Abstract
Purpose:
While clinical heart failure (HF) is recognized as an adverse effect from breast cancer (BC) treatment, sparse data exist on specific HF phenotypes in affected BC survivors. We examined risk of HF by left ventricular ejection fraction (LVEF) status in women with a history of BC.
Methods:
14,804 women diagnosed with all stages of invasive BC from 2005–2013 and with no history of HF were matched 1:5 to 74,034 women without BC on birth year, race, and ethnicity. LVEF values were extracted from echocardiography studies within 30 days before through 90 days after the HF clinical encounter. HF was stratified into HF with preserved ejection fraction (HFpEF, LVEF≥45%) and HF with reduced ejection fraction (HFrEF, LVEF<45%). Cumulative incidence rates (CIRs) were estimated with competing risk of overall death. Hazard ratios (HR) were calculated by multivariable Cox proportional hazards regression.
Results:
Mean time to HF diagnosis was 5.31 years (range 0.03–13.03) in cases and 5.25 years (range 0.01–12.94) in controls. 10-year CIRs were 1.2% and 0.9% for overall HF, 0.8% and 0.7% for HFpEF, and 0.4% and 0.2% for HFrEF in cases and controls, respectively. In fully-adjusted models, an overall significant increased risk of HF in cases versus controls was observed (HR: 1.31, 95% CI: 1.14, 1.51). The increased risk was seen for both HFrEF (HR: 1.59, 95% CI: 1.22, 2.08) and HFpEF (HR: 1.22; 95% CI: 1.03, 1.45).
Conclusion:
BC survivors experienced higher risk of HF compared with women without BC, and the risk persisted across LVEF phenotypes. Systematic cardio-oncology surveillance should be considered to mitigate this risk in BC patients.
Keywords: breast cancer, breast cancer survivors, heart failure, preserved ejection fraction, reduced ejection fraction, ejection fraction, left ventricular ejection fraction, prospective cohort study, breast cancer survivors, cardio-oncology
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for approximately 50% of incident heart failure (HF) overall in the general population [1]. HFpEF characteristics have been described to differ from HF with reduced ejection fraction (HFrEF) in that HFpEF patients are older, more often female, and have more cardiovascular and non-cardiovascular comorbidities [2].
Recently, a universal definition of HF was proposed to standardize identification and classification of HF for clinical practice, preventive and treatment strategies, performance measures, and research purposes [3]. The universal definition underscores that HF is a clinical syndrome with signs and/or symptoms caused by structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion. Within this framework, HFrEF is defined as HF with left ventricular ejection fracture (LVEF) ≤40%, while HFpEF includes LVEF ≥50%.
Identifying specific subpopulations at risk for HFpEF is crucial to develop effective screening due to the pathophysiological heterogeneity that exists within HFpEF cohorts [4]. Few studies to date have examined HF delineating LVEF phenotype in women with breast cancer, a population at higher risk of poor CVD outcomes due to cardiotoxic treatments [5]. Most cardio-oncology studies in breast cancer cohorts have focused on LVEF as a surrogate for HF [6], due to limited duration of follow-up and use of imaging-based metrics rather than hard endpoints for study feasibility. While this clinical framework may ascertain some cases of HFrEF, it largely neglects HFpEF, which can occur long after post-cancer treatment and is defined by normal LVEF. Herein, we provide a population-based estimate of HF by LVEF phenotype in breast cancer survivors, comparing risk of HF in women with and without a history of breast cancer history in Kaiser Permanente Northern California (KPNC), a large integrated health system that provides comprehensive medical care for more than 4.5 million members covering the San Francisco Bay Area and the Central Valley of California.
