Abstract
Objective:
Lung cancer is the leading cause of cancer-associated mortality worldwide. Central nervous system (CNS) metastasis is a prevalent and serious complication. The most common treatment for brain metastasis (BM) is still radiation therapy (RT). An increasing number of drugs have been shown to have intracranial activity or to sensitize tumours to radiotherapy.
Methods:
Consecutive advanced multiline therapy failure in patients with non-small-cell lung cancer (NSCLC) with BM at the authors’ hospital were retrospectively reviewed. Eligible patients were divided into two groups: Apatinib+RT group and RT group. Intracranial progression-free survival (PFS) and overall survival (OS) were analysed using the Kaplan–Meier method.
Results:
The median intracranial PFS for the RT group and Apatinib+RT group was 5.83 months and 11.81 months (p = 0.034). The median OS for the RT group and Apatinib+RT group was 9.02 months and 13.62 months (p = 0.311). The Apatinib+RT group had a better intracranial PFS, but there were no significant differences between the two arms in OS. The Apatinib+RT group had significantly reduced symptoms caused by BM.
Conclusion:
RT combined with apatinib could help to control intracranial metastases. The Apatinib+RT group had significantly reduced symptoms caused by BM and improved quality of life for patients, the safety of the two treatments was similar.
Advances in knowledge:
Here, we propose that RT combined with apatinib can significantly relieve brain symptoms and tolerate side-effects without affecting OS in patients with BM following failure of multiline therapy for NSCLC. Of course, this paper is a retrospective origin study, and more powerful evidence is needed to demonstrate.
Objectives
Lung cancer is the leading cause of cancer-associated mortality worldwide, and in China, non-small-cell lung cancer (NSCLC) represents approximately 80–85% of all lung cancers. 1,2 Central nervous system (CNS) metastasis is a prevalent and serious complication, with negative effects on quality of life (QoL) and overall survival (OS). 3 More than 10% of NSCLC patients present with brain metastasis (BM) at their first visit to hospital, 4,5 and approximately 30–40% of patients with NSCLC develop BM during the course of their disease, with a poor prognosis and a median survival of 1–4 months, without any treatment. 6 There are many therapeutic methods for BMs, including surgery, chemotherapy, targeted therapy and radiotherapy. Chemotherapy alone has been shown to extend survival by only 2–3 months in patients for whom immune or targeted therapy is not available. 7 There have some studies shown that with targeted therapy or immunotherapy could prolong survival to approximately 3–8 months. However, some patients eventually develop drug resistance after a median of 8–13 months of disease control. 8 Surgical resection or radiosurgery (SRS) combined with adjuvant WBRT prolong survival to approximately 8–11 months. 9
At present, the most common treatment for BM is still radiation therapy (RT), including stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT). WBRT is the most common method for the treatment of BMs because it is suitable for most patients and can rapidly relieve cranial nerve symptoms, with an effective rate of 70%. 10 This may be because BMs are often accompanied by brain oedema, and radiotherapy usually aggravates the oedema of the normal brain tissue to some extent. 10 Local approaches, such as surgery and SRS, are indicated in solitary or oligometastatic disease. Several chemotherapy drugs in combination with WBRT fail to improve survival because of the impenetrability of the blood–brain barrier (BBB). 11 Along with chemotherapy, many targeted agents have been developed to improve the typically dismal outcome associated with NSCLC. Irrespective of the origin and the site of metastases, the growth and survival of tumour cells depend on the establishment of an adequate blood supply, 12 which is mainly supported by neo-angiogenesis. Angiogenesis is regulated by several pro- and anti-angiogenetic factors. Among pro-angiogenetic factors, vascular endothelial growth factor (VEGF) is the most extensively studied and stimulates angiogenesis primarily through activation of vascular endothelial growth factor receptor-2 (VEGFR-2), 13 and both are commonly expressed in NSCLC. 14 The primary goal for using anti-angiogenetic therapies is to block the development of malignant neovasculature, to reduce oxygen availability in the tumour and to decrease its growth. Apatinib is an oral tyrosine kinase inhibitors (TKIs) with anti-angiogenic properties, and it is currently approved for the treatment of advanced gastric cancer. In addition, many studies have demonstrated that apatinib is effective in the treatment of advanced NSCLC, 15 and some studies suggest that apatinib has some synergistic effects with RT. 16 However, the mechanism of action of apatinib combined with RT for better control of BM is not completely clear.
With the development of targeted therapy and immunotherapy, an increasing number of drugs have been shown to have intracranial activity or to sensitize tumours to radiotherapy. Therefore, finding a highly efficient and relatively nontoxic radiosensitization drug is crucial to improving the therapeutic effects of radiotherapy for BMs. Our study aims to demonstrate the clinical efficacy of apatinib combined with radiotherapy vs radiotherapy alone in the treatment of patients with advanced multiline failure for non-small-cell lung cancer with BM.
