regular chow, electrodes for vagal nerve stimulation were implanted under general anesthesia. A longitudinal skin incision was made in the abdominal midline, both dorsal and ventral vagal trunks were localized in the subdiaphragmatic part of the esophagus; one pair of electrodes (Streamline 6494, Medtronic Inc., Minneapolis, MN) were circumferentially placed at the dorsal subdiaphragmatic vagus with an interval of 0.3 cm. In 10 of the rats, additional three electrodes were implanted subcutaneously for recording ECG, one at the heart apex, the other at the second intercostal space of right sternal border, and the third at about 0.5 cm below the xiphoid process. All electrodes were tunneled underneath the skin and externalized at the back of the neck.
In 10 rats fed with high-fat diet, the same method was used to implant VNS electrodes except that one additional electrode was implanted at the ventral subdiaphragmatic vagus.
Experimental Protocols
Experiment 1: Effects of VNS with Various Parameters on Blood Glucose in Normal Rats Treated with Glucagon
Eight of the rats were studied in six sessions randomly including one control session without stimulation and five sessions with VNS of different parameters. The oral glucose tolerance test (OGTT) was performed in a restrainer after a 5–6 h-fasting by oral feeding of 20% glucose (1 g/kg body weight). Glucagon (0.5 mg/kg) was intraperitoneal injected right after feeding glucose. About 5-μl blood sample was collected from the tail vein at following time points: 0 (baseline), 15, 30, 60, 90 and 120 min after the injection for the assessment of blood glucose level using a glucometer (Contour, Ascensia Diabetes Care, NJ, USA).
VNS Method and Parameters
VNS was performed during the entire OGTT period. Prior to the experiment, each rat was brought to the lab and stayed in the restrainer 2–3 h daily for at least 7 days. This reduced the potential stress effects attributed to the placement in the restrainer. During the test, each rat was put in the restrainer with electrode wires connected to a Universal Pulse Generator (Model DS8000, World Precision Instruments, Sarasota, FL, USA). Different VNS parameters were used: wide and short pulses (3 ms vs. 0.3 ms) and different frequencies (5, 14, 40, and 5000 Hz). Following parameter sets were tested: (1) frequency of 40 Hz, pulse width of 3 ms, pulse amplitude of 3 mA; (2) 40 Hz, 0.3 ms, 3 mA; (3) 14 Hz, 0.3 ms, 3 mA; (4) 5 Hz, 0.3 ms, 2 mA; and (5) 5000 Hz, 0.1 ms, 2 mA, biphasic. In the sham VNS session, the output was set as 0 mA.
Measurement of Autonomic Functions
Autonomic functions were assessed by the spectral analysis of heart rate variability (HRV) derived from the ECG in eight rats as follows: The ECG was recorded in the fasting state for 30 min on two separated days: one for sham-VNS and the other for VNS. Glucagon (0.5 mg/kg) was intraperitoneal injected right before the ECG recording. An HRV signal was derived from the original ECG recording by identifying R waves. Spectral analysis was performed on the HRV signal to compute the high-frequency component (HF; 0.8–4.0 Hz) reflecting cardiac vagal efferent activity and the low-frequency component (LF; 0.3–0.8 Hz) reflecting mainly sympathetic efferent activity [28].
Experiment 2: Effects and Mechanisms of VNS on Blood Glucose in Diabetic Rats
Ten type 2 diabetic rats were used in the following experimental sessions:
Effects of VNS with bilateral and unilateral VNS on blood glucose
Each rat was studied in three randomized sessions: sham-VNS, bilateral VNS (ventral-dorsal trunks), and unilateral VNS (dorsal trunk). The OGTT was performed as described in Exp. 1 except that no glucagon was injected and the testing duration was 3 h because prolonged hyperglycemia was observed in diabetic rats during the OGTT. VNS was performed using best parameters obtained from Exp.1 except that the pulse width was adjusted to 0.5 ms.
Effects of VNS with different stimulation patterns on blood glucose
Each rat was studied in three randomized sessions: sham-VNS, continuous VNS, and intermittent VNS. The stimulation parameters were set at pulse frequency of 5 Hz, pulse width of 0.5 ms, and pulse amplitude of 2 mA. The intermittent VNS was performed with a repeated process of 10 s on and 90 s off.
Experiment 3: VNS on Insulin Sensitivity in Diabetic Rats
Seven diabetic rats were used in this experiment. Each rat was studied in two randomized sessions: sham VNS and VNS with parameters optimized from Exp. 1 and Exp. 2. An insulin tolerance test (ITT) was performed after the rats were fasted for 5 h. Regular insulin (0.5 U/kg) in a saline solution was injected into the intraperitoneal cavity. About 5-μl Blood sample was then collected from the tail vein at the following time points: 0, 15, 30, 60, 90 and 120 min for the assessment of glucose level. VNS/sham VNS was performed during the entire 2-h ITT period.
Experiment 4: Role of GLP-1 on Hypoglycemic Effect of VNS
Seven diabetic rats were used in this study. Each rat was studied in four randomized sessions: sham VNS, VNS, exendin 9-39 (a GLP-1 antagonist, 100 μg/kg, i.p. Sigma) and VNS+ exendin-9-39. The OGTT was performed for 3 h as described previously; exendin was injected 30 min prior to the glucose gavage.
Statistical Analysis
Data are presented as means ± SE. A repeated measure ANOVA was used for multiple comparisons in each experiment; a non-parametric Mann-Whitney U test for between-groups comparisons with Bonferroni correction was used for any comparisons among three or more sessions. In the insulin tolerance test, the percentage of glucose change compared to the baseline at each time point was used to represent the insulin sensitivity. Significance was defined as P < 0.05.