Methods
The Pathways Heart Study is an ongoing National Cancer Institute (NCI)-funded cohort study within KPNC examining the incidence of CVD events and cardiometabolic risk factors in women with and without a history of breast cancer treatment (R01 CA214057). Administrative and clinical data were extracted from KPNC electronic health records (EHR) for this data-only analysis. All women diagnosed with incident American Joint Committee on Cancer 7th Edition (AJCC 7) Stage I-IV breast cancer from 2005–2013 with no prior history of invasive breast cancer were identified from the NCI’s Surveillance, Epidemiology, and End Results (SEER)-affiliated KPNC Cancer Registry. They were then matched 1:5 to women without a history of breast cancer on birth year, race (White, Black, Asian, Native Hawaiian and/or Pacific Islander, or American Indian and/or Alaska Native), and ethnicity (Hispanic or Non-Hispanic) identified from KPNC membership and demographic data sources.
Cardiometabolic risk factors and CVD events were ascertained according to the International Classification of Diseases (ICD) Ninth and Tenth diagnosis codes and Current Procedural Terminology codes from inpatient, ambulatory, and emergency department encounters and/or hospital discharge. Specifically, clinical HF diagnoses were determined by validated ICD-9 and ICD-10 diagnosis codes: 398.91, 402.01, 402.11, 402.91, 404.11, 404.13, 428.0, 428.1, 428.9, 404.01, 404.03, 404.91, 404.93, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, I09.81, I11.0, I13.0, I13.2, I50, I50.1, I50.2, I50.20, I50.21, I50.22, I50.23, I50.3, I50.30, I50.31, I50.32, I50.33, I50.4, I50.40, I50.41, I50.42, I50.43, I50.8, I50.81, I50.810, I50.811, I50.812, I50.813, I50.814, I50.82, I50.83, I50.84, I50.89, I50.9, I97.13. A physician-adjudicated validation study of ICD diagnosis codes for HF (428.0, 428.1, I11.0, I13.0, I50.21, I50.30, I50.31, I59.0) was completed in a small sample of KPNC patients (n=47) and found 91% (43 out of 47) positive predictive value of code ascertainment versus chart review. All events were independently reviewed by the two study cardiologists at KPNC (J.S.R. and C.I.), and any discrepancies were resolved via discussion and consensus.
LVEF values were obtained from echocardiography (ECHO) exams 30 days before the HF clinical encounter through 90 days after the encounter. Specifically, LVEF values were extracted from ECHO reports using text string searches and then supplemented by manual chart review by an experienced medical records abstractor for missing data. If any inconsistencies in dates of HF diagnosis relative to LVEF value were ascertained in the breast cancer cases, these patients were also manually adjudicated by a clinical cardiologist (JSR). Patients with an HF diagnosis for whom we could not ascertain their LVEF status, along with those having any prior history of HF, were excluded from the analysis. We recognize that measurement of LVEF is a continuum and requires clinical interpretation. For the present analysis, we selected LVEF status for HFpEF (LVEF≥45%) and HFrEF (LVEF<45%) based on prior major clinical trials testing the efficacy of emerging therapies for HFpEF [7, 8]. We also conducted a sensitivity analysis defining HFpEF as LVEF≥40% and HFrEF as LVEF<40% [9].
Baseline characteristics of the case and control populations were summarized by mean and standard deviation for continuous variables and frequency and percentage for categorical variables. Cumulative incidence rates of overall HF, HFpEF, and HFrEF were estimated using the cumulative incidence function accounting for the competing risk of overall death and censoring individuals at the end of follow-up.
Hazard ratios (HR) and 95% confidence intervals (CI) representing the association between having breast cancer and risk of incident HF by LVEF status were estimated from multivariable Cox proportional hazards regression. The index date was date of confirmed breast cancer diagnosis in the cases, usually from pathological confirmation, and the same (reference) date in the controls. The time scale was defined as time from the index date to earliest date of first incident HF, health plan disenrollment, death, or December 31, 2018. The proportional hazards assumption was tested using Schoenfeld residuals, and no evidence of violation was found. Models were adjusted for baseline body mass index; menopausal status; smoking status; prior history of hypertension, diabetes, and dyslipidemia; neighborhood median household income and education level; and prior history of CVD conditions (arrhythmia, HF, cardiomyopathy, myocarditis/pericarditis, stroke/transient ischemic attack (TIA), valvular disease, venous thromboembolic disease, ischemic heart disease) and chronic kidney disease within the two years before breast cancer diagnosis. Pre-specified case subgroups who received any cardiotoxic chemotherapy, left-sided radiation therapy, and/or endocrine therapy, were compared with their matched controls.