Methods
Patients
Consecutive advanced multiline therapy failure in patients with NSCLC with BM at the authors’ hospital from January 2016 to August 2020 were retrospectively reviewed. The eligibility criteria for this study were as follows: BM occurred in patients with NSCLC after failure of >2 lines of treatment; patients were historically diagnosed with NSCLC and new BM were confirmed by MRI; they had >3 measurable BM according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1; patients were diagnosed without a mutation in endothelial growth factor (EGFR), anaplastic lymphoma kinase (ALK), repressor of silencing 1 (ROS1), the RET and MET proto-oncogenes etc.; they had no serious dysfunction of major organs (e.g. heart failure or uraemia); and they had adequate haematologic function (absolute neutrophil ≥1.5*109 l−1 or platelet count ≥100*109 l−1).
Study design
Eligible patients were divided into two groups: Apatinib+RT group and RT group. In the Apatinib+RT group, patients with NSCLC received radiation to BM at the same time that apatinib was used to treat lung cancer or other metastatic lesions. Patients in this group continue to take apatinib unless the disease progresses or severe adverse events occured, and the mean duration of apatinib treatment was 7.25 months. In the RT group, patients only received RT to BM.
All patients were evaluated weekly during RT. Evaluation included a complete history, neurologic examination, blood counts, and biochemistry profile. Evaluation during follow-up was done monthly, including physical examination, neurologic examination, a complete blood count measurement, liver function test, and chest CT scan. Brain CT with and without contrast, abdominal CT, or bone scan, as well as MRI if necessary, was performed when there were relevant symptoms.
Statistical analyses
Pearson’s χ2 or Fisher’s exact test was used to compare the baseline characteristics between the apatinib+RT group and RT group. Tumour response was assessed according to RECIST 1.1. OS was defined as the interval from the date of initial BM diagnosis to the date of death. Intracranial PFS was defined as the interval between the WBRT initiation and the date of confirming CNS progression or death from CNS progression if death had occurred within 60 days of the last CNS assessment date. If the complete survival time of a patient was impossible to obtain or the disease did not progress, the patient’s status was assumed to be the last known survival and/or contact date. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v. 4.0.
Intracranial PFS and OS were analysed using the Kaplan–Meier method. Differences between groups were compared by the log-rank test. The Cox proportional hazards model was used for uni- and multivariate analyses to identify the independent prognostic factors for PFS and OS. Statistical analyses were carried out with SPSS 22.0 software. Tests were two-sided. A p-value < 0.05 was considered statistically significant, and robust estimates of the standard error were used in all regression analyses.
Results
Patient characteristics
Among the total of 63 NSCLC patients with advanced BM after undergoing multiline therapy in hospital from January 2016 to August 2021, 31 (49.2%) were in the RT group and 32 (50.8%) were in the Apatinib+RT group.
In the RT group, all patients received WBRT or SRS for BMs. According to the number of BMs, patients with three or fewer BMs received SRS witch has been used Varian EDGE machine. For patients with BMs, single segmental SRS limited V12 Gy brain tissue (normal brain+target vol) to ≤10 cm3. If brain tissue (normal brain+target vol) V12 Gy >10 cm3, fractional SRS considered to reduce the risk of radiation necrosis. Patients with BMs diameter ≤2 cm were treated with single segmental SRS, 20–24 Gy (located in or near a non-functional area). Patients with 2 cm ≤BMs diameter ≤3 cm were treated with single segment SRS, 18 Gy or fractionated SRS (27 Gy/3 fx or 30cGy/5 fx). For large lesions (usually BMs diameter>3 cm), a single dose of SRS is difficult to achieve good local control, and the therapeutic toxicity is significantly increased, Patients were treated with FSRS (27 Gy/3 fx or 30cGy/5 fx). And patients with more than four lesions were treated with WBRT (30 Gy/10 fx or 20 Gy/5 fx). In addition, the protection of organ at risk cannot be ignored: Brain stem: maximum tolerated dose≤54 Gy; Optic nerve and optic chiasma: maximum tolerated dose ≤60 Gy.; Crystal: maximum tolerated dose ≤9 Gy.; Temporal lobe: maximum tolerated dose ≤60 Gy.; Pituitary: maximum tolerated dose ≤50~54 Gy, and the maximum dose in hippocampus was limited to 9–16 Gy. 17
In the Apatinib+RT group, patients received radiotherapy in the same way as in the RT group, but they also received apatinib (500 mg/d) targeted therapy at the same time, until the disease progressed by restaging MRI or CT scan. When patients developed intolerable side-effects, the dose was reduced to 250 mg/d. Patients in the RT group and Apatinib+RT group were well balanced with regard to sex, age, smoking history, Karnofsky performance status (KPS), Lung Cancer Using Molecular Markers (Lung-molGPA), and histologic type (Table 1).
Table 1.