Results
Effects of VNS Pulse Width in Rats with Glucagon-Induced Acute Hyperglycemia
In rats with glucagon-induced acute hyperglycemia, VNS at pulse widths of 0.3 ms and 3 ms were studied. There was a significant difference among the sham VNS, VNS-0.3 ms and VNS-3 ms at both 60 min and 90 min (ANOVA, P = 0.017 and P = 0.021 respectively); VNS 0.3 ms significantly reduced the blood glucose level from 15 min to 30 min compared to the sham VNS, with a 38.4% reduction at 15 min (P = 0.043 vs. sham) and 26.9% at 30 min (P = 0.041 vs. sham, Fig. 1). The area under the curve (AUC) was marginally reduced with VNS at 0.3 ms (385.6 ± 14.6mg h/dl vs. 312.2 ± 34.7 mg h/dl, P = 0.06).
Fig. 1.
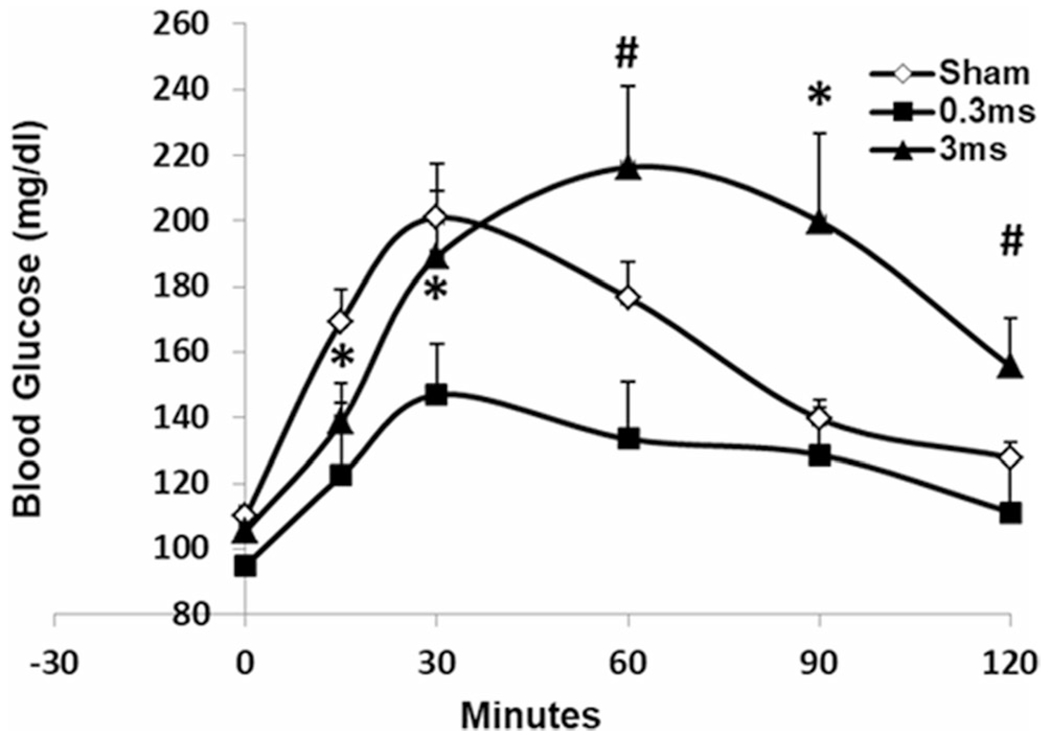
VNS at different pulse widths on blood glucose in rats induced by glucagon. VNS at 0.3 ms significantly reduced blood glucose at both 15 min and 30 min during OGTT (*P < 0.05 vs. sham); VNS at 3 ms increased blood glucose at 90 min (*P = 0.026 vs. sham); a significant difference was noted between VNS at 0.3 ms and 3 ms at both 60 min and 120 min (#P < 0.02)
Electrical stimulation with the pulse width of 3 ms at the gut was shown to decrease glucose level in rodent model of obesity [24]. However, an opposite effect was noted in the current study when the electrical stimulation was applied to the vagus nerve: after an initial glucose reduction at 15 min, VNS at 3 ms increased glucose level at 90 min compared to the sham-VNS (139.8 ± 5.5 vs. 199.9 ± 26.7, P = 0.026; Fig. 1); a significant difference was noted between VNS-3 ms and VNS-0.3 ms at both 60 min and 120 min (P = 0.005, P = 0.01 respectively).
Effects of VNS Frequency in Rats with Glucagon-Induced Acute Hyperglycemia
Different hypoglycemic effects of VNS with various stimulation frequencies were observed during the OGTT. As shown in Fig. 2a, VNS-5 Hz significantly decreased blood glucose level from 15 min to 120 min compared to the sham VNS; it decreased blood glucose level from 169.4 ± 3.2 to 137.6 ± 12.6 mg/dl at 15 min (P = 0.03), from 201 ± 16.3 to 147.4 ± 16.3 mg/dl at 30 min (P = 0.021), from 176.6 ± 10.9 to 132.1 ± 9.6 mg/dl at 60 min (P = 0.029), from 139.8 ± 5.5 to 114.1 ± 5.6 mg/dl at 90 min (P = 0.036), and from 127.9 ± 4.4 to 103.1 ± 5.9 mg/dl at 120 min (P = 0.047). In addition, the mean AUC was decreased from 385.6 ± 14.6 to 301.9 ± 17.2 mg h/dl with VNS during the 2-h OGTT (P = 0.02, Fig. 2b).
Fig. 2.
