Abstract
This guidance document provides a tiered framework for risk assessors and facilitates risk managers in making decisions concerning the approval of active substances (AS) that are chemicals in plant protection products (PPPs) and biocidal products, and authorisation of the products. Based on the approaches presented in this document, a conclusion can be drawn on the impact of water treatment processes on residues of the AS or its metabolites in surface water and/or groundwater abstracted for the production of drinking water, i.e. the formation of transformation products (TPs). This guidance enables the identification of actual public health concerns from exposure to harmful compounds generated during the processing of water for the production of drinking water, and it focuses on water treatment methods commonly used in the European Union (EU). The tiered framework determines whether residues from PPP use or residues from biocidal product use can be present in water at water abstraction locations. Approaches, including experimental methods, are described that can be used to assess whether harmful TPs may form during water treatment and, if so, how to assess the impact of exposure to these water treatment TPs (tTPs) and other residues including environmental TPs (eTPs) on human and domesticated animal health through the consumption of TPs via drinking water. The types of studies or information that would be required are described while avoiding vertebrate testing as much as possible. The framework integrates the use of weight‐of‐evidence and, when possible alternative (new approach) methods to avoid as far as possible the need for additional testing.
Keywords: transformation products, genotoxicity, disinfection, risk assessment, plant protection products, biocidal products, groundwater
Summary
This guidance document provides a framework for risk assessors and facilitates risk managers in making decisions concerning the approval of active substances (AS), which are chemicals in plant protection products (PPPs) and biocidal products, and authorisation of the products. Based on the approaches presented in this document, a conclusion can be drawn on the impact of water treatment processes on residues of the AS or its metabolites in surface water and/or groundwater abstracted for the production of drinking water.
This guidance enables the identification of actual public health concerns from exposure to harmful compounds generated during the processing of water for the production of drinking water, and it focuses on water treatment methods commonly used in the European Union (EU). This assessment is mainly based on existing information and guidance in this area to avoid the development of new test methods and divergent assessment approaches. To ensure a proportionate approach and avoid unnecessary testing, a tiered framework is developed. The framework allows for screening to determine whether residues from PPP use (according to good agricultural practice) or residues (from biocidal product use) can be present in water at water abstraction locations. Then it is assessed whether harmful transformation products (TPs) may form during water treatment and, if so, how to assess the impact of exposure to these water treatment TP (tTP) and other residues, including environmental TP (eTP), on human and domesticated animal health. The framework integrates the use of weight‐of‐evidence and, when possible alternative (new approach) methods to avoid as far as possible the need for additional testing, in particular unnecessary animal (vertebrate) testing. The guidance presents the method to address potential harm to human health through the consumption of TP via drinking water. The types of studies or information that would be required are described while avoiding unnecessary vertebrate testing as much as possible.
The guidance included in Chapter 2 provides background information and approaches to determine whether residues (AS and eTPs) from PPPs used according to good agricultural practices can be present in water at abstraction locations for the production of drinking water. If the use of a PPP results in direct or indirect emissions to surface water and/or direct or indirect emissions to soil and subsequently to groundwater, the formation of TPs during tTP to produce drinking water should be considered.
Whilst the main sources of pollution originate from agricultural areas, spray drift, foliar wash‐off into the soil, run‐off and drainage from the soil into surface waters and leaching into groundwater are the relevant routes of contamination following PPP use. Major factors affecting the fate of AS and their eTPs include the type of application, weather and climate, type of surface water at the field edge and its depth, soil type and its properties such as permeability, texture and organic matter transmissivity, field slope and overall topography, time and season, type of irrigation and product‐specific properties. Metabolites or eTP may not only concern drinking water (concerning human health) but may also harm non‐target organisms in the environment if critical thresholds are exceeded.
For the AS proposed for approval, and PPP proposed for authorisation, for the uses that may result in emissions to surface water and/or soil, the (predicted) concentrations in surface water and/or groundwater of the AS, their eTPs (if relevant) and any substances of concern present in the PPP or a treated article are needed. This information is available in the environmental risk assessment of the AS and PPP. Critical thresholds can then be compared to predicted environmental concentrations (PECs), which are calculated to estimate the exposure pattern of the AS and eTP (resulting from both abiotic and biotic transformation processes in the environment) based on their use pattern. PEC calculations differ between surface water and groundwater, both of which can serve as source waters for drinking water use. Practical guidance for calculating predicted environmental concentrations in the context of AS approval and PPP authorisation assessments, including models, tools and guidance on reporting, is included in Chapter 2 and Appendix A . For all substances estimated to be present at the abstraction points in both surface water and groundwater, at concentrations exceeding the trigger values specified in Chapter 2, their potential to form tTP during drinking water abstraction needs to be assessed. Guidance on how to identify the substances for which an assessment of the tTP formation is needed is included in this chapter. In specific cases, a qualitative assessment of the tTP and their fate during drinking water production may need to be performed.
In order to derive changed PEC consequent to the transport of AS and their eTPs from their point of entry into surface waters to their potential abstraction point for drinking water production, dilution factors need to be considered. Specific factors that drive dilution must be considered, such as landscape factors (slope of the soil or the proximity of the input area to the rivers), the type and characteristics of the soil, the timing and pattern of application, as well as weather such as heavy rainfall and dry periods which differ considering regional climates. In rivers, dilution depends on the width and depth of the river, the water level, the flow velocity and the length of the river from the entry to the point of withdrawal. Dilution factors per regulatory zone should reflect a realistic worst‐case scenario regarding flow conditions. The worst‐case dilution factors per regulatory zone are included in Chapter 2.
In Chapter 3, background and approaches are provided to determine whether biocidal AS and their metabolites, eTP and substances of concern in a biocidal product or a treated article need to be assessed for their potential to form TPs during the production of drinking water (tTP). Biocidal AS, their metabolites, and other substances of concern contained in the biocidal product or the treated article can reach surface water and groundwater through different pathways. Surface water can be exposed to the biocidal product emissions directly (e.g. from outdoor spray applications, leaching from treated wooden products, and antifouling agents) or indirectly when the releases are first directed to the sewer and the sewage treatment plant (STP) before discharge to the surface water. Groundwater is exposed indirectly as a result of the biocidal product emissions to the soil. Like surface water, soil can receive either direct emissions during the application or service life of a biocidal product or indirect emissions (via the soil application of manure or sludge containing biocidal product residues). The environmental compartments to which the emissions from the use of the biocidal products are directly or indirectly emitted are product type and use specific. Therefore, for each product type and emission scenario assessed, it is necessary to assess whether the emissions can reach surface water and/or groundwater and whether the recipient water body can be expected to be relevant as a drinking water source.
If the use of a respective biocidal product results in direct and/or indirect emissions to surface water and/or direct and/or indirect emissions to soil and subsequently to groundwater, the formation of TPs during water treatment (tTP) to produce drinking water should be considered.
For the AS proposed for approval and biocidal products proposed for authorisation for the uses that may result in emissions to surface water and/or soil, the concentrations in surface water and/or groundwater of the biocidal AS, their metabolites (if relevant) and any substances of concern present in the biocidal product or a treated article need to be determined. This information is available in the environmental risk assessment of the AS and the biocidal product. For substances estimated to be present at concentrations exceeding the trigger value at the point of abstraction in surface water, it is then necessary to assess their potential to form tTP during drinking water production. For groundwater, the environmental metabolites (i.e. major and ecotoxicologically relevant metabolites) estimated to be present at concentrations above the trigger value and shown to be toxicologically non‐relevant also need to be assessed for their potential to form tTP during drinking water production. Guidance on how to identify the substances for which an assessment of the tTP formation is needed, is included in this chapter. In specific cases, a qualitative assessment of the tTP and their fate during drinking water production may need to be performed. At the biocidal product authorisation stage, the risk assessment needs to be complemented to cover substances of concern in the product and potential new uses that may lead to additional routes of exposure to groundwater and surface water.
Chapter 4 provides background information on the formation of tTP during drinking water treatment processes and presents approaches to assess this experimentally in a laboratory setting. In Europe, drinking water is prepared from surface water, groundwater or bank filtrate. These may be contaminated by low‐level mixtures of chemicals, including AS from PPPs or biocides and their eTP. During drinking water treatment, AS and their eTP may be degraded in several processes, but in general, they are not fully mineralised. This can result in the formation of tTP that are potentially harmful (it cannot be assumed a priori that these are of lower toxicity than the parent substance).
Drinking water treatment processes that may result in the transformation of compounds include chlorination with sodium hypochlorite or chlorine, chlorination with chloramine, pre‐oxidation with chlorine dioxide, the combination of pre‐oxidation with chlorine dioxide and chlorination with sodium hypochlorite, chlorine or chloramine, ozonation, ultraviolet (UV) disinfection, photolysis, advanced oxidation processes and biodegradation during filtration over sand or granular activated carbon. This guidance focuses on the most common disinfection processes: rapid sand filtration, chlorination with sodium hypochlorite or chlorine, chlorination with chloramine, pre‐oxidation with chlorine dioxide, the combination of pre‐oxidation with chlorine dioxide and chlorination with sodium hypochlorite, chlorine or chloramine, ozonation and UV disinfection. Each of these processes can result in the formation of different types of tTPs. Appendix B presents the type of reactions that can occur during each of these processes and the types of TPs that can be formed. Modelling of transformation pathways and estimation of certain characteristics (such as Kow and pKa values) can be applied to predict the types of TPs that can be expected to be formed during such processes or that a compound will be stable. The literature can also be a source of specific evidence that an AS can be expected to be stable during these disinfection processes. The formation of TPs is not necessarily problematic. Whether it is problematic will depend on their properties and concentrations and the impact of any further treatment of the water, if applicable. Specific attention should be given to the potential formation of nitrosamines and chlorinated TPs in chlorination processes.
A stepwise approach is presented that can be used to identify which TP can be formed during drinking water treatment processes based on literature research, modelling and experimental approaches. This should consider not only the AS but also their main metabolites encountered in the aquatic environment (eTP).
Background and specific tools are presented for a modelling step, applying quantitative structure–activity relationships to predict the type of TP that may be formed in specific processes based on the molecular composition of the parent AS and eTP and the processes applied. This information can inform which type of analyses are required in the experimental approaches. In general, the identity of the predicted tTP formed must always be experimentally confirmed.
OECD guidelines 307, 308 or 309 may be used to determine the biodegradability of compounds and when results from all three of these guidelines are available give indications of tTP and their levels that have the potential to be formed during rapid sand filtration. A comparison of these guidelines is shown in Appendix C . In case these guidelines cannot be applied, OECD guideline 314 or an alternative method can be used to cover tTP formation during rapid sand filtration. This is described in Chapter 4.
Experimental procedures are described to determine on a laboratory scale whether TP may be formed (additional procedures are described in Appendix D ). Experiments are first to be carried out at high concentrations to allow the observation of the plausible generation of tTP. Not all of these will be formed under realistic conditions, where lower concentrations of the AS or eTP can be expected. Therefore, in the next step, the experiment is repeated at an environmentally relevant concentration in line with the environmental concentrations estimated following the guidance in Chapters 2 and 3. If tTP still appears to be formed, data from the high‐concentration experiment can be used to identify them. This information can then be used for further hazard assessment.
In Chapter 5, a tiered risk assessment approach for tTPs of biocides and PPPs formed in drinking water treatment processes in relation to human health and the health of food‐producing (domesticated) animals is provided. The approach presented in this chapter is in line with existing assessment schemes to identify relevant metabolites of PPPs and biocidal products in groundwater and with existing guidance on dietary risk assessment of residues. Any tTP that does not pass the criteria of the hazard and risk assessment is unacceptable at drinking water contamination levels exceeding thresholds set following this guidance. The risk assessment presented in this chapter consists of three tiers, starting with a hazard‐based assessment for genotoxicity (Tier 1) and progressing to additional, more complex tiers (Tier 2 and 3) if needed. The data requirements per tier and the evaluation process for proceeding to a higher tier are explained. This approach allows the tTP under consideration to be assessed at an appropriate level of complexity. Therefore, the general principle is to proceed from lower tiers which are relatively more conservative, to higher tiers which provide more realistic health risk assessments. Background, guidance and a flowchart decision scheme are provided for the tiered risk assessment of tTPs from biocides and PPPs formed in drinking water treatment processes.
The first step is to assess the formation of tTPs at a detectable level in drinking water during the treatment process (Chapter 4), paying special attention to the possible formation of nitrosamines. If the assessment shows that no tTPs are formed, no further risk assessment is required. All tTPs that have been identified to occur at a level exceeding 0.075 μg/L in drinking water are screened for their genotoxic potential as a first step in the tiered risk assessment.
Drinking water exposure may be estimated based on the information on environmental levels and on the formation of tTPs investigated experimentally. It should be evaluated whether exposure from all sources (including drinking water and dietary intake) to a TP or a group of structurally related TPs stays below or may exceed the health‐based guidance value (HBGV) or threshold of toxicological concern (TTC). If the concentration of a tTP does not exceed the HBGV or TTC, and if there is no health concern at the estimated levels of exposure, then the exposure is acceptable, and the tTP is exempted from further consideration. If not, tTP should be further evaluated in subsequent steps for potential toxicity. Based on the results of tier 2, tTP may need to be further evaluated in the next tier (Tier 3) for a more refined risk assessment to ensure that any contamination of water will not lead to unacceptable exposure of consumers and domesticated animals via drinking water. Guidance is presented on the approaches to derive values for the maximum (oral) exposure to a substance that is not expected to result in any appreciable health risk (HBGV) if these are not publicly available (Appendix F ).
In Chapter 6, the European context of the protection of water bodies, including drinking water sources, is described, and a recommendation on the use of effect‐based methods for water quality monitoring is provided. Different EU member states have different approaches to minimise the entry of PPPs and biocides into water bodies. Effect‐based monitoring of water samples using in vitro bioassays can be applied as an additional screening step in specific cases, e.g. if a TP cannot be appropriately identified and/or to investigate combined effects of mixtures of TP (in case multiple TP are formed simultaneously). In principle, this testing strategy should follow more or less the same decision scheme as proposed for the identified TP, and toxicological endpoints such as genotoxicity should be assessed, endocrine disruption and neurotoxicity might be assessed should relevant methods become established. Recommendation is included on sample preparation and specific in vitro test systems that can be applied for water quality monitoring.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
During the evaluation of AS in plant protection products evaluated under Regulation (EC) No 1107/2009 1 , a conclusion on the impact of water treatment processes on residues of the AS or its metabolites in surface water and/or groundwater abstracted for the production of drinking water, could not be drawn for almost all cases. As a result, the overall consumer risk assessment could not be completed as the full exposure profile from drinking water intake was not known. EFSA identified a corresponding data gap for information to address the effect of water treatment processes on the nature of residues present in surface water and/or groundwater, as applicable.
During the evaluation of AS in biocidal products under Regulation (EU) No 528/2012 2 , similar situations arose concerning the impact of water treatment processes on residues of AS and their metabolites in water abstracted for the production of drinking water.
The Commission requested that EFSA and ECHA jointly develop a guidance that provides a clear framework for risk assessors and facilitates decisions by risk managers concerning the approval of chemically AS. The framework should reflect the real situation in the EU as regards drinking water treatment; in this regard, a possible consequent consultation with the appropriate stakeholders involved in the treatment of water should be considered.
In particular, the following elements should be taken into account:
The objective of the guidance is to enable the identification of real concerns for public health from exposure to harmful by‐products in drinking water.
With the aim of having a proportionate approach and avoiding the need for unnecessary testing, a tiered framework should be considered. The framework should first allow for a screening to determine whether harmful residues may form during water treatment and, if so, how to assess the impact of exposure to the residues on human health.
The guidance should focus on the water treatment methods that are frequently used. At least chlorination, the most used methodology should be covered. The employment of slow sand filtration (or any other means used in practice to remove organic impurities) prior to the water treatment methods (e.g. chlorination) should be considered as standard practice in surface water treatment. It should be indicated how applicable the framework would be to other frequently used disinfection processes.
Existing information and guidance in the area should be used, wherever possible, to avoid the need for the development of new test methods and divergent assessment approaches.
When designing the framework, the weight of evidence and alternative methods should be promoted as far as possible to avoid the need for additional testing, in particular unnecessary vertebrate testing.
In case novel compounds may be expected to form during water treatment, guidance on how the risk to human health through the consumption of drinking water containing them would need to be addressed and should be provided in the guidance document. The type of studies or information that would be required in such cases to ascertain the safety for consumers should be outlined, taking into account the need to avoid unnecessary vertebrate testing.
1.2. Objectives
To develop guidance that enables the identification of real concerns for public health from exposure to harmful compounds generated during the processing of water for the production of drinking water.
A tiered framework is to be developed. The framework allows for a screening to determine whether residues from plant protection product (PPP) use according to good agricultural practice or residues from biocidal product use as directed can be present in water at water abstraction locations and if the presence of these residues cannot be excluded, if harmful compounds from these residues are formed during water treatment to produce drinking water. If formed, how to assess the impact of exposure to such compounds on human and domesticated animal (livestock) health.
The guidance has to focus on the water disinfection treatment methods of chlorination and ozonation (the most used disinfection methods). The employment of rapid sand filtration (used to remove organic impurities) prior to disinfection is to be included as a standard practice in surface water treatment. Groundwater is to be considered as not being subject to filtration before disinfection treatments.
Existing information and guidance in the area are to be identified and used to minimise the need to develop new test methods and avoid divergent assessment approaches.
When designing the framework, the weight of evidence and alternative methods should be promoted as far as possible to avoid the need for additional testing, in particular unnecessary vertebrate testing.
In cases where novel compounds are shown to be formed during water treatment, guidance on how the risk to human and animal health through the consumption of drinking water containing them would need to be addressed is to be provided in the guidance document. The type of studies or information that would be required in such cases to ascertain the safety for consumers is to be outlined.