Clinical characteristics of patients
| Characteristics | All patients N(%) |
RT group N(%) |
Apatinib+RT group N(%) |
p |
|---|---|---|---|---|
| All patients | 63(100) | 31(100) | 32(100) | |
| Gender | ||||
| Female | 21 (33.3) | 15 (48.4) | 9 (28.1) | |
| Male | 42 (66.7) | 16 (51.6) | 23 (71.9) | 0.098 |
| Age | ||||
| <65 | 49 (77.8) | 24 (77.4) | 25 (78.1) | |
| ≥65 | 14 (22.2) | 7 (22.6) | 7 (21.9) | 0.946 |
| Smoking | ||||
| Never | 30 (47.6) | 19 (61.3) | 12 (37.5) | |
| Current/former | 33 (52.4) | 12 (38.7) | 20 (62.5) | 0.059 |
| KPS | ||||
| <70 | 18 (28.6) | 8 (25.8) | 10 (31.2) | |
| 70–100 | 45 (71.4) | 23 (74.2) | 22 (68.8) | 0.633 |
| Lung-molGPA | ||||
| 0–1.5 | 42 (66.7) | 22 (71.0) | 20 (62.5) | |
| 2–3 | 21 (33.3) | 9 (29.0) | 12 (37.5) | 0.476 |
| Histology | ||||
| Adenocarcinoma | 39 (61.9) | 22 (71.0) | 17 (53.1) | |
| Non-adenocarcinoma | 24 (38.1) | 9 (29.0) | 15 (46.9) | 0.145 |
| WBRT/SRS | ||||
| WBRT | 28 (44.4) | 16 (51.6) | 12 (37.5) | |
| SRS | 35 (55.6) | 15 (48.4) | 20 (62.5) | 0.260 |
Lung Cancer Using Molecular Markers: Lung-molGPA;Radiation RT: radiation therapy; Karnofsky Performance Status: KPS.
Outcomes stratified by group
The median intracranial PFS for the RT group and Apatinib+RT group was 5.83 months (95% Cl, 2.99–8.67 months) and 11.81 months (95% Cl, 8.31–16.50 months, p = 0.034), respectively, as shown in Figure 1. The median OS for the RT group and Apatinib+RT group was 9.02 months (95% Cl, 6.30–11.70 months) and 13.62 months (95% Cl, 8.13–16.80 months, p = 0.311), respectively, as shown in Figure 2. The Apatinib+RT group had a better intracranial PFS (11.81 vs 5.83 months, p = 0.034), but there were no significant differences between the two arms in OS (13.62 vs 9.02 months, p = 0.311). The above results suggest that RT combined with apatinib could help to control intracranial metastases and delay the progression of intracranial metastases, but there was no significant effect on overall survival.
Figure 1.
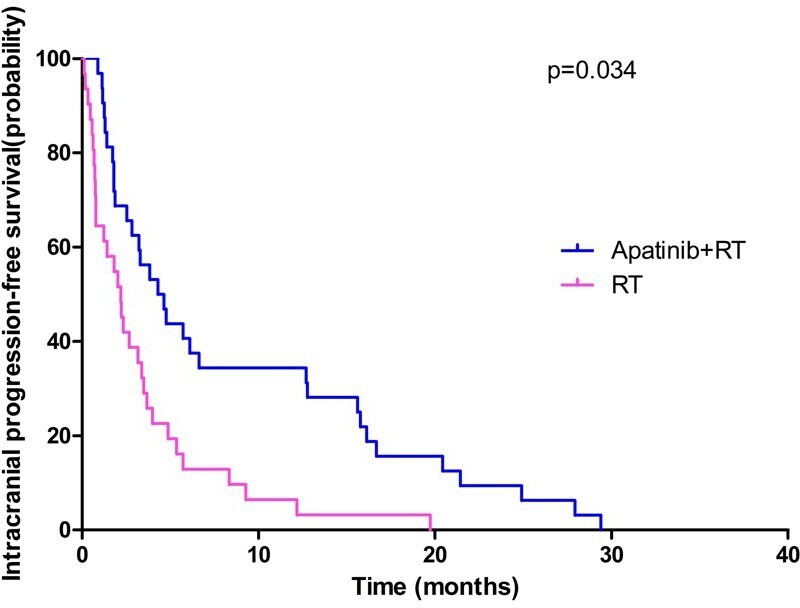
Intracranial progression-free survival of patients between RT group and Apatinib+RT group. RT, radiation therapy.
Figure 2.
