Abstract
Allostasis provides a supporting role to the homeostatic control of biological variables in mammalian species. While the concept of homeostasis is related to the control of variables within a set point or range that are essential to life, allostasis refers to systems that facilitate adaptation to challenges that the organism faces and the new requirements for survival. Essential for such adaptation is the role played by the brain in eliciting neural and neuroendocrine responses. Reproductive function is fundamental for the survival of species but is costly in energetic terms and requires a synchrony with an ever-changing environment. Thus, in many species reproductive function is blocked or delayed over immediate challenges. This review will cover the physiological systems and neuroendocrine pathways that supply allostatic control over reproductive neuroendocrine systems. Light, hypoxia, temperature, nutrition, psychosocial, and immune mediators influence the neuroendocrine control of reproductive functions through pathways that are confluent at the paraventricular nucleus; however, understanding of the integrative responses to these stimuli has not been clarified. Likely, the ultimate consequence of these allostatic mechanisms is the modification of kisspeptin and gonadotropin-releasing hormone neuronal activity, thus compromising reproduction function in the short term, while preserving species survivability.
Keywords: allostasis, reproduction, stress, homeostasis gonadotropin-releasing hormone, GnRH, pulses
Animals and humans face challenges during their existence and, usually, these challenges evoke an adaptive response that leads to the reestablishment of a state of constancy. Claude Bernard in his 19th century lectures on the phenomena of life common to animals and plants (1) stated, “there are really two environments for the animal, an external environment, in which the animal is placed, and an internal environment in which the elements of tissues live. Life does not run its course within an external environment.” This view implied the capacity of self-regulation or constancy of the internal environment and as such Bernard is considered the founder of the idea of a “milieu interior.” It was, however, Walter B. Cannon who coined the term homeostasis (2). Homeostasis can be considered as a control system in which the alteration of a variable leads to corrective measures, involving sensors, a regulator, and effectors to return the variable to a preset value. Since most biological variables are under constant change and the systems that regulate integrative responses are controlled by the brain, the concept of allostasis was proposed by Sterling and Eyer (3). In their seminal monograph, the authors stated that in physiology it is critical that “to maintain stability an organism must vary all the parameters of its internal milieu and match them appropriately to environmental demands.” Allostasis maintains the idea that the regulation of body function requires the anticipation of future needs and adaptation of organ function to a favorable or opposing environment. Thus, allostasis refers to the control and stabilization of body systems through change, as a support to homeostasis (4).
The primary component of allostatic mechanisms is the function of the brain to coordinate sensory information with the physiological response enabled by neural, behavioral, and/or neuroendocrine responses. A secondary component of the allostatic response is the capability to anticipate future needs. Anticipation is manifested by the brain via feedforward mechanisms. A feedforward mechanism is when the sensing pathway evokes responses without alteration of a measured variable. For instance, exposure of the skin to cold temperature does not change the body core temperature (reviewed later in this manuscript); rather, it elicits heat-generating and heat-preserving mechanisms such as piloerection and blood vessel contraction (5). The ability of anticipating future events can also span longer time frames, as illustrated by mammalian species in which changes in photoperiod or metabolic conditions induce embryonic diapause, which is the cessation of embryonic development due to the anticipation of adverse birth season or adverse development (6). The component of anticipation within allostasis is contrasted with homeostasis, which relies on feedback mechanisms that are characterized by the activation of an effector pathway, due to deviation from the expected range of a variable, followed by the deactivation of the corrective measure. Another key distinction between allostasis and homeostasis is that the control mechanisms in allostasis work in a wider range of activity than those of homeostasis (7), thus providing a complementary framework for adaptation.
Several important points arise with the concept of allostasis, as proposed by McEwen and Wingfield (4), and can be illustrated by considering grazing species such as sheep facing restricting environments. In one (favorable) scenario, sheep maintained in a pasture with high energy availability leads to constancy, with minimal changes in behavior or internal variables. Now consider that the sheep are moved to an environment (pasture) where the availability of pasture is 30% of the previous scenario. Given that the internal nutrient requirements of the sheep remain constant, each sheep must adapt to this new scenario by changing its grazing behavior; given low grass availability, the grazing time increases as well as the rate of bites during grazing. Internally, entering a state of negative energy balance results in the secretion of glucocorticoids, eliciting fat mobilization and muscle wasting. In this example, the allostatic load is the cost of maintaining glucose levels in the adverse environment while the allostatic overload encompasses the deleterious effects of glucocorticoid secretion and muscle wasting. A few tenets of allostasis can be emphasized for the purpose of this review. First, the brain is critical in the decision-making process. Given its ability to integrate and process sensory information from peripheral and central sources, the brain is the organ responsible for modulating and executing neuroendocrine responses. Second, maintaining an allostatic overload has deleterious effects. This response was first introduced in 1936 by Hans Selye in his general adaptation theory (8) in which he described experimental observations in rodents as a 3-stage process by which prolonged exposure to a factor led to a series of clinical signs. Finally, the concept of stress will be used in this review as a refection of an allostatic load/overload; however, it is important to point out that the concept of stress has been considered ambiguous in the past given that it is referred to as both the origin and the sequelae of an allostatic load/overload (4).
As detailed in this review, allostasis encompasses a variety of key systems influencing reproduction. The reproductive neuroendocrine system controls hormonal and reproductive cycles through changes in the pattern of gonadotrophin secretion. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are secreted from pituitary gonadotrophs on direct action of gonadotrophin-releasing hormone (GnRH) from the hypothalamus. Gonadal steroids are critical in modulating gonadotrophin (LH and FSH) and GnRH secretion. Among them, estradiol exerts both negative and positive feedback on GnRH secretion: The negative feedback induces a strong inhibition of LH pulses, while the positive feedback triggers a prolonged surge-like elevation of circulating LH concentrations preceding ovulation. For decades, a major question in the field of reproductive neuroendocrinology was how gonadal steroids regulated GnRH function since GnRH neurons were devoid of estrogen receptor α (9-11), while expressing the nonrelevant estrogen receptor β (12). Thus, it was concluded that the regulation of GnRH secretion is relayed onto afferent neurons. Several lines of evidence support the idea that kisspeptin neurons in the arcuate nucleus and the anteroventral periventricular area are part of the estrogen responsive circuit that is the determinant in the generation of GnRH/LH pulses and surges, respectively. Kisspeptin neuron populations display the estrogen receptor α (13), establish synaptic contacts with GnRH neurons (14), and display calcium dynamics that are highly associated with LH pulses as evaluated by fiber photometry studies in mice (15). In addition, the deficiency of kisspeptin or kisspeptin receptor leads to a hypogonadal-like state in developing mice (16, 17). Although the discovery of the kisspeptin signaling system provided an explanation for a variety of experimental observations (ie, multiunit activity of the infundibulum and its association with LH pulses), it also offered a new set of questions regarding its circuitry. For instance, what neurons/systems control the kisspeptin system that modulates GnRH and LH secretion? One such system is the so-called “stress response”(18), which in the context of this review is to be understood as an allostatic load/overload.