2. Exposure assessment in ground water and surface water under the plant protection products regulation
2.1. Factors influencing the fate of active substances (AS) and environmental transformation products (eTPs)
Pesticides can enter soil or water bodies in many ways. The most important sources are agricultural areas, where most pesticide products are used. The major non‐point sources are from field applications where the processes of spray drift, dust drift from non‐spray applications, foliar wash‐off to the soil, run‐off and drainage from soil to surface water, plus leaching to groundwater are important. The amount of spray drift or dust drift from non‐spray applications depends on the distance from the crop to the top of the bank and the water edges, the wind speed and direction and possibly used buffer zones. Spraying speed, volume, height and nozzle type of the used sprayer, the ditch layout and possible shielding also have influences on spray drift. Without mitigation measures, spray drift is often the most important component of the total loading of pesticides in surface water and can have acute effects on non‐target organisms. Run‐off can contribute to input in aquatic ecosystems. It depends on soil type, pesticide properties, the use of buffer zones, wetlands, landscape management and modification of application periods. Drainage systems lead to contamination in particular when pesticides are applied to areas just before the drains are flowing into surface water, in late spring or summer during the main application time of pesticides and if weakly absorbed compounds with high‐water solubility are used. The exposure depends on soil type and pesticide properties as well.
The term ‘eTP’ refers to metabolites (i.e. products formed by metabolic processes) and abiotic environmental TPs, more precisely degradation products formed by natural physical or chemical processes, other than TPs formed by reactions during drinking water treatment (tTPs).
The fate of AS and their eTPs depends on many factors:
The application type results in differing exposure routes predominating, such as spray drift or run‐off.
The weather and climate influence the temperature in soil and water, which is why it affects the activation energy of chemical reactions and transformation. Rainfall events and dry periods can cause different soil conditions and more run‐off, as well as different water‐content and soil moisture.
The type of edge‐of‐field surface water and its depth influence the predicted environmental concentration (PEC) values because during application time in spring and summer, the depth can be close to the minimum; therefore, spray drift might lead to high concentration values. Due to its proximity to water bodies, run‐off and spray drift can directly enter the surface water. The Strahler Order of edge‐of‐field rivers is also significant for the dilution factors and the concentrations in the area mix (see Section 2.3.1.2). A river with a high Strahler Order usually carries more water of different origins, so there is a higher dilution factor and a chance for a more complicated chemical mixture.
The soil type and its hydraulic conductivity influence run‐off and the amount of drained outwash. Run‐off can directly occur on account of saturation‐excess and infiltration excess. Additionally, chemical reactions and sorption of AS and eTP can depend on the type of soil and its pH value. Due to smaller infiltration capacities, a high‐clay content usually leads to heavier run‐off.
The slope of the field and overall topography are important factors that influence run‐off. FOrum for the Coordination of pesticide fate models and their USe (FOCUS) considers a slope of > 4% as the ‘worst case’ for run‐off scenarios.
Due to the influence of weather and climatic conditions on water and soil temperature and on the water content in soil and surface waters (see also point 2.), the timing and season of product application, as well as the number of applications and the interval between applications, can lead to variation in the concentration, in particular with respect to peak concentrations. Moreover, if pesticides are applied in winter just before winter drainage, environmental exposure often also rises.
The type and quantity of irrigation (e.g. via flood, furrow, drip or sprinkler) greatly affects the pesticide concentrations that reach water resources. It has to be considered whether the irrigation takes place under or over crop canopy and with or without surface run‐off.
Substance‐specific properties like persistency and sorption determine the chemical behaviour of an AS or eTP. The sorption to sediment or dispersed solids in water can lead to concentration peaks in surface water bodies. Furthermore, soil specific sorption and photolytic and anaerobic degradation rates influence the fate of PPP as well as the kinetics of transformation, non‐equilibrium sorption, plant uptake and volatilisation.
2.2. Consequences for water resources from using plant protection products
AS and eTP might not only concern drinking water with regard to human health. If their concentrations are above critical thresholds, they can also harm non‐target organisms in the environment. The groundwater quality standard is usually set to 0.1 μg/L for pesticide‐AS, and their relevant metabolites and the drinking water directive stipulates that the pesticide concentration in drinking water must not exceed 0.1 μg/L for a single pesticide and 0.5 μg/L for all pesticides (Directive 98/83/EC and Directive (EU), 2020/2184). In 2016 6.5% of the groundwater bodies failed to achieve ‘good chemical status’ because of pesticides (EEA, 2018). From 2007 to 2017, about 7% of European groundwater bodies showed exceedances by herbicides and below 1% by insecticides, whereas exceedances by fungicides were considered of lower prevalence (Mohaupt et al., 2020). AS and eTP can leach through the soil matrix into groundwater in periods when irrigation or precipitation exceeds evapotranspiration. Pesticides and their soil metabolites or eTP properties, such as sorption to the soil, persistence and degradation influence leaching from the topsoil layer into groundwater. The soil type and its properties, such as permeability, texture and organic matter, also play a major role in the pathway to groundwater. The leaching can take up to several decades, depending on the depth of groundwater and properties of the soil and the type of pesticide. They can enter surface waters directly through slow and fast interflow processes (including the drained outwash already discussed in Section 2.1) and surface run‐off.
2.3. Guidance on the calculation of predicted environmental concentrations (PECs)
The PEC value is calculated to estimate the exposure pattern of the AS and its eTPs based on its use pattern. Given that non‐agricultural uses cannot be adequately characterised due to their complexity, the assessments presented in this guidance document are not easily applicable to such uses. Knowing primary data such as the intrinsic substance properties, potential emission sources must be analysed, and releases to receive environmental compartments such as surface water and groundwater must be determined. Taking into account the complexity of diffuse substance inputs, particularly in surface waters, and in general due to the variety of potential processes during leaching of those of groundwater entries, simulations for the calculation of these entries and the resulting concentrations are stipulated as having to be provided in the registration process in the European Union according to Regulation 1107/2009 (and its predecessor Directive 91/414/EEC). Applicants should note that the PEC assessment outlined in this guidance is based on the current requirements at the time of writing. Considering the continuous adaptation of the respective requirements, PEC values based on representative uses are to be calculated according to the procedures set out in the pertinent FOCUS documents. In cases where intended uses are specified in product authorisation procedures other than those covered by the representative uses under the AS approval procedure, it is the responsibility of the authorising authorities to require the appropriate PECs and/or to consider product dossiers.
This section provides practical guidance for the calculation of PECs in the context of pesticide approval and member state authorisation assessments. In this respect, the assessment is based on the acceptance of certain ratios between expected exposure and observed toxicity, based on the underlying premise that low risk is to be expected if the expected exposure is sufficiently lower than the laboratory toxicity endpoints (or other trigger values).
AS thereby comprises all active substances as defined in PPP Regulation 1107/2009 (Art. 2), i.e. also chemical elements and compounds, substances whether of synthetic or natural origin, 3 or comparable in structure to a naturally occurring substance such as microorganisms and semiochemicals, which exert a general or specific effect against harmful organisms of agricultural production or against plants, plant parts or plant products. The risk and PEC assessment procedure outlined below is essentially applicable to each chemical AS, both of natural and synthetic origin and its eTPs, as well as to the metabolites of microorganism AS. But applicants, as well as competent authorities, are encouraged to consider if deviations might hold for the individual substance under assessment and whether additional guidance might apply. Examples of reference documents in this regard include the statement for the evaluation of transition metals (EFSA PPR Panel, 2021) or the guidance document for semiochemicals (European Commission, 2016). In the case of semiochemicals, for example, that can be released naturally by living organisms, natural exposure should be considered in comparison with the exposure resulting from the intended use of the PPP in the risk characterisation (European Commission, 2016). Also, following the ethos of the SANCO guidance on the assessment of the relevance of metabolites in groundwater (European Commission, 2021), consideration can be made that further assessment is not needed when one of the following conditions can be shown to have been met:
it is CO2 or an inorganic compound, not containing a heavy metal; or
it is a substance known to be of no toxicological or ecotoxicological concern and naturally occurring at much higher concentrations in surface or groundwater.
Irrespective of any scenario subsequently considered, an exposure assessment for the PPP or AS application dossier must initially clearly identify and indicate the chemicals and eTPs for which the PEC calculation is to be made and include necessary data on their relevant properties. Since AS in PPPs can be subjected to both abiotic and biotic transformation processes in the environment, Regulation (EC) No 1107/2009 defines a metabolite as ‘any metabolite or degradation product of an active substance, safener or synergist formed either in organisms or in the environment’. Accordingly, the terms ‘metabolite’ and ‘eTP’ are generally considered interchangeable in the present guidance and are hereinafter primarily referred to as the more inclusive eTP term (for degradation products from drinking water treatment, please refer to the explanation on tTP in Section 3.1). Metabolite will be used predominantly where necessary for explanatory purposes and ease of transferability, such as in model descriptions, etc. (see e.g. model descriptions in Sections 2.3.1 and 2.3.2).
For an eTP to be considered relevant for the general authorisation decision or the determination of risk reduction measures, it must (1) have a certain potential for occurrence in the respective compartment, to be determined through the PEC calculation of the exposure assessment and (2) if it is reasonably assumed:
to have intrinsic properties comparable to the parent substance in terms of its biological target activity,
to pose a higher or comparable risk to organisms than the parent substance,
to have certain toxicological properties considered unacceptable.
In addition, the identification of potentially relevant eTPs to be assessed needs to be based on scenarios that are representative of the environment and type of application. Fundamental prerequisites to identify those potentially relevant eTPs that require PEC calculation are incubation studies on the route and rate of degradation of AS in soil, water and sediment, which are also used to derive endpoints.
Regulatory degradation endpoints for AS and eTP include DT50 and DegT50 and DT90 and DegT90 values, which are needed as triggers and for PEC calculations (FOCUS, 2006). In this regard, the provided data and information, together with other relevant data and information, must be fundamentally sufficient to:
Identify the individual components present which at any time account for more than 10% of the amount of active substance added;
Identify, if possible, the individual components which, in at least two sequential measurements, account for more than 5% of the amount of active substance added;
Identify, if possible, the individual components which account for more than 5% of the amount of the active substance added and for which the maximum of formation is not yet reached at the end of the study.
A PEC assessment is required for all eTPs in addition to AS, meeting at least one of the above listed key criteria. The PEC also takes into account the range of uses authorised. Every applicant must provide PEC to cover the uses for which they are seeking authorisation which are usually defined by crop or other specified use situation. The uses are related to the specific and realistic combinations of cropping, soil, weather, field topography and aquatic bodies adjacent to fields by the scenarios that have been defined in the guidance available for calculating the PEC.
In principle, the information requirements for the PEC assessments for AS and eTPs in raw waters that may be abstracted as drinking water resources rely on the existing PEC assessments. However, refined PEC calculations (e.g. surface water higher Steps or national scenarios for surface and groundwater) can and should be included when triggered or if requested by competent authorities during the assessment of applications or if deemed helpful by the applicant to demonstrate safe use of PPP with respect to eTP. All these approaches may be used to calculate PEC.
Furthermore, it has to be ensured that the data to be submitted complies with Regulation (EC) No 1107/2009 and (EU) No 283/2013 (European Union Commission Regulation, 2013a) and (EU) No 284/2013 (European Union Commission Regulation, 2013b). All tests required in regard to providing compliant information on PPP, AS and eTP for further assessment shall be those referred to in Commission Communications 2013/C 95/01 and 2013/C 95/02 or their subsequent updates.
In terms of data requirements and implementation, a distinction must be made between PEC calculations for surface water and groundwater, both of which, either mixed or alone, can serve as water sources for drinking water production, so the most applicable and recent framework needs to be applied. Hence, the respective PEC calculations for both groundwater and surface water have to be conducted and provided in the dossier. A number of substance‐specific properties required in simulations of both sets are likely to recur; hence applicants may consider initially compiling an archive/database of basic information to provide all data required for the simulations to facilitate the assessments with the modelling tools.
The EU and EFSA, therefore, provide a set of agreed models and tools that allow the calculation of PECs for different scenarios.
For surface water, please see: https://esdac.jrc.ec.europa.eu/projects/surface-water
For groundwater, please see: https://esdac.jrc.ec.europa.eu/projects/ground-water
Other simulation models may also be used as long as the applicant demonstrates compliance with the data obtained with the governing legal requirements. Regional models may also be required in the individual Member States. An example of the use of regional models and scenarios is those that are used for product authorisations on the crop rice.
In order to provide clarity, this Guidance is based on the models (FOCUS) provided by the EU and EFSA.
In this regard, applicants should note that the types of concentrations differ in the PEC assessment for surface water versus groundwater. While the FOCUS methodology for groundwater calculates the 80th percentile of annual concentrations over 20 years at a soil depth of 1 m for each FOCUS scenario, the current FOCUS methodology for surface water (FOCUS 2015) reports PECsw as annual PECs based on the maximum concentration for each FOCUS scenario.
The flow charts below depict the necessary models to derive PECs for both surface water and groundwater. Further, more detailed information on data and software requirements needed for the use of the models is provided on the ESDAC JRC website (https://esdac.jrc.ec.europa.eu/projects/focus-dg-sante) subordinate to the overview pages (see above) and in the user manuals of the FOCUS versions of the models. Applicants may adopt other models, provided that evidence of compliance with EFSA requirements described in its opinions and documents on good modelling practice is demonstrated.
2.3.1. Surface water
In the EU FOCUS scenarios for surface waters, a tiered approach is applied to assess the exposure of aquatic organisms to pesticides. This is also transferable to the assessment of the exposure of surface waters potentially used as drinking water resources to pesticides and their eTPs. Here, in context with the formation of transformation products after drinking water treatment, the potential for the formation of further transformation products is of particular interest. In cases where PEC values derived from the models exceed the value that indicates a risk to aquatic organisms (as defined in EFSA PPR Panel, 2013b), applicants may consider emission reduction measures. When applying these emission reduction measures (when needed) and a trigger value of 0.1 μg/L derived from the Drinking Water Directive is exceeded, this guidance then introduces the consideration of applying dilution factors to the small FOCUS defined headwater surface water catchment PEC values to derive PEC surface water drinking water abstraction values (PECSW DW ABSTRACTION). The effect of drinking water treatment has to be assessed for all AS and eTPs with a PEC surface water drinking water abstraction value above 0.1 μg/L – a pragmatic approach adopted from the European Drinking Water directive. However, as far as indicated, specific drinking water values from the Drinking Water Directive may apply to individual substances. Values may be significantly lower than 0.1 μg/L for some eTPs, which applicants must consider prior to assessment.
The principle procedure to estimate PECs for AS and eTPs in the surface water is as follows:
simulation of exposure concentrations via the relevant entry pathways (including relevant exposure mitigation for each pathway);
simulation of the surface water fate of the substances once they entered the water body.
Simplified, the connection to drinking water treatment can be described as follows:

Due to the complexity of diffuse entries, together with possible transformation and metabolic processes, a number of scenarios need to be taken into account in the assessment.
This results in several processes that must be taken into account based on differing substance properties and application modes. Applicants need to use different entry models to assess the entry concentrations of substances in surface waters that result from pesticide loading via drift and either drainage or run‐off/erosion.
Furthermore, where calculated PEC values do not comply with the requirements to demonstrate a low risk to aquatic organisms, the applicant might assess the application of exposure mitigation processes, which is also enabled by a respective software tool.
In addition, they are required to simulate the fate of PPPs in surface waters at the edge of fields by using a further calculation model.
Within the framework of the FOCUS models provided, the following general flow chart therefore emerges (Figure 1):
Figure 1.
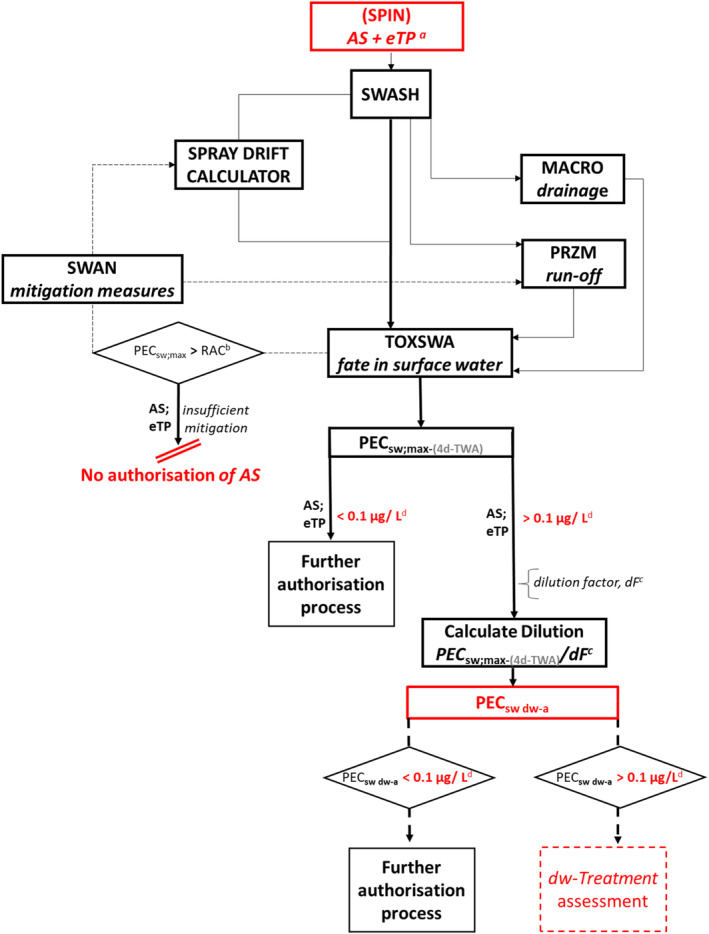
Flow chart with tests and models to be applied to derive surface water PECs.
aAll metabolites/eTPs that may occur under the respective conditions, other than metabolites/eTPs of no concern as set out in Sanco/221/2000 –rev.11 (European Commission, 2021) (According to the Guidance on the Assessment of Relevance of Metabolites (European Commission, 2021), only those substances can be considered of no concern to which one of the following conditions apply: – it is CO2 or an inorganic compound, not containing a heavy metal; or, – it is an organic compound of aliphatic structure, with a chain length of four or less, which consists only of C, H, N or O atoms and which has no ‘alerting structures’ such as epoxide, nitrosamine, nitrile or other functional groups of known toxicological concern. – it is a substance, which is known to be of no toxicological or ecotoxicological concern, and which is naturally occurring at much higher concentrations in the respective compartment.).