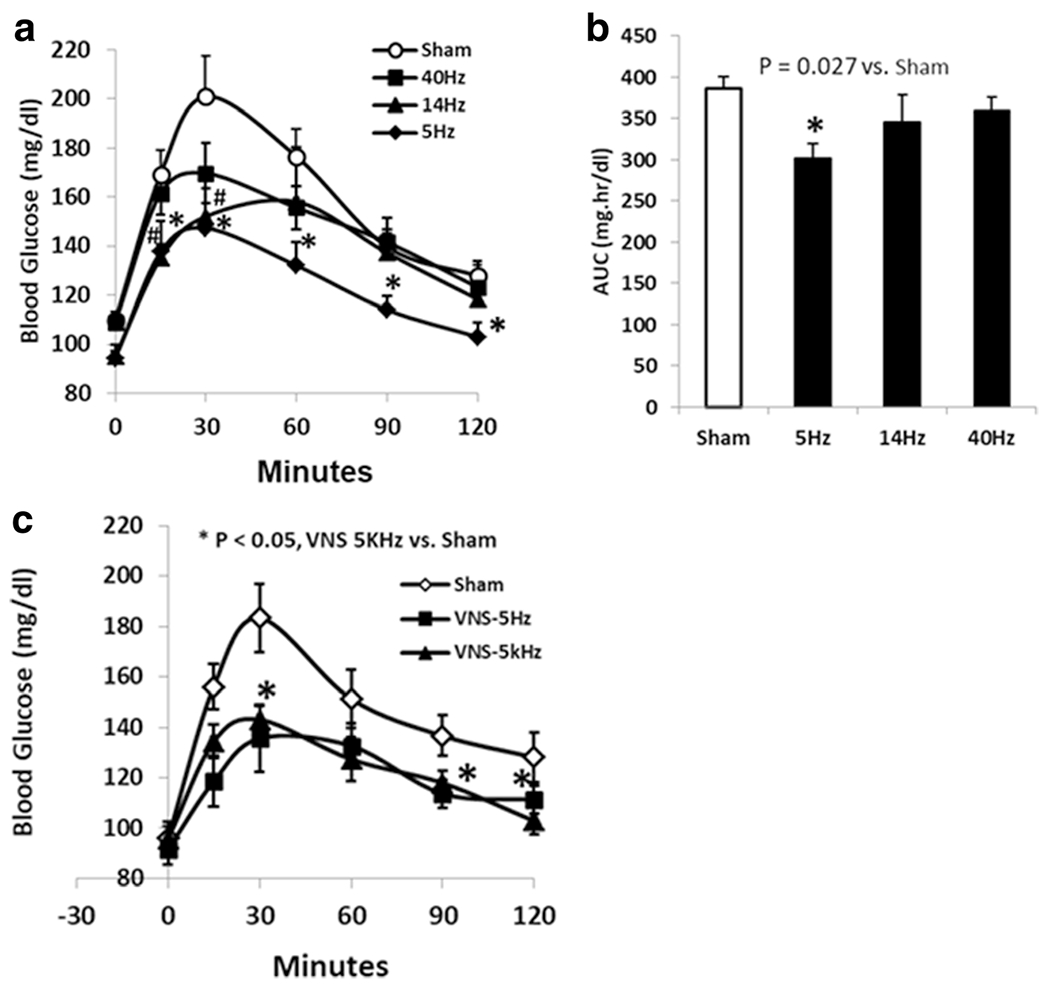
VNS at various frequencies on blood glucose in rats induced by glucagon: a VNS at 5 Hz substantially decreased the blood glucose level from 15 to 120 min (*P < 0.04 respectively vs. sham,). VNS at 14 Hz decreased the blood glucose only at 15 min and 30 min (*P < 0.04 respectively); VNS at 40 Hz had no effects on decreasing blood glucose during OGTT. b The area under the curve with VNS at 5 Hz was significantly decreased compared to the sham VNS (P = 0.027). c VNS at 5 kHz significantly decreased the blood glucose level at 30 min, 90 min, and 120 min (P < 0.05 vs. sham)
VNS-14 Hz decreased the blood glucose level only at 15 min and 30 min during the OGTT (Fig. 2a). The reduction was 23.7% at 15 min (P = 0.026) and 21.2% at 30 min (P = 0.039). There was no difference in the mean AUC between sham and VNS-14 Hz.
No significant difference was noted with VNS at 40 Hz on decreasing blood glucose level during OGTT (P > 0.2 at any time point, Fig. 2a) compared to the sham VNS, neither was on the AUC.
VNS at 5 kHz was tested in 10 rats to compare with VNS-5 Hz on glycemic control. There was a significant difference among the sham, VNS-5 Hz and VNS-5 kHz at 15 min, 30 min, and 60 min (ANOVA, P = 0.019, P = 0.015, P = 0.041 respectively). VNS at 5 kHz significantly decreased blood glucose level at 30, 90, and 120 min compared to the sham VNS; the reduction of glucose level was 25.7% at 30 min, 16.8% at 30 min, and 13.3% at 120 min (P = 0.02, P = 0.049, P = 0.02, respectively Fig. 2c). Although VNS-5 Hz seemed to be more potent on decreasing blood glucose level than VNS-5 kHz, no statistical difference was noted between the two methods (P > 0.1).
Bilateral vs. Unilateral VNS on Blood Glucose in Diabetic Rats
Both bilateral and unilateral VNS reduced blood glucose during the OGTT. The mean AUC was reduced from 936.3 ± 98.9 to 857.9 ± 90.9 mg h/dl with bilateral VNS, with the maximal reduction occurred at 90 min (13.7%, P = 0.01 vs. sham). Similar to bilateral VNS, unilateral VNS significantly reduced both AUC and blood glucose from 30 to 90 min (P < 0.02 for respectively); it decreased blood glucose by 21.2% at 90 min (P = 0.006). No significant difference was noted between bilateral or unilateral on decreasing blood glucose in diabetic rats.