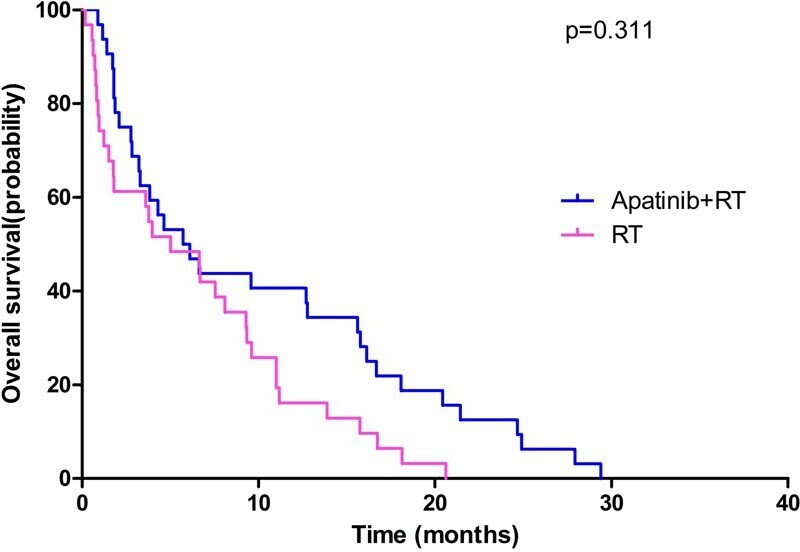
Overall survival of patients between RT group and Apatinib+RT group.
Multivariate analysis and toxicities
Multivariate analysis of intracranial PFS and OS for all NSCLC patients and for the RT group and Apatinib+RT group is shown in Table 2. Among all patients, sex (0.008), KPS (0.006), Lung-molGPA (0.038) and smoking history (0.008) were associated with OS. Within the Apatinib+RT group, sex (0.012) was associated with intracranial PFS, and sex (0.006) and smoking history (0.027) were associated with OS. In the RT group, no significant factors affected the intracranial PFS and OS.
Table 2.
Multivariate analysis of factors affecting intracranial PFS and OS in the patients
| Factors | Intracranial PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| All patients | |||||||
| Gender | 0.393 | 0.141–1.101 | 0.076 | 0.242 | 0.085–0.692 | 0.008 | |
| Age | 1.190 | 0.545–2.601 | 0.663 | 1.260 | 0.593–2.678 | 0.548 | |
| KPS | 1.065 | 0.979–3.357 | 0.057 | 1.950 | 0.370–5.349 | 0.006 | |
| Lung-molGPA | 0.721 | 0.496–1.048 | 0.086 | 0.665 | 0.452–0.978 | 0.038 | |
| Smoking | 0.430 | 0.180–1.026 | 0.057 | 0.297 | 0.121–0.728 | 0.008 | |
| Histology | 0.875 | 0.457–1.674 | 0.686 | 0.637 | 0.337–1.205 | 0.166 | |
| RT group | |||||||
| Gender | 0.824 | 0.221–3.071 | 0.773 | 0.464 | 0.100–2.161 | 0.328 | |
| Age | 1.751 | 0.576–5.317 | 0.323 | 1.748 | 0.529–5.774 | 0.360 | |
| KPS | 1.232 | 0.707–6.050 | 0.171 | 1.178 | 0.901–7.202 | 0.072 | |
| Lung-molGPA | 0.664 | 0.376–1.170 | 0.156 | 0.623 | 0.345–1.125 | 0.117 | |
| Smoking | 0.261 | 0.063–1.085 | 0.065 | 0.242 | 0.054–1.088 | 0.064 | |
| Histology | 0.502 | 0.183–1.375 | 0.180 | 0.675 | 0.229–1.992 | 0.476 | |
| Apatinib+RT group | |||||||
| Gender | 0.050 | 0.005–0.519 | 0.012 | 0.083 | 0.014–0.494 | 0.006 | |
| Age | 0.895 | 0.310–2.586 | 0.838 | 1.185 | 0.442–3.176 | 0.736 | |
| KPS | 1.587 | 0.708–3.555 | 0.262 | 1.300 | 0.941–4.622 | 0.068 | |
| Lung-molGPA | 0.737 | 0.437–1.243 | 0.252 | 0.691 | 0.411–1.162 | 0.163 | |
| Smoking | 0.463 | 0.139–1.536 | 0.208 | 0.277 | 0.089–0.862 | 0.027 | |
| Histology | 0.874 | 0.335–2.281 | 0.784 | 0.774 | 0.321–1.867 | 0.569 | |
progression-free survival: PFS; overall survival: OS; hazard ratio: HR; confidence interval: CI; Karnofsky Performance Status: KPS.
In the Apatinib+RT group, the disease control rate (DCR) and object response rate (ORR) were 56.3% (N = 18) and 38.7% (N = 12), respectively. In the RT group, the DCR and ORR were 19.4% (N = 6) and 37.5% (N = 12), respectively. The DCR and ORR were both higher in the Apatinib+RT group than in the RT group, but there were no statistically significant differences between the two groups (p = 0.163 and 0.111).