Analyses were conducted in R with the survival package (https://cran.r-project.org/web/packages/survival/index.html). This data-only study received human subjects research approval from the KPNC Institutional Review Board.
Results
A total of 14,804 women diagnosed with invasive breast cancer and with no history of HF were identified and matched to 74,034 women without breast cancer (Table 1). Women were mean 61.1 years (SD=12.8) at breast cancer diagnosis and 65% white, 15% Asian, 12% Hispanic, 7% Black, and 1% American Indian and/or Alaska Native. Breast cancer cases were comprised of more overweight and obese women and more non-smokers and former smokers compared with their matched controls. In the breast cancer cases, 182 women had HFpEF and 90 had HFrEF, whereas in the matched non-breast cancer controls, 732 women had HFpEF and 258 had HFrEF. In both groups, women with HFpEF were older and more likely to have hypertension (p<0.05) compared to those with HFrEF.
Table 1.
Characteristics of breast cancer cases and matched controls, overall and by incident heart failure with preserved ejection fraction (HFpEF, LVEF≥45%) and heart failure with reduced ejection fraction (HFrEF, LVEF<45%)
| Cases (n=14,804) | Controls (n=74,034)a | P-valuec | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n=14,804 | HFpEF n=182 | HFrEF n=90 | P-valueb | Total n=74,034 | HFpEF n=732 | HFrEF n=258 | P-valueb | ||||||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | ||||
| Age at diagnosis (years) | 61.1 | 12.8 | 70.2 | 9.8 | 65.7 | 10.5 | <0.01 | 61.1 | 12.8 | 73.3 | 10.1 | 71.5 | 9.8 | 0.01 | NA |
| Body Mass Index (kg/m 2 ) | 28.5 | 6.5 | 32.1 | 7.6 | 29.5 | 6.7 | 0.01 | 28.3 | 6.5 | 30.1 | 7.4 | 29.2 | 7.0 | 0.08 | <0.01 |
| Median neighborhood household income (% census tract) | 80,498 | 34,081 | 77,227 | 34,169 | 74,358 | 35,370 | 0.52 | 79,605 | 34,052 | 74,506 | 32,112 | 75,114 | 29,987 | 0.79 | <0.01 |
| Neighborhood education level (% census tract) | |||||||||||||||
| High school or less | 32% | 18% | 34% | 18% | 38% | 20% | 0.08 | 33% | 18% | 35% | 18% | 36% | 17% | 0.33 | <0.01 |
| Some college or associate degree | 31% | 10% | 31% | 11% | 30% | 11% | 0.66 | 31% | 10% | 31% | 10% | 31% | 10% | 0.81 | 0.96 |
| College degree | 23% | 11% | 23% | 12% | 20% | 12% | 0.15 | 23% | 11% | 22% | 11% | 21% | 11% | 0.59 | <0.01 |
| Graduate degree | 14% | 12% | 13% | 11% | 12% | 10% | 0.31 | 13% | 11% | 13% | 11% | 12% | 11% | 0.20 | <0.01 |
| n | % | n | % | n | % | P-valueb | n | % | n | % | n | % | P-valueb | P-valuec | |
| Race and/or ethnicity | 0.18 | 0.79 | NA | ||||||||||||
| White | 9,601 | 65% | 127 | 70% | 51 | 57% | 48,026 | 65% | 516 | 70% | 188 | 73% | |||
| Black | 1,105 | 7% | 17 | 9% | 17 | 19% | 5,528 | 7% | 73 | 10% | 21 | 8% | |||