Intermittent vs. Continuous VNS on Blood Glucose in Diabetic Rats
In the diabetic rats, continuous VNS decreased the blood glucose level from 30 to 90 min compared to the sham stimulation; the blood glucose was decreased from 294.3 ± 15.9 to 246.1 ± 25.8 mg/dl at 30 min (P = 0.03), from 336.3 ± 24.1 to 249.6 ± 20.0 mg/dl at 60 min (P = 0.02), and from 284.3 ± 25.2 to 223.6 ± 19.7 mg/dl at 90 min (P = 0.03). Intermittent VNS at 5 Hz with 10 s on and 90 s off significantly decreased glucose level during OGTT from 15 to 90 min compared to the sham VNS. The reduction of the blood glucose level was 34.2% at 15 min (P = 0.006), 39.6% at 30 min (P < 0.001), 38% at 60 min (P < 0.001), and 29.2% at 90 min (P < 0.001) (Fig. 3a). Intermittent VNS was shown to have a more potent hypoglycemic effect compared to the continuous VNS (P = 0.01, at 15 min); the AUC during 3-h OGTT significantly reduced from 734.1 ± 59.7 to 532.1 ± 32.4 mg h/dl (P < 0.01 vs. sham, Fig. 3b).
Fig. 3.
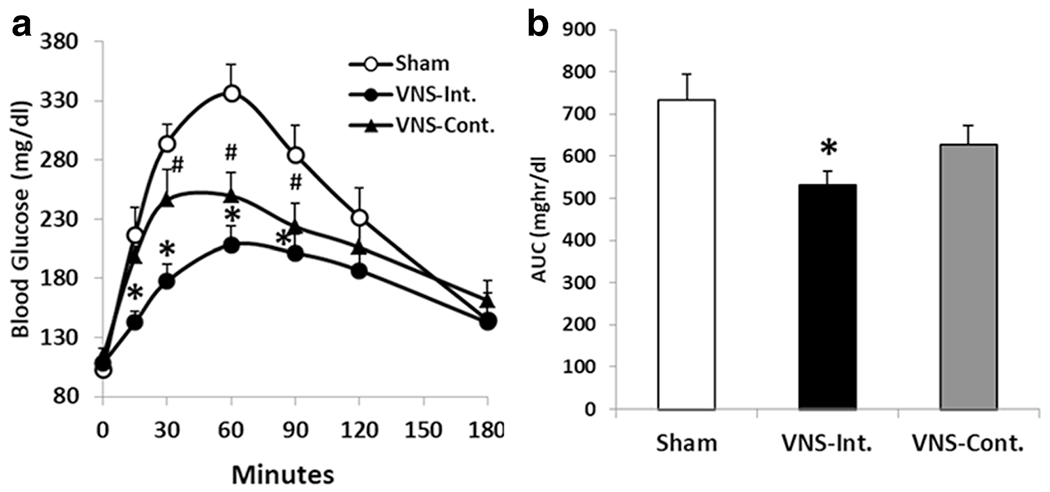
VNS at different stimulation patterns on blood glucose in diabetic rats. a Intermittent VNS at 5 Hz significantly reduced blood glucose from 15 to 90 min (*P < 0.04 respectively vs. sham). Continuous VNS significantly decreased the blood glucose from 30 to 90 min (#P < 0.04 respectively). b The area under the curve during OGTT was significantly reduced with intermittent stimulation (*P < 0.01 vs sham). VNS-Int., intermittent VNS; VNS-Cont., continuous VNS.
VNS Enhanced Vagal Activity in Rats Treated with Glucagon
VNS-5 Hz enhanced vagal activity in the fasting state in rats treated with glucagon. HF, representing vagal activity, was elevated from 0.61 ± 0.03 to 0.74 ± 0.02 with VNS-5 Hz (P = 0.005); LF was decreased from 0.39 ± 0.03 to 0.26 ± 0.02 with VNS (P = 0.005 vs. sham); accordingly, the LF/HF was decreased from 0.69 ± 0.07 in the sham to 0.33 ± 0.04 in VNS (P = 0.003), suggesting the enhancement of vagal tone (Fig. 4).
Fig. 4.