The objective of this review is to present an overview of important allostatic pathways that play a role in shaping the neuroendocrine control of reproductive function. First, we will review the mechanisms and actions of environmental variables (ie, temperature, nutrient availability) on body function and discuss the current evidence for their effects on neuroendocrine function. Second, we will review the gaps in knowledge regarding the circuitry linking environmental changes and reproductive function. Finally, examples will be presented to illustrate specific conditions in which species can evade the suppressive effects of allostatic load on reproductive function.
Temperature
Changes in environmental temperature affect species living in all kinds of environments. Mammalian species are homeothermic, maintaining the internal temperature at a relatively constant level, regardless of external temperatures. Temperature is sensed at the level of the skin through neuronal projections that contain receptors that are activated due to cold (eg, TRPM8 (19)) or warmth (eg, TRPV1 (20)). These neurons are located in the dorsal root ganglia and are pseudomonopolar; that is, a single neuronal projection emerging from the cell body, which divides soon into two with one directed to a target organ and the other directed to the dorsal horn of the spinal cord. While skin (or the shell) temperature is reflective of environmental temperature, the regulated variable in the body is the temperature of the core (21). The core is an inner area in the body including the viscera, spinal cord, and brain, and is kept relatively constant, so that small changes in temperature elicit global regulatory responses (5). Stimuli are processed at the dorsal root ganglia and the dorsal horn of the spinal cord, and depending on intensity or length of exposure, the information can be transmitted to the brain via ascending the spinal cord or can induce a reflex response (eg, muscle contraction, sweat). Thermal stimuli from the spinal cord reach the lateral parabrachial nuclei in the pons. Glutamatergic neurons in the lateral parabrachial nucleus (22) express both proenkephalin and dynorphin and are implicated in triggering heat-defensive physiological (eg, vasodilation, suppression of brown fat metabolism) or behavioral responses at the preoptic area (23). Importantly, experimental models in which the preoptic area of the hypothalamus was thermally stimulated resulted in heat dissipation mechanisms (24), suggesting that the preoptic area is the part of the brain that controls thermal responses (5).
The excess of heat, often denominated as heat stress, is used as a descriptor for the physiological effects resulting from the inability to maintain efficient heat dissipation. This concept has become popular in livestock industries in the equatorial zones to illustrate the effect of environmental high temperature. However, cold stress is also a cause of concern, particularly in areas of higher latitude. The effects of heat stress on reproductive function have been examined carefully in reviews focusing on gonadal and oocyte function (25, 26). Regarding the effects on neuroendocrine secretion, the picture is less clear. In an early study, cows exposed to a high temperature (33.5 °C) demonstrated reduced amplitude LH surges and shorter estrous phases than cows maintained without heat stress (27). Exposure to an environment of 36 °C without access to shade led to a 45% reduction in LH pulse frequency when compared to cows that were exposed to an air conditioned barn, but no differences in follicular-phase estradiol concentrations were observed (28). Similarly, rats exposed for 9 days to 40 °C or 42 °C showed an approximately 50% reduction in circulating LH concentrations when compared to room temperature (26 °C) control rats (29). Moreover, cycling ewes showed decreased estrous behavior and a 70% suppression in the incidence of the LH surge when exposed to high temperature (30). It is currently unknown if these effects are mediated due to neural interaction between thermal sensitive neurons in the preoptic area or parabrachial nucleus and GnRH or kisspeptin neurons. Another potential explanation for this inhibitory effect is the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the secretion of glucocorticoids, as elevated corticosterone has been reported after exposure to high environmental temperature in humans (31). Importantly, corticosterone administration suppresses LH secretion rapidly through a mechanism involving both pituitary and hypothalamic mechanisms (32-34).
Although cold environmental temperature is a considerable harm to the livestock industry in several parts of the world, its effects are poorly understood in a physiological context. During maintained cold climate a substantial portion of the energy intake will be destined for heat generation. In addition, to maintain heat generation, stored energy is mobilized in mammalian species in response to the secretion of hypothalamic thyrotropin-releasing hormone, which leads to the secretion of thyrotropin from the pituitary and 3,5,3′-triiodothyronine (T3) and thyroxine (T4) from the thyroid gland. Both thyroid hormones will increase basal metabolism to counteract the negative energy balance. In rats, cold exposure caused activation of the paraventricular nucleus (PVN) and the locus coeruleus (35) and cold stress induced a specific increase in thyrotropin-releasing hormone gene expression at the PVN (36). Since anterograde and retrograde projections have been described between neurons in the medial preoptic area and the PVN (37, 38), a direct connection may exist between thermosensitive neurons in the preoptic area and neurons in the PVN to elicit this thyrotropin-releasing hormone response. It remains to be established if thyrotropin-releasing hormone neurons in the PVN communicate directly with GnRH neurons in the hypothalamus. However, there is potential for direct thyroid hormone actions (T3 and T4) on GnRH neurons since GnRH neurons in both golden hamster and sheep contained the α nuclear form of the thyroid hormone receptor at a rate of 28% and 46%, respectively (39). In addition, experimental evidence incorporates kisspeptin neurons as targets for thyroid hormones. In sheep, around 90% of kisspeptin neurons express the thyroid hormone receptor α regardless of photoperiod (40). In rats that were treated with propylthiouracil (an agent that induces a hypothyroid state), arcuate kisspeptin messenger RNA and peptide were downregulated in association with irregular estrous cycles (41). Conversely, when rats induced into a hypothyroid state were treated with kisspeptin, ovarian cyclicity was restored (42). These findings support the hypothesis that cold stress may affect reproductive function through actions of thyroid hormones on kisspeptin and GnRH function.
A brain-to-ovary neural pathway has been proposed by the use of a chronic intermittent cold stress in rats (43). This intermittent stress paradigm induced the activation of ovarian nerves with no change in the adrenal secretion of corticosterone (43, 44), allowing the evaluation of the peripheral sympathetic nerve actions. In this model, cold stress was associated with activation of magnocellular PVN neurons and activation of noradrenergic nerves at the ovarian level (45, 46) and such activation was mediated by glutamate at the PVN (47). Importantly, cold stress–induced effects such as induction of Fos expression (a commonly used marker of neuronal activation) in tyrosine hydroxylase neurons increased circulating estradiol and ovarian noradrenaline content, as well as irregular estrous cycles, and reduced ovulation rates were prevented by locus coeruleus lesions (48), supporting the idea that the locus coeruleus is part of the pathway that innervates the ovary. These findings suggest a complementary mechanism whereby cold stress impairs reproductive function, via pathways independent of kisspeptin and GnRH, and thus reinforces the role of peripheral nerves modulating allostatic responses.