| Asian | 2,147 | 15% | 13 | 7% | 10 | 11% | 10,722 | 15% | 68 | 9% | 19 | 7% | |||
| Hispanic | 1,769 | 12% | 22 | 12% | 11 | 12% | 8,844 | 12% | 66 | 9% | 27 | 10% | |||
| Native Hawaiian and/or Pacific Islander | 65 | 0% | 1 | 1% | 0 | 0% | 324 | 0% | 1 | 0% | 0 | 0% | |||
| American Indian and/or Alaska Native | 117 | 1% | 2 | 1% | 1 | 1% | 590 | 1% | 8 | 1% | 3 | 1% | |||
| Smoking status at baseline | 0.37 | 0.01 | <0.01 | ||||||||||||
| Never smoker | 8,128 | 55% | 84 | 46% | 37 | 41% | 39,961 | 54% | 335 | 46% | 102 | 40% | |||
| Current smoker | 1,320 | 9% | 21 | 12% | 6 | 7% | 6,804 | 9% | 79 | 11% | 49 | 19% | |||
| Former smoker | 4,223 | 29% | 63 | 35% | 39 | 43% | 16,243 | 22% | 212 | 29% | 71 | 28% | |||
| Unknown | 1,133 | 8% | 14 | 8% | 8 | 9% | 11,026 | 15% | 106 | 14% | 36 | 14% | |||
| Post-menopausal | 11,087 | 75% | 173 | 95% | 78 | 87% | 0.03 | 56,010 | 76% | 709 | 97% | 250 | 97% | 1.00 | 0.05 |
| History of diabetes before breast cancer d | 1,977 | 13% | 58 | 32% | 25 | 28% | 0.58 | 9,536 | 13% | 269 | 37% | 80 | 31% | 0.11 | 0.12 |
| History of dyslipidemia before breast cancer e | 6,434 | 43% | 127 | 70% | 46 | 51% | <0.01 | 30,670 | 41% | 518 | 71% | 174 | 67% | 0.36 | 0.42 |
| History of hypertension before breast cancer f | 6,240 | 42% | 149 | 82% | 59 | 66% | <0.01 | 30,937 | 42% | 593 | 81% | 190 | 74% | 0.02 | <0.01 |
| History of chronic kidney disease before breast cancer | 2,378 | 21% | 84 | 50% | 24 | 33% | 0.02 | 11,610 | 21% | 348 | 51% | 117 | 52% | 0.91 | 0.71 |
| Prevalent CVD at baseline | |||||||||||||||
| Arrhythmia | 143 | 1% | 4 | 2% | 2 | 2% | 1.00 | 697 | 1% | 29 | 4% | 8 | 3% | 0.66 | 0.81 |
| Cardiac arrest | 2 | 0% | 0 | 0% | 0 | 0% | NC | 8 | 0% | 0 | 0% | 0 | 0% | NC | 1.00 |
| Cardiomyopathy | 0 | 0% | 0 | 0% | 0 | 0% | NC | 10 | 0% | 1 | 0% | 1 | 0% | 1.00 | 0.32 |
| Heart failure | 0 | 0% | 0 | 0% | 0 | 0% | NC | 0 | 0% | 0 | 0% | 0 | 0% | NC | NC |
| Carotid disease | 0 | 0% | 0 | 0% | 0 | 0% | NC | 0 | 0% | 0 | 0% | 0 | 0% | NC | NC |
| Ischemic heart disease | 83 | 1% | 5 | 3% | 2 | 2% | 1.00 | 516 | 1% | 26 | 4% | 9 | 3% | 1.00 | 0.07 |
| Myocarditis pericarditis | 4 | 0% | 0 | 0% | 0 | 0% | NC | 7 | 0% | 0 | 0% | 0 | 0% | NC | 0.18 |
| Stroke | 107 | 1% | 4 | 2% | 0 | 0% | 0.38 | 552 | 1% | 21 | 3% | 5 | 2% | 0.56 | 0.81 |
| Transient Ischemic Attack (TIA) | 5 | 0% | 0 | 0% | 0 | 0% | NC | 63 | 0% | 4 | 1% | 0 | 0% | 0.54 | 0.06 |
| Valvular disease | 11 | 0% | 0 | 0% | 0 | 0% | NC | 69 | 0% | 2 | 0% | 2 | 1% | 0.60 | 0.58 |
| Venous thromboembolic disease | 28 | 0% | 0 | 0% | 0 | 0% | NC | 163 | 0% | 2 | 0% | 0 | 0% | 0.97 | 0.52 |
| Received chemotherapy (case only) | 6,229 | 42% | 51 | 28% | 50 | 56% | <0.01 | NA | NA | NA | NA | NA | NA | NA | NA |