Applicants should note that the term ‘metabolite’ is commonly referred to in most models rather than degradation product, eTP or similar. In order to prevent ambiguities, applicants need to verify which input data have to be provided accordingly.
bRAC: Regulatory acceptable concentration, resulting from respective effect assessment.
cdF: Dilution Factors – refer to Table 1 for the respective appropriate factor.
dThe criterion for surface water intended for drinking water abstraction is that the concentration of each AS and its eTP formed from that AS must be below 0.1 μg/L, or even lower for a certain eTP if there is a lower parametric value (maximum permissible concentration ‐ MAC) as defined in the Drinking Water Directive (Directive (EU), 2020/2184).
For authorisation, applicants need to demonstrate that the risk posed by the substance is likely to be sufficiently low to be acceptable. That is, the calculated PECsw values for all AS and eTPs to be assessed when compared to relevant (eco‐)toxicity data are below toxic concentrations and is indicated using the PEC/RAC concept. According to the Generic guidance for FOCUS surface water Scenarios (FOCUS, 2015), if the substance passes one of the scenarios for surface water, it can, in principle, be included in the European Commission database for substances approved under Regulation (EC) No 1107/2009. However, the proposed approach in this guidance is for applicants to demonstrate that the PECsw simulated values for all substances to be evaluated, and indeed for all relevant scenarios and field applications, are below the RAC value. However, even if all relevant scenarios are passed, which in principle means that the substance can be trusted to be used safely in the large majority of situations in the EU, this does not absolutely exclude the possibility of risk in specific local situations in certain regions. Therefore, at the latest at the Member State authorisation stage of PPP, all scenarios considered relevant at the national level must be passed through, and any such vulnerable situations need to be assessed at the Member State level. Furthermore, after adding a relevant dilution factor to the PECsw to calculate the PECSW DW ABSTRACTION for any concentration above 0.1 μg/L, the effect of drinking water treatment on these residues has to be assessed.
In order to identify the AS and eTPs under assessment and access their intrinsic properties for the models, applicants have to provide the necessary information data and feed them into the models unless they already exist in databases. This can be done by adding the required data to the model or using an appropriate database.
SPIN was designed as a tool for this very purpose; it serves as a repository for substance properties of AS and eTPs and is where the transformation pathway used in simulations is defined.
For more information on required data, consult:
https://esdac.jrc.ec.europa.eu/projects/swash
https://esdac.jrc.ec.europa.eu/projects/macro-0
https://esdac.jrc.ec.europa.eu/projects/przmsw
https://esdac.jrc.ec.europa.eu/projects/toxswa
https://esdac.jrc.ec.europa.eu/projects/swan
and the respective user manuals).
As a key tool and an overall user shell, SWASH (Surface WAter Scenarios Help) assists in calculating pesticide exposure concentrations using and linking the EU FOCUS Surface Water Scenarios. These comprise a specific set of models to account for the different contamination routes of the surface waters under consideration.
Within the FOCUS framework, MACRO is to be used to calculate the contribution of drainage inputs to surface water bodies.
The FOCUS PRZM_SW (Pesticide Root Zone Model) is a one‐dimensional, dynamic model to determine the PPP contribution of run‐off and erosion events and for the prediction of chemical movement in unsaturated soils using vertical chromatographic leaching. In the EU‐evaluation process, PRZM_SW should be used within the framework of the Step 3 FOCUS surface water models and the SWASH shell to simulate chemical movement within or right below the root zone in unsaturated soils.
The TOXSWA (TOXic substances in Surface WAters) simulation is part of the pesticide exposure assessment used in the EU evaluation. TOXSWA can calculate PECs in water and sediment, using geometric mean degradation rates in water and sediment at 20°C, partitioning to and from sediment and the vapour pressure as well as solubility of the PPP in water and its molecular mass. The program calculates daily concentrations in surface water and sediment and the maximum time‐weighted average concentrations for specific time periods. To obtain realistic estimates of environmental concentrations suitable for the context of further assessment under this guidance, the 4‐day PEC is proposed to be used, rather than, e.g. initial PEC values (PECini). Transport, transformation, sorption and volatilisation are considered during the calculation since AS, and their eTPs are transported by advection and dispersion in water. To simulate the flow dynamics in edge‐of‐field surface waters, the field‐scale system is defined as the downstream part of a smaller catchment basin.
Most promising mitigation measures targeting entries into surface waters are, by their very nature, based on emission controls, although this varies widely in implementation for the main entry pathways. This includes:
technical and landscape measures, such as adjustments in application technology or no‐spray buffer zones targeting drift entries to water bodies at the edge of the field.
erosion mitigation measures, such as adjustments in slope requirements and vegetation cover management.
and can encompass adjustments to application rate and frequency.
Mitigation measures may be necessary if a risk to the (aquatic) environment is indicated by the predictable environmental concentrations under the current application parameters compared to the effects. If mitigation measures are required to proceed with the assessment, applicants are encouraged to consult the guidelines and recommendations of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment for guidance (FOCUS, 2007).
In principle, within the FOCUS Step 3 scenarios relatively simple changes can be made by refining input parameters for the chemical or scenario to make them more precisely reflect the potential exposure being assessed. Additionally, new scenarios could be developed for use in Step 4 to more precisely reflect the range of environmental and agronomic conditions for the use of a PPP at a local or regional scale.
SWAN (Surface Water Assessment eNabler) is a tool to assess the application of exposure mitigation processes that are based on FOCUS Surface Water Step 3 as implemented in SWASH. SWAN allows for the consistent application of mitigation measures in accordance with available guidance derived data (e.g. FOCUS, 2007) that can be used as the basis for a Step 4 simulation.
2.3.1.1. Reported data
Finally, applicants need to compile the obtained data in the registration report. In addition to all data and result files obtained from the simulations, this report should also include the following information:
Input parameters according to the latest valid List of Endpoints.
Input parameters used (+ justification).
Management‐related parameters obtained from the intended Good Agricultural Practice (GAP): crop, number of applications, application rate, interval, interception, application time, application window (dates and days), application date and application method.
Versions of the models used.
In this guidance, dilution factors are applied for edge‐of‐field surface water bodies to decide which diluted compounds require further assessment. If, despite dilution, the concentration value of the compound or substance is higher than 0.1 μg/L, further assessment is required. The dilution factors to be used are determined for each regulatory zone in Europe (North, Central Europe and South) by selecting an agricultural land use within a 50 m buffer around rivers using a GIS programme. For details on dilution factors that the applicants need to consider, please refer to Section 2.3.1.2.
Mitigation measures required to demonstrate low aquatic risk must be implemented in the Step 4 FOCUS calculations, which should be completed for the AS and all eTPs that reach levels in soil and/or surface water and sediment specified in Regulation (EU) No. 283/2013 as needing to be assessed. If risk is already demonstrated as low at lower FOCUS Steps, when PEC are higher than 0.1 μg/L, it is advisable to at least provide FOCUS Step 3 PEC. Dilution factors for edge‐of‐field surface water bodies will then be applied for AS and eTPs for FOCUS PEC higher than 0.1 μg/L to produce PECSW DW ABSTRACTION. For national product authorisations, national scenarios with national mitigation measures can be needed in addition to the calculations for FOCUS scenarios. The effect of drinking water treatment has to be assessed for all AS and eTPs with PECSW DW ABSTRACTION higher than 0.1 μg/L.
2.3.1.2. Determination of dilution factors for the three regulatory zones in Europe
In order to derive changes in PEC during the fluvial transport of AS and eTPs from entry to surface waters at the edge‐of‐field to their potential abstraction point for drinking water production, dilution factors need to be considered. These can vary within the individual regulatory zones that have been defined for PPP authorisation in Europe. The factors, which drive dilution, must be considered. These include land use, landscape factors, such as the slope of the land or the proximity of the input area to the rivers. Also, the type and characteristics of the soil, the timing and pattern of application, etc., can be relevant. Furthermore, weather and climatic factors such as heavy rainfall and dry periods must be considered. All these factors determine the amount or concentration of pesticides when they enter surface waters. After AS and eTPs have entered rivers and lakes, evaluating the concentration after dilution is important.
The three European regulatory zones have significantly different conditions. For example, in the number and length, as well as Strahler Order (SO) of the rivers or the agricultural use of soils. These factors also vary greatly between countries, and dilution varies throughout the year (e.g. rainfall changes affecting water levels and different pesticide use patterns). Determination of dilution factors is typically required as discharge permits for point source emissions must ensure compliance with water quality standards for the aquatic environment. Dilution factors must be based on the flow of the effluent and the stream. Low flow means less water is available for dilution, so dilution factors must be based on ‘worst case scenarios’ to protect the aquatic environment successfully. Therefore, determining a dilution factor per zone is necessary to reflect a realistic worst‐case scenario. For example, a worst case scenario exists when AS and their eTPs enter a small brook or river just upstream of a drinking water abstraction point during a dry period, as low flow conditions will limit the dilution of the PPP.
Furthermore, the zones differ in their agricultural use. Northern Europe features far less agricultural area than Central and Southern Europe, whereas Central Europe has slightly more arable land than southern Europe.
Worst‐case dilution factors to be applied:
-
Central European Regulatory Zone: 2
Dilution factors have been published for multiple countries within the Central European Regulatory Zone. For the Netherlands, dilution factors below 5 (De Greef and De Nijs, 1990) and 2 (Adriaanse et al., 2008) have been reported. For Germany, 5 has been reported (Link et al., 2017).
-
Southern European Regulatory Zone: 5
Gros et al. (2007) conducted a study to derive dilution factors for five WWTPs (waste water treatment plants) in Spain, whereas the lowest dilution factor reported was 5. Studies from other countries included in the Southern European Regulatory Zone show worst‐case dilution factors somewhat higher, e.g. 19 (Italy, Verlicchi et al., 2014).
-
Northern European Regulatory Zone: 10
No measured or modelled dilution factors have yet been reported for the Northern European Regulatory Zone. Due to the dense river and lake network in the related countries, a higher dilution factor of 10 can be applied. According to Keller et al. (2014) and Link et al. (2017), this dilution factor can be considered a standard factor.
Most of these dilution factors have been calculated, measured or modelled for the input of wastewater effluents into surface waters. As dilution processes are very similar once substances have entered surface water and as they are modified according to land use type and Regulatory Zone, these dilution factors can also be applied for PPP (AS and eTPs).
The dilution factors described above are further disaggregated for different land use types within each European Regulatory Zone. Applicants need to pick the dilution factors relevant to their AS and eTPs under assessment. If a combination of land‐use types has to be considered, referring to the worst‐case risk approach described above, applicants have to apply the lowest dilution factor of the relevant land use types.
These worst‐case dilution factors have been derived by integrating research‐based publications (see above), characteristics from the three European Regulatory Zones (see Appendix A), and expert knowledge. As a baseline, the dilution factors from research‐based publications have been applied. They were then adjusted to the specific characteristics of each Regulatory Zone (Central Europe 2, Southern Europe: 5, Northern Europe 10). This included the area of surface water and the length of river networks. In a final step, the dilution factors for surface water were adjusted to the relevance of different land use types within each Regulatory Zone (see Table 1 and Appendix A).
Table 1.
Dilution factors (worst case) for different European Regulatory Zones and land use types
| Land use type | European Regulatory Zone | ||
|---|---|---|---|
| Central | South | North | |
| Arable | 2 | 3 | 9 |
| Tree crops | 2 | 3 | 9 |
| Grassland | 2 | 4 | 10 |
| Forests | 2 | 4 | 10 |
When the standard dilution factors result in further assessment being triggered, the types of approaches set out in the GIS work in Appendix A can be used to show that land use (including the area that has the potential to be treated due to specificities of the potential use pattern considering the authorisations in place and or requested for relevant plant protection products) would allow the use of a different dilution factor.
2.3.2. Groundwater
Through the application of PPPs, various substances can enter the groundwater via leaching processes. In this respect, the leaching of a substance, either AS itself or an eTP, strongly depends on its persistence and mobility in soil, as well as on the physical transport properties of the soil and the transport processes. In terms of substance properties, persistence represents a measure of a pesticide's resistance to chemical transformation and is usually described by the soil DegT50 value, with mobility usually described by soil‐water partition coefficients (European Commission, 2014).
In particular, for AS, unfavourable intrinsic substance properties such as high‐water solubility, high leaching potential and stability in conjunction with high precipitation and permeable soil types elevate the risk of AS entry. At the same time, AS/PPP that are degradable can lead to eTP entries into groundwater that must also be covered in the assessment.
As a general rule, PECGW calculations using simulations are required according to European Commission (2014) and have to be submitted in the dossier as part of the authorisation process for AS in products, and each potentially relevant eTP expected to be present in soil under the required use conditions as described in European Commission (2014) based on the results of soil degradation studies or lysimeter studies, to quantitatively assess their ability to contaminate groundwater.
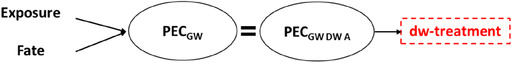
Thus, in addition to the substance‐specific properties, essential criteria which must be covered in the simulations are soil properties, weather conditions and, since both the type of pesticide used and the potential for plant uptake also depend on this, the respective arable crop.Whereas in surface water assessment, the PECSW is diluted almost without exception prior to drinking water treatment, the same does not necessarily apply to groundwater. The worst‐case scenario requires consideration of spatial entries in the abstraction zone, thus equating the PECGW to the PECGW DW ABSTRACTION (further referred to as PECGW DW ABSTRACTION) in principle. Therefore, simplified, the connection to drinking water treatment can be described as follows:
In principle, all tiers of the European Commission (2014) approaches may be used to derive PECGW. Similarly to the PEC assessment for surface water, the EU regulatory exposure assessment scheme provides a set of FOCUS models to simulate the leaching of AS and their eTPs to groundwater. The above key criteria are thereby covered by a set of nine standard combinations of weather, soil and crop data, which together represent agriculture in the EU. In total, there are 125 realistic worst‐case scenarios to be run (FOCUS, 2014; EFSA PPR Panel, 2013a,b; European Commission, 2014).
In accordance with point 3.10 of Annex II to Regulation (EC) No 1107/2009 on the fate and behaviour in groundwater, an AS shall only be approved if it has been demonstrated for one or more representative uses that the predicted concentration of the AS or its eTP after application of the PPP under realistic conditions of use complies with the relevant criteria referred to in Article 29(6). These shall ensure a high level of protection of human and animal health and the environment. Thus, passing one of the groundwater scenarios would be, in principle, sufficient for the AS to be included in the European Commission database for substances approved under Regulation (EC) No 1107/2009. However, in line with the prescribed approach for PECSW and in consideration of the precautionary principle, the herein proposed approach is for applicants to demonstrate that the PECGw simulated values for all substances to be evaluated, and indeed for all relevant scenarios and field application use patterns, are below the trigger value. Applicants are encouraged to consider the assessment requirements for the member state they are submitting to, as the particular combination of models and, more specifically, scenarios chosen by the member state may differ.
For clarity, this document provides guidance as to which substances require a PECGW calculation under the pesticide authorisation and how to calculate the PECGW using the tools provided by the EU.
The basic FOCUS PECGW assessment scheme comprises all PECGW modelling assessments based on data according to the requirements in Regulation (EC) 1107/2009 in combination with standard scenarios (European Commission, 2014). Regarding eTPs to be considered, applicants may refer to FOCUS (2006) and EFSA (2014) for detailed guidance on the selection of appropriate degradation kinetics, also briefly mentioned in Section 1.3, and on the averaging procedure for the representative modelling endpoints and to FOCUS (2000, 2003) and European Commission (2014) as well as FOCUS (2014) for principal guidance on the selection of pesticide input parameters. The scenarios have been implemented as sets of input files for four simulation models ‐ MACRO, PEARL, PELMO & PRZM. These input files and the simulation models which are needed to run them form an important part of the leaching assessment process. And while the methods applied and results achieved often overlap, this is not the case for every scenario. Therefore, in line with European Commission (2014) and EFSA PPR Panel (2013a,b), applicants and rapporteurs are advised to provide simulations with PEARL and PELMO or PRZM. In case a crop of interest is defined for Châteaudun, the MACRO model needs to be run in addition (European Commission, 2014).
Unlike the PEC calculation for surface waters, the models in the groundwater simulation run in parallel. Since both PEARL and PELMO (based on PRZM) or PRZMGW are required, applicants are advised to run simulations with both models and include the results of running both models in the report and, in accordance with the procedure outlined in European Commission (2014), provide the highest PECGW for the submissions. Unless, in one of the two models, PECGW results to be < 0.001 μg/L for all relevant scenarios and all substances triggering groundwater assessment, then it is not necessary to perform simulation runs with the other model.
Appropriate information on transformation and metabolism pathways in the soil, as well as the soil eTP properties, have to have been surveyed by using appropriate tests and have been included by the applicant in the dossier as required by Regulation (EU) No 283/2013.
Within the framework of the FOCUS models provided, the following general flow chart therefore emerges (Figure 2):
Figure 2.