Circadian Regulation and Seasonality
In several species, natural light and changes in day length synchronize biological rhythms through mechanisms involving endocrine and neural components (49). Light information is transmitted from the retina through the retinohypothalamic tract to the suprachiasmatic nucleus (SCN), which is considered the central circadian clock. Electrolytic lesions in portions of the SCN led to an anovulatory state in rats (50, 51), demonstrating the necessity of this brain region for coordinating reproduction in some species. The SCN is composed of neurons expressing arginine vasopressin (AVP), vasoactive intestinal peptide (VIP), neuromedin S, and prokineticin 2 (52), and putative suprachiasmatic AVP and VIP neurons project to kisspeptin and GnRH neurons, respectively (53, 54). Electrophysiological studies combined with optogenetic stimulation of AVP neurons showed an AVP excitatory stimulation on the day of proestrus followed by inhibitory actions on the day of estrous (55), supporting the idea that AVP innervation of kisspeptin neurons undergoes estrous cycle-dependent plasticity. The importance of circadian light/dark cycles to reproductive function is illustrated by the occurrence of the LH surge shortly after the initiation of the dark cycle on the day of proestrus in the mouse and human (56, 57). Interestingly, in cattle, the LH surge also tends to occur during the nighttime (58). Further evidence for the role of light/dark cycles is supported by observations from decades ago whereby exposure to constant light suppressed ovulation in albino rats (59) and either acute or chronic circadian disruption led to an impairment of the LH surge and reproductive success in mice (60). Finally, mice exposed to a short-day photoperiod had delayed expression of arcuate kisspeptin and delayed puberty occurrence (61). Beyond reproduction, the disruption of light/dark cycles has broad deleterious effects on behavioral and metabolic functions (62, 63), potentially through an SCN to PVN pathway. Indeed, SCN regulation of corticotrophin-releasing hormone (CRH) and AVP secretion from the PVN, which triggers the release of adrenocorticotropin from the pituitary to induce secretion glucocorticoids, is a key driver of diverse physiological rhythms (62). However, there is still a need to define directly the effect of light vs “photoperiod” stress in the control of biological and reproductive rhythms.
Seasonal reproduction driven by circannual light/dark cycles is a good example of an allostatic mechanism in which the animal prepares for future needs. For instance, rams in the transition from nonbreeding to breeding season exhibit a series of physiological changes, such as increases in LH pulses (64) and testicular diameter (65) in preparation for the requirements of the future breeding season. Seasonal breeding species necessitate strategies to align nutrient availability in the environment with moments of high caloric demand to achieve the highest probability of successful reproduction. Photoperiod signals provide a cue to anticipate future changes in the environment, with the control of melatonin as one pathway by which day-length information is conveyed to the reproductive axis. When darkness is detected, the SCN transmits information to the PVN, which itself relays information through the intermediolateral column of the spinal cord to the superior cervical ganglia. Noradrenergic neurons in the superficial cervical ganglia innervate the pineal gland and induce the synthesis of melatonin (66). Melatonin is a hormone derived from the amino acid tryptophan that is secreted from the pineal gland in the epithalamus in the absence of light. Species that reproduce seasonally are classified as long-day or short-day breeders, depending on whether lengthening or shortening of day favor or suppress reproductive rhythms. In short-day breeders, melatonin secretion exerts a permissive effect on sexual activity, whereas in long-day breeders melatonin exerts a suppressive effect (67). It has been proposed that secreted melatonin may be acting at the pars tuberalis to modify thyrotropin expression, and to induce a increase in T4 to T3 conversion, due to regulation of deiodinase expression, to activate nearby receptors at the infundibulum (67, 68). This increase in local T3 activity may be leading to changes in the GnRH secretory pattern (67, 68). However, current evidence in long-day breeders such as hamsters suggests that LH secretion is stimulated through a mechanism involving RFRP-3, an RF-amide peptide synthetized in the dorsomedial hypothalamus of several species (69). Additionally, in some short-day breeders such as sheep, dopaminergic neurons in the retrochiasmatic area may be involved in the long-day inhibition of reproductive cycles (67, 70). Future work is required to delineate the pathway by which RFRP-3, melatonin, and dopaminergic neurons influence seasonal reproduction as well as the degree of interaction between these neural circuits across species.
Nutrition
In the wild, animals and humans undergo cycles of abundance and scarcity of food availability. To avoid the deleterious effect of prolonged periods of undernutrition, animals have evolved to cope with such deficit periods through a mixture of behavioral and hormonal mechanisms. Circulating glucose is fundamental for all organs in the body, especially the brain, consuming approximately 30% of circulating body glucose, yet representing only 1% to 3% of total body weight. Changes in circulating glucose are rapidly sensed peripherally and centrally and trigger a cascade of physiological responses to correct such deviations, each coordinated by the brain via mechanisms still undergoing investigation. An early study in rats carrying ventromedial hypothalamic (VMH) nuclei lesions showed that these rats had no counterregulatory responses to the stress of hypoglycemia, supporting a role of the hypothalamus in mediating glucose control (71). Our current understanding suggests that glucose sensing occurs centrally at the ventromedial portion of the arcuate nucleus/median eminence as well as the nucleus of tractus solitarius (NTS)/area postrema (72) through innervating neurons at the lateral parabrachial nucleus neurons (73). It is a matter of debate if the glucoresponsive neurons in the ventromedial hypothalamus/median eminence and the NTS/area postrema are sensing glucose concentrations in cerebrospinal fluid or are sensing glucose in the extracellular fluid outside the blood-brain barrier (ie, through circumventricular organs (74)). Glucose sensing also occurs peripherally at the vena porta among other peripheral sensors such as the gastrointestinal tract and carotid bodies (74-76). Collectively, these neurons convey glucose information to VMH neurons that display “glucoexcitability” or “glucoinhibition” properties and are responsible for coordinating the effector responses to glucose changes (74).
The control of glucose concentrations after an allostatic challenge such as hypoglycemia is achieved by the central nervous system’s influence over peripheral glands and endocrine mechanisms. One mechanism is the secretion of epinephrine from the adrenal medulla, triggered by a descending pathway from the spinal cord and brainstem and hypothalamus (77), inducing blood glucose concentrations. In addition, glucagon secretion from pancreatic α cells in Langerhans islets also aids in countering a hypoglycemic state. It is important to point that these two mechanisms are mediated by a neural stimulus that includes both sympathetic and parasympathetic components (78). A third mechanism of counteracting hypoglycemia is the secretion of glucocorticoids by the adrenal cortex to induce glucose release from glycogen storage or enhance hepatic gluconeogenesis.
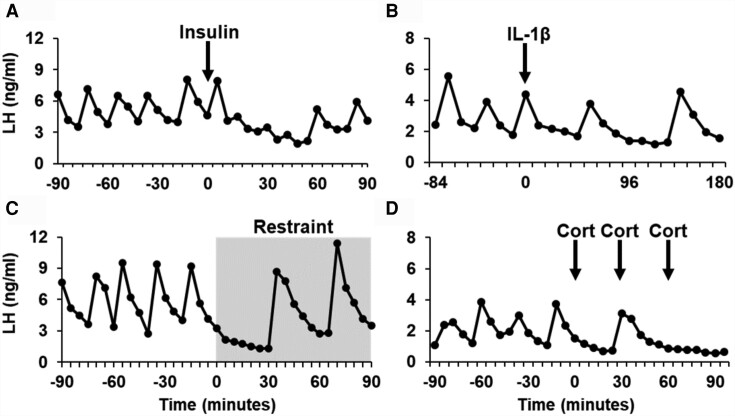
Work in several species has shown that inadequate nutritional fuels impair reproductive function by a central mechanism. Studies using feed-restriction models demonstrate reproductive impairment involves impaired pulsatile and surge modes of LH via disruption of kisspeptin circuits (79, 80). An important experimental approach to understand the neural circuits elicited by low glucose has been the insulin-induced hypoglycemia paradigm that suppressed LH in a variety of species including human, monkey, sheep, rat, and mouse (81-85). In sheep, administration of insulin led to a decrease in LH pulses regardless of estradiol status, and infusion of glucose led to the observation of an LH pulse (83), suggesting that impairment was due to low glucose and not the administration of insulin per se. Importantly, Fos gene expression was observed mainly in the PVN of sheep, suggesting the PVN as a potential site of the modulatory response. Studies in the rat have shown that administration of insulin decreased LH secretion and was associated with suppressed electrical activity of the GnRH pulse generator (82, 86). Recently, a study in mice that received insulin led to a decrease in mean LH and LH pulse frequency during the 90-minute observation period (85). Since no differences were observed in LH concentration after kisspeptin administration between insulin- vs saline-treated mice, it was suggested that the inhibitory effect was due to a mechanism upstream of kisspeptin neurons. In addition, the authors observed a decrease in the degree of activation of kisspeptin neurons in the arcuate nucleus in insulin-treated mice (85), suggesting that kisspeptin neurons may be one of the targets of the glucostatic pathway in the hypothalamus during an allostatic challenge. An example of the LH response to insulin in mice is presented in Fig. 1A.