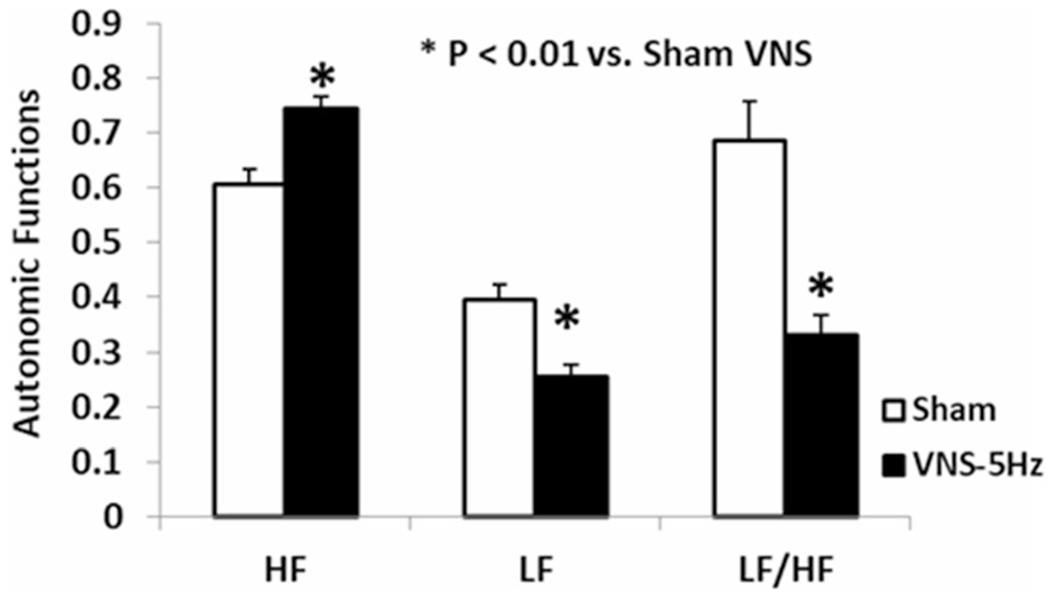
VNS at 5 Hz on autonomic functions derived from heart rate variability in rats treated with glucagon. VNS-5 Hz significantly enhanced vagal activity (HF), decreased sympathetic activity (LF), therefore enhanced sympathovagal balance (LF/HF). (*P < 0.01 vs. sham)
VNS on Insulin Sensitivity in Diabetic Rats
Insulin sensitivity, represented by the percentage of glucose change during ITT, was significantly improved by intermittent VNS at 5 Hz. The blood glucose from baseline was increased by 10.66 ± 3.89% in sham VNS but decreased by 6.79 ± 2.52% with VNS at 15 min (P = 0.02), then was decreased by 23.92 ± 3.47% in sham VNS and 39.23 ± 4.08% with VNS at 60 min (P = 0.009), 37.06 ± 3.84% with sham-VNS and 52.83 ± 3.64% with VNS at 90 min (P = 0.005) and 54.35 ± 3.01% with sham-VNS and 61.19 ± 4.02% with VNS at 120 min (P = 0.006) (Fig. 5).
Fig. 5.
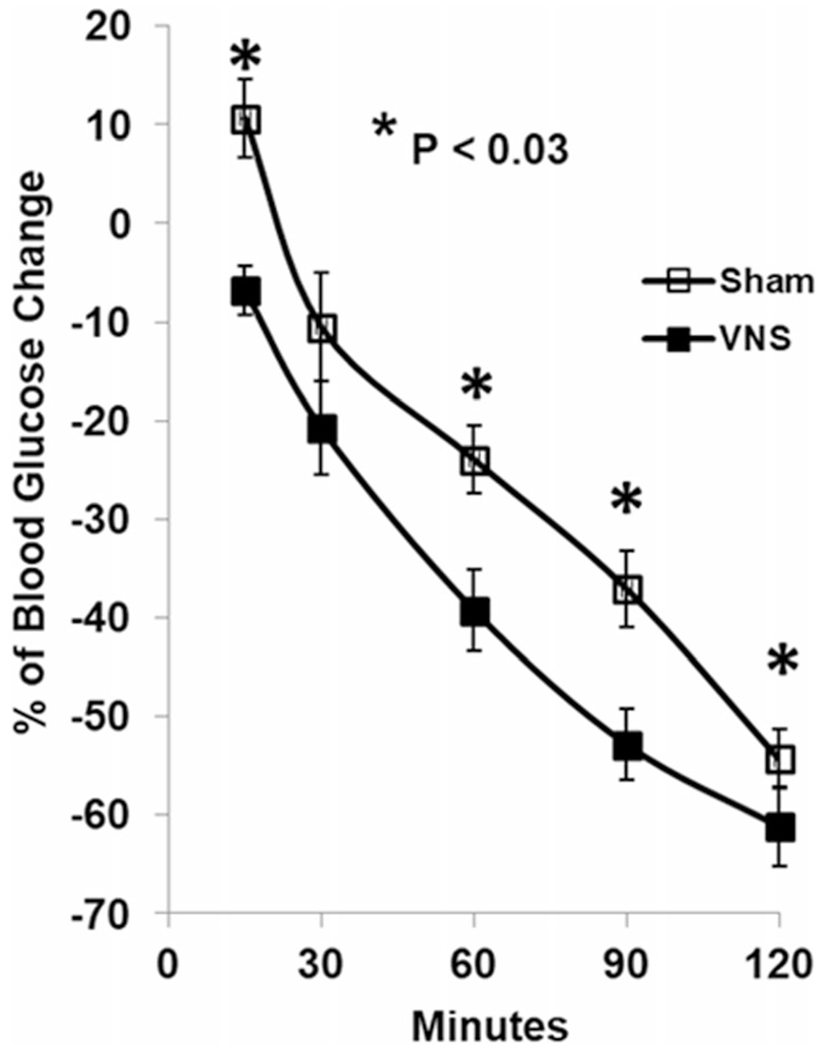
VNS at 5 Hz on insulin sensitivity in diabetic rats. VNS significantly increased insulin sensitivity during insulin tolerance test at 15, 60, 90, and 120 min (*P < 0.03 vs. sham)
Involvement of GLP-1 in Hypoglycemic Effect of VNS
GLP-1 receptor antagonist exendin 9-39 significantly blocked the hypoglycemic effect of VNS. VNS significantly decreased the blood glucose from 60 to 180 min with an about 10% reduction at each time point compared to the sham VNS. Exendin itself increased blood glucose, especially at 30 min (216 ± 16 mg/dl vs. 253 ± 14 mg/dl, P = 0.02 vs. sham) and 60 min (243 ± 13 mg/dl vs. 266 ± 14 mg/dl, P = 0.005 vs. sham). No difference was noted between the exendin and exendin + VNS sessions from 90 to 180 min (P > 0.05) during OGTT or the AUC, suggesting partially blockage of the hypoglycemic effect of VNS. No difference was noted either between exendin + VNS or the sham VNS during OGTT or the 3-h AUC (P > 0.1) (Fig. 6a,b).