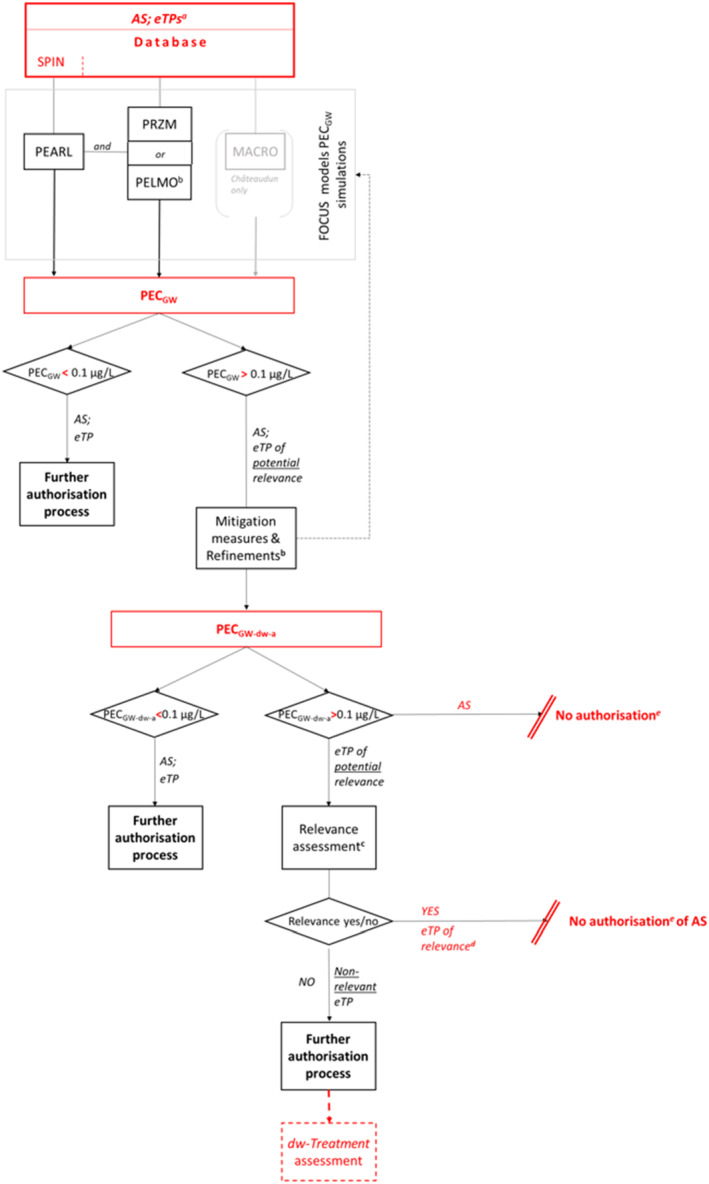
Flow chart with tests, models and triggers to be applied to derive groundwater PECs
a: All metabolites/eTPs that may occur under the respective conditions, other than metabolites/eTPs of no concern.
Applicants should note that the term ‘metabolite’ is commonly referred to in most models rather than degradation product, eTP or similar. In order to prevent ambiguities, applicants need to verify which input data have to be provided accordingly.
b: Refinements may be performed by data adjustment at the parameter level, at the scenario level, or in mixed modes. Besides refinement steps, mitigation (measures to adjust or restrict pesticide use to reduce the risk of leaching to an acceptable level) may be included at each tier of the assessment process.
c: Relevance assessment includes 3‐steps: (i) Screening for biological activity against the target organism, (ii) Screening for genotoxicity, (iii) Screening for toxicity, and in case of acceptable screening results in addition: Exposure assessment – threshold of concern approach. In addition, for eTP that have passed the hazard assessment but are estimated to be present at levels exceeding the toxicological threshold of 0.75 μg/L in groundwater but are below 10 μg/L, the relevance assessment also requires a refined assessment of their potential toxicological significance. For detailed guidance on the step‐by‐step approach to assessing the relevance of eTP, applicants should refer to Sanco/221/2000 – rev.11 (European Commission, 2021) (Applicants should note that the limit value of 10 μg/L in accordance with European Comission (2021) is chosen for pragmatic reasons and is adopted in the figure and the approach description for simplification. However, some eTPs may also belong to other categories defined in the Drinking Water Directive and therefore are subject to different limit values as defined in Annex I, Part B of this Directive. This has to be assessed on a case‐by‐case basis and the limit value adjusted accordingly.).
d: eTP is either considered relevant in at least one of three screening steps (see c) or for which the refined exposure assessment to be provided if an acceptable hazard has been identified ‐ with the threshold of concern approach – exceeds the acceptable threshold value (of maximum 0.75 μg/L, or, if other sources of exposure are added, possibly below) and do not pass in the refined risk assessment (European Commission, 2021).
e: Where authorisation of any use is not possible because ‘safe’ situations for groundwater exposure have not been identified, the active substance is not approved for use in the EU.
Thus, whether and to what extent further assessment of each eTP is required depends on its predicted concentration and its classification as a metabolite or eTP resp. of relevance, non‐relevance and non‐concern (European Commission, 2021). In this regard, assessments of the eTPs of AS are required to be of comparable transparency, scientific validity and an equivalent level of regulatory scrutiny to that of the parent AS.
PEARL (Pesticide Emission Assessment at Regional and Local scales) is a one‐dimensional numerical model for the behaviour of pesticides in the soil–plant system. A number of possible lower boundary conditions are included in the simulated soil water flux (e.g. groundwater levels that fluctuate depending on precipitation), with soil evaporation and plant transpiration also factored into the simulation.
PEARL provides an assessment of pesticide leaching in all nine EU groundwater scenarios in which plant growth is simulated using a simple growth model wherein both leaf area index and rooting depth are a function of plant development stage.
For further information on input data requirements, applicants may refer to European Commission (2014) and https://esdac.jrc.ec.europa.eu/projects/pearl.
PELMO is a one‐dimensional simulation model. Using chromatographic leaching, it can simulate the vertical movement of pesticides in soil matrices. PELMO and PRZM are largely interchangeable in the EU authorisation process and applicants opt for either PELMO or PRZM_GW in addition to PEARL.
FOCUS's main objective is to implement a harmonised approach for European Tier 1 risk assessments as required by Regulation 1107/2009.
For further information on input data requirements, applicants may refer to EC (2014) and https://esdac.jrc.ec.europa.eu/projects/pelmo
and for PRZM_GW to https://esdac.jrc.ec.europa.eu/projects/przmgw
For PEC groundwater calculations, MACRO was parameterised for the Châteaudun scenario only. No MACRO simulations are necessary if the PECGW values calculated with FOCUS PEARL and FOCUS PELMO are < 0.001 μg/L for all substances which trigger groundwater assessment. For further information on input data requirements, applicants may refer to European Commission (2014) and https://esdac.jrc.ec.europa.eu/projects/macro.
2.3.2.1. eTPs to be assessed
In addition to the requirements and definitions for metabolites/eTPs given in Section 2.3, this section provides the applicant with further details on the categorisation of eTPs and the PECGW assessment procedure in accordance with European Commission (2014, 2021).
As a general principle, all eTPs that are not excluded because they are proved to be of no concern 4 and that are found during soil degradation and/or in available lysimeter or field leaching studies should be characterised and identified by the applicants to the extent that this is technically feasible. A PECGW calculation and assessment shall be provided for each of these substances similar to procedures for AS; however, in the course of assessment, procedural distinctions may be applied, depending on the estimated concentration and the relevance of the respective eTP (European Commission, 2014, 2021).
According to the SANCO guidance on the assessment of the relevance of metabolites in groundwater (European Commission, 2021) Step1, a metabolite/eTP is of no concern and therefore requires no further assessment if one of the following conditions is met:
It is CO2 or an inorganic compound, not containing a heavy metal; or,
It is an organic compound of an aliphatic structure with a chain length of 4 or less. It consists only of C, H, N or O atoms and has no ‘alerting structures’ such as epoxide, nitrosamine, nitrile or other functional groups of known toxicological concern.
It is a substance known to be of no toxicological or ecotoxicological concern and naturally occurring at much higher concentrations in the respective compartment.
This implies that a PECgw calculation is generally required for the parent substance/AS as well as for all metabolites that are not of no concern, with a cut‐off value of 0.1 μg/L for active substances, and a trigger value of 0.1 μg/L for further investigation for metabolites/eTP.
An eTP is considered not relevant if it does not meet the criteria for relevant metabolites/eTPs of the hazard and exposure assessments (including, if necessary, a refined risk assessment based on more toxicological data) but is neither classified as ‘not of concern’ according to the criteria listed above. For non‐relevant eTPs, the 0.1 μg/L limit does not apply or may not be obligatory, and they may be subject, on a case‐by‐case approach, to an individual groundwater limit concentration, as outlined in detail in European Commission (2021) and they also have to be assessed concerning the effects of drinking water treatment (FlowChart PECGW).
In general, much of the data required for eTPs of individual AS under assessment may already be available since, according to the data requirements for AS set out in Regulation (EU) No 283/2013, several studies have to be performed by the registrant on the metabolism of the AS in the different relevant environmental compartments.
In detail and also regarding required triggers and endpoints, applicants may refer to the Annex of REGULATION (EU) No 283/2013 Part A Section 7.1 and Part B Section 7 for the soil testing and information requirements that are of particular importance concerning the groundwater assessment of metabolites and other TPs and for further details on the tests, they can refer to the associated Commission Communications 2013/C 95/01 or subsequent updates. Consequently, it is reasonable to assume that at least some information on metabolism, rate, pathway and kinetics is available for all compartments and can thus be used for the PECGW assessment of the AS and its eTPs. Therefore, prior to conducting additional testing, applicants are encouraged to examine existing studies to determine whether the respective eTPs have previously been covered by studies required for the AS or metabolites/eTP that have reached levels that trigger assessment, taking into account the use pattern and fate of the compounds under investigation.
2.3.2.2. Lysimeter
Lysimeter studies may not be required in all settings but may be included as a valid resource in refinements. However, the results of the degradation and mobility tests and the calculated PECGW shall be considered when deciding whether to conduct lysimeter tests as experimental field tests as part of a leaching assessment.
However, relevant lysimeter tests have to be used in the groundwater exposure assessment when available. They shall be performed to investigate the mobility of the substance in the soil, the potential for leaching to groundwater, and the potential distribution in the soil. Compounds/resolved chromatographic fractions present in annual average lysimeter leachate above 0.1 μg/L have to be identified and assessed for their groundwater relevance (European Commission, 2021).
Applicants need to discuss the respective type of tests to be performed with the relevant national authorities. Tests shall:
cover the realistic worst case;
be of sufficient duration to allow observation of possible seepage.
In general, information derived from lysimeter tests must provide sufficient data to evaluate the mobility and leaching propensity of the active ingredient and eTPs ((EU) No.283/2013).
2.3.2.3. Mitigation measures regarding groundwater leaching
Unlike surface water simulation, groundwater simulation provides no dedicated tool to automatically evaluate mitigation measures. Mitigation measures must therefore be recommended and, if necessary, simulated or tested in real scenarios on the basis of rational judgement, understanding of the substance properties as well as the factors affecting them.
Most appropriate mitigation measures addressing leaching to groundwater will often differ from those for surface water discharges. Appropriate mitigation measures to prevent AS or eTPs leaching into groundwater often relate to the GAP, but will often differ from those for surface water entries. In general, buffer zones or amended application equipment are not relevant.
Instead, appropriate mitigation measures include limiting use patterns on the crops to be assessed, i.e. application timing, number and rate. In addition, site characteristics could be included, such as approved or restricted soil properties on which to apply or restrict application in vulnerable areas and limiting application to certain times of the year.
For efficient mitigation measures regarding groundwater entries, please refer to European Commission (2014).
2.3.2.4. Reported data
In accordance with the surface water assessment, the registration report should, in addition to all data and result files obtained from the simulations, also comprise the following information:
Input parameters according to the latest valid List of Endpoints.
Input parameters and justifications.
Relevant values related to the GAP: crop, number of applications, application rate, interval, interception, application dates, application method.
Versions of the models used.
3. Exposure assessment in groundwater and surface water under the biocidal products regulation
3.1. Summary
Biocidal active substances (AS) and their metabolites/transformation products (hereafter referred to as environmental transformation products, eTPs), 5 as well as other components in a biocidal product or a treated article, 6 when present in the water abstracted for drinking water production, can be a source of harmful substances generated during the processing of water to produce drinking water. As explained in Chapter 2, in the context of this document, substances are designated as tTPs, i.e., treatment transformation products when formed during the water treatment.
Regulation (EU) No 528/2012 concerning the placing on the market of biocidal products (BP), in the following, referred to as ‘BPR’, stipulates in Article 19(1)(b)(iii) that biocidal products shall be authorised provided that ‘the biocidal product has no immediate or delayed unacceptable effects itself, or as a result of its residues, on the health of humans, … directly or through drinking water …’.
During the AS approval procedure, the concentrations of the AS and eTPs need to be determined in groundwater and surface water, whenever appropriate. This assessment needs to be revised and complemented at BP authorisation stage, when new uses are proposed for authorisation. In addition, substances of concern in a biocidal product or in a treated article need to be considered at BP authorisation stage.
Regarding the formation of tTPs, an ECHA guidance on disinfection by‐products (DBPs) 7 generated during water treatment processes using halogenated active substances in swimming pools has been made available. Priority was given to swimming pools since this was considered the most relevant use from the point of human exposure to DBPs and its associated possible risk to health. However, the guidance does not cover all situations and, so far, focuses on the by‐products generated from the active substance used as a disinfectant. Furthermore, there is no ECHA guidance on DBPs generated during water treatment processes for the production of drinking water.
To address this gap by means of a proportionate approach, a tiered framework has been proposed that identifies emission pathways and components in the biocidal products with relevance to tTPs formation during surface and groundwater treatment. The framework builds on existing guidance and aims to avoid unnecessary testing whenever possible. The following key steps should be followed:
Step 1 – identify whether the product type (PT) and uses lead to direct/indirect emissions of residues to surface water and/or soil (groundwater) that is likely to be used for drinking water production.
Step 2 – identify active substances, major metabolites/transformation products, ecotoxicologically relevant metabolites/transformation products and substances of concern. The definitions of major metabolites/transformation products, ecotoxicologically relevant metabolites/transformation products and substances of concern are provided in the BPR and/or the Guidance on BPR, part B + C, Volume IV, 8 as well as in Section 3.5 of this Guidance.
Step 3 – calculate exposure concentrations and identify active substances, major metabolites/transformation products, ecotoxicologically relevant metabolites/transformation products, substances of concern with PECsurface water and/or PECgroundwater above 0.1 μg/L. Refinement of the exposure assessment is possible in line with the Guidance on BPR,10 based on expert judgement and consideration of other relevant information, including the TAB entries.
Step 4 – note that if PECgroundwater of the AS, substances of concern and eTPs that are toxicologically relevant (Sanco/221/2000 – rev.11, European Commission (2021)) is above 0.1 μg/L, the approval for the active substance/authorisation of a biocidal product is not granted; thus only eTPs with PECgroundwater above 0.1 μg/L that are not toxicologically relevant remain to be assessed in further steps for groundwater.
Step 5 – assess the potential of compounds identified in steps 3 and 4 for the formation of tTPs during water treatment processes. If tTPs are estimated to be formed above 0.075 μg/L, continue with human health and animal health risk assessment (more information in Chapter 5).
The following sub‐sections intend to assist applicants and competent authorities in the identification of substances for which an assessment of the formation of tTPs may be needed. More details are provided in connection to the five steps listed above, including: the identification of relevant emission pathways, prediction of concentrations in water, thresholds, possible refinement options and the subsequent steps to be made in case tTPs may be formed during the water treatment processes for the production of drinking water.
3.2. Routes of exposure
Biocidal AS and their eTPs, as well as substances of concern in a BP or a treated article, can reach surface water and groundwater through different pathways (Figure 3).
Figure 3.
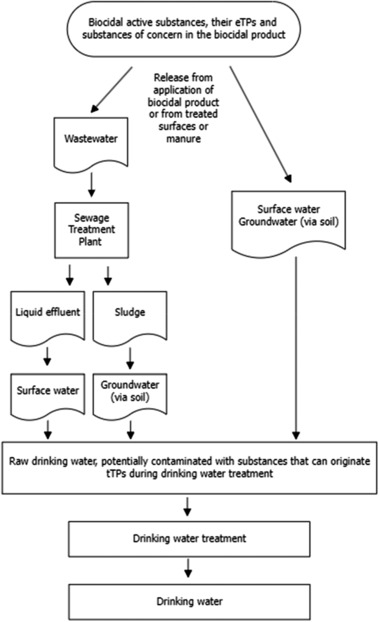
Routes of entry of AS, their eTPs and other substances of concern contained in the biocidal product into surface water and groundwater
Surface water can be directly exposed to the BP emissions (e.g. from outdoor spray applications against insects or by leaching from treated wooden commodities or antifouling agents applied on ships) or can be an indirect recipient when the releases are first directed to the sewer and STP before discharge to surface water.
Groundwater is exposed indirectly via the emissions to the soil. Soil can receive either direct emissions during the application or service life of a biocidal product (e.g. emissions during outdoor in‐situ applications or leaching from a house treated with wood preservatives) or indirect emissions from the application of manure from treated animal housings or STP sludge containing the active substance and its eTPs.
3.3. Biocidal product types relevant for the assessment
Biocidal products are divided into 22 different PTs. Within a given PT, not all scenarios contribute to the same compartments. For each assessed scenario, it needs to be evaluated whether the emissions can reach surface water and/or groundwater and whether it is reasonable to expect that the recipient water body is relevant as drinking water source. E.g. the pond in PT 8 ‘bridge over pond’ scenario may be considered not relevant since drinking water is not abstracted from garden ponds.
It should always be assessed on a case‐by‐case basis whether, under normal conditions, the use of the substance in a respective biocidal product leads to exposure to surface water and groundwater compartments and whether the exposed water fractions are likely to be used as sources for drinking water. It should be noted that the aim of the present document is to complement rather than duplicate existing guidelines, such as the Guidance on Disinfection By‐Products. 7
3.4. Exposure assessment of biocides (Tier 1 and 2)
The ECHA Guidance on biocides legislation available on the ECHA website 9 describes how to perform the required assessments. In particular, for the environmental exposure assessment of surface water and groundwater, the Guidance on BPR: Vol IV Environment Parts B + C (v2.0, 2017) 8 should be followed. Section 2 . Exposure assessment covers the determination of the emissions and fate of an active substance or a substance of concern, in a biocidal product or in a treated article, and its transformation or degradation, to predict their likely concentrations in the environment (predicted environmental concentrations, PECs). The calculation of the local PEC values for the aquatic compartment is covered in Section 2.3.7.3 of the Guidance, and the calculation of concentrations in groundwater in Section 2.3.7.6.