| Anthracycline | 4,112 | 28% | 35 | 19% | 43 | 48% | <0.01 | NA | NA | NA | NA | NA | NA | NA | NA |
| Trastuzumab or pertuzumab | 1,521 | 10% | 11 | 6% | 11 | 12% | 0.13 | NA | NA | NA | NA | NA | NA | NA | NA |
| Received left-sided radiation therapy (case only) | 4,813 | 33% | 62 | 34% | 31 | 34% | 1.00 | NA | NA | NA | NA | NA | NA | NA | NA |
| Received endocrine therapy (case only) | 7,874 | 53% | 104 | 57% | 42 | 47% | 0.13 | NA | NA | NA | NA | NA | NA | NA | NA |
NA = not applicable, NC = not calculable
Breast cancer cases matched 1:5 to non-breast cancer controls on birth year and race/ethnicity.
Chi-square or Fisher exact test for categorical variables, one-way ANOVA for continuous variables across two HF groups within case/control group
Chi-square or Fisher exact test for categorical variables, one-way ANOVA for continuous variables across case/control groups
Diabetes status was determined by the KPNC diabetes registry which uses a combination of inpatient and outpatient diagnosis codes, pharmacy, and blood test results to identify diabetic patients.
Dyslipidemia was determined by a combination of diagnosis codes, pharmacy records, and blood lipid test.
Hypertension was determined by a combination of diagnosis codes, blood pressure values, and number of clinical encounters.
Mean time to HF diagnosis was 5.31 years (range 0.03–13.03) in the cases and 5.25 years (range 0.01–12.94) in the controls over a mean follow-up period of 7.0 years (range <1.0–13.4). In cases and controls, 10-year cumulative incidence rates were 1.2% and 0.9% for overall HF, 0.8% and 0.7% for HFpEF, and 0.4% and 0.2% for HFrEF in cases and controls, respectively.
In fully adjusted models, an overall significant increased risk of HF in breast cancer cases compared with controls was observed (HR: 1.31, 95% CI: 1.14, 1.51). When examined by LVEF phenotype, the risk was higher for HFrEF (HR: 1.59, 95% CI: 1.22, 2.08) compared with HFpEF (HR: 1.22; 95% CI: 1.03, 1.45). In further analyses by breast cancer treatment and HF phenotype, women treated with chemotherapy with or without any radiation therapy and/or endocrine therapy were 3.1 times more likely to develop HFrEF (HR: 3.10, 95% CI: 2.06, 4.67) and over 1.6 times more likely to develop HFpEF (HR=1.67, 95% CI: 1.19, 2.35) compared with their controls (Figure 1). Women who received left-sided radiation therapy with or without any chemotherapy and/or endocrine therapy compared with their controls had 1.6 times the risk of developing HFrEF (HR=1.61, 95% CI: 1.00, 2.60) and 1.4 times the risk of developing HFpEF (HR=1.40; 95% CI: 1.04, 1.87). In women who received endocrine therapy compared with their controls, no significant associations were observed for risk of HFrEF (HR=1.30; 95% CI: 0.88, 1.92) or HFpEF (HR=1.15; 95% CI: 0.92, 1.44).
Figure 1.
