Abstract
We report a case of cerebral phaeohyphomycosis in a 36-year-old male caused by the neurotropic fungus Ramichloridium obovoideum (Matushima) de Hoog 1977 (Ramichloridium mackenziei Campbell et Al-Hedaithy 1993). This man resided in the Middle East, where the fungus appears to be endemic and, possibly, geographically restricted, since all previous reports of brain abscesses due to this organism have been for patients indigenous to this area. As a servant of the Saudi Arabian royal family, he appeared in the United States seeking treatment for chronic weight loss, fatigue, decreased memory, and a more recent 2-week history of right-hand weakness which worsened to involve the entire right upper extremity. On the day prior to his admission, he had a focal motor seizure with rotation of the head and eyes to the right, followed by secondary generalization. A computerized tomogram showed a ring-enhancing hypodense lesion in the left parietal subcortical region with associated edema and mass effect. Diagnosis of a fungal etiology was made following a parietal craniotomy and excisional biopsy by observation of septate, dematiaceous hyphal elements 2 to 3 μm in width on hematoxylin-and-eosin-stained sections from within areas of inflammation and necrosis. Culture of the excised material grew out a dematiaceous mould which was subsequently identified as R. obovoideum. At two months postsurgery and with a regimen of 200 mg of itraconazole twice a day, the patient was doing well and returned to Saudi Arabia. His condition subsequently deteriorated, however, and following a 7-month course of itraconzole, he expired. We use this case to alert clinicians and personnel in clinical mycology laboratories of the pathogenicity of this organism and its potential occurrence in patients with central nervous system signs and symptoms who have resided in the Middle East and to review and/or compare R. obovoideum with other neurotropic, dematiaceous taxa and similar nonneurotropic, dematiaceous species.
The numbers and types of saprobic, opportunistic, and dematiaceous moulds that have been documented as etiologic agents of phaeohyphomycosis continue to escalate. Clinical presentations may include superficial, cutaneous, subcutaneous, and systemic disease. Dematiaceous fungi that occur in compromised hosts may go on to disseminate hematogenously, inciting disease in various organs. Some that disseminate appear neurotropic, i.e., have a predilection for central nervous system (CNS) tissue, where they may localize, causing brain lesions and/or abscesses. These neurotropic organisms are frequently thermotolerant or thermophilic, growing at 40°C or higher. Dematiaceous filamentous taxa known to be neurotropic include the hyphomycetous fungi Cladophialophora bantiana (Saccardo) de Hoog, Kwon-Chung et McGinnis (4, 12, 20, 24, 25, 30, 44, 52), Exophiala dermatitidis (Kano) de Hoog (Wangiella dermatitidis McGinnis) (21, 23, 24, 27, 35, 46, 52), and Ochroconis gallopavum (Cooke) de Hoog (24, 47, 51, 52), as well as the dematiaceous ascomycete Chaetomium atrobrunneum Ames (1, 39). Bipolaris spicifera (Bainer) Subramanian (5, 24, 53) and Bipolaris hawaiiensis (M. B. Ellis) Uchida et Aragaki (24, 31, 41) may also invade the CNS via paranasal sinus extension. Additional dematiaceous agents incriminated include Rhinocladiella atrovirens Nannfeldt (10, 24), Curvularia pallescens Boedijn (16, 24, 26), and Fonsecaea pedrosoi (Brumpt) Negroni (2, 17, 18, 24, 52). Most of these species have a rather widespread distribution and are encountered in various ecological niches. Based on cases previously reported as either Ramichloridium obovoideum or Ramichloridium mackenziei, however, this etiologic agent appears to be geographically restricted to the Middle East (7, 22, 28, 32). Nonneurotropic species sharing macroscopic and microscopic morphologies similar to those of R. obovoideum include Ramichloridium schulzeri Saccardo de Hoog (8, 9, 13, 14, 40), Rhinocladiella aquaspersa (Borelli) Schell et al. (8, 42), and Veronaea botryosa Ciferri et Montemartini (3, 9, 35). A comparison of culture characteristics, microscopic morphologies, and in vitro susceptibilities may aid in differential diagnosis for patients with CNS signs and/or symptoms of a presumed, dematiaceous fungal etiology.
MATERIALS AND METHODS
Case report.
The patient was a 36-year-old male servant of the Saudi Arabian royal family who presented at the Allegheny General Hospital in Pittsburgh, Pa., in May of 1995 with a 2-week history of right-hand weakness which worsened to involve the entire right upper extremity. On the day prior to admission, he had a focal motor seizure with rotation of the head and eyes to the right, followed by secondary generalization.
His past medical history was significant for stage IIIb mixed-cellularity Hodgkin’s disease diagnosed by biopsy of the left axillary lymph node in 1983. The patient was treated with alternating cycles of MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) until complete remission was achieved. In 1986, the patient suffered a relapse which required six cycles of MOPP and ABVD. The patient relapsed again, was treated with CMVP-16 (cyclophosphamide, methotrexate, etoposide), and had been in complete remission since 1987. Past medical history was also significant for schistosomiasis as a child and chronic hepatitis B with portal hypertension, hepatomegaly, and splenomegaly. Since 1993, the patient had noticed a 10- to 12-kg weight loss, was chronically fatigued and apathetic, and had a decreased memory.
Upon admission on 6 May 1995, the patient was fully conscious, had a bitten tongue, normal fundi, and normal visual fields. There was increased weakness in the right upper extremity which was more pronounced distally than proximally, hyperactive deep tendon reflexes, and normal sensation. During his hospital stay, there was deterioration of normal lower-extremity strength over a period of 2 weeks. Except for hepatosplenomegaly, the remainder of the physical examination was within normal limits. The clinical impression was of upper neuron weakness of the right upper and lower extremities with right focal motor seizure with secondary generalization. A space-occupying lesion in the left midrolandic gyrus was suspected.