The Apatinib+RT group had significantly reduced symptoms caused by BM, mainly headache (21.9 and 19.4%) and vomiting (28.1 and 16.1%). Toxicities were reported in all patients in the RT group and Apatinib+RT group, such as headache (54.8 and 28.1%, respectively), nausea (67.7 and 37.5%) and vomiting (61.3 and 34.4%) (Table 3). Myelosuppression was also one of the more common adverse reactions in both groups, manifesting as anaemia (51.6 and 50.0%, respectively), neutropaenia (45.2 and 53.1%) and thrombocytopaenia (45.2 and 46.9%) in the RT group and Apatinib+RT group.
Table 3.
Toxicity profile for all patients
| Side-effects | RT gruop (%) (N = 31) | Apatinib+RT group (N = 32) | ||
|---|---|---|---|---|
| All grades, N. (%) | Grade III/IV, N. (%) | All grades, N. (%) | Grade III/IV, N. (%) | |
| Fatigue | 17 (54.8) | 3 (9.7) | 18 (56.3) | 4 (12.5) |
| Anorexia | 13 (41.9) | 2 (6.5) | 13 (40.6) | 2 (6.3) |
| Diarrhoea | 3 (9.7) | 0 (0) | 3 (9.4) | 1 (3.1) |
| Nausea | 21 (67.7) | 5 (16.1) | 12 (37.5) | 2 (6.3) |
| Vomiting | 19 (61.3) | 4 (12.9) | 11 (34.4) | 1 (3.1) |
| Headache | 17 (54.8) | 3 (9.7) | 9 (28.1) | 2 (6.3) |
| Anaemia | 16 (51.6) | 1 (3.2) | 16 (50.0) | 1 (3.1) |
| Neutropaenia | 14 (45.2) | 3 (9.7) | 17 (53.1) | 2 (6.3) |
| Thrombocytopaenia | 14 (45.2) | 1 (3.2) | 15 (46.9) | 2 (6.3) |
Radiation therapy: RT.
Most patients tolerated the side-effects well. Overall, all toxicities were generally brief, reversible, and manageable. They were well tolerated after symptomatic treatments.
Quality of life
QOL refers to the subjective feeling and total satisfaction of human beings in physical, psychological, spiritual and social aspects. American medical doctor Wenger think QoL is composed of three parts: 1. Functional state, i.e. patients with a variety of daily life ability and whether they can carry out normal people need a variety of routine activities, including the ability of daily life, social work ability, intelligence, emotional state that is the economic status of the five aspects. 2. The perception state, that is, the patient’s own evaluation of the above cognition varies from person to person. 3. Symptoms can be changed by symptoms caused by the disease itself and adverse reactions caused by various treatments. Therefore, survival therapy, as a response to the physical function, psychological state, social adaptation and environmental factors of patients, can fully reflect the health level of patients. 18,19
According to the tumour patient QoL score standard scores in China 1990, from loss of appetite, spirit, sleep, fatigue, pain, understanding and co-operation with family, understand and co-operate with colleagues, self-understanding of cancer, attitude towards treatment, daily life, treatment side-effects, facial expression Grade 12 aspects, full marks for 60 min, 51–60 was divided into excellent, 41–50 was divided into good, 31–40 is divided into general, 21–30 divided into poor, divided into terrible less than 20. 19 The results were shown in Table 4. There was no significant difference between the RT group and Apatinib+RT group. The score of most patients was 31–50. Patients enrolled in this study were NSCLC with BM after the failure of multiline therapy, so most patients were physically weak, while a few patients with a score less than 20 were in very poor condition, mainly due to long-term bed rest caused by serious adverse reactions. The mean score of RT group was 33.8, and the mean score of Apatinib+RT group was 38.1, which was significantly higher than that of RT group. The proportion of patients with 31–50 scores in Apatinib+RT group (78.1) was significantly higher than that in RT group (67.7%), mainly because the symptoms related to BM were reduced.
Table 4.
QoL profile for all patients
| QoL | All patients% (N = 63) |
RT group % (N = 31) | Apatinib+RT group% (N = 32) |
|---|---|---|---|
| AVERAGE | 36.0 | 33.8 | 38.1 |
| 51–60 | 3 (4.7) | 1 (3.2) | 2 (6.3) |
| 41–50 | 21 (33.3) | 8 (25.8) | 13 (40.6) |
| 31–40 | 25 (39.7) | 13 (41.9) | 12 (37.5) |
| 21–30 | 10 (15.9) | 6 (19.3) | 3 (9.4) |
| <20 | 4 (6.3) | 3 (9.7) | 2 (6.3) |
Quality of QoL.quality of life.
Discussion and conclusions
CNS metastasis is a prevalent and serious complication of NSCLC, with negative effects on quality of life and OS. 3 BM from NSCLC remains a difficult problem in clinical practice. The purpose of this study was to investigate the clinical efficacy of apatinib added to RT in patients with BM after failure of multiline therapy.