Figure 1.
Comparison of luteinizing hormone (LH) profiles in mice treated with A, insulin; B, interleukin 1β; C, physical restraint; or D, corticosterone. Blood samples were collected from ovariectomized (insulin, interleukin 1β, restraint) or estrogen-treated ovariectomized (cortisol; Cort) mice before and after exposure to a stressor or a stress level of corticosterone (34, 85, 87, 88). Blood samples were analyzed at the University of Virginia assay core or an inhouse assay developed in our laboratory (89). Panels A, C, and D were originally published in refs. 34, 85, and 108 and has been adapted and reproduced by permission of Oxford University Press (http://global.oup.com/academic). Panel B was originally published in ref. 106 and has been adapted and reproduced by permission of Bioscientifica (https://www.bioscientifica.com/publishing/journals/).
On the flip side, studies using chronic hyperglycemia induced by streptozotocin (a drug that targets and ablates insulin-producing cells in the pancreas) showed that elevated glucose was associated with decreased LH concentrations and pulse frequency in female rats (90), as well as lowered mean LH concentration and testosterone secretion in male streptozotocin-treated rats (91). Furthermore, streptozotocin administration led to a decrease in estrous cyclicity and LH concentrations, which was associated with lower expression of the kisspeptin gene in the arcuate nucleus, but, interestingly, not in the anteroventral periventricular area (92). Despite the evidence available and considering the importance of nutrition for brain function, it is unknown how glucosensitive neurons in the VMH or the NTS influence kisspeptin or GnRH neuronal function.
Altitude
The effects of high altitude are manifested through the establishment of a hypoxic state, which initiates compensatory changes. Oxygen chemoreceptors are located mainly in the carotid bodies, an organ located in the bifurcation of the common carotid arteries. Given their abundant irrigation, carotid bodies are well positioned to sense oxygen and carbon dioxide pressure, and a depression in oxygen content leads to an increase in neuronal activity in these structures. The carotid body is innervated by the carotid sinus nerve, a branch of the glossopharyngeal nerve (93), which innervates the retrotrapezoidal nuclei in the medulla oblongata. Evidence that ablation of retrotrapezoidal nuclei neurons led to a lack of responsiveness to accumulation of circulating CO2 (hypercapnia) in mice (94) suggests that the retrotrapezoidal nuclei is an important part of the hypoxia-responsive pathway. In addition, the NTS also acts as an integrator of chemoreceptor and baroreceptor activity in the brainstem, along with the medullary raphe and cerebellar structures that provide support to hypercapnic responses (95). Mice maintained in hypoxia compared to normoxia for 2 hours showed an increase in Fos expression in PVN neurons that projected to the NTS with concomitant activation of NTS neurons (96), suggesting a direct functional communication between these nuclei. On the establishment of hypoxia, both short-term and long-term compensatory response system are established. Short-term regulation is focused on cardiometabolic compensation (ie, maintaining blood pressure), while long-term regulation is accomplished by changes in blood composition either by increasing blood volume (reabsorption of salts and water) or increasing the production of red blood cells (secretion of erythropoietin (97)).
A few studies suggest a negative correlation between altitude and reproductive neuroendocrine function. In one study, sheep were either maintained at low altitude (500 meters above sea level), moved from low altitude to high altitude (3589 meters above sea level), or maintained at high altitude and monitored; sheep that were maintained at low altitude had higher estradiol concentrations at estrous onset and higher LH concentration at the periovulatory stage (98). Similarly, female mice housed at 8% oxygen exhibited a decrease in LH, prolactin, and progesterone concentrations, and had lower uterine weight and fewer implantation sites compared to mice at 20% oxygen (99). As for a potential mechanism of reproductive suppression, the PVN is activated during hypoxia (96) and hypoxia-induced glucocorticoid secretion (100), suggesting the suppressive effect of hypoxia may be attributed to circulating glucocorticoids. One challenging factor to consider is that the effects of low atmospheric pressure are poorly studied in the literature, and it is difficult to dissect such influence from hypoxia that occurs at high altitude. A lesson can be drawn from the studies in male astronauts that were sampled before and after a trip to outer space (101). All participants had lower urinary testosterone levels and lower sexual drive after the flight. Unexpectedly, LH concentrations were elevated from baseline (101).
Immunologic
Local and general immunological challenges induce changes in tissues and systems in response to mediators secreted by the host, including interleukins, tumor necrosis factors, prostaglandins, and leukotrienes among others. Similarly, substances produced by microorganisms induce immunological effects. Exotoxins are molecules produced by bacteria that are secreted into the invaded tissue and help either defeat the immune response or to induce cytolysis. Endotoxins are products of the internal bacteria cell wall released on bacterial lysis. The result of immunological challenges is the production of general inflammation including fever and cardiovascular failure and is associated with general dysfunction that is coordinated by the nervous system.
Lipopolysaccharide (LPS) has been used as an experimental model to test the effects of endotoxemia on a variety of physiological systems. An early study in sheep showed that the intravenous administration of LPS led to an immediate blockade of portal GnRH secretion and pituitary blockade while stimulating the secretion of cortisol in a abrupt manner (102). More recently, administration of LPS in sheep led to a decrease of both kisspeptin and GnRH expression in the hypothalamus (103). Similarly, intrauterine administration of LPS in rats led to endometrial recruitment of polymorphonuclear cells, suppression of cyclicity, reduction in LH secretion, and reduction in kisspeptin expression in the arcuate nucleus (104). In a recent review, authors presented a potential pathway for LPS-induced deleterious effects on GnRH/LH secretion via circumventricular organs, such as the choroid plexus and the median eminence (105). These organs constitute an interphase between cerebrospinal fluid and the extracellular fluid, allowing access to surrounding neurons; however, the question remains: Where is the site of action of LPS, cytokines, or other immune mediators in the mammalian brain?
Interleukins are a group of local mediators that are produced by white blood cells on immunogenic stimulation. Studies in sheep have shown that administration of interleukin 1β led to fever, activation of PVN neurons, and increase of the expression of CRH (106). Studies in the rat have shown that intracerebroventricular delivery of interleukin 1β led to a reduction in LH, GnRH, and testosterone in male rats (107, 108), and the effect was reversed by inhibitors of prostaglandin synthesis (107). Administration of interleukin 1β in estradiol-treated mice resulted in an acute reduction of LH secretion and concomitant reduction of kisspeptin neuron activation and was associated with an activation of neurons that are involved in mediating stress (ie, PVN, NTS (87)). An example of the LH response to interleukin 1β in mice is presented in Fig. 1B.