For the emission estimation of most of the product‐types a respective Emission Scenario Document (ESD) and additional related documents are available on the ECHA webpage dedicated to ESDs. 10 For instance, PT specific amendments to the ESDs need to be considered as well during the assessment. During the ongoing review programme for biocides, decisions were taken for several PTs that specify the emission estimation and should be considered when performing the exposure assessment. These decisions are included in the Technical Agreements for Biocides (TABs). 11
In some cases, it may not be possible to establish a PEC value and a qualitative assessment of exposure in surface water is needed (e.g. for very reactive and/or oxidative substances). Likewise, for inorganic rapidly reacting substances no groundwater assessment is needed. However, it needs to be discussed case‐by‐case if the substance is rapidly reacting. When it is impossible to quantify the exposure to the active substance or its eTPs, a qualitative assessment of the eTPs and their fate during drinking water production should be performed. The TAB document provides more detailed information on the qualitative assessment (e.g. TAB entries ENV 208 and ENV 229).
Once the environmental risk assessment is concluded, those uses should be identified that:
are proposed for approval/authorisation, and
result in emissions to surface water and/or to soil (and subsequently in groundwater).
For these uses, the concentrations of AS, its eTPs and substances of concern in a biocidal product or in a treated article need to be identified in surface water and/or groundwater.
3.4.1. PECsurface water determination
In Section 2.3.7.3 of the Guidance on BPR, Part B + C, Volume IV 8 , calculation of the predicted environmental concentration in surface water due to indirect release (the effluent of the sewage treatment plant is diluted into the surface water, run‐off from fields and/or re‐circulation of contaminated groundwater into surface water) and direct release (direct emissions to surface water, for example, in the case of PT8: wood preservatives – use classes 3, 4b, 5, etc.) is described. In addition, as specified in Section 2.3.1 Surface water of the present document, FOCUS Surface Water Scenarios are available for usage in the EU evaluation process under 91/414/EEC, EC Document Reference SANCO/4802/2001‐rev2. The respective simulation models are used for predicting emissions to surface water due to drift, drainage and run‐off from soil are combined with the fate model TOXSWA. In order to minimise user influence and possible mistakes, a general model shell, SWASH, has been developed to ensure that the correct and relevant FOCUS scenarios are being defined to run the required calculations.
3.4.2. PECgroundwater determination
According to Section 2.3.7.6 of the Guidance on BPR Part B + C, Volume IV 8 , the concentration in groundwater is calculated for indirect exposure of humans through drinking water. As an indication for potential groundwater levels, the concentration in porewater of agricultural soil is taken.
If no data on degradation in soil are available for exposure modelling in groundwater, the result is a worst‐case PECporewater estimate as the substance is assumed to accumulate over a 10‐year period. In case data on degradation in soil are used, PEClocalagr. soil, porew is a realistic worst‐case estimate since biodegradation, leaching and volatilisation are taken into account over a 10‐year period and over a limited soil depth (DEPTHi depending on the ESD, see Table 10 of the Guidance on BPR, Part B + C, Volume IV 8 ). If the risk assessment for the groundwater compartment indicates an inacceptable risk based on this first tier PECporewater and data on degradation in soil had been taken into account, refinement of PEClocalgw is the next step to be taken.
As a refinement option, the PEClocalgw can be estimated by using available groundwater simulation models developed for the assessment of pesticide mobility in soil, reflecting more realistic groundwater conditions, or by using measured data (lysimeter studies or monitoring data).
The following tiered approach to biocide groundwater assessments is proposed in the Guidance on BPR, Part B + C, Volume IV 8 :
-
–
Tier 1: Estimation of PECgw as soil pore water concentration.
-
–
Tier 2: Consideration of parent and all major metabolites against the cut‐off criteria listed below.
-
–
Tier 3: Refinement of Tier 1 estimates using FOCUS PEARL (or PELMO) and relevant PT specific guidance. Regarding the input parameters and scenarios to be considered in the refinement steps for biocides in FOCUS PEARL, more details can be found in the TAB document especially (but not exclusively) in entries ENV 22–23, 36, 165, 166 and 208.
The following basic cut‐off criteria are applicable to avoid the need for a full formal refinement of FOCUS groundwater assessment (Guidance on BPR: Vol IV Environment Parts B + C (v2.0, 2017 8 ; ENV 20 in TAB):
For active substance only assessments (i.e. where no major metabolites are formed), the standard cut‐off criteria (DT50 < 21 days at 20°C and Koc > 500 L/kg) could be used for biocide application rates up to 100 kg a.s./ha per year.
For assessments including metabolites, the standard cut‐off criteria could be used if (a) both parent and metabolites meet the standard cut‐off criteria and (b) the biocide application rates are less than 10 kg a.s./ha per year
Where a parent assessment is triggered based on the cut‐off criteria (i.e. because it has a DT50 > 21 days at 20°C or Koc < 500 L/kg), metabolites should always be included irrespective of their properties.
The applicability of the cut‐off criteria should be discussed case‐by‐case and well justified. TAB ENV 20 provides further information on the proposed cut‐off criteria for groundwater assessment and on the limitations of this approach, which should be taken into account in the decision‐making process. If there are any doubts that the PECgroundwater of a substance may exceed the 0.1 μg/L threshold and/or an appropriate margin of safety cannot be demonstrated, the full formal assessment of groundwater exposure should be performed.
3.5. Assessment of transformation products (tTPs)
When the use of a biocidal product results in direct or indirect emissions to surface water and/or direct or indirect emissions to soil and subsequently to groundwater, the formation of tTPs during water treatment to produce drinking water may need to be considered.
In the stepwise approach presented in Chapter 3, it is recognised that certain substances emitted to water and soil (groundwater) may not require the formal tTPs formation assessment because it may not be scientifically necessary. This may include, for example, rapidly reacting active substances which, due to their fast reaction with soil organic matter, are unlikely to reach groundwater and eTPs, such as carbon dioxide and other inorganic compounds not containing heavy metals and/or chemicals that are known to not be of (eco)toxicological concern and occur naturally at significantly higher concentrations in surface water and/or groundwater (Technical Agreements for Biocides, ENV 208, 2022; Sanco/221/2000 – rev.11, European Commission, 2021). Such substances may be omitted from the formal tTP formation assessment; however decisions to do this need to be supported with a justification.
The assessment of the tTPs formation follows a stepwise approach (Figure 4 ):
Figure 4.
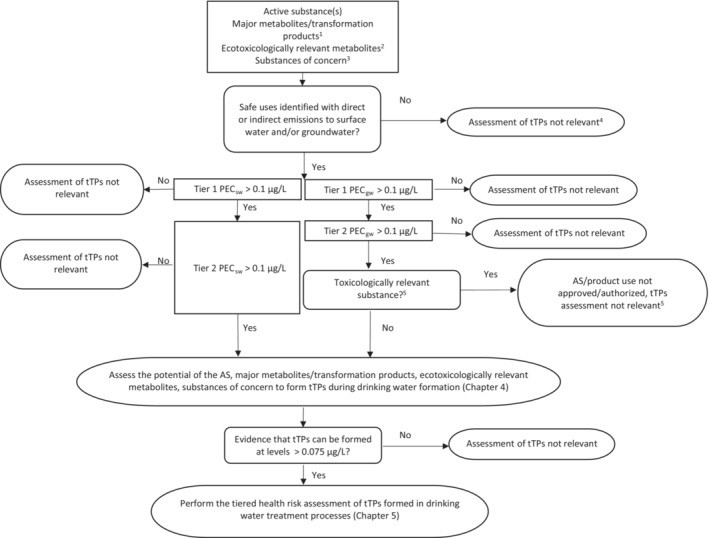
Stepwise approach to the assessment of tTPs formation
Step 1 – Identification of the uses with emissions to surface water and/or soil (and subsequently to groundwater) and corresponding emission concentration values.
The basis for this step is the outcome of the environmental risk assessment performed in the context of applications for active substance approval and product authorisation. More details are provided in Section 3.4 above.
Step 2 – Identification of substances that require tTPs formation assessment.
Following the drinking water directive (DIRECTIVE (EU) 2020/2184) for pesticides and their metabolites, the trigger value for the assessment of tTPs formation is the concentration limit of 0.1 μg/L for a single substance. The threshold value is applied to AS, its eTPs and substances of concern in a biocidal product or in a treated article that reach surface water and/or groundwater.
Surface water: for the uses identified in step 1, substances estimated to be present at concentrations above 0.1 μg/L (i.e. PECsurface water > 0.1 μg/L) require tTPs formation assessment. If a concentration exceeds the threshold 0.1 μg/L, it may be preferable to refine the exposure assessment (Tier 2 exposure assessment) before carrying out the tTPs formation assessment described in Chapter 4.
Groundwater: for the uses identified in step 1, eTPs estimated to be present at concentrations above 0.1 μg/L (i.e. PECgroundwater > 0.1 μg/L) and shown to be toxicologically non relevant 12 require tTPs formation assessment. In case eTPs are estimated to be present at concentrations above 0.1 μg/L and shown to be toxicologically relevant, the AS will not be approved, and the formation of tTPs does not need to be assessed.
For the assessment of the potential of substances to form tTPs during drinking water production, Chapter 4 of this Guidance is to be followed.
For the tTPs, which are estimated to be formed above 0.075 μg/L during drinking water production, it is then necessary to assess their hazard and subsequently perform the risk assessment according to Chapter 5 of this Guidance.
1Formed ≥ 10% on a molar basis, of the active substance in any relevant environmental compartment or appearing at two consecutive sampling points at amounts ≥ 5% on a molar basis, or if at the end of the study, the maximum of formation is not yet reached but accounts for ≥ 5% on a molar basis, of the active substance at the final time point.
2A metabolite/transformation product which poses a higher or comparable hazard to any organism as the active substance.
3Article 3(f) of the BPR specifies that a substance of concern would, unless there are other grounds for concern, normally be:
-
–
A substance classified as dangerous or that meets the criteria to be classified as dangerous according to Directive 67/548/EEC, and that is present in the biocidal product at a concentration leading the product to be regarded as dangerous within the meaning of Articles 5, 6 and 7 of Directive 1999/45/EC, or
-
–
A substance classified as hazardous or that meets the criteria for classification as hazardous according to Regulation (EC) No 1272/2008, and that is present in the biocidal product at a concentration leading the product to be regarded as hazardous within the meaning of that Regulation,
-
–
A substance which meets the criteria for being a persistent organic pollutant (POP) under Regulation (EC) No 850/2004, or which meets the criteria for being persistent, bio‐accumulative and toxic (PBT) or very persistent and very bio‐accumulative (vPvB) in accordance with Annex XIII to Regulation (EC) No 1907/2006;
-
–
‘Other grounds for concern’: potential substances of concern.
AS from other PTs contained in the product (e.g. in‐can preservatives) for which a draft final Competent Authority Report (CAR, with an agreed risk assessment) is available. This criterion identifies other active substances in the biocidal product that act as co‐formulants. Those substances should be regarded as substances of concern because they potentially affect environmental organisms due to their intrinsic biological activity. They should be considered as substances of concern if they are present in the biocidal product at a concentration ≥ 0.1%. This concentration limit is not applicable to PBT‐ or vPvB‐ substances and endocrine disrupting chemicals (EDs), as safe concentration limits cannot be derived for those substances. 13 No concentration limit applies to substances that are classified as such. It needs to be checked whether this concentration limit is valid for the respective substance as highly toxic substances may contribute to the overall toxicity of the product even when contained to very small amounts in the product, i.e. a co‐formulant should be regarded as substance of concern if the PNEC of the respective substance is lower than the PNEC of the a.s. even though its concentration in the product is below the 0.1% criterion. However, exemptions are possible under the following condition: the substance is contained in Annex I of the BPR.
-
–
Substances that enhance the effect of the active substance in the product, e.g. synergists. For synergists, information/data shall be provided related to the interaction between the active substance and the synergist, not only for the synergist itself. For such substances, an appropriate evaluation of the risks posed by the active substance in the presence of the synergist rather than an evaluation of the risks posed by the synergist itself should be undertaken. A generic concentration cut‐off value for the presence of a synergist in a product, applicable to all synergists cannot be specified. On a case‐by‐case basis, a synergist should be considered a substance of concern, if it is present at a concentration that enhances the toxicity of the active substance, as indicated by the available data. Furthermore, the hazard profile, potency and exposure potential of the substance enhancing the effects of the active substance should be taken into account. Further details on the identification of synergists can be found in Section 10.2.3 in the Guidance on BPR: Vol IV Environment Parts B + C (v2.0, 2017) 8 . Also, diluents, (lipophilic) organic solvents and surfactants like e.g. naphtha may influence the toxicity of a mixture by enhancing the bioavailability of the active substance(s) and should therefore be regarded carefully. In principle, all co‐formulants need to be checked for a potential influence on the toxicity of the other product components.
-
–
Substances that have been included in the candidate list established in accordance with the REACH Regulation (1907/2006/EC, as amended), Article 57 (f) and 59(1) or fulfil the criteria for inclusion in the candidate list, if not already covered by the criteria of Article 3(f) of the BPR (see above). This criterion will capture the clearly‐defined substances of concern specified in Article 3(f) of the BPR as well as EDs and PBT‐substances which are not covered by Article 57 (d‐e) of the REACH Regulation.
-
–
Substances which meet two of the criteria for being PBT in accordance with Annex XIII to Regulation (EC) No 1907/2006, as amended.
-
–
Substances for which an Environmental Quality Standard (EQS) has been derived under Directive 2000/60/EC (Water Framework Directive; according to paragraph 67, Annex VI, BPR).
4In principle, uses found to pose unacceptable risks are not authorised, so assessment of tTPs will not be needed. In case of a derogation, the scheme should be followed as for safe uses whenever appropriate and relevant.
5For safe uses is already ensured that groundwater concentrations of AS and substances of concern are below 0.1 μg/L (for the single substance). Otherwise, the AS and product will not be approved/authorised. In case major and ecotoxicologically relevant metabolites present at concentrations above 0.1 μg/L are shown to be toxicologically relevant, the AS/product will not be approved/authorised, and the formation of tTPs does not need to be assessed.
3.6. Assessment at biocidal product authorisation stage when relevant
A representative biocidal product needs to be assessed in the context of active substance approval. For a product type covered by the active substance application to be approved, the risk assessment must show acceptable risks for at least one use scenario for that given product type. In addition, the assessment of tTPs, as described in this guidance, must be performed.
At the biocidal product authorisation stage, the full composition of the product has to be assessed as described in Part II of the Guidance on BPR 8 . A new assessment of the tTPs will have to be completed then, taking into account the full composition of the product as well as additional uses not covered in the representative biocidal product assessment.
4. Transformation products formation
4.1. Transformation products formation during drinking water treatment
In Europe, drinking water is prepared from surface water, groundwater or bank filtrate. In all cases, these sources may be contaminated by low‐level mixtures of chemicals, including AS from PPPs or biocides and their metabolites, even when used in the way intended, i.e. according to good agricultural practice and used as directed, respectively. During drinking water treatment, substances may be degraded in several processes, but in general, they are not fully mineralised. This can result in the formation of TPs, that are potentially harmful (it cannot be assumed a priori that these are of lower toxicity than the parent substance). Therefore, it is important to determine what (type of) TPs may be formed during drinking water treatment to establish whether these involve risks to either humans or animals that consume the treated water. This chapter provides guidance to establish whether harmful compounds from the PPPs and biocides may be formed during water treatment to produce drinking water.
Drinking water treatment processes that may result in the transformation of compounds are:
Chlorination with sodium hypochlorite (NaOCl) or Cl2 gas
Chlorination with chloramine (NH2Cl)
Pre‐oxidation with chlorine dioxide (ClO2)
Combination of Pre‐oxidation with ClO2 and Chlorination with NaOCl or NH2Cl
Ozonation (O3)
Ultraviolet (UV) disinfection
Photolysis
Advanced oxidation processes: UV/hydrogen peroxide (H2O2), O3/H2O2, UV/O3, UV/Cl
Biodegradation during filtration over sand or granular activated carbon (GAC)
In line with the terms of reference, this guidance focuses on the formation of TPs during the most common disinfection processes:
Rapid sand filtration
Chlorination with NaOCl or Cl2 gas
Chlorination with NH2Cl
Pre‐oxidation with ClO2
Combination of Pre‐oxidation with ClO2 and Chlorination with NaOCl or Cl2 or Chloramination with NH2Cl
Ozonation
UV disinfection
For each of these processes, the formation of several types of TPs can be expected and differ between processes. The processes applied may differ between EU member states or areas, and therefore an extensive list is given. If the application under consideration will result in uses in a territory or territories where any of the particular treatment processes listed are not applied in drinking water treatment, then that process or those processes may be omitted. In Appendix B, an extensive literature overview is given of the type of reactions that can occur during each of these processes and the types of TPs that can be formed. The cited and additional literature sources can also be a resource for specific evidence that an AS can be expected to be stable during these disinfection processes. Modelling of transformation pathways and estimations of certain characteristics (like Kow and pKa values) can also be applied to predict the types of TPs that can be expected to be formed during such processes or that a compound will be stable. In general double bonds (including aromatic structures) will be broken, and often acids, ketones and aldehydes will be formed. Furthermore, the molecular weight will decrease when chain scissions occur. When applying chlorination processes, chlorine atoms can be incorporated in organic molecules, resulting in chlorinated TPs, which may be carcinogenic or mutagenic (Bletsou et al., 2015; Matsushita et al., 2018). In some cases, depending on the structure of the parent compounds and/or the eTPs, nitrosamines may be formed, which also require special attention due to their carcinogenity (Kadmi et al., 2015). The presence of bromide ions in water resources can also lead to bromination reactions during oxidation treatment by chlorine, monochloramine and ozone. The formation of TPs does not necessarily have to be problematic. Whether that is the case depends on their characteristics and concentrations (Brunner et al., 2020) and the impact of further treatment of the water, if applicable. To make sure transformation products are formed by reaction of the parent compound in question, it is important to carry out experiments in pure solutions, not containing mixtures of several compounds (apart from natural organic matter, NOM). To determine whether a certain transformation product can also be formed from NOM, it is recommended to also carry out similar experiments with a solution containing only NOM. In case experiments show that certain TPs only originate from NOM, no further evaluation has to be carried out. However, in case the eTPs appear to contribute to their concentrations, further evaluation will be required.