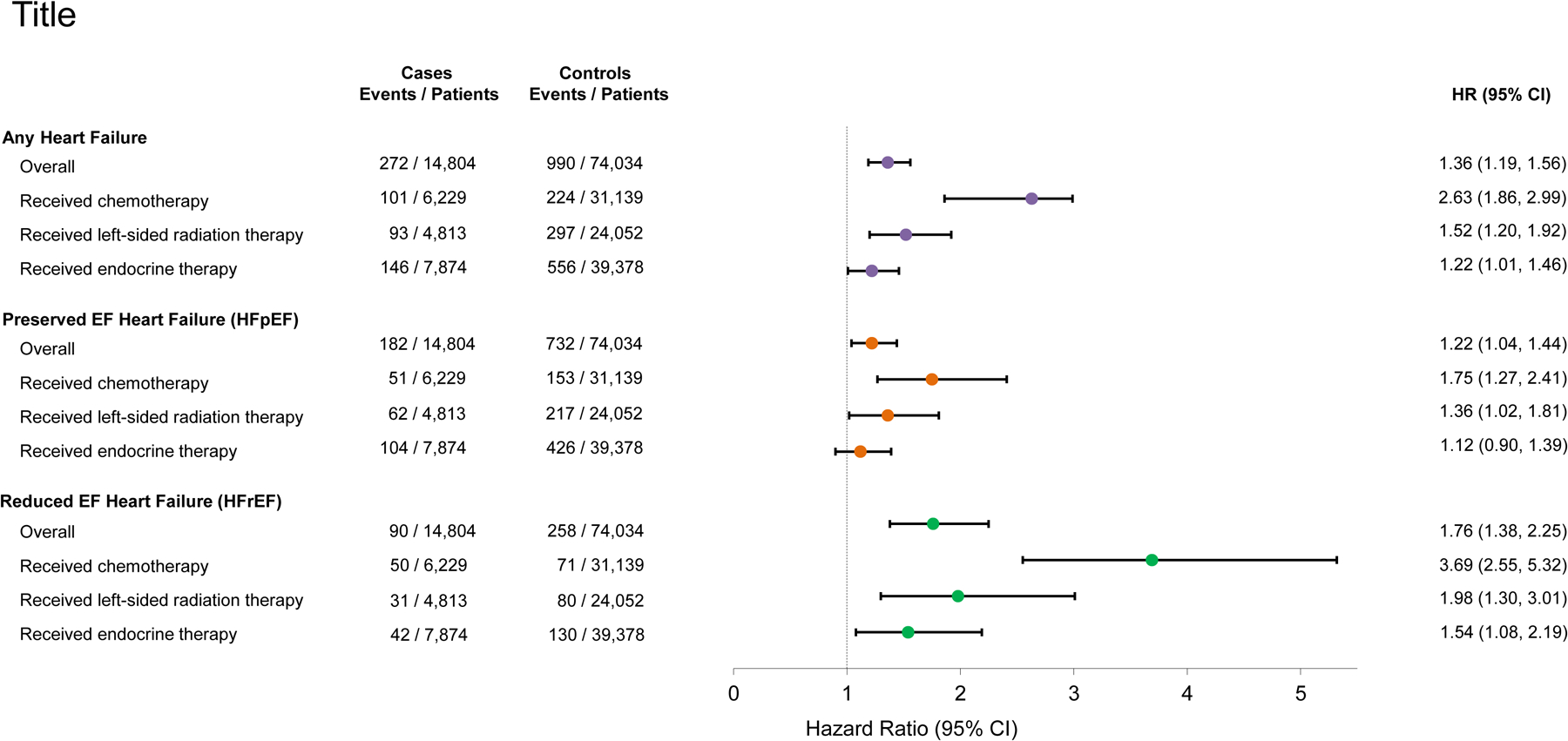
Relative risks estimated from multivariable Cox proportional hazards regression of any incident heart failure, heart failure with preserved ejection fraction (HFpEF, ≥45%), and heart failure with reduced ejection fraction (HFrEF, LVEF<45%), overall and by primary breast cancer treatment received. Models were adjusted for baseline body mass index, menopausal status, smoking status, prior history of hypertension, diabetes, and dyslipidemia, neighborhood median household income and education level, and prior history of CVD conditions (arrhythmia, HF, cardiomyopathy, myocarditis/pericarditis, stroke/TIA, valvular disease, venous thromboembolic disease, ischemic heart disease) and chronic kidney disease within the two years before breast cancer diagnosis. The treatment subgroups presented are not mutually exclusive (i.e., they can overlap).