Fig. 6.
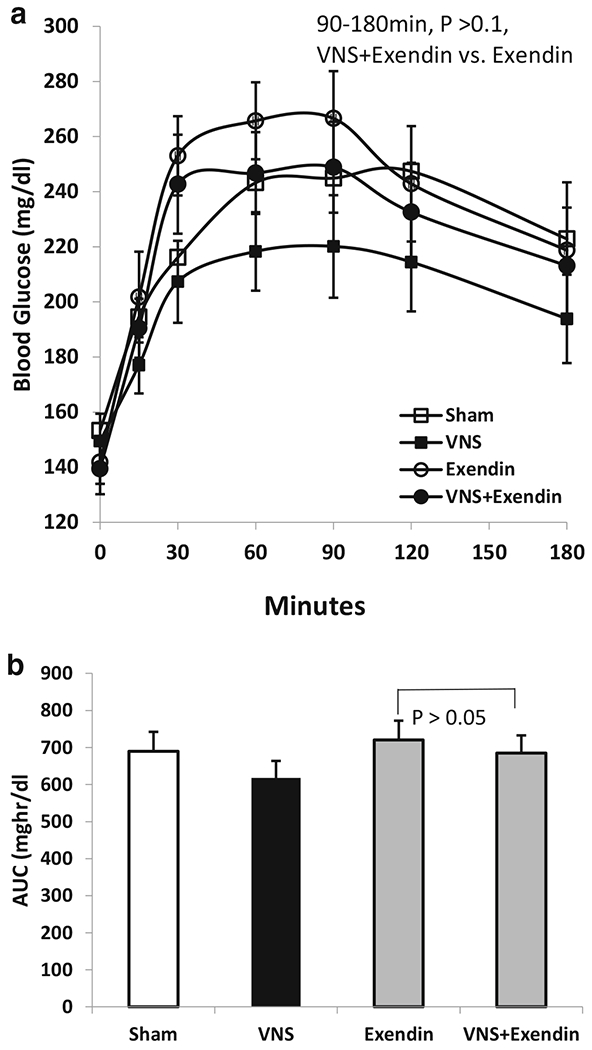
Involvement of GLP-1 in VNS in diabetic rats. a VNS at 5 Hz decreased the blood glucose from 60 to 180 min. However, GLP-1 antagonist exendin 9-39 blocked the hypoglycemic effect of VNS (P > 0.05, exendin vs. exendin + VNS). b No significant difference was noted of the area under the curve between exendin and exendin + VNS sessions, suggesting the involvement of GLP-1 on hypoglycemic effect of VNS
Discussion
In this study, we have found that low-frequency VNS (frequency < 40 Hz) reduced blood glucose in rats; VNS at 5 Hz resulted in a more potent hypoglycemic effect in both normal and diabetic rats. VNS with short pulse (0.3 ms) was better than wide pulse (3 ms) and the intermittent stimulation was more effective than the continuous stimulation. The hypoglycemic effect of VNS was possibly attributed to the improvement of vagal activity and the modulation of GLP-1.
Two animal models were used in this study: an acute hyperglycemia model with glucagon was used during VNS parameter optimization and a type 2 diabetic model was used for validating the effectiveness of VNS with optimized stimulation parameters and for studying mechanisms of VNS involving the autonomic functions and GLP-1. Glucagon has been used in the animal models to induce a state of hyperglycemia and hyperglucagonemia which are present in the diabetic setting [23, 29, 30]. In an early study by McCallum et al., glucagon was injected in the dogs underwent vagotomy to induce a canine model of diabetic gastroparesis; gastric emptying was delayed and the serum glucose level rose to a mean of 240 mg/dl and maintained above the normal range for at least 120 min [29]. In the current study, glucagon induced hyperglycemia to a peak of 201 mg/dl at 30 min during OGTT in rats (Fig. 1, sham). The diabetic model was developed based on previous studies in the literature in both rats and dogs [25, 31]. The combination of HFD and low-dose STZ that decreases but not completely destroys pancreatic μ-cells results in diabetes that resembles syndromes of type 2 diabetes. In the rats treated with HFD and low-dose STZ, the mean fasting blood glucose increased to > 300 mg/dl 5 days after STZ injection, then dropped to 168 mg/dl 10 days after the injection. The elevated fasting blood glucose was maintained for at least 3 months during the experimental period.
Recently, there is a rising interest on VNS for diabetes. However, different VNS methods have been reported in the literature, including different stimulation parameters, stimulation locations, and stimulation durations. Both high and low frequencies were used ranging from 0.05 Hz [12, 17] to 5000 Hz [32, 33]; stimulation sites included cervical vagus nerve, subdiaphragmatic vagus nerve (bilateral and unilateral), and auricular vagus nerve; daily stimulation duration varied from 10 min to 12 h [20–22, 33]. VNS at 0.01–10 Hz was reported to reduce excess weight gain in rats fed with high-fat diet [17, 34, 35]. VNS at 30 Hz decreased weight gain, food consumption and improved insulin sensitivity in diet-induced obese pigs [22, 36]. A combination of 2- and 15-Hz auricular VNS was reported to prevent the progression of hyperglycemia in Zucker fatty rats [21]. All these frequencies were within the category of low frequency; the question was which direction should go to achieve a better hypoglycemic effect: to the lower or higher scale? In the current study, 5 Hz, 14 Hz, and 40 Hz were tested. Five-hertz VNS was commonly used for treating epilepsy, depression, and intestinal inflammation [11, 15, 16]. The 14 Hz was used in a gastric electrical stimulation (GES) method called “Enterra® therapy” for treating nausea and vomiting in gastroparetic patients [37, 38]; vagally mediated neural mechanisms are believed to be involved in the anti-emetic effect of Enterra® therapy [38, 39]. GES at Enterra parameters reduced vasopressin-induced emesis in dogs and increased vagal activity assessed by heart rate variability in rats and this effect was blocked either by vagotomy or by denervation of vagal afferent fibers via capsaicin [39, 40]. Different from 14 Hz targeting on the symptoms, GI stimulation at 40 Hz was more effective on the alteration of GI motility including gastric contractions and gastric emptying [41–43]. Neither of these low-frequency stimulations has been studied on vagus nerve for diabetes. Current results indicated that VNS at both 5 Hz and 14 Hz decreased blood glucose during the OGTT, whereas 5 Hz was the most potent among the three low frequencies.