Psychosocial Challenges
Psychosocial stress is processed through a complex neural circuitry that involves memory, behavioral, and neuroendocrine responses and is dependent on olfactory, visual, tactile, and auditive stimuli. In female mice, psychosocial stress induced by acute isolation and restraint led to an abrupt suppression of LH pulses in association with a reduction in Fos content in kisspeptin neurons in the arcuate nucleus; interestingly, the authors also observed an increase in Fos content in RFRP neurons in the dorsomedial hypothalamus (Fig. 1C (88)). The hypothalamic peptide RFRP-3, a member of the RF-amide peptide family that includes kisspeptin, has been proposed as a central mediator of the neuroendocrine effects of both acute and chronic stress. For instance, chronic restraint of rats for 3 hours/day for 18 days led to an overexpression of RFRP-3 in the dorsomedial hypothalamus and reproductive impairment while no changes in kisspeptin and GnRH gene expression were found (109). When the RFRP-3 gene was silenced in rats and treated with the same stress paradigm, the effects of stress were ameliorated (109), consistent with studies involving acute psychosocial stress in mice (110). Interestingly, a recent study proposed that the posterodorsal medial amygdala provided a pathway that influences the hypothalamus. The authors demonstrated that chemogenetic inhibition of the posterodorsal medial amygdala prevented psychosocial stress–induced LH inhibition through the PVN (111). These findings suggest that part of the limbic system is involved in relaying sensory information to the neuroendocrine hypothalamus.
Conclusions and Future Directions
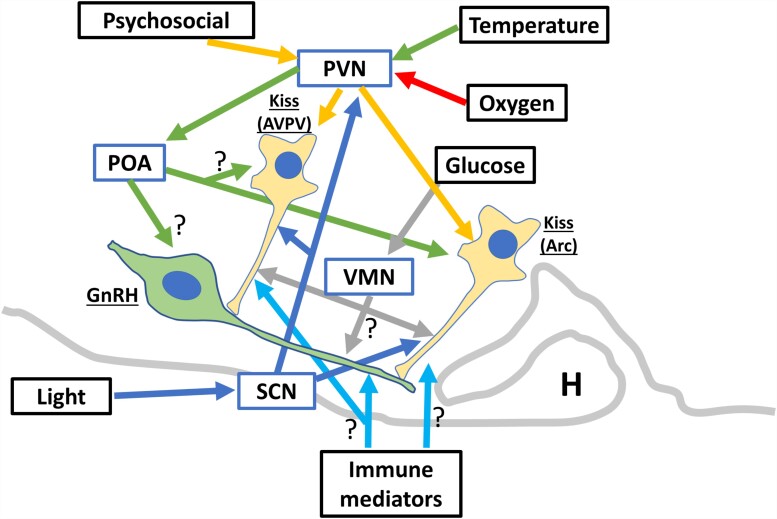
Great progress in the understanding of reproductive neuroendocrinology has occurred in the last 20 years, particularly with the discovery of kisspeptin, which is a potent regulator of GnRH secretion. Most of the allostatic systems reviewed here showed that there is a direct or indirect influence on kisspeptin neurons. Therefore, further investigation is warranted to dissect the pathways linking the underlying neuronal circuitry in addition to the chemical mediators at play (refer to McCosh et al (112) for a thorough review of stress molecules influencing the GnRH pulse generator). The allostatic response to a stressor leads to a variety of physiological and behavioral responses. The behavioral effects lead to the prevention of “normal” behavior (eg, estrous manifestation; 27) or the display of coping behaviors (eg, self-grooming behavior). In addition, the “stress” neurocircuitry in the hypothalamus inhibits the activity of the GnRH pulse and surge generator, which itself can be further inhibited by mediators of the HPA activation. The collective literature supports the premise that the PVN is at the center of the allostatic response, representing both the site for activating the stress response and the site integration of most of the aforementioned systems. An example of the organization of the mechanistic influences of allostatic systems in reproductive neuroendocrinology is presented in Fig. 2.
Figure 2.
Schematic of allostatic factors (black boxes) suppressing GnRH and LH secretion through different hypothalamic nuclei (blue boxes). The involvement of the arcuate nucleus (Arc) or the anteroventral periventricular area (AVPV) kisspeptin neurons in the “stress” networks determines whether the LH pulse or surge secretion pattern are affected during an allostatic challenge. GnRH, gonadotrophin-releasing hormone; H, hypophysis; Kiss, kisspeptin; LH, luteinizing hormone; POA, preoptic area; PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus; VMN, ventromedial nucleus.
Since the stimulation of the PVN and the HPA axis leads to the activation of several mediators that are components of the stress response, it remains necessary to understand the local circuitry at the PVN and its downstream relevance. For instance, the relevance of CRH in controlling GnRH function remains unclear given (i) variable and even contradictory effects (87), and (ii) its receptor CRH1R (CRH high-affinity receptor) seems to not have a role in GnRH suppression (18, 113, 114). Further, a recent report showed that activation of γ-aminobutyric acid–producing neurons at the PVN were critical in suppressing pulsatile LH secretion in mice, even when CRH neurons were activated (115). Thus, the role of other neuropeptides and neurotransmitters in the PVN, such as urocortins, AVP, oxytocin, γ-aminobutyric acid, or glutamate, will provide guidelines to understand the mechanism of GnRH suppression by stress. On the other hand, the final product of this pathway, corticosterone, induces an estrogen-dependent suppression of pulsatile LH that occurs rapidly (within minutes) and is comparable to other stressors (Fig. 1D (34)). Chronic corticosterone can inhibit both the pulsatile and surge secretion of LH via a mechanism that may be mediated by genomic signaling within hypothalamic kisspeptin neurons (116, 117).
Although the effects of allostasis or stress are mostly deleterious to reproductive function, depending on the biology of the species, there are instances in which the neuroendocrine system controlling reproduction becomes “resistant” or overrides such influence. In a review, Wingfield and Sapolsky (118) identified certain conditions in which species experience successful reproduction during the establishment of an allostatic state. Such scenarios include species in which males become dominant for a short tenure and face continuous challenges to their hierarchical position (olive baboons), species that reproduce only once after extreme challenges and just before death (migrating salmon), and species in which the breeding season is highly competitive and very short in spring (Artic squirrel). The mechanisms involved may include the blockade of stressor sensing or blockade of the secretory activity from the hypothalamus and the activation of the HPA axis or novel effects of glucocorticoids to enable reproduction during unfavorable conditions (118).
On a final note, an existing challenge to the understanding of stress or allostatic biology is the overreliance on the vivarium-housed laboratory mouse as an experimental model. Although the value of rodents in biomedical research is undeniable, this issue neglects the multiple biological specializations that exist to cope with or compensate for stress outside of this species. Thus, a comparative approach would greatly enhance understanding of the diversity of pathways enabling modulation of information during an allostatic state. Indeed, inputs from thermal, nutritional, light, psychosocial, and altitude sensors within the body modulate and, in most instances, suppress reproductive function through mediation by the brain. This reinforces the idea that the allostasis paradigm allows the exploration of biological strategies to adapt and prepare for adaptation to the environment.