When defining HFpEF as LVEF≥40% and HFrEF as LVEF<40%, the results were very similar in magnitude to those observed using the 45% LVEF cutoff, overall and within treatment subgroups for breast cancer cases compared with controls (Supplemental Table 1).
Discussion
As expected, breast cancer survivors experienced significantly higher 1.3 fold risk of incident HF compared to women without breast cancer. Moreover, the risk persisted by LVEF phenotype, being 1.6 fold for HFrEF and 1.2 fold for HFpEF. When examining by primary breast cancer treatment received, women who had chemotherapy or left-sided radiation therapy were at even higher risk for HFrEF (2.0–3.0 fold) and HFpEF (1.5–2.0 fold) compared with women without breast cancer. Receipt of any endocrine therapy was not associated with HF of either LVEF phenotype.
Our results for overall HF risk are consistent with a large retrospective study of 78,318 early-stage breast cancer patients and 234,954 matched non-cancer control subjects in Ontario, Canada using health care administrative data [10]. They reported a 1.2 fold higher risk of hospitalized HF in women with breast cancer compared with women without breast cancer. Previous studies have also reported elevated risk of overall HF in women with breast cancer [11, 12]. However, to our knowledge, we are one of the first to examine risk of HF in these clinically distinct HF phenotype subgroups, as most of these prior studies did not have data on LVEF.
Our findings are important for breast cancer survivors because HFpEF is common in older women, yet this higher risk for HFpEF is often not considered in the context of a breast cancer diagnosis and survivorship [2]. Furthermore, incident breast cancer and cardiovascular disease have shared risk factors and mechanisms, making the breast cancer patient population particularly susceptible and vulnerable to HFpEF [13]. Similarly, a bidirectional risk exists between the two diseases, partially led by a pro-inflammatory environment underlying both cancer and/or HF [14]. Therefore, it is paramount to understand the risk for HFpEF in addition to the traditional focus on reduced LVEF with resultant HFrEF in breast cancer survivors.
Our analysis might be subject to selection bias as only HF events with an associated LVEF value were included. Also, analyses by breast cancer treatment focused on receipt of any chemotherapy, radiation therapy, and endocrine therapy and did not examine combinations of treatments due to limited sample size. Further, chemotherapy regimens have changed somewhat since the inception of our cohort in 2005. However, exposure to cardiotoxic chemotherapy drugs such as anthracyclines continues to be common [15, 16], lending more support and relevance of this study on subsequent risk of HF subtypes for today’s breast cancer patients. Despite these limitations, our study is the largest to date of breast cancer patients to examine LVEF phenotypes, especially HFpEF, as well as consider not only the impact of chemotherapy but also radiation therapy and endocrine therapy.
Improving the care experience and clinical outcomes of patients with HF remains challenging in the patient’s ecosystem of clinician teams, caregivers, payers, and clinical research findings [17]. Our study is one of few studies to characterize HF risk by LVEF phenotype in breast cancer survivors, a population at higher risk from exposure to cardiotoxic cancer treatments, thus prompting more focus on precision medicine and population health. Importantly, we found that women who had chemotherapy or left-sided radiation therapy were also at higher risk of HFpEF, a phenotype that has been under-recognized and under-characterized in breast cancer cohorts. In fact, in our study, the overall cumulative incidence of HFpEF at 10 years was higher than HFrEF. Therefore, timely recognition and diagnosis of HFpEF is critical to mitigate morbidity and mortality in an era of emerging therapies for HFpEF. These include definitive benefit with sodium-glucose cotransporter-2 inhibitors [18] and potential reduction of hospitalization for HF with angiotensin receptor-neprilysin inhibitors and mineralocorticoid inhibitors [2].