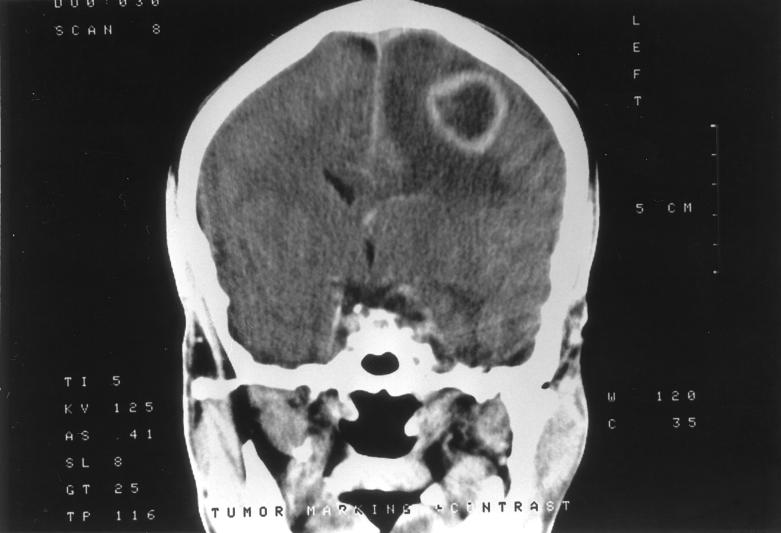
An electroencephalogram showed a normal pattern of waking and sleeping. A preoperative computerized tomogram with and without contrast showed a ring-enhancing hypodense lesion in the left parietal subcortical region with associated edema and mass effect, but no significant midline shift (Fig. 1). Radiographically, the diagnostic possibilities included solitary brain metastasis, high-grade glioma, and brain abscess.
FIG. 1.
A computerized tomogram scan with contrast showing a ring-enhancing hypodense lesion in the left parietal subcortical region with surrounding edema.
Leukocyte count decreased to 3,900 per mm3, with a normal differential. Total amounts of protein and globulins in serum were decreased to 5.9 and 2.25 g%, respectively. Levels of alkaline phosphatase, gamma glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, creatine phosphokinase, and lactate dehydrogenase were 393, 108, 46, 61, 865, and 309 IU/liter, respectively. The serum cholesterol level was elevated to 203 mg/dl. Chest and dorsal spine radiographs were within normal limits. Ultrasound of the abdomen showed mild hepatosplenomegaly but no enlarged retroperitoneal or periaortic lymph nodes. Treatment of the patient with 100 mg of phenytoin was begun.
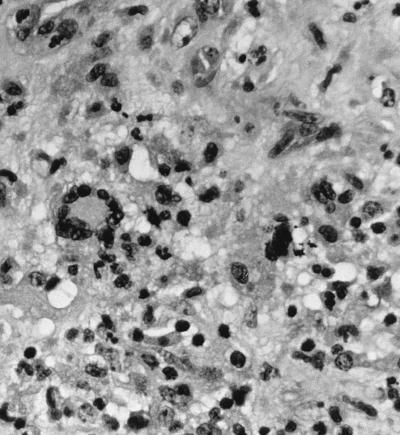
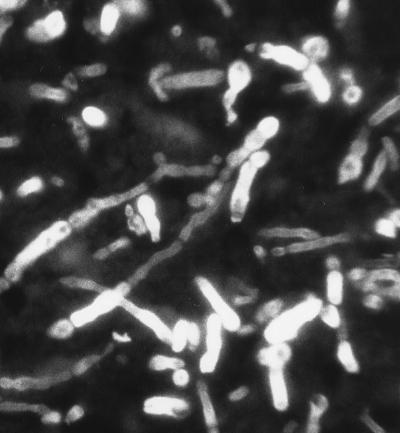
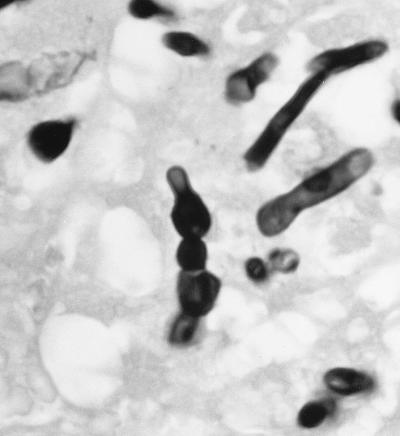
On 18 May, 12 days postadmission, the patient underwent a parietal craniotomy and an excisional biopsy. At operation, an extremely thick capsule was encountered which, upon penetration, contained 3 cm3 of thick, grossly purulent material. The ball of the cavity was dissected free of the surrounding white matter and extended several centimeters below the cortex. Approximately 2 by 2 by 2 cm of white and brown tissue submitted for frozen sectioning and hematoxylin and eosin staining showed a severe mixed acute and granulomatous infiltrate with large areas of tissue necrosis (Fig. 2). Within the areas of inflammation and tissue necrosis were occasional brown pigmented hyphal forms approximately 2 to 3 μm in width, with the presence of septa in some areas. The fungal elements were also readily apparent in paraffin-embedded Congo red tissue sections (Fig. 3). Although the hyphal form predominated, there were also moniliform elements (hyphal elements with swellings a regular intervals) measuring up to 4 μm in diameter at their widest point. These were most easily visualized in the Gomori methenamine silver-stained sections (Fig. 4). Following histopathological confirmation of a dematiaceous mould as the etiologic agent, a regimen of 200 mg of itraconazole (ITRA) twice a day (BID) was begun.
FIG. 2.
Brain lesions showing mixed acute and granulomatous inflammation with hematoxylin and eosin staining. Magnification, ×300.
FIG. 3.
Paraffin-embedded Congo red tissue sections containing hyphal elements of R. obovoideum. Magnification, ×630.
FIG. 4.
Gomori methenamine silver stain showing moniliform hyphal elements of R. obovoideum. Note the moniliform (bead-like) hyphae characteristic of agents of phaeohyphomycosis. Magnification, ×1,200.
The fungal culture was referred to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center at San Antonio (accession no. 95-1147) for identification and susceptibility studies.