If the literature clearly indicates that the active substance or eTP needing assessment is stable during one or more of the processes, then experimental testing of the potential formation of tTPs will not be needed for that process. A theoretical study based on first principles of chemical reactions supported by the literature associated with these reactions demonstrating that the active substance or eTP is stable is considered included under this provision.
4.2. Identification of transformation products
When PPPs and biocides are applied, residues from these AS and their TPs can be expected to end up in the water system. Surface water, groundwater and bank filtrate are used as sources for drinking water production. Therefore, residues of these AS may also be subject to drinking water treatment processes, where they may be converted into TPs. The aim of this chapter is to present an approach that can be used to identify which TPs can be formed during drinking water treatment processes and thus will have to be taken into account in a risk assessment process.
As a result of degradation in soil and water, not only parent compounds but also some eTPs 14 may be encountered as residues in the water. Both the parent AS and their eTPs have to be addressed in literature research, modelling and experimental approaches, as all of these parent and eTPs compounds may be present in water that is used as a source for drinking water. Even if eTPs are considered to be not harmful, they may cause the formation of harmful transformation products during subsequent processes. A literature search is required for all active substances and eTP that originate from the uses assessed and are predicted to have PEC with predicted concentrations > 0.1 μg/L. If literature provides clear evidence that a compound will be stable under the required conditions in the relevant processes, no further testing will be required.
All experiments should be carried out at least in water with a defined composition (‘standard water C' according to CIPAC standard waters, with additional dissolved organic carbon (DOC); see experimental part) to avoid divergent assessments and enable comparability between studies. A two‐step approach is applied, in which, at first instant, experiments will be carried out at a relatively high concentration to facilitate detection of possible tTPs. Then, the experiment should be repeated at a lower concentration, which corresponds to concentrations that may actually be observed in the environment. Only if at low concentrations tTPs can still be observed in significant concentrations (> 0.075 μg/L) the results of the high concentration experiments should be used for identification of the relevant tTPs. Analyses of TPs can be done using radiolabelled AS to track the TPs by the labelling, but this is not strictly required. If AS has different functional groups and radiolabelled AS is used, consider a different radiolabel for each functional group. It is also possible to perform suspect analysis or non‐target analysis, to study which TP may be formed. As these chemical analyses are not straightforward, a scheme is proposed to facilitate the process (see Figure 5). As a first step, we suggest applying modelling, e.g. based on QSARs (quantitative structure activity relationships), to predict the type of TP that may be formed, based on the molecular composition of the parent AS and eTP and the processes applied. QSAR approaches used should be reported as described in the ECHA (2016b) practical guide and any subsequent updates. This information is used to determine what types of chemical analyses are required. If the type of TP is known (based on literature information or prediction), suspect screening analysis can be applied, which is less elaborate in comparison to non‐target analysis. However, if modelling gives insufficient information to apply suspect screening analyses, a non‐target analysis will be required. This applies to both the high and low concentration experiments. In any case, modelling alone will not be sufficient to determine the TP formed, except for when the modelling outcome is supported by robust evidence from the literature that a compound will be stable to the disinfection process reactions, including consideration of organic material present in sources for drinking water. In general, the identity of TP formed according to modelling predictions always has to be experimentally confirmed.
Figure 5.
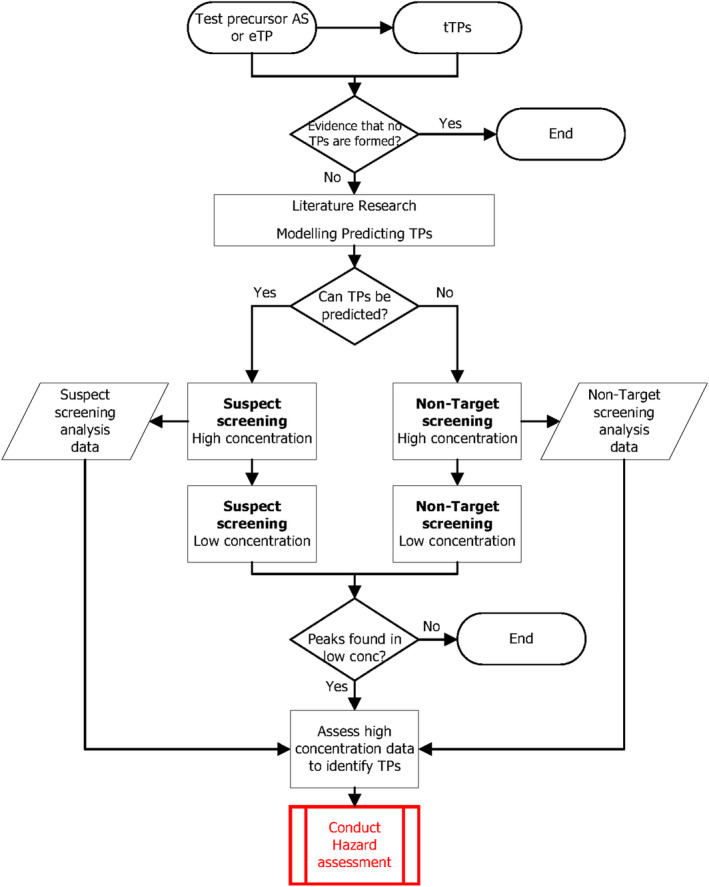
Flow scheme for TP identification. AS = active substance, eTP = environmental transformation products, TP = transformation product, tTP = treatment transformation product, conc = concentration. The TP observed after experiments at high concentrations do not have to be identified. Only, if experiments at low concentrations have demonstrated that they may be present in relevant concentrations, data from the high concentration experiments can be used to identify the relevant TP.
Identification of TPs is easiest at high concentrations, but these concentrations will not be representative for the actual situation in water bodies. Therefore, a two‐step procedure is used, one at a high concentration (to facilitate observation of possible tTPs) and one at a concentration in line with the PEC from Chapters 2 and 3. At first, experiments are carried out at a relatively high concentration of about 1,000 times the limit of quantification (LOQ) 15 of the parent active substance or eTP compound (can be more than one), typically between 100 and 1,000 μg/L. At such a high concentration, the plausible generation of TP can be experimentally observed, even though it does not have to be representative of the actual situation. Therefore, at this moment, identification of the signals will not yet be necessary. By repeating the experiment at an environmentally relevant concentration, in line with the PEC, it should be confirmed which signals are relevant and should be characterised in more detail. Chromatographic analysis will produce a chromatogram showing signals of all compounds present in the formed mixture of tTPs, originating from one test precursor. For this purpose, non‐target analysis can be carried out. The principles of non‐target analyses have been described by Hollender et al. (2017 and 2019), Bletsou et al. (2015) and Béen et al. (2021). The specific application of non‐target analyses for the identification of TPs has been described by Wang et al. (2020), Schollée et al. (2018), Di Marcantonio et al. (2020) and Brunner et al. (2019). According to the procedures described there, it is possible to determine the molecular structures of TPs that have been formed. This type of experiment will enable the identification of tTPs where in the chromatograms, the signals of any tTP are to be expected. At this stage, it will not be necessary to identify the tTPs themselves, although it is expected that this should be possible in this concentration range. Not all tTPs will have to be identified in this manner, nor will have to be considered in the hazard assessment, as they may not be formed under realistic conditions, where lower concentrations of the AS or eTP can be expected. In the next step, the experiment can thus be repeated at an environmentally relevant concentration (see Section 4.2.5), typically at a concentration around 1–10 μg/L, which should be in line with the results of the approaches in Chapters 2 and 3. Since it is now known where in the chromatogram the signals of the tTP are to be expected, it can be determined whether these tTP can still be detected at a relevant concentration, i.e. > 0.075 μg/L. Such detection indicates whether the TP still are formed at environmentally relevant concentrations of the AS or eTP. If the signals cannot be observed in this case (i.e. the signal cannot be distinguished from noise and is at a concentration < 0.075 μg/L), it means that the tTP is not formed in a concentration that can be measured in ‘drinking water’ produced from surface water and/or groundwater with trace levels of the AS under investigation. In this case, it may not be necessary to identify them. Only if they still appear to be formed data from the first experiment, in combination with information from models and literature, can be used to identify the tTPs. This information can then be used for further hazard assessment.
An overview of the stepwise approach for the assessment of the formation of TPs is given below:
In step 1, it is identified which eTP or AS will also have to be considered with regard to the formation of tTPs. Further testing is required for those compounds that can be expected to be present in a relevant concentration in sources for drinking water (as defined in Chapters 2 or 3). If from the literature, it is undoubtedly clear that no transformation products can be formed, further testing will not be necessary. This should be made clear with proper references to this literature.
Subsequently, in step 2, modelling of the compounds identified in step 1 is carried out to obtain information on the type of tTP that may be formed during drinking water treatment processes. Any data known from literature will have to be included in this step. This information will be used as input for suspect screening analyses.
Then, TP formation experiments are carried out at a concentration of about 1,000 times the limit of quantification (‘high’ concentration) for the compounds identified in step 1. If, from theory, it is undoubtedly clear that similar compounds will result in similar tTPs, only one of each category of compounds will have to be further examined experimentally. Suspect screening methods will be applied for analyses. In this stage, the tTP will not have to be identified yet. The same test conditions will have to be applied to a blank (standard water) to determine whether some compounds may be formed by reaction of the compounds already present in the standard water.
Afterwards, the TP formation experiments are repeated at a concentration that has been predicted in the environment (see Chapters 2 and 3), typically in the order of magnitude of 1–10 μg/L (‘lower’ concentration).
If the TP observed in high concentration experiments can still be observed in low concentration experiments at a concentration that is expected to be ≥ 0.075 μg/L, the compound in question will have to be identified using information obtained in the ‘high’ concentration experiments, combined with information from literature and modelling.
After the relevant tTPs have been identified, a hazard assessment will be carried out. The procedures required for this hazard assessment are defined in Chapter 5.
Schematically the process is shown in Figure 5.
4.2.1. Step 1: Presence in the environment before water treatment
When AS are applied as a PPP and/or biocide, they may enter the water system in their original form, as the parent compound. However, it is also possible that some TPs are already formed during or after use in the environment as a result of degradation in soil and/or water, and these may be present in relevant concentrations (see Chapters 2 and 3). With the information in the regulatory dossier, including calculation and/or environmental modelling approaches, the expected concentrations in water sources used for the production of drinking water may be estimated (see Chapters 2 and 3). If these calculations indicate that compounds may be present in concentrations above 0.1 μg/L (which is the threshold for AS), the investigation of the effect of water treatment processes is triggered in the sources for drinking water.
The potential presence of environmental metabolites/TPs (referred to in this guidance as ‘environmental transformation products; eTP’) in the (aquatic) environment should be available in regulatory dossiers for active PPP and biocides. Metabolite identification usually involves radio labelled test substances (see OECD guidelines 307, 308 and 309 for transformation and degradation of chemicals (https://www.oecd-library.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-3-degradation-and-accumulation_2074577x)) for details of these methods).
4.2.2. Step 2: Prediction of the formation of transformation products during drinking water treatment processes
The formation of tTPs should be determined in experimental tests under different circumstances in the relevant processes, mimicking actual drinking water production processes. Identification of the tTPs formed can be facilitated if it would be known what type of tTP may be formed beforehand. This will depend on three different parameters: (a) the molecular structure of the parent compounds or eTP, (b) the type of treatment process applied and (c) the circumstances (like matrix composition, pH, temperature). As a result, it will not be possible to accurately predict all tTPs that may be formed, but it is possible to at least obtain a general idea of plausible tTP. This evaluation can be based on information in literature (see Appendix B) or modelling. Table 2 gives an overview of the type of reactions that may be involved in different drinking water treatment processes, and some examples of tTPs that have been detected. Note that this list is incomplete and can only be used for illustration. Modelling can also be applied to facilitate the identification of tTPs formed during the realistic concentration tests described in step 3. By carrying out the experiments, the formation of tTPs can be determined. If the type of tTP that are to be expected is unclear, non‐target analyses can be carried out to identify the tTP. However, analyses can be easier and less expensive if, on the forehand, the type of tTP to be expected are known to some extent. In that case, instead of non‐target analyses, suspect analyses can be carried out. To identify some suspects, literature studies and in some cases, modelling can be applied. If this does not result in sufficient information, non‐target analyses will have to be performed to screen for tTP formed during treatment.
Table 2.
Overview of examples of reactions and examples of TP that may occur during disinfection of drinking water. Details are given in Appendix B
| Process | Reactions | Some examples of tTPs |
|---|---|---|
| Chlorination with free chlorine |
Substitution of a hydrogen atom or e.g. an alkylamine group in an aromatic ring (multiple) chlorine addition to a double bond or aromatic structure Ring‐opening and scission reactions Decarboxylation, dehydration, deamination, demethylation and cyclisation reactions Chlorination of amine containing compounds Hydrolysis of C‐N bonds Oxidation of thioethers Chlorination of sulfonamides may result in chlorine substitution, S‐C cleavage, S‐N hydrolysis, desulfonation, cyclisation, oxidation/hydroxylation and conjugation reactions Organophosphorous compounds |
Chlorinated TP Non‐chlorinated TP N‐chloramines Carbonyl derivatives Sulfoxides Oxons, replacing the P=S bond with a P=O bond |
| Reactions with chloramines |
Halogenations Hydroxylations Dealkylations Ring opening reactions |
N‐nitrosodimethylamine Iodo‐trihalomethanes Brominated compounds |
| Ozonation |
Reactions with double bonds, aromatic ring structures, deprotonated structures, sulfur, phosphorus, nitrogen and oxygen atoms Hydrogen abstraction, radical‐radical interaction, de‐alkylation, ring cleavage, electrophilic addition or electron transfer by hydroxyl radicals Reactions of amides Reactions of phosphorous containing compounds |
Phenol and naphtol like compounds Aldehydes, ketones and carboxylic acids Compounds containing a N‐O bond (originating from amines) Other phosphorous containing compounds Brominated compounds N‐Nitrosodimethylamine |
| UV disinfection | Photolysis | |
| Biodegradation |
Oxidation of alkanes, alkenes, aromatic and alicyclic hydrocarbons, halogenated carbons and sulfonated hydrocarbons N‐dealkylation (dealkylation of a quaternary ammonium moiety), hydroxylation, amide hydrolysis and N‐dearylation (cleavage of the bond between nitrogen in the amide moiety and the aryl group) |
Carboxylates N‐dealkylated products and amides |
Drinking water disinfection processes that may result in the transformation of compounds are:
Chlorination with NaOCl or Cl2
Chloramination with NH2Cl
Pre‐oxidation with ClO2
Combination of Pre‐oxidation with ClO2 and Chlorination with NaOCl or NH2Cl
Ozonation
UV disinfection
Rapid sand filtration (biodegradation) for surface water resources.
Often processes are combined in drinking water treatment. A combination of pre‐oxidation with ClO2, followed by chlorination with NaOCl or chloramination with NH2Cl, is often applied. Therefore, this combination should also be considered in the evaluation.
The first five processes are oxidation processes. UV disinfection may result in the formation of tTP by photolysis. Sand filters are applied to remove particles. Often coagulants are added to surface water, to coagulate particulate matter. For anaerobic groundwater, aeration is applied. This results in the oxidation of dissolved iron and subsequent precipitation of iron(hydr)oxides. During both oxidation and precipitation, organic matter may also be incorporated into the flocks that are formed during the coagulation/flocculation process, which can be removed by rapid sand filtration. Slow sand filtration is also a well‐known water treatment technique, which significantly contributes to the total disinfection of the drinking water. As sand filters also reduce nitrogen and carbon sources, they may contribute to reducing the amounts of tTPs formed. On the other hand, in both types of sand filters, micro‐organisms will grow, forming TP through biodegradation processes. In practice, not all processes will be (subsequently) carried out. However, as beforehand it generally is not clear which treatment processes may be applied, all different experiments (see Section 4.2.4) will usually have to be carried out to make sure that no harmful tTPs will be present in the water after the use of products containing the parent compounds. Though as indicated in Section 4.1, there may be exceptions where it may be known that a specific treatment process is not applicable in a territory. In this case, a process can be omitted from assessment. This procedure does not aim to mimic the real situation in a certain treatment but to determine which tTPs may be formed in real treatment processes.
4.2.3. Modelling of chemical reactions: literature and QSAR approach
Step 2 involves modelling of possible TP during these processes. For this purpose, several models may be applied. Examples are:
BioTransformer: https://biotransformer.ca/; provides information on some oxidation and biodegradation processes
EnviPath: https://envipath.org/; provides information on some oxidation and biodegradation processes
US EPA's Chemical Transformation Simulator: https://www.epa.gov/chemical-research/users-guide-chemical-transformation-simulator-cts; gives information on chlorination, ozonation, UV treatments and oxidation processes
Programme Epi Suite; gives information on biodegradation processes.
If information from OECD 307, 308, 309, 303 and 314 is available, this should be used to predict biodegradation products potentially formed in sand filters. However, when this information is not available (which may be the case depending on the uses being assessed), QSAR modelling may help to predict certain TPs, based on statistical relations between molecular structures and properties. Furthermore, as many biodegradation reactions are oxidation reactions, models predicting biodegradation TPs may also be useful to obtain information on possible TP in a more general way. Through suspect or non‐target analyses, the actual TP formed should be identified in a subsequent step.
The type of reactions a molecule can undergo depends on the chemical composition of the molecule, in which its three‐dimensional steric composition plays an important role. For example, the distance between two atoms in the molecule or the steric hindrance caused by other atoms or functional groups can play a very important role in whether and how a reaction may take place. Besides, environmental conditions like matrix composition, temperature and pH, may affect the reactions.
Examples of reactions that may take place and TP that can be formed are given in Table 2. A detailed overview of the literature is given in Appendix B.