Abbreviations
- AVP
arginine vasopressin
- CRH
corticotrophin-releasing hormone
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- LH
luteinizing hormone
- LPS
lipopolysaccharide
- NTS
tractus solitarius
- PVN
paraventricular nucleus
- SCN
suprachiasmatic nucleus
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- VIP
vasoactive intestinal peptide
- VMH
ventromedial hypothalamus
Contributor Information
Rodrigo A Carrasco, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Diego, La Jolla, CA 92093-0674, USA.
Kellie M Breen, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Diego, La Jolla, CA 92093-0674, USA.
Funding
This work was supported by the National Institutes of Health (grant Nos. R01 HD103725, R21 HD105103, and P50 HD012303).
Disclosures
Kellie M. Breen is an editorial board member for Endocrinology.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Bernard C. Lectures on the Phenomena of Life Common to Animals and Plants, Translation by Hebbel E. Hoff, Roger Guillemin [and] Lucienne Guillemin. Thomas; 1974. [Google Scholar]
- 2. Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9(3):399‐431. [Google Scholar]
- 3. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. John Wiley & Sons; 1988:629‐649. [Google Scholar]
- 4. McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2‐15. [DOI] [PubMed] [Google Scholar]
- 5. Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenelon JC, Banerjee A, Murphy BD. Embryonic diapause: development on hold. Int J Dev Biol. 2014;58(2-3-4):163‐174. [DOI] [PubMed] [Google Scholar]
- 7. McEwen BS, Wingfield JC. What's in a name? Integrating homeostasis, allostasis and stress. Horm Behav. 2010;57(2):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138(3479):32‐32. [DOI] [PubMed] [Google Scholar]
- 9. Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50(2):283‐298. [DOI] [PubMed] [Google Scholar]
- 10. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887‐895. [DOI] [PubMed] [Google Scholar]
- 11. Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304(5924):345‐347. [DOI] [PubMed] [Google Scholar]
- 12. Hrabovszky E, Shughrue PJ, Merchenthaler I, et al. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141(9):3506‐3509. [DOI] [PubMed] [Google Scholar]
- 13. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225‐230. [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Coolen LM, Kriegsfeld LJ, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770‐5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216‐E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nandankar N, Negrón AL, Wolfe A, Levine JE, Radovick S. Deficiency of arcuate nucleus kisspeptin results in postpubertal central hypogonadism. Am J Physiol Endocrinol Metab. 2021;321(2):E264‐E280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 18. McCosh RB, Breen KM, Kauffman AS. Neural and endocrine mechanisms underlying stress-induced suppression of pulsatile LH secretion. Mol Cell Endocrinol. 2019;498:110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almeida MC, Hew-Butler T, Soriano RN, et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32(6):2086‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang X. Molecular sensors and modulators of thermoreception. Channels (Austin). 2015;9(2):73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jessen C. Thermal afferents in the control of body temperature. Pharmacol Ther. 1985;28(1):107‐134. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107(19):8848‐8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norris AJ, Shaker JR, Cone AL, Ndiokho IB, Bruchas MR. Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. Elife. 2021;10:e60779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishikawa Y, Nakayama T, Kanosue K, Matsumura K. Activation of central warm-sensitive neurons and the tail vasomotor response in rats during brain and scrotal thermal stimulation. Pflugers Arch. 1984;400(3):222‐227. [DOI] [PubMed] [Google Scholar]
- 25. Hansen PJ. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci. 2009;364(1534):3341‐3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Wettere WHEJ, Kind KL, Gatford KL, et al. Review of the impact of heat stress on reproductive performance of sheep. J Anim Sci Biotechnol. 2021;12(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madan ML, Johnson HD. Environmental heat effects on bovine luteinizing hormone. J Dairy Sci. 1973;56(11):1420‐1423. [DOI] [PubMed] [Google Scholar]
- 28. Wise ME, Armstrong DV, Huber JT, Hunter R, Wiersma F. Hormonal alterations in the lactating dairy cow in response to thermal stress. J Dairy Sci. 1988;71(9):2480‐2485. [DOI] [PubMed] [Google Scholar]
- 29. Zheng M, Nagaoka K, Watanabe G. Pre-pubertal exposure to high temperature impairs ovarian and adrenal gland function in female rats. J Vet Med Sci. 2019;81(2):279‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hill TG, Alliston CW. Effects of thermal stress on plasma concentrations of luteinizing hormone, progesterone, prolactin and testosterone in the cycling ewe. Theriogenology. 1981;15(2):201‐209. [DOI] [PubMed] [Google Scholar]
- 31. Collins KJ, Few JD, Forward TJ, Giec LA. Stimulation of adrenal glucocorticoid secretion in man by raising the body temperature. J Physiol. 1969;202(3):645‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145(2):692‐698. [DOI] [PubMed] [Google Scholar]
- 33. Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150(1):341‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kreisman MJ, McCosh RB, Breen KM. Role of CORT duration and estradiol dependence for stress-level of CORT to inhibit pulsatile LH secretion in female mice. J Endocr Soc. 2021;5(Suppl 1):A552. [Google Scholar]
- 35. Jiang XH, Guo SY, Xu S, et al. Sympathetic nervous system mediates cold stress-induced suppression of natural killer cytotoxicity in rats. Neurosci Lett. 2004;358(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 36. Zoeller RT, Kabeer N, Albers HE. Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology. 1990;127(6):2955‐2962. [DOI] [PubMed] [Google Scholar]
- 37. Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246(3):312‐342. [DOI] [PubMed] [Google Scholar]
- 38. Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci. 2006;23(12):3284‐3296. [DOI] [PubMed] [Google Scholar]
- 39. Jansen HT, Lubbers LS, Macchia E, DeGroot LJ, Lehman MN. Thyroid hormone receptor (alpha) distribution in hamster and sheep brain: colocalization in gonadotropin-releasing hormone and other identified neurons. Endocrinology. 1997;138(11):5039‐5047. [DOI] [PubMed] [Google Scholar]
- 40. Dufourny L, Gennetay D, Martinet S, Lomet D, Caraty A. The content of thyroid hormone receptor α in ewe kisspeptin neurones is not season-dependent. J Neuroendocrinol. 2016;28(2):12344. [DOI] [PubMed] [Google Scholar]
- 41. Tomori Y, Takumi K, Iijima N, Takai S, Ozawa H. Kisspeptin expression is decreased in the arcuate nucleus of hypothyroid female rats with irregular estrus cycles. Neurosci Res. 2017;117:35‐41. [DOI] [PubMed] [Google Scholar]
- 42. de Oliveira LS, da Silva TQM, Barbosa EM, et al. Kisspeptin treatment restores ovarian function in rats with hypothyroidism. Thyroid. 2022;32(12):1568‐1579. [DOI] [PubMed] [Google Scholar]