In July 1995, 2 months postsurgery, the patient was doing well and the weakness, involving his right arm and leg had improved considerably. He returned to Saudi Arabia, continuing his regimen of 200 mg of ITRA BID. However, his condition subsequently deteriorated, requiring rehospitalization. He expired on 1 January 1996, after having received approximately 8 months of itraconazole. The patient did not garden and had no occupational exposure to the soil. Interestingly, he did walk barefoot in the desert. He had formerly been employed as a telephone operator before his most recent employment as a servant for the royal family.
Histopathology.
Approximately 2 by 2 by 2 cm of white and brown tissue was submitted for frozen sections and hematoxylin and eosin and Gomori methenamine silver stains. Deparaffined histological sections with fluorescent calcofluor white Fungi-Fluor stains (Polysciences, Inc., Warrington, Pa.) and Congo red stains (Sigma Chemical, St. Louis, Mo.) were also examined (48).
Mycology.
Brain tissue from the excisional biopsy was submitted to the mycology laboratory for fungal cultures. The specimen was plated on Sabhi and brain heart infusion agars with 5% sheep blood (Becton Dickinson Microbiology Systems, Cockeysville, Md.), with incubation at 30°C. These primary plates had been sealed with Shrink Seal (Scientific Devise Laboratory, Inc., Glenview, Ill.) to maintain adequate humidity.
The isolate was referred to the Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio, San Antonio, Tex., for identification and susceptibility testing. The isolate was plated onto potato flakes agar (PFA), which was prepared in-house (37), and Mycobiotic Agar (Remel, Lenexa, Kans.) at 25°C (ambient air, alternating daylight and darkness), at 35°C, and at 42°C (ambient air in the dark). Subcultures at 25 and 35°C were held for 4 weeks, while those at 42°C were maintained for 2 weeks. Slide cultures, maintained for 7 days at 25°C, were prepared on PFA blocks and examined in lactophenol cotton blue (Poly Scientific Research and Development Corp., Bay Shore, N.Y.) mounts.
Antifungal susceptibility testing.
The case isolate was tested to determine its susceptibilities to antifungal agents. Tests were performed by previously described macrodilution methods (34, 38). Briefly, the case isolate and the Paecilomyces control strain (UTHSC 90-459) were grown on PFA, which was prepared in-house, for 14 days at 25°C to induce conidial formation. Mature PFA cultures were overlaid with sterile, distilled water, and suspensions were made by gently scraping the colonies with the tip of a Pasteur pipette. Heavy hyphal fragments were allowed to settle, and the upper, homogeneous conidial suspensions were removed. Conidial suspensions were adjusted spectrophotometrically to 95% transmittance at 530 nm and were further diluted 1:10 in medium. Final drug concentration ranges were as follows: for amphotericin B (AMB; E.R. Squibb & Sons, Princeton, N.J.), 0.03 to 16 μg/ml; for 5-fluorocytosine (5-FC; Hoffmann-La Roche Inc., Nutley, N.J.), 0.125 to 64 μg/ml; for fluconazole (FLU; Pfizer, Inc., New York, N.Y.), 0.125 to 64 μg/ml; and for ITRA (Janssen Pharmaceutica, Titusville, N.J.), 0.015 to 8 μg/ml. AMB was tested in Antibiotic Medium 3 (Difco, Detroit, Mich.); other antifungal agents were tested in RPMI-1640 with l-glutamine and morpholinepropanesulfonic acid (MOPS) buffer at a concentration of 165 mM and without sodium bicarbonate (American Biorganics, Inc., Niagara Falls, N.Y.). Previously prepared, frozen drug tubes containing 0.1 ml of drug were allowed to thaw and were inoculated with 0.9 ml of the conidial medium suspension. A drug-free growth control tube was included with the case isolate and control organism. The tubes were incubated at 35°C, and MICs were read at the first 24-h interval when growth was observed in the drug-free growth control. MICs were defined in terms of the first tube that gave a score of 0 (optically clear) for AMB and a score of 2 (reduction in turbidity of ≥80% in contrast to the drug-free control tube) for 5-FC, FLU, and ITRA.
RESULTS
Mycology.
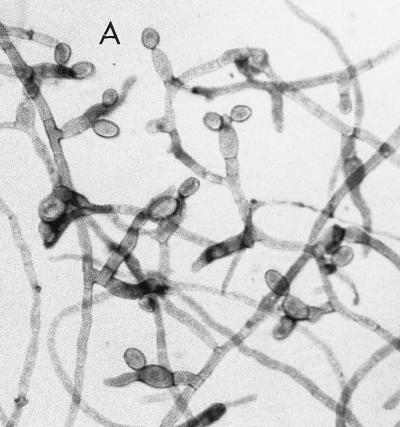
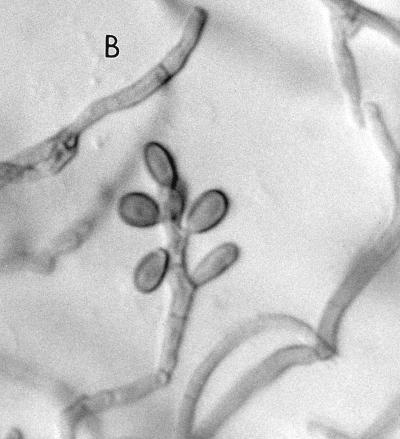
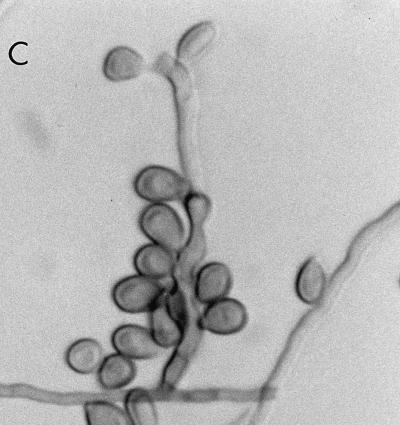
At the Allegheny General Hospital, the mould was first detected on Sabhi and brain heart infusion agars as small, gray, lanose colonies at 4 days of incubation at 30°C. After 7 days of incubation, colonies were 8 to 10 mm, lanose, and gray black. The reverse color on Sabhi agar was gray black, with the tops of the colonies on both media being black, woolly, and domed. The microscopic morphology, by tape and teased mounts in lactophenol cotton blue (Becton Dickinson Microbiology Systems), was described as similar to that seen in F. pedrosoi. Often two smooth-walled and lightly pigmented conidia were seen projecting from both sides of the relatively thick-walled rachis, giving a “Mickey Mouse” appearance (Fig. 5A). Cultures in the Fungus Testing Laboratory on PFA at 25°C revealed colonies that were dark brown to black with a jet-black reverse, woolly, and slow growing, reaching only 2 to 4 mm in 10 days. Six weeks of incubation at 25°C produced colonies with a high-domed central area and a submerged margin throughout. Growth was better at 35 than 25°C with diameters reaching 12 to 20 mm in 17 days. Growth also occurred at 42°C and on media containing cycloheximide, although measurements were not taken. Hyphae were septate and brown, measuring 1.3 to 2.0 μm in diameter. Conidiophores arose at more or less right angles to the vegetative hyphae and were not strongly differentiated from it (micronematous). The conidiogenous areas elongated sympodially, becoming flexous (Fig. 5B). The conidial rachis, 3 to 12 μm long by 3 to 5 μm in diameter, was somewhat larger than the lower part of the conidiogenous cells. Relatively few conidia per fertile axis were present. They were pale brown, ellipsoid to obovoid, single celled, smooth, 5.85 to 8.7 μm long by 2.7 to 4.8 μm wide, with a protuberant hilum or a flat secession scar up to 1.3 μm wide (Fig. 5C).