Molecular modelling refers to theoretical and/or statistical methods (based on empirical data), which can be implemented in computational systems that can be used to model or mimic the behaviour of molecules in certain reactions (see e.g. Molecular Modelling for Organic Chemistry). There are several computer models available that can be applied to predict the type of reactions that can take place, depending on the type of molecules involved and the circumstances during a treatment process. There are many developments in the field of organic chemistry modelling, which are e.g. published in the Journal of Molecular Modeling. Some examples were given by Lee et al. (2017) and Tentscher et al. (2019).
Measured and predicted chemical and physical constants for existing compounds can be found on sites like chemicalize (Chemicalize ‐ Instant Cheminformatics Solutions) and PubChem (PubChem [nih.gov]). These constants will help to predict and understand the behaviour of compounds in a certain process and evaluate the likelihood of certain transformation steps and subsequent products that are formed.
Measuring and quantifying molecular properties may be very elaborate and expensive, depending on the properties. Therefore, models have been developed which predict such properties and behaviour based on QSARs and QSPRs: quantitative structure activity and property relationship models. These can also be applied to the chemical behaviour of compounds in different treatment processes and the types of reactions they can undergo. In order to determine QSARs, a large amount of experimental data on molecular structures and related behaviour c.q. reactions is required. Statistical techniques determine a relation between a certain structure and a property. This mathematical relation can be used to predict the behaviour of compounds whose properties have not been investigated. E.g. an ionised acid (represented by the pKa value) may behave differently in oxidation processes than its neutral counterpart. Log Kow values can indicate whether the compound is likely to occur in an aqueous solution or whether it would more likely sorb to soil or sediment and thus be less liable to changes due to drinking water treatment.
The EPI Suite Estimation Programme was developed by the United States Environmental Protection Agency and contains predictive models and tools for assessing chemicals under the Toxic Substance Control Act: EPI Suite™‐Estimation Programme Interface|US EPA. It provides estimates of physical/chemical and environmental fate properties. The model can estimate log octanol–water partition coefficients, water solubility, gas‐phase reactions rates for reactions with hydroxyl radicals and ozone, Henry's law constants, melting and boiling points, aerobic and anaerobic biodegradability and sorption coefficients for soil and sediment. It also estimates hydrolysis rate constants and half‐lives for esters, carbamates, epoxides, halomethanes, selected alkyl halides and phosphorus esters. It identifies a variety of chemical structure classes for which hydrolysis may be significant.
Furthermore, literature information from Appendix B may be used to predict the expected TP type, as it gives an overview of possible reactions taking place during various water treatment processes.
With modelling tools, estimating which types of TPs can reasonably be expected to be formed is possible. For example: in some cases, acids or ketones are likely to be formed, and this knowledge may facilitate analyses and identification of specific TP. Modelling, however, cannot be applied as a substitute for experimental tests. Although there are no models available yet to predict the exact outcome of chlorination or ozonation processes, models that predict oxidation and biodegradation TP may also indicate what type of TP may be formed, as in all cases, oxidation reactions will be involved. Experiments will have to be carried out to confirm whether the predicted types of TPs can indeed be observed.
4.2.4. Step 3: Experimental procedures at high concentrations
Analytical procedures
Two types of analyses can be applied: suspect or non‐target screening. This depends on whether or not it was possible to predict the formation of certain TP (suspects). For the analyses, the initial concentration should be 1,000 × LOQ, to facilitate the identification of relevant TPs. If some TPs are found, concentrations can be decreased to e.g. 1–10 μg/L, which should be in the relevant order of magnitude for contaminants in surface water (see Chapters 2 and 3). In this way, it can be checked whether the TP that may be formed are detectable at environmentally relevant concentrations. If the limit of detection of a compound is so low that 1,000 × the LOD would be in the same order of magnitude as the environmentally relevant concentration, both experiments will coincide. In that case, it should be substantiated whether this concentration will give sufficient information to continue, or whether a higher concentration should be applied to be able to identify possible tTPs.
For the detection of TPs, a non‐target screening approach using liquid chromatography‐mass spectrometry (LC–MS) and/or gas chromatography–mass spectrometry (GC–MS) will be used. To cover a wide polarity range of TPs, multiple chromatographic techniques should be used (e.g. reversed phase and hydrophilic interaction chromatography) as well as varying detection techniques (e.g. positive and negative mode mass spectrometry, UV/Vis detection). Besides variation in chromatographic techniques, sample preparation should be minimised to prevent loss of TPs. Nowadays, most water samples containing micropollutants are analysed by direct injection (LC–MS). To cover a wide polarity range of TP, multiple pre‐treatment steps like solid phase extraction (SPE) can be used (for instance, lipophilic/hydrophilic affinity, cation exchange, anion exchange). A concentration step like SPE is only used when the sensitivity for a compound is low, or very low detection limits are required (e.g. < 1 ng/L) or for clean‐up in case of a complex matrix. A detection limit of ≤ 0.075 μg/L is required. For GC–MS analysis, sample pre‐treatment (e.g. SPE) is still necessary.
Apart from the suspect and non‐target methods described above, also radio labelling (e.g. using radiolabelled ozone) may be applied to facilitate analyses. It will depend on the individual cases whether radio labelling or suspect/non‐target analysis methods is preferred.
Preparation of standard water
Standard water has properties that represent characteristics in water sources found across the EU. Using a standardised water should result in the reproducibility of results.
Standard water is prepared by dissolving CaCO3, MgO, NaHCO3, NH4OH, HCl and NaOH in de‐ionised water according to the recipe for Standard Water C in CIPAC standard waters. This water has a hardness of 500 ppm, a pH between 7.0 and 8.0 and a Ca2+/Mg2+ ratio of 4:1. Apart from these inorganic compounds, Suwannee River DOC should also be dissolved to a DOC‐concentration of 3 mg C/L. If similar organic materials (demonstrated by analyses) are available, these can also be used.
Experimental procedures
Drinking water production process conditions strongly depend on local circumstances, like the actual source water quality, series of treatment processes applied and specific circumstances (like space available and historic developments at the production site). Only the final quality parameters have been standardised, but not the processes to achieve these standards. This paragraph provides a guidance to designing experiments, based on best practices as documented in literature.
Experiments should be carried out to determine which TP may be formed. For this purpose, experiments are recommended twice, first at a high, then at an environmentally relevant concentration, in line with PEC, mentioned in Chapters 2 and 3. Experiments at high concentrations should make it possible to see where signals of the most important TPs (occurring at concentrations that can be measured and identified) are to be expected. However, identification will only be necessary in case the second experiment, at low concentrations, indicates these signals refer to compounds that can be formed under environmentally relevant conditions.
All experiments are carried out at room temperature (20–25°C) unless stated otherwise. Where the tested active substance or eTP has not been radiolabelled, a blank control experiment needs to be set up to confirm that TPs identified originated from the test substance and not the Suwannee River DOC.
The following procedures have to be followed:
4.2.4.1. Chlorination with NaOCl; description of experiments
Materials
1 beaker (1 L)
Magnetic stirrer
NaOCl solution neutralised to pH = 9 with a NaOCl concentration up to 18% w/w containing ~ 500–2,500 g Cl2/L should be prepared and analysed just before use.
N.B. In practice, Ca(OCl)2 can also be applied, but for testing, the active free chlorine content is the important parameter. Therefore, either NaOCl or Ca(OCl)2 should be applied to the required free chlorine content.
Experimental procedure
Add NaOCl to 800 mL of drinking water to a free chlorine concentration with a molar ratio free chlorine/[AS or eTP) > 10 at the start of the experiment. This concentration has to be experimentally confirmed, and the residual concentration should be measured after a reaction time of 12 h.
Carry out the experiment at a starting pH of 6.5, 7.5 and 8.5 at room temperature.
Add the compound under investigation (the active substance and/or its eTP) to a concentration of 1,000 × the LOQ (also see https://ec.europa.eu/food/system/files/2021-02/pesticides_mrl_guidelines_2020-12830.pdf).
Stir for at least 12 consecutive hours, with a maximum of 24 h.
Analyse the added compound(s) and mark the signals of possibly relevant TPs.
4.2.4.2. Chloramination with NH2Cl; description of experiments
Materials
1 beaker (1 L)
Magnetic stirrer
NH2Cl‐stock solution, prepared from NaOCl and NH4Cl solution.
Experimental procedure
First, the NH2Cl stock solution (with a concentration of NH2Cl of about 50–250 mg H2Cl/L) has to be prepared by slowly adding NaOCl into a rapidly stirred NH4Cl solution adjusted to pH = 8.5 at a Cl2:N molar ratio of 1: 1.2 (procedure described by Le Roux et al. (2012). Experiments are carried out at room temperature.
Add NH2Cl to 800 mL drinking water to a concentration > 5–10 mol/mol.
Carry out the experiment at a pH between 6.5 and 7.0.
Add the compound under investigation to a concentration of 1,000 × the LOQ.
Stir for at least 24 h, measuring the NH2Cl concentration at t = 0 and t = 24 h.
Analyse the added compound(s) and mark the signals of possibly relevant TPs.
4.2.4.3. Oxidation with ClO2 ; description of experiments
For all experiments, apply the required safety measures for handling ClO2.
The ClO2 solution can be prepared from gaseous ClO2 by slowly adding dilute H2SO4 to a NaClO2‐solution following the standard method APHA, 1998. The ClO2 concentration can be determined, e.g. using a Hach DR2800 portable photometer (Hash, Loveland, CO, USA) by the diethyl‐p‐phenylene diamine colourimetric method (see APHA, 1998). Experiments should be carried out applying a ClO2/compound (parent compound or eTP) ratio of 2.5 (at least ClO2/DOC = 0.5–1.5).
After at least 12 h of reaction time at room temperature, the residual ClO2 concentration should be measured before removing the excess ClO2 by bubbling N2 through the solution. In this way, it is proven that an excess of oxidant has been applied.
Analyse the added compound(s) and mark the signals of possibly relevant TPs. The presence of nitrogen has a strong effect on the TP formed (see Appendix B).
4.2.4.4. Combined processes, pre‐oxidation followed by chlorination or monochloramination; description of experiments
For all experiments, apply the required safety measures for handling ClO2.
ClO2 often is applied as pre‐oxidation, followed by chlorination by either NaClO or NH2Cl.
Therefore, it is necessary to also carry out the experimental procedures for ClO2 oxidation followed by NaClO treatment and for ClO2 oxidation followed by NH2Cl treatment, according to the procedures described for the separate processes.
Analyse the added compound(s) and mark the signals of possibly relevant TPs. The presence of nitrogen has a strong effect on the TP formed.
4.2.4.5. Ozonation; description of experiments
Materials
One closed beaker or bubble column (1 L).
Magnetic stirrer.
O3 dosing.
Experimental procedure
For all experiments, apply the required safety measures for handling O3.
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the LOQ. In a subsequent stage, if certain TP will have been identified, this may be decreased to an environmentally relevant concentration in the order of about 1–10 μg/L. O3 can be dosed as a gas in the solution by means of a bubble column and by using either air or oxygen in an ozone generator. For water treatment, ozone is typically generated from ambient air or oxygen using the DBD method (dielectric barrier discharge). A preferable method is to dose directly from a concentrated, freshly prepared O3 solution in ultrapure water. The ozone concentration to be dosed is 1.5 mg/L.
Stir the solution for at least 1 h, both at a pH of 6.5 and 9.5 at room temperature and at low alkalinity, to prevent radical scavenging by bicarbonate ions. In principle, the solution can be quenched with H2SO3 to remove the excess ozone. However, since this compound may interfere with the analyses and ozone reacts very quickly, it will not be necessary to quench the solution before analysis.
Analyse the added compound(s) and mark the signals of possibly relevant TPs.
4.2.4.6. UV disinfection; description of experiments
Materials
Collimated beam device (see Figure 6; [Bolton et al., 2005]). In this device, UV radiation can be applied under well‐defined circumstances.
Figure 6.
Collimated beam set‐up
Petri dish with a magnetic stirrer.
Experiments are carried out in a continuously stirred volume of 150 mL, with a water depth of 1.6 cm.
Experimental procedure
Prepare a solution of the compound under investigation in the defined standard water, preferably at a concentration of 1000x the LOQ. Later, if certain TPs require identification, this may be decreased to a concentration in the order of 1–10 μg/L.
Make a UV absorption scan by measuring the UV‐transmission of the solution at every wavelength from 200 to 300 nm.
Apply a UV dose of 100 mJ/cm2, using a medium pressure (MP) UV lamp (equipped with quartz sleeves to prevent radiation < 240 nm to be involved) 16 and room temperature. The irradiation time required can be calculated using the spreadsheet of J. Bolton (Bolton and Cater, 1994, Bolton and Stefan, 2002, Bolton and Linden, 2003, Bolton, 2010). Low pressure (LP) lamps can also be applied for disinfection, but MP lamps have a higher chance for TP formation, and therefore should be used in this test.
Analyse the added compound(s) and mark the signals of possibly relevant TPs.
4.2.4.7. Biodegradation during sand filtration
Biodegradation can occur both in the environment and in filter beds applied in drinking water treatment processes (sand filtration and filtration over activated carbon). Biodegradation in the environment is addressed by four OECD guidelines:
OECD Guideline test no. 307: aerobic and anaerobic transformation in soil.
OECD Guideline test no. 308: aerobic and anaerobic transformation in aquatic sediment systems.
OECD Guideline test no. 309: Aerobic mineralisation in surface water – simulation biodegradation test.
OECD Guideline test no. 314: Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater.
A comparison of the first three tests is given in Appendix C. OECD 307, 308 and 309 test systems typically use radiolabelled test substance and are all carried out in batch configuration. It is mentioned in OECD 308 that this test is unsuitable to simulate flowing water, and the same is true for the other two guidelines. Sand filtration is a process where the water flows through a system with specific hydraulic retention times. This may mean different biodegradation products form during sand filtration than those determined in these three OECD guidelines.
Apart from the differences in retention times mentioned in the previous paragraph, the microbial communities and characteristics of the sand in sand filters may also be different from the soil, sediment and water tests in OECD 307, 308 and 309. As a result, adsorption kinetics and biodegradation kinetics (and possible TPs) can be different for different compounds.
It is difficult to test the occurrence of biodegradation in a sand (or GAC) filter, as biodegradation will only start after the filter has been operated for some time. The microbial community needs time to adapt to the water composition. This would severely complicate the experiments, require long‐term processes and still might not show what kinds of TP may be formed. It can be assumed that, in general, the microbially mediated transformation products in rapid sand filters will be comparable to those already identified in OECD 307, 308 and 309 when appropriately radiolabelled test substance was used. If all these tests are not available in the dossier (which can be the case for some PPP or biocide product uses), another test has to be carried out. For this purpose, OECD 314 may be used. This test guideline also requires the use of radiolabelled (14C) compounds. In cases where radiolabelled test compounds are not available, the following procedure may be applied. Furthermore, in all the studies, it has to be that tTPs have to be determined, and not only total removal mineralisation rates. As the main goal of this guidance is to establish which TP, in principle, may be formed, the following describes a kind of ‘worst case’ biodegradation experiment, in which chances are high that different biodegradation products may be formed. For this ‘worst case’ experiment approach, activated sludge from a municipal wastewater treatment plant should be used. Although the activated sludge is microbiologically very different from sand filter communities and sediments, it does contain a wide variety and a high number of microorganisms inherently adapted to degrading organic contaminants. It thus offers a possibility to show whether biodegradation might occur and which type of TP, in that case, might be formed. In this respect, where all three of reliable OECD 307, 308 and 309 results or results from OECD 314 are not available, it can be used as a worst‐case model system for potential TP formation due to microbial activity in different systems.
Materials
Beaker with stirrer
Activated sludge. Activated sludge from a municipal wastewater treatment plant (WWTP) should be as fresh as possible (≤ 48 h, stored at a temperature of 4–7°C). It should be transported safely (avoid pressure build‐up) and in a clean vessel.
Experimental procedure
Prepare a solution of the compound under investigation in drinking water, preferably at a concentration of 1,000 × the LOQ. Later, if certain TPs are identified, this may be decreased to an environmentally relevant concentration in the order of about 1 μg/L.
Clean the beaker glass apparatus with tap water.
Add activated sludge to the solution. The volume ratio between sludge and the solution should be 1:4.
Adjust pH to 7.4 if necessary. Start stirring at 100 RPM and close the beaker (preferably with a cap, but Styrofoam would also be acceptable). The temperature should be kept between 15–20°C.
During the first runs, check the oxygen and pH every hour and determine if further action is required. One could consider bubbling nitrogen through the mixture to suppress oxygen if oxygen is present. If pH is below 7.0 or above 8, it will have to be adjusted by adding acid/caustic (drops of 1 M NaOH or HCl).
Stir the mixture for 24 h. Analyse the mother compound and if possible TPs, and mark the signals of possibly relevant TPs.
4.2.5. Step 4: experimental procedures at low concentrations
The same procedures described above have to be repeated at a more realistic concentration for the environmental presence of the compounds involved (based on the modelling in Chapters 2 and 3). This generally is in the order of magnitude of about 1–10 μg/L.
4.2.6. Step 5: Identification of possible TPs
If the signals of the TPs observed in step 3 can still be observed (indicating that significant concentrations of this compound may be encountered during drinking water treatment), the TPs will have to be identified. This identification can be based on the results obtained in steps 2 and 3 and is part of Tier 1 of the risk assessment of tTP (Chapter 5, Stage 2 in Figure 7).
Figure 7.