Interestingly, stimulation at 5 kHz reduced the blood glucose similar to 5 Hz VNS. Contrast to low-frequency VNS, a kilohertz frequency current was capable of blocking vagus nerve signaling. In an initial preclinical study, both Aδ and C components of the vagus compound action potentials were attenuated with 5-kHz stimulation for 1 min [44]. In early 2015, VNS at a high frequency of 5 kHz was approved for treating obesity (VBLOC) [32]. The vagal blockade has also been applied for treating diabetes among obese patients with T2DM. Glycemic control was improved by the VBLOC therapy with a decrease of 0.6% on A1C after 2 years of treatment; however, no control data were available in the study [33]. If the finding of this study could be translated into humans, VNS could be performed at 5 Hz instead of 5 kHz, which would be very attractive in clinical applications.
While gastric or intestinal electrical stimulation at 3 ms was found more potent in altering gastrointestinal motility functions [24, 39, 42]. VNS with 3 ms was found to increase blood glucose, an effect opposite to VNS with 0.3 ms/0.5 ms. It is conceivable that nerve stimulation at 3 ms is effective; however, further mechanistic studies are needed to explore why VNS with 3 ms increased the blood glucose level.
The most commonly used location for VNS is the cervical (left) vagal nerve, which has been used for treating epilepsy, depression, and intestinal inflammation [11, 15, 16]. Bilateral vagus nerve stimulation was used for treating diabetes in a swine model [22]; it was also used in the vagal blockage for treating obesity in humans [32, 45]. The selection of stimulation location is seemingly based on the nerve innervation and the feasibility of surgical technique. Branches that leave the cervical vagal trunks predominantly supply organs associated with cardiorespiratory function, whereas the branches that leave the subdiaphragmatic vagal trunks innervate organs associated with metabolism [12]. Due to the possible cardiorespiratory side effects, cervical vagus nerve was not selected in the current study. Moreover, unilateral cervical vagal stimulation was reported to have a minimal effect on body weight and did not affect fasting glycemia [22, 46, 47]. In the current study, VNS at the dorsal trunk and VNS bilateral trunks were compared in diabetic rats; both of the locations reduced blood glucose and no significant difference was noted during the OGTT, suggesting whether unilateral or bilateral stimulation might not be crucial for subdiaphragmatic vagal nerve stimulation.
The mechanism underlying the low-frequency VNS for treating diabetes is unclear. The following actions might have been involved: firstly, VNS might improve or normalize sympathovagal imbalance in diabetes by suppressing sympathetic overactivity and/or elevating diminished vagal activity. In the current study, vagal activity was enhanced, leading to a decreased sympathovagal balance in rats with hyperglycemia. Secondly, VNS might increase insulin release or sensitivity in diabetes. Previous studies have shown that VNS increased fasting insulin release from the pancreas [20] and increased whole-body insulin sensitivity, reflected as substantial improvement in brain, hepatic and skeletal muscle glucose uptake [22]. The current data from the insulin tolerance test also suggested an increase in insulin sensitivity with VNS. Thirdly, the hypoglycemic effect of VNS might be related to the intestinal release of GLP-1. GLP-1 is a gut hormone that is secreted from the small intestine in response to enteric nutrients. Vagal afferent neurons express GLP-1 receptors [48–50] and terminate in the lamina propria of the intestinal mucosa as well as in the wall of the hepatic portal vein [48]. Vagal afferent neurons may therefore relay the gut GLP-1-derived signals to the brain and mediate satiating and glucoregulatory responses [48]. The GLP-1 receptor agonist is used as a new pharmacotherapy for type 2 diabetes. In the current study, the hypoglycemic effect of VNS was blocked by GLP-1 antagonist exendin 9-39, suggesting a major role of GLP-1. The limitation of the current study was that VNS was performed acutely and no chronic data were available at this time. Future chronic study with daily treatment of VNS is needed to investigate mechanisms involving GLP-1, insulin secretion, and β-cell functions.
In conclusion, VNS at low frequencies of 5- and 14-Hz reduce blood glucose in a rodent model of type 2 diabetes and intermittent stimulation is more potent than continuous stimulation. Compared to other VNS methods used for treating obesity or diabetes, the VNS method optimized in this study consumes much less energy and is therefore more feasible for future clinical applications. The hypoglycemic effect of 5 Hz VNS may be attributed to the improvement of sympathovagal imbalance, increased insulin sensitivity, and the release of GLP-1.
Funding
Jieyun Yin was partially supported by a grant from NIH-SPARC (Stimulating Peripheral Activity for Relieving Conditions) (1U18TR001926).
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
Statement of Animal Rights/Ethical Approval All applicable institutional and /or national guidelines for the care and use of animals were followed.