FIG. 5.
(A) Cellophane tape preparation of R. obovoideum showing the “Mickey Mouse” appearance of immature, sympodially proliferating conidiogenous cells with mostly two conidia. Magnification by the Hoffman modulation contrast system, ×630. (B) Conidiogenous cells of R. obovoideum with few conidia per fertile axis. Magnification by Nomarski optics, ×920. Reprinted from reference 50 with permission of the publisher. (C) Numerous conidia of R. obovoideum showing a protuberant hilum (arrow) or flat secession scar. Magnification by Nomarski optics, ×920. Reprinted from reference 50 with permission of the publisher.
In vitro antifungal susceptibility.
The results of in vitro susceptibility tests, based on drug concentrations normally achievable in patients receiving recommended dosages, indicated that the isolate was susceptible to AMB and ITRA (19, 45). MICs were read initially at 192 h (when growth control was first positive) and again at 216 h (24- to 48-h intervals) were 0.5 and 1 μg/ml and ≤0.015 and ≤0.015 μg/ml, respectively. MICs of 5-FC, an antifungal agent used primarily in combination therapy, were 8 and 8 μg/ml. FLU MICs were 16 and 16 μg/ml, concentrations which may be achieved at elevated dosing regimens (36). Both 5-FC and FLU MICs were read after 192 and 216 h of incubation.
DISCUSSION
Case report.
Although the patient’s past medical history was significant for stage IIIb mixed-cellularity Hodgkin’s disease (in complete remission since 1987), chronic hepatitis B with portal hypertension, hepatomegaly, and splenomegaly, and shistosomiasis as a child, it is difficult to establish whether these conditions were contributory to acquisition of the fungus. The patient denied any occupational exposure to the soil, but he did walk barefoot in the desert. Cerebral phaeohyphomycosis due to other neurotropic agents, particularly C. bantiana, is also frequently seen in patients without obvious (detectable), immunosuppressive, coincidental patterns of preexisting illness and without an unambiguous occupational predisposition, although culturally proven cases do have a male-to female ratio of about 3:1 (12, 24). One exception appears to be the relationship between preexisting Nocardia asteroides and phaeohyphomycotic brain abscesses due to C. bantiana (24, 30, 32, 43). In the 8 cases of R. obovoideum referenced here, in which the sex of the patient was known, the male-to-female ratio was 1:1.
Therapy.
Cerebral phaeohyphomycosis is one of the most serious clinical manifestations caused by dematiaceous fungi and has a high degree of morbidity and mortality, requiring early and aggressive therapy. The patient in this case report was admitted on 6 May 1995, was diagnosed on 18 May 1995, was given an initial dose of 200 mg of ITRA BID on 22 May 1995, and remained on this regimen until 26 December 1995, 6 days prior to expiring on 1 January 1996. Although the patient was diagnosed rather quickly after admission, he had experienced a weight loss of 10 to 12 kg, was chronically fatigued and apathetic, and had had a decrease in memory since 1993. Seventeen cases of intracranial mycoses requiring neurosurgical intervention were reviewed by Jamjoom et al. (22). Citing a 41% mortality, they indicated that reasons for a fatal outcome included (i) failure to consider a fungal etiology, (ii) failure to obtain an early tissue diagnosis because of late referral, and (iii) failure to respond to antifungal therapy. Naim-ur-Rahman et al. reported three cases with a 100% mortality due to rupture of recurring abscesses into the ventricles despite adequate antifungal chemotherapy and multiple surgical procedures (33). The fatal outcome in this patient may have been attributed, in part, both to his seeking medical attention late in the course of the disease to possible failure of ITRA therapy. However, he did appear to receive an adequate dosing regimen for an isolate demonstrating in vitro susceptibility to this agent (MICs of less than 0.015 μg/ml) (45). Although levels in serum documenting adequate absorption of the drug were not measured, the patient did initially respond to the drug, permitting his return home to Saudi Arabia. ITRA, like other azole antifungals, is fungistatic rather than fungicical and may have merely suppressed the organism. Factors in addition to in vitro susceptibility which may have influenced the outcome, as outlined by Rex et al., include the pharmacokinetics of the drug, general host factors, site of infection, and virulence of the pathogen (36). In vivo-in vitro drug studies with other neurotropic agents, C. bantiana, E. dermatitidis, and O. gallopavum (Dactylaria constricta var. gallopava), prior to the introduction of ITRA, suggested that the greatest protection in experimentally infected mice was offered by 5-FC followed by AMB, FLU, and ketoconazole and that in vitro susceptibility testing had no predictive value (11). In vivo animal studies with R. obovoideum versus ITRA were not available at the time of the patient’s therapy.