In conclusion, understanding the extent to which breast cancer survivors are at risk of preserved and reduced HF is clinically important to guide tailored cardiac therapies. Our findings lend support for systematic cardio-oncology surveillance and care of breast cancer patients receiving cardiotoxic treatments for managing risk of both HFpEF and HFrEF.
Supplementary Material
Acknowledgements
We thank the Kaiser Permanente Northern California patients who provided the data for this study. This work was supported by the National Cancer Institute, Bethesda, MD 20814 (R01 CA214057 and U01 CA195565).
Footnotes
Conflicts of interest: All authors have no conflicts of interest to declare.
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Human Subjects Institutional Review Board (IRB) of Kaiser Permanente Northern California (Current approval until August 3, 2023).
Prior presentation: 2020 American College of Cardiology Virtual Meeting (J.S. Rana)
Statements and Declarations
Consent to Participate: This is a data-only study which used existing protected health information in the Kaiser Permanente Northern California electronic health record. Therefore, waiver of informed consent was not required as data analysis is regulated by the HIPAA Privacy Rule.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available due to potentially identifiable information (e.g., dates of diagnoses) and KPNC privacy regulations. They are available from the corresponding author upon reasonable request contingent on appropriate human subjects approval and data use agreements.
References
- 1.Dunlay SM, Roger VL, and Redfield MM, Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol, 2017. 14(10): p. 591–602. [DOI] [PubMed] [Google Scholar]
- 2.McDonagh TA, et al. , 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J, 2021. 42(36): p. 3599–3726. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B, et al. , Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GA, et al. , Biological Phenotypes of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol, 2017. 70(17): p. 2186–2200. [DOI] [PubMed] [Google Scholar]
- 5.Florescu DR and Nistor DE, Therapy-induced cardiotoxicity in breast cancer patients: a well-known yet unresolved problem. Discoveries (Craiova), 2019. 7(1): p. e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaduganathan M, et al. , Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients with Cancer Undergoing Chemotherapy. JACC CardioOncol, 2019. 1(1): p. 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt B, et al. , Spironolactone for heart failure with preserved ejection fraction. N Engl J Med, 2014. 370(15): p. 1383–92. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, et al. , Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med, 2019. 381(17): p. 1609–1620. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, et al. , Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New England Journal of Medicine, 2021. 385(16): p. 1451–1461. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Qadir H, et al. , The Risk of Heart Failure and Other Cardiovascular Hospitalizations After Early Stage Breast Cancer: A Matched Cohort Study. J Natl Cancer Inst, 2019. 111(8): p. 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles EJA, et al. , Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst, 2012. 104: p. 1293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinder MC, et al. , Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol, 2007. 25(25): p. 3808–15. [DOI] [PubMed] [Google Scholar]
- 13.Mehta LS, et al. , Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation, 2018. 137(8): p. e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Boer RA, et al. , A new classification of cardio-oncology syndromes. Cardiooncology, 2021. 7(1): p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braybrooke J, et al. , Abstract GS2–06: Taxane with anthracycline versus taxane without anthracycline: An individual patient-level meta-analysis of 16,500 women with early-stage breast cancer in 13 randomised trials. Cancer Research, 2022. 82(4_Supplement): p. GS2–06–GS2–06. [Google Scholar]
- 16.NCCN Guidelines for Breast Cancer (Version 2.2022). 2022.
- 17.Califf RM, The Ecosystem to Support People with Heart Failure. J Card Fail, 2021. [DOI] [PubMed] [Google Scholar]
- 18.Anker SD, et al. , Sodium-glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: reasons for optimism. Eur J Heart Fail, 2021. 23(8): p. 1250–1255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to potentially identifiable information (e.g., dates of diagnoses) and KPNC privacy regulations. They are available from the corresponding author upon reasonable request contingent on appropriate human subjects approval and data use agreements.