Systemic therapies have been deemed ineffective in BM under the hypothesis that the BBB limits their delivery to the brain. 11 The BBB serves as a functional and structural barrier, limiting the passive diffusion of hydrophilic and charged compounds into the brain. Its tight junctions limit the passage of large molecules from the blood to the brain. Lockman et al suggested that the BBB and the blood-tumour barrier present a significant obstacle in the treatment of BMs by limiting drug uptake to subtherapeutic levels. 20 RT is one of the most effective treatments for BMs. 21–23 Here, the patients with three or fewer BMs received SRS, 21 and patients with more than four lesions were treated with WBRT. 23 However, there are few studies on whether VEGFR inhibitor combined with radiotherapy has better clinical efficacy. Our study showed that the Apatinib+RT group had a better intracranial PFS (11.81 vs 5.83 months, p = 0.034). RT combined with apatinib could help to control intracranial metastases and delay the progression of intracranial metastases, but it did not significantly extend OS. In addition, the safety of the two treatments was similar, and apatinib combined with RT did not increase the toxic effects or side-effects compared with RT alone. One point worth mentioning was that, there was no such toxicity facing the radiation oncologists, using Radiation alone. It was true that radiotherapy alone rarely causes myelosuppression. This phenomenon occurs because most of the patients included in this paper were patients who have failed multiline treatment, and the general condition of the patients was poor. Most of the patients have been treated with second-line or third-line chemotherapy, so many patients have myelosuppression.
Apatinib is an oral TKI with anti-angiogenic properties, and many studies have demonstrated that apatinib was effective in the treatment of advanced NSCLC. 15 Tang et al demonstrated that apatinib combined with systemic cytotoxic chemotherapy had clinical efficacy in patients with disease-refractory metastatic NSCLC and provides evidence for further studies investigating apatinib-based combination regimens. Xu et al pointed out that apatinib was effective and well tolerated in patients with advanced NSCLC and had a good clinical effect in the treatment of BMs. 24 Xiaofang Ying et al reported the first case of apatinib combined with brain radiotherapy in EGFR Wild-Type and ALK-Negative Lung Adenocarcinoma With Multiple BMs, which achieved rapid clinical response. They propose that apatinib combined with brain radiotherapy may be an alternative treatment option for BM from NSCLC, especially for those without driver mutations. 25 In our study, when using apatinib for advanced NSCLC, we found that apatinib combined with RT for BM prolonged the intracranial PFS and significantly reduced the symptoms caused by BM and improved QoL for patients, such as intracranial oedema, severe headache, nausea and vomiting. Apatinib, a first-generation oral anti-angiogenic drug, selectively inhibits VEGFR-2, leading to decreased vascular endothelial cell proliferation and migration and tumour microvascular density. 24 Apatinib targets VEGFR-2, RET, platelet-derived growth factor-β (PDGFR-β), v-Src sarcoma viral oncogene homologue (c-Src), and stem cell factor receptor (c-Kit). 26–28 Apatinib can effectively inhibit the proliferation, migration, and tube formation of human umbilical vein endothelial cells, can block the budding of rat aortic rings and can inhibit the growth of several established human tumour xenograft models with little toxicity. 26 Previous studies reported that apatinib could reverse ATP-binding cassette transporter (ABC) subfamily B member 1 (ABCB1/MDR1/P-glycoprotein)- and ABC subfamily G member 2 (ABCG2/BCRP)-mediated multidrug resistance, which suggested the potential usefulness of combining apatinib with other chemotherapy drugs. 27,29
Some studies suggest that apatinib has some synergistic effects with RT. 16 The mechanism through which apatinib combined with RT achieved better control of BM was not completely clear. It may be related to the following points. First, some studies suggest that VEGFR drugs can interfere with tumour metastasis pathways. Angiogenesis, which is mainly mediated by the VEGF pathway, is crucial for tumour survival, growth and invasion both in primary and metastatic brain lesions. As a primary driver of angiogenesis, VEGF is secreted by tumour cells in response to decreased vessel density and hypoxia. VEGF is highly expressed in breast, colorectal, and non-small-cell lung carcinomas. 30–32 Therefore, VEGFR inhibitors (such as apatinib) downregulate this pathway and reduce the number of tumour cells entering the brain, which may enhance the sensitivity to RT. Furthermore, several studies have shown that VEGFR promotes the normalisation of blood vessels, which can improve the delivery of drugs to the brain and play a role in RT sensitisation. 33,34 Tong et al 33 indicated that inhibition of VEGF signalling by a monoclonal antibody that binds to the VEGF ligand, preventing receptor phosphorylation, has been shown to improve drug delivery through vascular normalisation. Jain et al 34 also found that VEGFR inhibition reduces tumour angiogenesis. In addition, a rationale for the use of VEGFR inhibition in BM was the concept of vascular normalisation. 35,36
Other studies have suggested that apatinib can penetrate the blood–brain barrier and play a synergistic role in radiotherapy. 37,38 BM can induce neovascularisation, with leaky vessels, but can also co-opt existing brain vasculature, with a near-normal BBB, particularly in a tumour-infiltrated brain around tumour (BAT). Clinically, BMs can show highly variable permeability, and this has been recapitulated in haematogenous metastases in animal models. 36 Blocking VEGF signalling in systemic tumours produces a morphologically and functionally normalised vasculature by pruning immature vessels and improving perivascular cell and basement membrane coverage and function. 38 It was hypothesised that normalisation of existing tumour vasculature will improve chemotherapy delivery and chemotherapy/radiotherapy efficacy. All of these mechanisms imply that an anti-angiogenic agent would always augment the response to radiation or chemotherapy.
