Abstract
The revised World Health Organization guidelines on multidrug-resistant tuberculosis include linezolid in the core drugs group. Consequently, the use of linezolid for patients with multidrug-resistant tuberculosis is increasing. Common adverse events of long-term linezolid use include bone marrow suppression and neuropathies. However, there is limited information on a rare adverse event, black hairy tongue. Here, we report a case of linezolid-induced black hairy tongue in a patient with multidrug-resistant tuberculosis. The etiology, pathogenesis, diagnosis, and treatment of black hairy tongue are also discussed.
Keywords: Linezolid, tongue, tuberculosis
Introduction
The treatment of multidrug-resistant tuberculosis (MDR-TB) is challenging because it requires the long-term use of second-line anti-TB drugs that are more toxic and less effective than first-line drugs. 1 The treatment outcomes of patients with MDR-TB remain unsatisfactory. Only 56% of MDR-TB patients in a 2016 global cohort completed treatment successfully. 2 To overcome this problem, the World Health Organization (WHO) revised the guidelines on MDR-TB in 2019. 3 The most important change was a new priority ranking of the anti-TB drugs available for MDR-TB based on a balance between expected efficacy and side effects. As a result, linezolid was reclassified as a core drug.
Linezolid is an oxazolidinone antibiotic that was originally approved for drug-resistant Gram-positive bacterial infection. After the excellent antimycobacterial activity of linezolid became known, its use for MDR-TB patients increased gradually. 4 With the revised WHO guidelines, the use of linezolid for MDR-TB treatment is expected to increase. Common adverse events of long-term linezolid use include bone marrow suppression and neuropathies. 5 However, rare adverse events with the increased long-term use of linezolid for MDR-TB may raise concerns. Here, we describe the case of an MDR-TB patient with a linezolid-induced back hairy tongue (BHT).
Case report
In September 2019, a 60-year-old human immunodeficiency virus-negative Korean woman was referred to our hospital for treatment of MDR-TB. She had a history of hepatitis B virus infection. MDR-TB treatment was initiated with levofloxacin, amikacin, prothionamide, cycloserine, and pyrazinamide. One month after starting the treatment, the anti-TB drugs were discontinued temporarily due to hepatotoxicity (the serum alanine aminotransferase (ALT) level was 457 U/L). After the ALT level normalized, a re-challenge test for anti-TB drugs was performed; prothionamide and pyrazinamide were found to be the causes of her hepatotoxicity. In November 2019, the MDR-TB treatment regimen was modified to levofloxacin, amikacin, cycloserine, and delamanid. In December 2019, linezolid 300 mg once daily orally was added to strengthen the regimen.
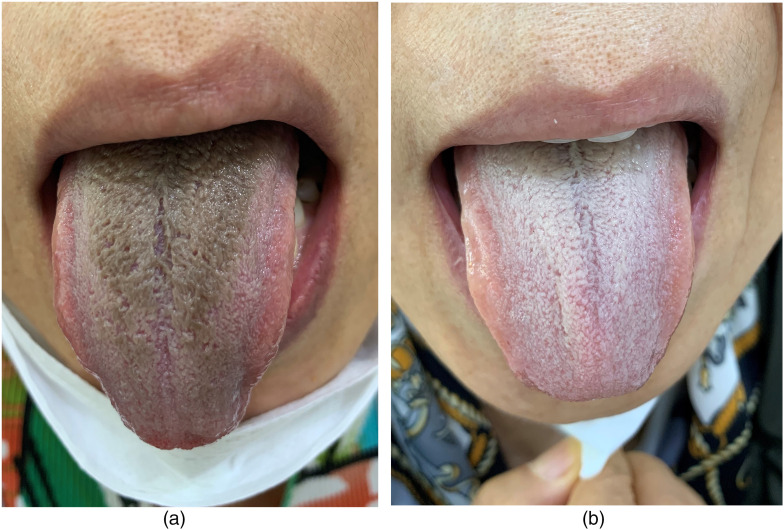
Eleven weeks after starting linezolid, she complained of tongue discoloration (Figure 1(a)). On observation, brown to black discoloration was seen on the middle to the posterior dorsum of the tongue, with carpet-like elongated filiform lingual papillae. She did not complain of any symptoms related to the tongue lesion. Her vital signs, complete blood count, and chemistry were normal. She denied drinking alcohol, smoking, consuming coffee, tea, or other colored beverages, or using mouthwash. Reviewing her medications, no drug had been added since starting linezolid. The patient's concomitant medications have not been reported to cause such a tongue lesion. Therefore, her tongue lesion was suspected of being linezolid-induced BHT. She was advised to stop taking the linezolid, and a follow-up visit 3 weeks later showed complete resolution of the BHT (Figure 1(b)).
Figure 1.
Tongue in a 60-year-old woman with multidrug-resistant tuberculosis: (a) 11 weeks after starting linezolid and (b) 3 weeks after discontinuing linezolid.
Linezolid (300 mg once daily orally) was reintroduced 1 week after the tongue lesion resolved and the BHT recurred 2 months later. However, the degree of discoloration was not as severe as in the first episode and she does not complain of any related symptoms. With reassurance, she continued taking the linezolid and maintained good oral hygiene. She is currently treating her MDR-TB with linezolid and the BHT waxes and wanes. Naranjo's Adverse Drug Reaction Probability Scale score was eight, which revealed a probable adverse drug reaction of linezolid intake, to BHT. 6
Discussion
There have been several case reports on linezolid-induced BHT.7–11 However, there are limited data for patients with MDR-TB who need long-term linezolid. 12 Compared to previous reports, our case differed in that (1) BHT occurred relatively late in the course of linezolid treatment (in previous reports, BHT usually occurred 2 weeks after starting linezolid) and (2) BHT recurred at the reintroduction of linezolid. The lower dose of linezolid in our patient (300 mg daily) may have contributed the late onset of BHT. Most adverse events of linezolid are dose dependent. Little data are available on the impact of long-term linezolid use on the recurrence of BHT. More case reports and further investigations are needed to clarify this issue.