References
- 1.Introduction: standards of medical care in diabetes-2018. Diabetes Care. 2018. Jan;41(Suppl 1):S1–S2. Epub 2017/12/10. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28. 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metrics. 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–36. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Alberti KG. Surgery or medical therapy for obese patients with type 2 diabetes? N Engl J Med. 2012;366(17):1635–6. [DOI] [PubMed] [Google Scholar]
- 8.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. [DOI] [PubMed] [Google Scholar]
- 10.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016. Jan;39 Suppl 1:S4–5. Epub 2015/12/24. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–82. [DOI] [PubMed] [Google Scholar]
- 12.de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimonprez A, Raedt R, Baeken C, et al. The antidepressant mechanism of action of vagus nerve stimulation: evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34. [DOI] [PubMed] [Google Scholar]
- 14.Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276–86. [DOI] [PubMed] [Google Scholar]
- 15.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713–28. [DOI] [PubMed] [Google Scholar]
- 16.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9. [DOI] [PubMed] [Google Scholar]
- 17.Bugajski AJ, Gil K, Ziomber A, et al. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J Physiol Pharmacol. 2007;58(Suppl 1):5–12. [PubMed] [Google Scholar]
- 18.Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes. 2007;31(11):1756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobocki J, Fourtanier G, Estany J, et al. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006;139(2):209–16. [DOI] [PubMed] [Google Scholar]
- 20.Ahren B, Taborsky GJ Jr. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118(4):1551–7. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Zhai X, Rong P, et al. Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One. 2014;9(11):e112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malbert CH, Picq C, Divoux JL, et al. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes. 2017;66(4):848–57. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang X, Li S, Foreman R, et al. Hyperglycemia-induced small intestinal dysrhythmias attributed to sympathovagal imbalance in normal and diabetic rats. Neurogastroenterol Motil. 2015;27(3):406–15. [DOI] [PubMed] [Google Scholar]
- 24.Ye F, Liu Y, Li S, et al. Hypoglycemic effects of intestinal electrical stimulation by enhancing nutrient-stimulated secretion of GLP-1 in rats. Obes Surg. 2018;4 [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20. [DOI] [PubMed] [Google Scholar]
- 26.de Bem GF, Costa CA, Santos IB, et al. Antidiabetic effect of Euterpe oleracea Mart. (acai) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: a positive interaction. PLoS One. 2018;13(6):e0199207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu W, Lin L, Lin Z, et al. Duodenum exclusion alone is sufficient to improve glucose metabolism in STZ-induced diabetes rats. Obes Surg. 2018;22 [DOI] [PubMed] [Google Scholar]
- 28.Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298(4):G563–70. [DOI] [PubMed] [Google Scholar]
- 29.Bellahsene BE, Lind CD, Schirmer BD, et al. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Phys. 1992;262(5 Pt 1):G826–34. [DOI] [PubMed] [Google Scholar]
- 30.Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1190–5. [DOI] [PubMed] [Google Scholar]
- 31.Ionut V, Liu H, Mooradian V, et al. Novel canine models of obese prediabetes and mild type 2 diabetes. Am J Physiol Endocrinol Metab. 2010;298(1):E38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312(9):915–22. [DOI] [PubMed] [Google Scholar]
- 33.Shikora SA, Toouli J, Herrera MF, et al. Intermittent vagal nerve block for improvements in obesity, cardiovascular risk factors, and glycemic control in patients with type 2 diabetes mellitus: 2-year results of the VBLOC DM2 study. Obes Surg. 2016;26(5):1021–8. [DOI] [PubMed] [Google Scholar]
- 34.Gil K, Bugajski A, Kurnik M, et al. Physiological and morphological effects of long-term vagal stimulation in diet induced obesity in rats. J Physiol Pharmacol. 2009;60(Suppl 3):61–6. [PubMed] [Google Scholar]
- 35.Gil K, Bugajski A, Thor P. Electrical vagus nerve stimulation decreases food consumption and weight gain in rats fed a high-fat diet. J Physiol Pharmacol. 2011;62(6):637–46. [PubMed] [Google Scholar]
- 36.Val-Laillet D, Biraben A, Randuineau G, et al. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite. 2010;55(2):245–52. [DOI] [PubMed] [Google Scholar]
- 37.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25(10):815–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin J, Abell TD, McCallum RW, et al. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15(3):224–31. discussion 31 [DOI] [PubMed] [Google Scholar]
- 39.Chen JD, Qian L, Ouyang H, et al. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124(2):401–9. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Qiao X, Chen JD. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004;49(5):729–37. [DOI] [PubMed] [Google Scholar]
- 41.Chen JD, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol. 2017;11(5): 407–18. [DOI] [PubMed] [Google Scholar]
- 42.Song GQ, Zhu H, Lei Y, et al. Gastric electrical stimulation optimized to inhibit gastric motility reduces food intake in dogs. Obes Surg. 2015;25(6):1047–55. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H, Sallam H, Chen DD, et al. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Phys Regul Integr Comp Phys. 2007;293(5):R1875–81. [DOI] [PubMed] [Google Scholar]
- 44.Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8(5):056013. [DOI] [PubMed] [Google Scholar]
- 45.Morton JM, Shah SN, Wolfe BM, et al. Effect of vagal nerve blockade on moderate obesity with an obesity-related comorbid condition: the ReCharge study. Obes Surg. 2016;26(5):983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banni S, Carta G, Murru E, et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLoS One. 2012;7(9):e44813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George MS, Sackeim HA, Rush AJ, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47(4):287–95. [DOI] [PubMed] [Google Scholar]
- 48.Krieger JP, Arnold M, Pettersen KG, et al. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa A, Satake H, Nakabayashi H, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110(1):36–43. [DOI] [PubMed] [Google Scholar]
- 50.Ronveaux CC, de Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav. 2014;135:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