There are some limitations to this study. First, this study was done retrospectively at a single institution, which may have led to inherent bias, and need more powerful evidence is needed to demonstrate. Second, the number of patients enrolled in this study may be insufficient. Factors that may impact the outcomes could not be fully evaluated. The follow-up period may not be long enough. External validation should be done using another large database to further evaluate the value RT combined with apatinib for patients with BM after failure of multiline therapy.
RT combined with apatinib could help to control intracranial metastases and delay the progression of intracranial metastases, but there was no significant effect on overall survival. The Apatinib+RT group had significantly reduced symptoms caused by BM and improved QoL for patients, mainly headache (21.9% and 19.4%) and vomiting (28.1% and 16.1%). In addition, the safety of the two treatments was similar, and apatinib combined with RT did not increase the toxic effects or side-effects compared with RT alone.
Footnotes
Funding: The study was founded by Science and Technology Cooperation Project of Wenzhou Science and Technology Bureau. (grant number 2018H0003); The key R&D Project of the Department of Science and Technology of Zhejiang Province. (2020C03028); Major Project of Wenzhou Science and Technology Bureau. (2020ZY0013).
Ethics approval: It has passed the review of Ethics Committee, and the code of ethics approval is KY2021-R127.
The authors Huanle Pan and Xiaobo Zhou contributed equally to the work.
Contributors: HP, LS and XZ acquired and analyzed the data, XC and CX drafted the manuscript. YL and WD made contributions to follow up the patients. All authors read and approved the final manuscript.
Contributor Information
Huanle Pan, Email: wenzhoucxn@126.com.
Xiaobo Zhou, Email: cxnscx@163.com.
Lanxiao Shen, Email: feicuibinjiang2022@163.com.
Yida Li, Email: ccaicai202203@163.com.
Wenjun Dong, Email: wz569026911@163.com.
Saijun Wang, Email: www20200927@126.com.
Yuyue Zhang, Email: wzcaianna@163.com.
Wenkai Pan, Email: 471413779@qq.com.
Congying Xie, Email: xiecongying@wmu.edu.cn.
Xiaona Cai, Email: 569026911@qq.com.
REFERENCES
- 1. Chen W, Zhang S, Zou X. Estimation and projection of lung cancer incidence and mortality in China. Zhongguo Fei Ai Za Zhi 2010; 13: 488–93. doi: 10.3779/j.issn.1009-3419.2010.05.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steuer CE, Ramalingam SS. Targeting EGFR in lung cancer: lessons learned and future perspectives. Mol Aspects Med 2015; 45: 67–73. doi: 10.1016/j.mam.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ampil F, Caldito G, Milligan S, Mills G, Nanda A. The elderly with synchronous non-small cell lung cancer and solitary brain metastasis: does palliative thoracic radiotherapy have a useful role? Lung Cancer 2007; 57: 60–65. doi: 10.1016/j.lungcan.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 4. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23: 6207–19. doi: 10.1200/JCO.2005.03.145 [DOI] [PubMed] [Google Scholar]
- 5. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007; 12: 884–98. doi: 10.1634/theoncologist.12-7-884 [DOI] [PubMed] [Google Scholar]
- 6. Huang Q, Ouyang X. Predictive biochemical-markers for the development of brain metastases from lung cancer: clinical evidence and future directions. Cancer Epidemiol 2013; 37: 703–7. doi: 10.1016/j.canep.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators . Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol 2015; 1: 457–64. doi: 10.1001/jamaoncol.2015.1145 [DOI] [PubMed] [Google Scholar]
- 8. Yang J-J, Chen H-J, Yan H-H, Zhang X-C, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013; 79: 33–39. doi: 10.1016/j.lungcan.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 9. Zabel A, Debus J. Treatment of brain metastases from non-small-cell lung cancer (NSCLC): radiotherapy. Lung Cancer 2004; 45: S247–52. doi: 10.1016/j.lungcan.2004.07.968 [DOI] [PubMed] [Google Scholar]
- 10. Bradley KA, Mehta MP. Management of brain metastases. Semin Oncol 2004; 31: 693–701. doi: 10.1053/j.seminoncol.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 11. Postmus PE, Smit EF. Chemotherapy for brain metastases of lung cancer: a review. Ann Oncol 1999; 10: 753–59. doi: 10.1023/a:1008318515795 [DOI] [PubMed] [Google Scholar]
- 12. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435–39. doi: 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- 13. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. doi: 10.1016/s0092-8674(00)80108-7 [DOI] [PubMed] [Google Scholar]