A review by Dixon et al. of 26 cases of CNS fungal infections due to C. bantiana suggested that the neurosurgical resection of the lesion, with or without antifungal therapy, was the most important determinant for cure and survival (12). A resectable lesion was defined as a discrete mass demarcated by a peripheral gliotic capsule surrounding necrotic infected debris, in contrast to abscesses having poorly delimited margins and satellite abscesses. Complete resection of the lesion in this case was not thought possible.
Antifungal susceptibility.
While standardization in antifungal susceptibility testing has been attained for the major, clinically significant yeasts with publication of the National Committee for Clinical Laboratory Standards document no. M27-A (34), standardization of susceptibility testing for filamentous fungi is only commencing (15). Parameters previously defined for yeast testing, with some modification, also appear to be useful for mould testing. The case isolate in the present study was tested by a modified M27-A method. Although standardized antifungal susceptibility testing now permits large surveys of yeast isolates for correlating MIC data with clinical response, such correlations for infrequently encountered moulds are not possible, still making empiric therapy necessary. Clinical outcomes with R. obovoideum have been dismal, regardless of antifungal therapy, and appear to lack correlation with in vitro data (7).
Taxonomy and identifying features.
The genus Ramichloridium, causing leaf-spots on banana, was first described by Stahel (49). However, the description was invalidly published, lacking a Latin description. In 1977, de Hoog reviewed a number of dematiaceous hyphomycetes that produce holoblastic conidia from a sympodial proliferating axis, including Ramichloridium and Rhinocladiella (8). These genera were separated mainly on the basis of (i) macronematous conidiophores in Ramichloridium versus micronematous conidiophores in Rhinocladiella and (ii) human pathogenicity, lacking in Ramichloridium but present in Rhinocladiella species. A third, less stable characteristic mentioned was the presence of a yellow or an orange diffusable pigment, often present in Ramichloridium but lacking in Rhinocladiella. R. obovoideum (Matsushima) de Hoog comb. nov. was included in Stahel’s review and was originally described as R. obovoideum. In the late 1980s, case reports incriminating R. obovoideum as an agent of cerebral phaeohyphomycosis emerged. In 1993 Campbell and Al-Hedaithy published a review of eight cases due to an organism they named R. mackenziei (Table 1) (7). Characteristics which deviated from the previously-described genus Ramichloridium included (i) a lack of yellow or orange diffusable pigment, (ii) conidiophores not obviously differentiated from the vegetative hyphae, and (iii) the unambiguous human pathogenicity of the organism. Despite these deviations, they felt the most appropriate genus to accommodate these fungi was Ramichloridium. Because Matsushima’s isolate was not available for comparison with the case isolates and conidia from the brain abscess isolates were smaller than those for R. obovoideum (4.7 to 9.0 by 2.7 to 4.0 μm versus 8.8 to 12 by 3.8 to 5 μm), the new name R. mackenziei was proposed in honor of D. W. R. Mackenzie. Our case isolate also had conidial dimensions smaller than those originally described. Until further isolates are reported for comparison and current and future isolates are evaluated at the molecular level, it is difficult to ascertain whether the size of the conidia is a stable characteristic, a view held by Campbell and Al-Hedaithy (7), or whether this variability in conidial dimensions is within the limits for the species (8, 29).
TABLE 1.
Review of cases of cerebral phaeohyphomycosis due to R. obovoideuma
| Patient no. | Age | Sex | Type or no. of abscesses | Histopathology | Underlying disease | Therapy | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 55 | F | Several | Pus; septate, branching hyphae | — | AMB, 5-FC, and KETO | Death |
| 2 | 70 | M | Solitary | Pus; septate hyphae | AMB | Death | |
| 3 | 70 | M | Three | Pus; septate, branching, brown-walled hyphae | Extensive bowel surgery | AMB and 5-FC | — (discharged against medical advice) |
| 4 | 60 | F | Unilateral | Aspirated pus | Prior surgery | — | — |
| 5 | 55 | M | Thalamic while on AMB | Pus; brown-walled, branching hyphae | Renal transplant 5 months prior | AMB | Death |
| 6 | 32 | F | Solitary | Pus; yellow-brown hyphae | Renal transplant 5 months prior | — | — |
| 7 | — | — | Solitary (—) | — | — | — | Death |
| 8 | 75 | F | Two | Pus; branched, brown-walled, septate hyphae | — | — | — |
| 9 (UTHSC no. 97-1147) | 36 | M | Solitary | Pus; brown, septate hyphae; moniliform fungal elements | Hodgkin’s disease (in remission since 1987) | ITRA (200 mg/BID for 8 mo) | Death |
F, female; M, male; —, data unknown; KETO, ketoconazole.
Other dematiaceous hyphomycetes with potential or genuine cerebral pathogenicity include C. bantiana (also named Xylohypha bantiana, Cladosporium bantianum, Cladosporium trichoides, Cladosporium trichoides var. chlamydosporum, and Xylohypha emmonsii), E. (Wangiella) dermatitidis, and O. gallopavum (Dactylaria gallopava and Dactylaria constricta var. gallopava). The darkly pigmented ascomycete C. atrobrunneum is an additional taxon causing cerebral phaeohyphomycosis. Characteristics of these five dematiaceous neurotropic taxa, including their in vitro susceptibility data, are outlined in Table 2. A closely related species, Chaetomium strumarium, although not dematiaceous, is also an agent of brain abscesses (1). Additional organisms which have been reported to invade the CNS include the graminicolous (living on grass) species B. spicifera (5, 24, 53), B. hawaiiensis (31, 41), and C. pallescens (16, 26). R. atrovirens was also incriminated in CNS involvement in a human immunodeficiency virus-positive male drug abuser (10).