- 43. Dorfman M, Arancibia S, Fiedler JL, Lara HE. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol Reprod. 2003;68(6):2038‐2043. [DOI] [PubMed] [Google Scholar]
- 44. Bhatnagar S, Mitchell JB, Betito K, Boksa P, Meaney MJ. Effects of chronic intermittent cold stress on pituitary adrenocortical and sympathetic adrenomedullary functioning. Physiol Behav. 1995;57(4):633‐639. [DOI] [PubMed] [Google Scholar]
- 45. Fiedler J, Jara P, Luza S, et al. Cold stress induces metabolic activation of thyrotrophin-releasing hormone-synthesising neurones in the magnocellular division of the hypothalamic paraventricular nucleus and concomitantly changes ovarian sympathetic activity parameters. J Neuroendocrinol. 2006;18(5):367‐376. [DOI] [PubMed] [Google Scholar]
- 46. Luza SM, Arancibia S, Venegas M, Lara HE. Thyrotropin-releasing hormone as a mediator of the central autonomic pathway controlling ovarian function. Neuroendocrinology. 2003;77(4):273‐281. [DOI] [PubMed] [Google Scholar]
- 47. Jara P, Rage F, Dorfman M, et al. Cold-induced glutamate release in vivo from the magnocellular region of the paraventricular nucleus is involved in ovarian sympathetic activation. J Neuroendocrinol. 2010;22(9):979‐986. [DOI] [PubMed] [Google Scholar]
- 48. Bernuci MP, Szawka RE, Helena CVV, Leite CM, Lara HE, Anselmo-Franci JA. Locus coeruleus mediates cold stress-induced polycystic ovary in rats. Endocrinology. 2008;149(6):2907‐2916. [DOI] [PubMed] [Google Scholar]
- 49. Malpaux B, Thiéry JC, Chemineau P. Melatonin and the seasonal control of reproduction. Reprod Nutr Dev. 1999;39(3):355‐366. [DOI] [PubMed] [Google Scholar]
- 50. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153(6):2839‐2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34(6):395‐404. [DOI] [PubMed] [Google Scholar]
- 52. Piet R. Circadian and kisspeptin regulation of the preovulatory surge. Peptides. 2023;163:170981. [DOI] [PubMed] [Google Scholar]
- 53. Horvath TL, Cela V, van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res. 1998;795(1-2):277‐281. [DOI] [PubMed] [Google Scholar]
- 54. Vida B, Deli L, Hrabovszky E, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22(9):1032‐1039. [DOI] [PubMed] [Google Scholar]
- 55. Jamieson BB, Bouwer GT, Campbell RE, Piet R. Estrous cycle plasticity in the central clock output to kisspeptin neurons: implications for the preovulatory surge. Endocrinology. 2021;162(6):bqab071. [DOI] [PubMed] [Google Scholar]
- 56. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794‐4802. [DOI] [PubMed] [Google Scholar]
- 57. Kerdelhué B, Brown S, Lenoir V, et al. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75(3):158‐163. [DOI] [PubMed] [Google Scholar]
- 58. Ginther OJ, Pinaffi FLV, Khan FA, Duarte LF, Beg MA. Circadian influence on the preovulatory LH surge, ovulation, and prolactin concentrations in heifers. Theriogenology. 2013;79(3):528‐533. [DOI] [PubMed] [Google Scholar]
- 59. Brown-Grant K. The role of the retina in the failure of ovulation in female rats exposed to constant light. Neuroendocrinology. 1974;16(5-6):243‐254. [DOI] [PubMed] [Google Scholar]
- 60. Bahougne T, Kretz M, Angelopoulou E, Jeandidier N, Simonneaux V. Impact of circadian disruption on female mice reproductive function. Endocrinology. 2020;161(4):bqaa028. [DOI] [PubMed] [Google Scholar]
- 61. Bohlen TM, Silveira MA, Buonfiglio DC, et al. A short-day photoperiod delays the timing of puberty in female mice via changes in the kisspeptin system. Front Endocrinol (Lausanne). 2018;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oster H. The interplay between stress, circadian clocks, and energy metabolism. J Endocrinol. 2020;247(1):R13‐R25. [DOI] [PubMed] [Google Scholar]
- 63. Rumanova VS, Okuliarova M, Zeman M. Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int J Mol Sci. 2020;21(15):5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robinson JE, Radford HM, Karsch FJ. Seasonal changes in pulsatile luteinizing hormone (LH) secretion in the ewe: relationship of frequency of LH pulses to day length and response to estradiol negative feedback. Biol Reprod. 1985;33(2):324‐334. [DOI] [PubMed] [Google Scholar]
- 65. Lincoln GA, Lincoln CE, McNeilly AS. Seasonal cycles in the blood plasma concentration of FSH, inhibin and testosterone, and testicular size in rams of wild, feral and domesticated breeds of sheep. J Reprod Fertil. 1990;88(2):623‐633. [DOI] [PubMed] [Google Scholar]
- 66. Drijfhout WJ, van der Linde AG, de Vries JB, Grol CJ, Westerink BH. Microdialysis reveals dynamics of coupling between noradrenaline release and melatonin secretion in conscious rats. Neurosci Lett. 1996;202(3):185‐188. [DOI] [PubMed] [Google Scholar]
- 67. Weems PW, Goodman RL, Lehman MN. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front Neuroendocrinol. 2015;37:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ikegami K, Refetoff S, Cauter EV, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019;15(10):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Henningsen JB, Ancel C, Mikkelsen JD, Gauer F, Simonneaux V. Roles of RFRP-3 in the daily and seasonal regulation of reproductive activity in female Syrian hamsters. Endocrinology. 2017;158(3):652‐663. [DOI] [PubMed] [Google Scholar]
- 70. Weems P, Smith J, Clarke IJ, Coolen LM, Goodman RL, Lehman MN. Effects of season and estradiol on KNDy neuron peptides, colocalization with D2 dopamine receptors, and dopaminergic inputs in the ewe. Endocrinology. 2017;158(4):831‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest. 1994;93(4):1677‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J Neurophysiol. 2015;114(2):999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meek TH, Nelson JT, Matsen ME, et al. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci U S A. 2016;113(14):E2073‐E2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bentsen MA, Mirzadeh Z, Schwartz MW. Revisiting how the brain senses glucose—and why. Cell Metab. 2019;29(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, Donovan CM. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes. 2014;63(8):2866‐2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Donovan CM, Watts AG. Peripheral and central glucose sensing in hypoglycemic detection. Physiology (Bethesda). 2014;29(5):314‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Verberne AJM, Korim WS, Sabetghadam A, Llewellyn-Smith IJ. Adrenaline: insights into its metabolic roles in hypoglycaemia and diabetes. Br J Pharmacol. 2016;173(9):1425‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Loewy AD, Haxhiu MA. CNS cell groups projecting to pancreatic parasympathetic preganglionic neurons. Brain Res. 1993;620(2):323‐330. [DOI] [PubMed] [Google Scholar]
- 79. Knuth UA, Friesen HG. Starvation induced anoestrus: effect of chronic food restriction on body weight, its influence on oestrous cycle and gonadotrophin secretion in rats. Acta Endocrinol (Copenh). 1983;104(4):402‐409. [DOI] [PubMed] [Google Scholar]
- 80. Kreisman MJ, Tadrousse KS, McCosh RB, Breen KM. Neuroendocrine basis for disrupted ovarian cyclicity in female mice during chronic undernutrition. Endocrinology. 2021;162(8):bqab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86(10):4913‐4919. [DOI] [PubMed] [Google Scholar]
- 82. Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic “stress” and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666‐673. [DOI] [PubMed] [Google Scholar]