BHT, also called “lingua villosa nigra,” is a benign condition characterized by abnormally hypertrophied, elongated filiform papillae on the dorsal tongue surface with discoloration. Historically, the discoloration was described as “black”; however, it may be brown, yellow, green, or blue. 13 The incidence of BHT in patients receiving linezolid for skin and soft tissue infections was 0.2% in controlled phase III trials. 14 The etiology of BHT remains unclear. However, many factors can contribute to or aggravate BHT. These include smoking, alcohol consumption, excessive black tea or coffee consumption, poor oral hygiene, drugs of abuse (e.g. crack cocaine), oxidizing mouthwashes, recent head, and neck radiotherapy, trigeminal neuralgia, cancer, acquired immunodeficiency syndrome, drugs causing xerostomia (e.g. antipsychotics or anticholinergics), and certain antibiotics (e.g. penicillin, erythromycin, doxycycline, neomycin, etc.).13,15 BHT is caused by defective desquamation of the tongue, which results in excessive growth and thickening of the filiform papillae. Debris, bacteria, fungus, or other foreign materials then accumulate in the filiform papillae and ultimately cause discoloration. 15 However, it is still unclear how the predisposing factors contribute to the pathogenesis of BHT.
Patients with BHT are usually asymptomatic. However, it may raise aesthetic concerns. 13 The diagnosis of BHT relies on a visual examination (the typical discoloration and elongated filiform papillae of the tongue). Microscopic examination may help to show the elongated filiform papillae. Investigation of precipitating factors and recent medication change is also important. 13 BHT is a self-limiting benign disease with an excellent prognosis. Reassurance about the benign nature of this condition helps the patient. The treatment consists of discontinuing offending drugs or predisposing factors and maintaining good oral hygiene. Drug-induced BHT usually resolves within 1–2 weeks after stopping the offending drug. Gentle debridement with a soft toothbrush and rinsing with baking soda or diluted (3%) hydrogen peroxide may assist desquamation of the tongue.13,15
Physicians may ignore BHT because it causes mainly aesthetic problems and is reversible. Nevertheless, BHT may place an additional burden on patients and the healthcare system. 13 Physicians need to be aware of this rare adverse event.
Author biographies
Jaemin Lee is a pulmonology doctor in Busan Adventist Hospital, Busan, Republic of Korea. He completed his fellowship in pulmonology at Pusan National University Hospital in 2021. His research interests include tuberculosis and other infectious respiratory diseases.
Hyun Sung Chung is a pulmonology doctor in the National Cancer Center, Goyang, Republic of Korea. He completed his fellowship in pulmonology at Pusan National University Hospital in 2021. His research interests include lung cancer and interventional bronchoscopy.
Jiyeon Roh is a pulmonology doctor. She completed her fellowship in pulmonology at Pusan National University Hospital in 2021. Her research interests include tuberculosis and non-tuberculous mycobacterial disease.
Yeseul Oh is a pulmonology doctor. She completed her fellowship in pulmonology at Pusan National University Hospital in 2021. Her research interests include pneumonia and COPD.
Jeongha Mok is an associate professor in the Department of Internal Medicine, Pusan National University Hospital, Busan, Republic of Korea. His research interests include tuberculosis, non-tuberculous mycobacterial disease and latent tuberculosis infection.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics statement: This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB approval number: H-2009-009-095). The patient's written informed consent was obtained for publication of the clinical details and images in this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jeongha Mok https://orcid.org/0000-0001-7406-1373
References
- 1.Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J 2017; 49: 1600803. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2019. Geneva: WHO, WHO/CDS/TB/2019.15, 2019. [Google Scholar]
- 3.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: WHO, WHO/CDS/TB/2019.3, 2019. [PubMed] [Google Scholar]
- 4.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367: 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifan Z, Sainan B, Feng S, et al. Linezolid for the treatment of extensively drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2019; 23: 1293–1307. [DOI] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 7.Khasawneh FA, Moti DF, Zorek JA. Linezolid-induced black hairy tongue: a case report. J Med Case Rep 2013; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braggio C, Bocchialini G, Ventura L, et al. Linezolid-induced black hairy tongue. Acta Biomed 2018; 89: 408–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jover-Diaz F, Cuadrado-Pastor JM, Talents-Bolos A, et al. Black tongue associated with linezolid. Am J Ther 2010; 17: e115–e117. [DOI] [PubMed] [Google Scholar]
- 10.Balaji G, Maharani B, Ravichandran V, et al. Linezolid induced black hairy tongue. Indian J Pharmacol 2014; 6: 653–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga-Simões J, Santos LA. Linezolide-induced hairy tongue. Oxf Med Case Reports 2016; 12: 295–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain AK, Puri MM, Sarin R. Black brown discoloration and hairy tongue – A rare linezolid side effect. Indian J Tuberc 2017; 64: 44–46. [DOI] [PubMed] [Google Scholar]
- 13.Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol 2014; 20: 10845–10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hau T. Efficacy and safety of linezolid in the treatment of skin and soft tissue infections. Eur J Clin Microbiol Infect Dis 2002; 21: 491–498. [DOI] [PubMed] [Google Scholar]
- 15.Thompson DF, Kessler TL. Drug-induced black hairy tongue. Pharmacotherapy 2010; 30: 585–593. [DOI] [PubMed] [Google Scholar]