TABLE 2.
Dematiaceous neurotropic taxaa
| Species | Macroscopic morphology on PFAb | Microscopic morphologyc | Ecology | Physiologyd | % Susceptible in vitro (n) (drug)e | Comment(s) | References |
|---|---|---|---|---|---|---|---|
| R. obovoideum (R. mackenziei) | Black, woolly, domed colony; slow growth | Sympodial; few conidia per fertile axis; brown conidia; 4.7–9.6 by 2.7–6.0 | Middle East; soil? plants? | 40°C, +; cyclo, V | 100 (1) (AMB) | Domed colony occurs at maturity | 7, 22, 28, 32, 50 |
| 100 (1) (5-FC) | |||||||
| 100 (1) (FLU) | |||||||
| 100 (1) (ITRA) | |||||||
| C. (Xylohypha) bantiana | Olivaceous to black; woolly; moderate growth | Long, poorly branched, non- fragile chains of conidia; hila absent | Wide distribution in soil and plants | 40°C, +; cyclo., +; urease, + | 83 (6) (AMB) | Other names include Cladosporium trichoides | 12, 20, 24, 25, 30, 44, 50, 52 |
| 100 (4) (5-FC) | |||||||
| 100 (1) (FLU) | |||||||
| 100 (6) (ITRA) | |||||||
| 100 (1) (MON) | |||||||
| E. (Wangiella) dermatitidis | Olivaceous to black; mucoid; moderate growth | Black yeast synanamorph present; ellipsoidal conidia; 2.5–4 by 2–3 | Wide distribution in soil, plants, and water | 40°C, +; nitrate, − | 100 (1) (AMB) 100 (2) (FLU) 100 (3) (ITRA) | Both annellides and phialides may be present; colony may be more filamentous on Sabouraud dextrose agar | 21, 23, 24, 27, 35, 46, 50, 52 |
| O. gallopavum, (Dactylaria gallopava) | Reddish-brown, velvety, maroon diffusing pigment; slow growth | Two-celled, clavate conidia; 11–18 by 2.5–4.5; on denticles | Wide distribution in thermal areas | 42°C, +; cyclo., −; urease, + | 100 (2) (AMB) 100 (1) (5-FC) 100 (2) (ITRA) | Distinguished from other agents by being brownish rather than gray | 24, 47, 50–52 |
| C. atrobrunneum | Gray to black with production of perithecia; moderate growth | Perithecia diam, 70–150; subglobose, dark brown; few straight setae; brown ascospores; fusoidal; 9.3–10.8 by 4.9–6.2 | Wide distribution in soil, plants, and air | 40°C, + | None available | Fatal in a leukemic patient | 1, 39 |
This list is not all inclusive. B. spicifera and B. hawaiiensis may invade the CNS via paranasal sinus extension. Additional dematiaceous agents reported include R. atrovirens, C. pallescens, and F. pedrosoi. In some instances, C. bantiana may have been reported as F. pedrosoi.
PFA was prepared in-house for 10 days at 25°C.
Dimensions are given in micrometers.
+, growth; V, variable growth; −, no growth; cyclo, cycloheximide.
n, number of isolates tested by the National Committee for Clinical Laboratory Standards Reference method for broth dilution antifungal susceptibility testing of yeasts (proposed standard M27-A, modified to accommodate mould testing). Mould susceptibility testing is currently nonstandardized, and breakpoints have not been established. Percent susceptibility and percent resistance, respectively, are based upon the following MICs (in micrograms per milliliter): AMB, ≤1 and ≥2; 5-FC, ≤16 and ≥32; FLU, ≤32 and ≥64; ITRA, ≤0.5 and ≥1.0; and miconazole (MON), ≤8 and ≥16.
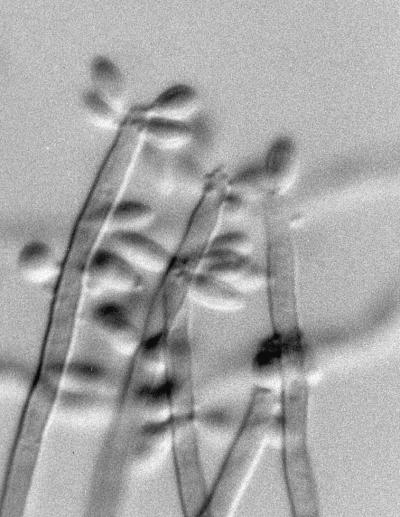
Nonneurotropic dematiaceous fungi sharing macroscopic and microscopic morphologies similar to those of R. obovoideum include R. schulzeri, R. aquaspersa, and V. botryosa. R. schulzeri, which was reported in a case of “golden tongue” in a leukemic patient, is also sometimes seen from respiratory isolates. It more closely matches the original description of the genus Ramichloridium in that the conidiophores are markedly differentiated from the vegetative hyphae, there are numerous conidia per fertile axis (Fig. 6), and a distinct yellow diffusable pigment is usually present. R. aquaspersa, an agent of human chromoblastomycosis, bears closely packed hyaline-to-subhyaline and ellipsoid conidia on crowded denticles. V. botryosa, an agent of subcutaneous phaeohyphomycosis in China, produces predominately two-celled, crowded conidia on a sympodially proliferating fertile axis.
FIG. 6.
R. schulzeri with dark conidiophores and numerous conidia per fertile axis. Magnification by Nomarski optics, ×920.
The numbers and types of opportunistic dematiaceous fungi continue to escalate. While most have occurred in immunocompromised hosts, patients without obvious detectable immunosuppression may also be at risk for disseminated disease. R. obovoideum (R. mackenziei) should be considered as a possible etiologic agent in cerebral phaeohyphomycosis, particularly in patients indigenous to Middle Eastern countries. It is also prudent for laboratory workers studying this organism to bear in mind its likely neurotropic nature. Because of its potential respiratory acquisition and until the route of infection is clearly elucidated, all studies with this mould, as well as with all other filamentous fungi, should be accomplished with a biological safety hood.