- 83. Adam CL, Findlay PA. Inhibition of luteinizing hormone secretion and expression of c-fos and corticotrophin-releasing factor genes in the paraventricular nucleus during insulin-induced hypoglycaemia in sheep. J Neuroendocrinol. 1998;10(10):777‐783. [DOI] [PubMed] [Google Scholar]
- 84. Clarke IJ, Horton RJ, Doughton BW. Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology. 1990;127(3):1470‐1476. [DOI] [PubMed] [Google Scholar]
- 85. McCosh RB, Kreisman MJ, Tian K, Ho BS, Thackray VG, Breen KM. Insulin-induced hypoglycemia suppresses pulsatile LH secretion and arcuate Kiss1 cell activation in female mice. J Neuroendocrinol. 2019;31(12):e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. He D, Funabashi T, Sano A, Uemura T, Minaguchi H, Kimura F. Effects of glucose and related substrates on the recovery of the electrical activity of gonadotropin-releasing hormone pulse generator which is decreased by insulin-induced hypoglycemia in the estrogen-primed ovariectomized rat. Brain Res. 1999;820(1-2):71‐76. [DOI] [PubMed] [Google Scholar]
- 87. Makowski KN, Kreisman MJ, McCosh RB, Raad AA, Breen KM. Peripheral interleukin-1β inhibits arcuate Kiss1 cells and LH pulses in female mice. J Endocrinol. 2020;246(2):149‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang JA, Song CI, Hughes JK, et al. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology. 2017;158(11):3716‐3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kreisman MJ, McCosh RB, Breen KM. A modified ultra-sensitive ELISA for measurement of LH in mice. Endocrinology. 2022;163(9):bqac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dong Q, Lazarus RM, Wong LS, Vellios M, Handelsman DJ. Pulsatile LH secretion in streptozotocin-induced diabetes in the rat. J Endocrinol. 1991;131(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 91. Castellano JM, Navarro VM, Fernández-Fernández R, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55(9):2602‐2610. [DOI] [PubMed] [Google Scholar]
- 92. Enomoto H, Iwata K, Matsumoto K, Otsuka M, Morita A, Ozawa H. Hypothalamic KNDy neuron expression in streptozotocin-induced diabetic female rats. J Endocrinol. 2022;253(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology (Bethesda). 2015;30(5):340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dubreuil V, Thoby-Brisson M, Rallu M, et al. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29(47):14836‐14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 96. Ruyle BC, Klutho PJ, Baines CP, Heesch CM, Hasser EM. Hypoxia activates a neuropeptidergic pathway from the paraventricular nucleus of the hypothalamus to the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1167‐R1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. 2019;10:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Parraguez VH, Diaz F, Cofré E, et al. Fertility of a high-altitude sheep model is compromised by deficiencies in both preovulatory follicle development and plasma LH availability. Reprod Domest Anim. 2014;49(6):977‐984. [DOI] [PubMed] [Google Scholar]
- 99. Rattner BA, Michael SD, Brinkley HJ. Plasma gonadotropins, prolactin and progesterone at the time of implantation in the mouse: effects of hypoxia and restricted dietary intake. Biol Reprod. 1978;19(3):558‐565. [DOI] [PubMed] [Google Scholar]
- 100. Hwang GS, Chen CC, Chou JC, et al. Stimulatory effect of intermittent hypoxia on the production of corticosterone by zona fasciculata-reticularis cells in rats. Sci Rep. 2017;7(1):9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Strollo F, Riondino G, Harris B, et al. The effect of microgravity on testicular androgen secretion. Aviat Space Environ Med. 1998;69(2):133‐136. [PubMed] [Google Scholar]
- 102. Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology. 1997;138(10):4273‐4281. [DOI] [PubMed] [Google Scholar]
- 103. Lee CY, Li S, Li XF, et al. Lipopolysaccharide reduces gonadotrophin-releasing hormone (GnRH) gene expression: role of RFamide-related peptide-3 and kisspeptin. Reprod Fertil Dev. 2019;31(6):1134‐1143. [DOI] [PubMed] [Google Scholar]
- 104. Magata F, Toda L, Sato M, et al. Intrauterine LPS inhibited arcuate kiss1 expression, LH pulses, and ovarian function in rats. Reproduction. 2022;164(5):207‐219. [DOI] [PubMed] [Google Scholar]
- 105. Magata F, Tsukamura H, Matsuda F. The impact of inflammatory stress on hypothalamic kisspeptin neurons: mechanisms underlying inflammation-associated infertility in humans and domestic animals. Peptides. 2023;162:170958. [DOI] [PubMed] [Google Scholar]
- 106. Vellucci SV, Parrott RF, da Costa AC, Ohkura S, Kendrick KM. Increased body temperature, cortisol secretion, and hypothalamic expression of c-fos, corticotrophin releasing hormone and interleukin-1 beta mRNAs, following central administration of interleukin-1 beta in the sheep. Brain Res Mol Brain Res. 1995;29(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 107. Rivest S, Rivier C. Centrally injected interleukin-1 beta inhibits the hypothalamic LHRH secretion and circulating LH levels via prostaglandins in rats. J Neuroendocrinol. 1993;5(4):445‐450. [DOI] [PubMed] [Google Scholar]
- 108. Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology. 1997;138(3):1008‐1013. [DOI] [PubMed] [Google Scholar]
- 109. Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. eLife. 2015;4:e04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mamgain A, Sawyer IL, Timajo DAM, et al. RFamide-related peptide neurons modulate reproductive function and stress responses. J Neurosci. 2021;41(3):474‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ivanova D, Li XF, McIntyre C, Liu Y, Kong L, O’Byrne KT. Urocortin3 in the posterodorsal medial amygdala mediates stress-induced suppression of LH pulsatility in female mice. Endocrinology. 2021;162(12):bqab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McCosh RB, O’Bryne KT, Karsch FJ, Breen KM. Regulation of the gonadotropin-releasing hormone neuron during stress. J Neuroendocrinol. 2022;34(5):e13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Phumsatitpong C, De Guzman RM, Zuloaga DG, Moenter SM. A CRH receptor type 1 agonist increases GABA transmission to GnRH neurons in a circulating-estradiol-dependent manner. Endocrinology. 2020;161(11):bqaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yip SH, Liu X, Hessler S, Cheong I, Porteous R, Herbison AE. Indirect suppression of pulsatile LH secretion by CRH neurons in the female mouse. Endocrinology. 2021;162(3):bqaa237. [DOI] [PubMed] [Google Scholar]
- 115. McIntyre C, Li XF, Ivanova D, Wang J, O’Byrne KT. Hypothalamic PVN CRH neurons signal through PVN GABA neurons to suppress GnRH pulse generator frequency in female mice. Endocrinology. 2023;164(6):bqad075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kreisman MJ, McCosh RB, Tian K, Song CI, Breen KM. Estradiol enables chronic corticosterone to inhibit pulsatile LH secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2020;110(6):501‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Luo E, Stephens SBZ, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157(3):1187‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J Neuroendocrinol. 2003;15(8):711‐724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.