- 14. Bonnesen B, Pappot H, Holmstav J, Skov BG. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: relation to prognosis. Lung Cancer 2009; 66: 314–18. doi: 10.1016/j.lungcan.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 15. Tang J, Li XY, Liang JB, Wu D, Peng L, Li X. Apatinib plus chemotherapy shows clinical activity in advanced NSCLC: a retrospective study. Oncol Res 2019; 27: 635–41. doi: 10.3727/096504018X15288447760357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadota K, Huang C-L, Liu D, Ueno M, Kushida Y, Haba R, et al. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer 2008; 44: 1057–67. doi: 10.1016/j.ejca.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 17. Yuankai S, Yan S, Jinming Y, Cuimin D, Zhiyong M, et al. Expert consensus on diagnosis and treatment of brain metastases in lung cancer in China. Chin J Lung Cancer 2017. Available from: No.1:1009-3419 [Google Scholar]
- 18. GANZ PA . Quality of life and the patient with cancer. individual and policy implications. Cancer 1994; 74: 1445–52. doi: [DOI] [PubMed] [Google Scholar]
- 19. Wenger NK. Improved quality of life after PTCA: Generalizability and concerns. Cathet Cardiovasc Diagn 1992; 27: 95–96. doi: 10.1002/ccd.1810270203 [DOI] [PubMed] [Google Scholar]
- 20. Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010; 16: 5664–78. doi: 10.1158/1078-0432.CCR-10-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ursino S, Montrone S, Cantarella M, Menghini V, Matteucci F, Mazzotti V, et al. Stereotactic body radiotherapy of bone metastases in oligometastatic disease: prognostic factors of oncologic outcomes. Tumori 2016; 102: 59–64. doi: 10.5301/tj.5000441 [DOI] [PubMed] [Google Scholar]
- 22. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014; 15: 346–55. doi: 10.1016/j.cllc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 23. Khuntia D, Brown P, Li J, Mehta MP. Whole-Brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006; 24: 1295–1304. doi: 10.1200/JCO.2005.04.6185 [DOI] [PubMed] [Google Scholar]
- 24. Xu J, Liu X, Yang S, Zhang X, Shi Y. Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol 2018; 14: 264–69. doi: 10.1111/ajco.12834 [DOI] [PubMed] [Google Scholar]
- 25. Ying X, Liu H, Wang M, Peng M, Ruan P, Verma V, et al. Clinical response to apatinib combined with brain radiotherapy in EGFR wild-type and ALK-negative lung adenocarcinoma with multiple brain metastases. Front Oncol 2020; 10: 517. doi: 10.3389/fonc.2020.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374–80. doi: 10.1111/j.1349-7006.2011.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tong X, Wang F, Liang S, Zhang X, He J, Chen X, et al. Apatinib (yn968d1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and abcb1-overexpressing leukemia cells. Biochem Pharmacol 2012; 83: 586–97. doi: 10.1016/j.bcp.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 28. Geng R, Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother 2015; 16: 117–22. doi: 10.1517/14656566.2015.981526 [DOI] [PubMed] [Google Scholar]
- 29. Mi Y, Liang Y, Huang H, Zhao H, Wu C-P, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010; 70: 7981–91. doi: 10.1158/0008-5472.CAN-10-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee T-H, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med 2007; 4(): e186. doi: 10.1371/journal.pmed.0040186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kadota K, Huang C-L, Liu D, Ueno M, Kushida Y, Haba R, et al. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer 2008; 44: 1057–67. doi: 10.1016/j.ejca.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 32. Barresi V, Di Gregorio C, Regiani-Bonetti L, Ponz-De Leon M, Barresi G, Vitarelli E. Stage I colorectal carcinoma: VEGF immunohistochemical expression, microvessel density, and their correlation with clinical outcome. Virchows Arch 2010; 457: 11–19. doi: 10.1007/s00428-010-0933-5 [DOI] [PubMed] [Google Scholar]
- 33. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004; 64: 3731–36. doi: 10.1158/0008-5472.CAN-04-0074 [DOI] [PubMed] [Google Scholar]
- 34. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62. doi: 10.1126/science.1104819 [DOI] [PubMed] [Google Scholar]
- 35. Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999; 284: 1994–98. doi: 10.1126/science.284.5422.1994 [DOI] [PubMed] [Google Scholar]
- 36. Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005; 19: 7–16. [PubMed] [Google Scholar]
- 37. Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010; 16: 5664–78. doi: 10.1158/1078-0432.CCR-10-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004; 64: 3731–36. doi: 10.1158/0008-5472.CAN-04-0074 [DOI] [PubMed] [Google Scholar]