REFERENCES
- 1.Abbott S P, Sigler L, McAleer R, McGough D A, Rinaldi M G, Mizell G. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J Clin Microbiol. 1995;33:2692–2698. doi: 10.1128/jcm.33.10.2692-2698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hedaithy, S. S. A., Z. A. B. Jamjoom, and E. S. Saeed. 1988. Cerebral phaeohyphomycosis caused by Fonsecaea pedrosoi in Saudi Arabia. Acta Pathol. Microbiol. Scand. 96(Suppl. 3):94–100. [PubMed]
- 3.Ayadi A, Huerre M-R, de Bievre C. Phaeohyphomycosis caused by Veronea botryosa. Lancet. 1995;346:1703–1704. doi: 10.1016/s0140-6736(95)92866-9. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J E, Bonner H, Jennings A E, Lopez R I. Chronic meningitis caused by Cladosporium trichoides. Am J Clin Pathol. 1973;59:398–407. doi: 10.1093/ajcp/59.3.398. [DOI] [PubMed] [Google Scholar]
- 5.Biggs P J, Allen R L, Powers M M, Holley H P. Phaeohyphomycosis complicating compound skull fracture. Surg Neurol. 1986;25:393–396. doi: 10.1016/0090-3019(86)90216-8. [DOI] [PubMed] [Google Scholar]
- 6.Borelli D. Acrotheca aquaspersa nova species agente de cromomicosis. Acta Cient Venez. 1972;23:193–196. [PubMed] [Google Scholar]
- 7.Campbell C K, Al-Hedaithy S S A. Phaeohyphomycosis of the brain caused by Ramichloridium mackenziei sp. nov. in middle eastern countries. J Med Vet Mycol. 1993;31:325–332. [Google Scholar]
- 8.de Hoog G S. Rhinocladiella and allied genera. Stud Mycol. 1977;15:1–140. [Google Scholar]
- 9.de Hoog G S, Rahman M A, Boekhout T. Ramichloridium, Veronaea, and Stenella: generic delimitation, and new combinations and two new species. Trans Br Mycol Soc. 1983;81:485–490. [Google Scholar]
- 10.del Palacio-Hernanz A, Moore M K, Campbell C K, del Palacio-Medel A, Del Castillo R. Infection of the central nervous system by Rhinocladiella atrovirens in a patient with acquired immunodeficiency syndrome. J Med Vet Mycol. 1989;27:127–130. [PubMed] [Google Scholar]
- 11.Dixon D M, Polak A. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy. 1987;33:129–140. doi: 10.1159/000238485. [DOI] [PubMed] [Google Scholar]
- 12.Dixon D M, Walsh T J, Merz W G, McGinnis M R. Infections due to Xylohypha bantiana (Cladosporium trichoides) Rev Infect Dis. 1989;11:515–525. doi: 10.1093/clinids/11.4.515. [DOI] [PubMed] [Google Scholar]
- 13.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. London, United Kingdom: Academic Press; 1980. pp. 698–699. [Google Scholar]
- 14.Ellis M B. More dematiaceous hyphomycetes. Kew, United Kingdom: Commonwealth Mycological Institute; 1976. pp. 163–165. [Google Scholar]
- 15.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman A D, Campos J M, Rorke L B, Bruce D A, Arbeter A M. Fatal recurrent Curvularia brain abscess. J Pediatr. 1981;99:413–415. doi: 10.1016/s0022-3476(81)80331-9. [DOI] [PubMed] [Google Scholar]
- 17.Fukushiro R. Chromomycosis in Japan. Int J Dermatol. 1983;22:221–229. doi: 10.1111/j.1365-4362.1983.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukushiro R, Kagawa S, Nishiyama S, Takahashi H. Un cas de chromoglastomycose cutanée avec métastase cérébrale mortelle. Presse Méd. 1957;65:2142. [PubMed] [Google Scholar]
- 19.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 20.Heney C, Song E, Kellen A, Raal F, Miller S D, Davis V. Cerebral phaeohyphomycosis caused by Xylohypha bantiana. Eur J Clin Microbiol Infect Dis. 1989;8:984–988. doi: 10.1007/BF01967570. [DOI] [PubMed] [Google Scholar]
- 21.Hiruma M, Kawanda A, Ohata H, Ohnishi Y, Takahashi H, Yamazaki M, Ishibashi A, Hatsuse K, Kakihara M, Yoshida M. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses. 1993;36:1–7. doi: 10.1111/j.1439-0507.1993.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 22.Jamjoom A B, al-Hedaithy S A, Jamjoom Z A, Al-Hedaithy M, el-Waridy S F, Rahman N, Al-Moallern M. Intracranial mycotic infections in neurosurgical practice. Acta Neurochir. 1995;137:78–84. doi: 10.1007/BF02188786. [DOI] [PubMed] [Google Scholar]
- 23.Kenney R T, Kwon-Chung J K, Waytes A T, Melnick D A, Pass H I, Merino M J, Gallin J I. Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin Infect Dis. 1992;14:235–242. doi: 10.1093/clinids/14.1.235. [DOI] [PubMed] [Google Scholar]
- 24.Kwon-Chung K J, Bennett J E, editors. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 620–677. [Google Scholar]
- 25.Lacy M, Farr W. Focus on Fungal Infections 6. Alpharetta, Ga: Imedex USA, Inc.; 1996. Cladophialophora bantiana brain abscess unresponsive to itraconazole and amphotericin B: case report, abstr. 41; p. 41. [Google Scholar]
- 26.Lampert R P, Hutto J H, Donnelly W H, Shulman S T. Pulmonary and cerebral mycetoma caused by Curvularia pallescens. J Pediatr. 1977;91:603–605. doi: 10.1016/s0022-3476(77)80511-8. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T, Padhye A A, Ajello L, Standard P G. Critical review of human isolates of Wangiella dermatitidis. Mycologia. 1984;76:232–249. [Google Scholar]
- 28.Matsushima T. Icones microfungorum a Matsushima lectorum. 1975. p. 123. . Kobe, Japan. [Google Scholar]
- 29.McGinnis M R, Rinaldi M G. Some medically important fungi and their common synomyms and obsolete names. Clin Infect Dis. 1997;25:15–17. doi: 10.1086/514507. [DOI] [PubMed] [Google Scholar]
- 30.Middleton F G, Jurgenson P F, Utz J P, Shadomy S, Shadomy H J. Brain abscess caused by Cladosporium trichoides. Arch Intern Med. 1976;136:444–448. [PubMed] [Google Scholar]
- 31.Morton S J, Midthun K, Merz W G. Granulomatous encephalitis caused by Bipolaris hawaiiensis. Arch Pathol Lab Med. 1986;110:1183–1185. [PubMed] [Google Scholar]
- 32.Musella R A, Collins B H. Cerebral chromoblastomycosis. J Neurosurg. 1968;35:219–222. doi: 10.3171/jns.1971.35.2.0219. [DOI] [PubMed] [Google Scholar]
- 33.Naim-ur-Rahman, El Sheikh Mahgoub, Chagla A H. Fatal brain abscesses caused by Ramichloridium obovoideum: report of three cases. Acta Neurochir. 1988;93:92–95. doi: 10.1007/BF01402887. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 35.Nishimura K, Miyaji M, Taguchi H, Wang D L, Li R Y, Meng Z H. Proceedings of the 4th International Symposium of the Research Center of Pathogenic Fungi, Microbiology, and Toxicology. Tokyo, Japan. 1989. An ecological study of pathogenic dematiaceous fungi in China; pp. 17–20. [Google Scholar]
- 36.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldi M G. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982;15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldi M G, Howell A W. Antifungal antimicrobics: laboratory evaluation. In: Wentworth B, editor. Diagnostic procedures for mycotic and parasitic infections. 7th ed. Washington, D.C: American Public Health Association; 1988. pp. 325–356. [Google Scholar]
- 39.Rinaldi M G, Inderlied C B, Mahnovski V, Monforte H, Lam B L, Fothergill A W, McGough D A. Abstracts of the 11th Congress of the International Society for Human and Animal Mycology. 1991. Fatal Chaetomium atrobrunneum Ames, 1949, systemic mycosis in a patient with acute lumphoblastic leukemia, abstr. PS2.69; p. 107. [Google Scholar]
- 40.Rippon J W, Arnow P M, Larsoon R A, Zang K L. “Golden tongue” syndrome caused by Ramichloridium schulzeri. Arch Dermatol. 1985;121:892–894. [PubMed] [Google Scholar]
- 41.Ruben S J, Scott T E, Seltzer H M. Intracranial and paranasal sinus infection due to Drechslera. South Med J. 1987;80:1057–1058. doi: 10.1097/00007611-198708000-00031. [DOI] [PubMed] [Google Scholar]
- 42.Schell W A, McGinnis M R, Borelli P. Rhinocladiella aquaspersa, a new combination for Acrotheca aquaspersa. Mycotaxon. 1983;17:341–348. [Google Scholar]
- 43.Seaworth B J, Kwon-Chung K J, Hamilton J D, Perfect J R. Brain abscess due to a variety of Cladosporium trichoides. Am J Clin Pathol. 1983;79:747–752. doi: 10.1093/ajcp/79.6.747. [DOI] [PubMed] [Google Scholar]
- 44.Sekhon A S, Galbraith J, Mielke B W, Garg A K, Sheehan G. Cerebral phaeohyphomycosis caused by Xylohypha bantiana, with a review of the literature. Eur J Epidemiol. 1992;8:387–390. doi: 10.1007/BF00158573. [DOI] [PubMed] [Google Scholar]
- 45.Sharkey P K, Rinaldi M G, Dunn J F, Hardin T C, Fetchick R J, Graybill J R. High dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;35:707–713. doi: 10.1128/aac.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimazono Y, Isaki K, Torii H, Otsuka R. Brain abscess due to Hormodendrum dermatitidis (Kano) Conant, 1953. Report of a case and review of the literature. Folia Psychiatr Neurol Jpn. 1963;17:80–96. doi: 10.1111/j.1440-1819.1963.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 47.Sides E H, Benson J D, Padhye A A. Phaeohyphomycotic brain abscess due to Ochroconis gallopavum in a patient with maligmant lymphoma of a large cell type. J Med Vet Mycol. 1991;29:317–322. [PubMed] [Google Scholar]
- 48.Slifkin M, Cumbie R. Congo red as a fluorochrome for the rapid detection of fungi. J Clin Microbiol. 1988;26:827–830. doi: 10.1128/jcm.26.5.827-830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahel G. The banana leaf speckle in Surinam caused by Chloridium musae nov. spec. and other related banana disease. Trop Agric (Trinidad) 1937;14:42–44. [Google Scholar]
- 50.Sutton D A, Fothergill A W, Rinaldi M G. Guide to clinically significant fungi. Baltimore, Md: Williams & Wilkins; 1997. [Google Scholar]
- 51.Terreni A A, DiSalvo A F, Baker A S, Jr, Crymes W B, Morris P R, Dowda H., Jr Disseminated Dactylaria gallopava infection in a diabetic patient with chronic lymphocytic leukemia of the T-cell type. Am J Clin Pathol. 1990;94:104–107. doi: 10.1093/ajcp/94.1.104. [DOI] [PubMed] [Google Scholar]
- 52.Walsh T J, Dixon D M, Polak A, Salkin I F. Comparative histopathology of Dactylaria constricta, Fonsecaea pedrosoi, Wangiella dermatitidis, and Xylohypha bantiana in experimental phaeohyphomycosis of the central nervous system. Mykosen. 1987;30:215–225. doi: 10.1111/j.1439-0507.1987.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 53.Young C N, Swart J G, Ackerman D, Davidge-Pitts K. Nasal obstruction and bone erosion caused by Drechslera hawaiiensis. J Laryngol Otol. 1978;92:137–143. doi: 10.1017/s0022215100085145. [DOI] [PubMed] [Google Scholar]