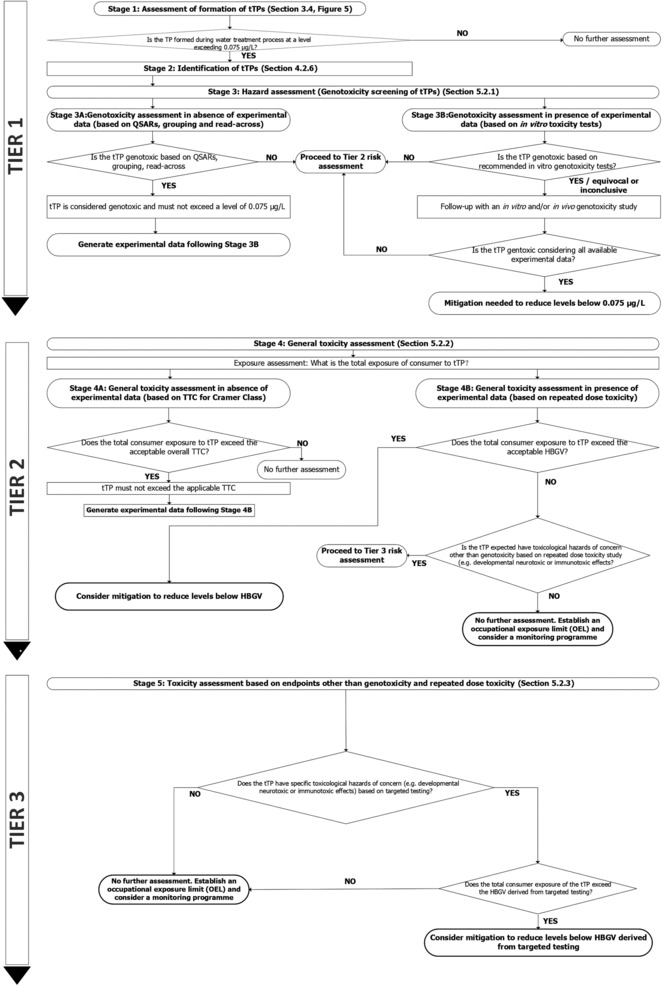
Flowchart decision scheme for the tiered risk assessment of tTPs of biocides and PPPs formed in drinking water treatment processes in relation to human health or food‐producing domesticated animals; a similar decision scheme can be prepared based on Appendix F. In Tier 2, stage 4B: If in vivo general toxicity data are not suitable to set a HBGV, proceed to Tier 3 risk assessment
4.2.7. Step 6: Hazard and risk assessment
Hazard and risk assessments (see Chapter 5) have to be carried out for the relevant tTPs identified in step 5. In practice, a structure/identity is needed for all tTPs that are present at a concentration exceeding 0.075 μg/L (level above which screening for genotoxicity is necessary, see paragraph 5.2.1). It can be difficult to determine the concentration of unknown compounds. An estimation can be made in these cases based on reference compounds. These reference compounds depend on the laboratory involved, the type of compounds under investigation and the matrix composition. Examples are atrazine D5 and bentazone D6, PFOA‐13C8 and isotope labelled pharmaceuticals to study their metabolites. 17 After identification, the real concentration observed after treatment should be established.
5. Hazard and risk assessment of transformation products of biocides and plant protection products
5.1. Introduction
This chapter provides a pragmatic approach to support the regulatory decision‐making concerning the toxicological hazard of identified experimentally confirmed tTPs formed in drinking water treatment processes (Chapter 4) and a tiered approach for the health risk assessment of tTPs of biocides and PPPs formed in drinking water treatment processes in relation to human health and the health of domesticated animals (food‐producing animals). The approach proposed in this chapter is based on the guidance on the establishment of the residue definition for dietary risk assessment (EFSA PPR Panel, 2016) and the risk assessment scheme to identify relevant metabolites of PPPs in groundwater (European Commission, 2021) combined with components from other relevant human health hazard and risk assessment documents published by EFSA and ECHA. In addition, peer‐reviewed scientific literature and documents from other international authorities were also considered. The risk assessment is a tiered approach involving (i) genotoxicity screening (Tier 1), (ii) general toxicity assessment (Tier 2) and toxicity assessment for endpoints other than genotoxicity and general toxicity (Tier 3). In Tiers 1 and 2, there are two situations possible for a tTP, i.e. (i) no experimental data available and (ii) experimental data available. In case of exceedance of a corresponding threshold in the absence of experimental data, experimental data can be generated following this guidance. The data requirements per tier and the evaluation process for proceeding to a higher tier are explained below in Sections 5.2–5.5. This approach allows the tTP under consideration to be assessed at an appropriate level of complexity. The general principle is to proceed from lower tiers which are relatively more conservative, to higher tiers which provide more realistic health risk assessments (EFSA PPR Panel, 2013b).
The proposed approach requires that the chemical identity of a TP is known and that hazard assessment can be performed on the individual tTP (i.e. the tTP is available in sufficient quantity and sufficiently stable for testing). In the case of the occurrence of any mixtures (either intentional or unintentional), the approaches described in the EFSA statement on genotoxicity assessment of chemical mixtures (EFSA Scientific Committee, 2019a) and the EFSA guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019b) can be considered.
Any tTP that does not pass the criteria of the hazard and risk assessment is unacceptable at drinking water contamination levels exceeding thresholds set following this guidance.
5.2. Risk assessment tiers
5.2.1. Tier 1: genotoxicity of transformation products
Tier 1 of the risk assessment corresponds to an early assessment that uses a conservative approach to identify the specific tTP and to determine whether exposure to TP has the potential to harm human health and/or domesticated animal health by genotoxic mechanisms. Two situations are possible for a tTP, i.e. (i) no experimental genotoxicity data available and (ii) experimental genotoxicity data available. Information obtained from the preliminary risk assessment based on the genotoxic hazard of the TTC can be used to decide whether or not to proceed to higher tier assessments. A flow chart for Tier 1 is shown in Figure 7.
All individual tTPs that have been identified to occur at a level exceeding 0.075 μg/L in drinking water should be appropriately assessed to investigate their genotoxic potential. Special attention should be paid to the possible formation of nitrosamines, as some nitrosamines, primarily N‐nitrosodimethylamine (NDMA), are recognised as disinfection by‐products (DBP) of health concern (Kadmi et al., 2015; ATSDR, 2022). If the assessment shows no tTPs are formed, no further risk assessment is required. However, if the assessment indicates the formation of tTPs, the next stage should be the identification of (known and/or characterisation of unknown) tTPs as described in Chapter 4 (Section 4.2.6) of this guidance document.
Non‐identified substances (i.e. substances characterised as chromatographically resolved peaks) at a level exceeding 0.075 μg/L in drinking water, should be considered genotoxic (worst‐case) unless demonstrated otherwise. Consequently, mitigation would be needed to ensure levels would be below 0.075 μg/L or for the pertinent uses, a negative decision made for the product authorisation.
Genotoxicity assessment in absence of experimental data: QSARs, grouping and read‐across (Stage 3A in Figure 7 )
In the absence of experimental data on the genotoxicity of the TP, the use of QSARs, grouping and read‐across approaches should be considered first (ECHA, 2008, 2017a, 2022; EFSA External Scientific Report, 2019; EFSA PPR Panel, 2016; OECD, 2017), while taking into account the context of tTPs and their parent compounds. 18 QSARs, grouping and read‐across approaches should be considered between tTPs and precursors, where experimental data are available on the precursors. Detailed information on the application of QSAR and read‐across for the prediction of genotoxicity can be found in Section 2.3 of the guidance on the establishment of the residue definition for dietary risk assessment (EFSA PPR Panel, 2016). QSAR approaches used should be reported as described in the ECHA (2016b) practical guide and any subsequent updates. Read‐across must be carried out in accordance with the requirements of the Read‐Across Assessment Framework (RAAF) (ECHA, 2017a). More information on in silico approaches can be found in Appendix E.
The exposure to potential genotoxic tTPs must not exceed the threshold of 0.15 μg/person per day set by the EFSA for the ‘Threshold of Toxicological Concern’ with regards to potential DNA‐reactive mutagens and/or carcinogens (EFSA Scientific Committee, 2019c). Assuming a consumption of 2 l of water per day, this corresponds to a threshold concentration of 0.075 μg/L in drinking water. This TTC value when expressed as a water concentration can only be considered, if drinking water is the only source of exposure by ingestion (i.e. the tTP is not present in food). In other cases, potential exposure related to the authorisation of an active substance from possible sources other than drinking water must be taken into account in order to ensure that total exposure of consumers to a tTP is appropriately assessed and compared to the TTC of 0.15 μg/person per day (EFSA PPR Panel, 2016). In case of exceedance of the threshold for potential genotoxic tTPs in absence of experimental data, experimental data can be generated following stage 3B of this guidance.
If the exposure to potential genotoxic tTPs does not exceed the threshold of 0.15 μg/person per day, no further testing is required at this stage.
Genotoxicity assessment based on experimental data: the testing battery for genotoxicity assessment (Stage 3B in Figure 7 )
For an adequate assessment of the genotoxic potential of tTPs based on experimental data, the following endpoints must be assessed in a testing battery:
-
–
gene mutation;
-
–
structural and numerical chromosomal alterations (i.e. clastogenicity and aneugenicity).
A combination of a bacterial reverse mutation (Ames) test (OECD 471, 2020) and an in vitro micronucleus test (OECD 487, 2016) fulfils the requirements to cover the genetic endpoints listed above with a minimum number of in vitro tests (EFSA Scientific Committee, 2011). A test battery also including the mammalian cell gene mutation test (using the Hprt and xprt genes (OECD 476, 2016) or the Thymidine Kinase (TK) gene (OECD 490, 2016)) would also cover the endpoints listed above (ECHA, 2017b, 2022; European Commission, 2021). Accepted modifications to the standard test guidelines/methods have been developed to enhance test sensitivity to specific classes of substances. Expert judgement should be applied to judge whether any of these are appropriate for a substance under investigation (ECHA, 2017b). Any alternative test batteries for genotoxicity assessment must fully cover the endpoints listed above (ECHA, 2017b,c, 2022; European Commission, 2021; EFSA Scientific Committee, 2011, 2017a, 2021a).
When choosing the tests to investigate the above endpoints, the latest scientific and technical knowledge should be taken into account. If there are indications that the tTP is not compatible with one or more recommended in vitro tests, e.g. if specific metabolic pathways for the tTP would be lacking in the standard in vitro systems, or it is known that the in vitro test system is inappropriate for that substance or its mode of action, testing may require appropriate modification of the in vitro test(s) (European Commission, 2021; EFSA Scientific Committee, 2011). For example, protocol modifications for the Ames test might be appropriate for substances such as gases, volatile liquids, azo‐dyes, diazo compounds, glycosides and petroleum oil derived products, which should be regarded as special cases (ECHA, 2017b).
If all in vitro results are clearly negative in adequately conducted tests, no further testing is required in this stage (European Commission, 2021; EFSA Scientific Committee, 2011).
Follow‐up of positive results from in vitro genotoxicity tests
In the case of inconclusive, equivocal results from in vitro tests (or contradictory results if several in vitro studies of the same type are available), it may be appropriate to conduct further testing in vitro, either by repetition of a test already conducted, for example under different conditions or by conducting a different in vitro test, to obtain reliable and conclusive results for a clear interpretation of the genotoxic potential. It may be necessary to follow up with an in vivo genotoxicity study (European Commission, 2021; EFSA Scientific Committee, 2011).
Positive results of in vitro test(s) may be followed up with appropriate in vivo test(s) to address the concerned endpoint(s). Evidence, either from the test itself or from other toxicokinetic or repeated dose toxicological studies with the tTP, that the target tissue(s) have been exposed to the tTP is essential for the reliability and interpretation of results (EFSA Scientific Committee, 2011, 2017a).
Depending on the results obtained in vitro, the following in vivo genotoxicity tests are recommended:
-
–
a mammalian erythrocyte micronucleus test (OECD 474, 2016) or a mammalian bone marrow chromosome aberration test (OECD 475, 2016);
-
–
transgenic rodent somatic and germ cell gene mutation assays (OECD 488, 2022);
-
–
an in vivo mammalian alkaline comet assay (OECD 489, 2016).
More information on the conduct of these and other in vivo tests is available in the abovementioned OECD test guidelines and in guidance documents describing regulatory genotoxicity test batteries (ECHA, 2017b,c; EFSA Scientific Committee, 2011, 2017a).
The approach to in vivo testing should be step‐wise. If the first test is positive, no further test is needed, and the substance should be considered as an in vivo genotoxin. If the test is negative, it may be possible to conclude that the substance is not an in vivo genotoxin. However, in some cases, a second in vivo test may be necessary as there are situations where more than one endpoint regarding gene mutations and structural and numerical chromosomal aberrations in the in vitro tests is positive and if the first in vivo test is negative. It may also be necessary to conduct a further in vivo test on an alternative tissue if, for example, it becomes apparent that the substance did not reach the target tissue in the first test. The combination of assessing different endpoints in different tissues in the same animal in vivo or combining with a repeated dose toxicity study should be considered for animal welfare reasons (ECHA, 2017b, 2022; EFSA Scientific Committee, 2011, 2017a, 2021b).
Evaluation criteria for genotoxic tTPs
tTPs are considered genotoxic based on their hazard, which includes positive, equivocal or inconclusive results from experimental data of appropriate tests or exceedance of the TTC value of 0.15 μg/person per day (corresponding to a threshold of 0.075 μg/L in drinking water based on a daily water consumption of 2 L) for potential genotoxic tTPs in drinking water in the absence of experimental data. Non‐identified tTPs are also considered genotoxic (worst‐case) unless demonstrated otherwise. This TTC value applies to water if drinking water is the only source of exposure by ingestion. In case of exceedance of the threshold for potential genotoxic tTPs in absence of experimental data (stage 3A), experimental data can be generated following this guidance (stage 3B). If the exposure level of a tTP exceeds the TTC, testing might be considered for a lead compound within the group (EFSA PPR Panel, 2016).
tTPs which are demonstrated not to be genotoxic, are considered to have passed Tier 1 and should be subjected to a general toxicity assessment (Tier 2).
5.2.2. Tier 2 risk assessment
Tier 2 of the risk assessment aims at comparing the exposure to a tTP to a level that is not expected to result in an appreciable health risk. This can be achieved through application the TTC approach and/or setting a substance‐specific health‐based guidance value (HBGV, see Section 5.3) for tTP. All tTPs identified according to Chapter 4 to occur at a level exceeding 0.075 μg/L in drinking water and that are not identified as genotoxic should be appropriately assessed to investigate their general toxicity in Tier 2. Two situations are possible for a tTP, i.e. (i) no experimental general toxicity data available and (ii) experimental general toxicity data available. Tier 2 also includes exposure assessment to estimate the total intake of a tTP.
Exposure assessment
Exposure assessment should include the total estimated intake of the tTPs, aggregating all sources of oral intake (dietary and water). Details on exposure assessment can be found in Chapter 5 of the EFSA Guidance on the establishment of the residue definition for dietary risk assessment (EFSA PPR Panel, 2016) and Chapter 5 of the ECHA Guidance on the Biocidal Products Regulation Volume III Human Health ‐ Assessment & Evaluation (Parts B + C) (ECHA, 2017c).
General toxicity assessment in the absence of experimental data, exposure to tTP against the TTC for Cramer Class (Stage 4A of Figure 7)
The TTC approach is a pragmatic, scientifically valid methodology to assess the safety of substances of unknown toxicity that avoids extensive and expensive evaluation and assessment (WHO/IPCS, 2009) and establishes a level of exposure at which a chance of adverse effects is considered to be low (i.e. below which there would be no appreciable risk to human health) (EFSA Scientific Committee, 2019b). In this stage, the concentrations of tTP to which exposure is likely to occur should be determined. Drinking water exposure may be estimated based on the information on environmental levels (Chapters 2 and 3) and on the formation of tTP investigated experimentally (Chapter 4). Exposure from other sources and aggregate exposure to similar tTP belonging to the same TTC class should be taken into account to ensure that the total exposure of consumers to TP will not exceed the TTC.
The combined exposure of all identified tTPs belonging to a similar group of toxicity (or structurally similar tTPs belonging to the same TTC class) without toxicity data available should be added up and compared to the respective TTC threshold per Cramer class (EFSA PPR Panel, 2016). In order to apply the TTC in a cumulative way, the ratio between the exposure of each tTP and the corresponding Cramer Class TTC needs to be added. If the ratio of the sum is ≥ 1, a specific hazard and/or comparative risk assessment needs to be conducted. If the resulting sum is < 1, no further assessment is necessary.
The thresholds of 18 μg/person per day (for organophosphate and carbamate with anti‐cholinesterase activity) or 90 μg/person per day (Cramer Class III), 540 μg/person per day (Cramer Class II) and 1,800 μg/person per day (Cramer Class I) as set by the EFSA for the ‘Threshold of Toxicological Concern’ should be used (EFSA Scientific Committee, 2019c). Assuming a consumption of 2 l of water per day, this corresponds to drinking water concentrations of 9 μg/L (for organophosphate and carbamate with anti‐cholinesterase activity), 45 μg/L (Cramer Class III), 270 μg/L (Cramer Class II) and 900 μg/L (Cramer Class I).
The TTC approach in this stage should be seen as a screening and prioritisation tool for the risk assessment of chemicals when hazard data are incomplete (EFSA Scientific Committee, 2019c), which is restricted to cases where reliable and robust exposure estimates can be made. Significant uncertainties due to limited knowledge about the identity and/or the magnitude of the concentration in drinking water of tTPs would hamper reliable and robust exposure estimates required for waiving the toxicological characterisation of tTPs.
The abovementioned TTC values can be considered if drinking water is the only source of exposure by ingestion (i.e. the tTP is not present in food). In other cases, potential exposure related to the authorisation of an active substance from possible sources other than drinking water must be taken into account in order to ensure that total exposure of consumers to a tTP is appropriately assessed (EFSA PPR Panel, 2016).
It must be noted that TTC for Cramer Classes do not apply to substances with specific properties, chemical structures not represented in the database used to derive the respective TTC value or substances outside the domain of applicability (EFSA Scientific Committee, 2012a, 2019c).
In general, the TTC approach is applicable to the whole population. However, when exposure in infants below the age of 16 weeks is in the region of the relevant TTC, special considerations apply, as outlined in the guidance on the risk assessment of substances present in food intended for infants under 16 weeks of age (EFSA Scientific Committee, 2017c, 2019c).
If the exposure level of a tTP, or if the sum of the group that the tTP belongs to, appears to be above the TTC, grouping and testing might be considered for a lead compound within the group (EFSA PPR Panel, 2016). Experimental data can be generated following stage 4B of this guidance.
If the exposure level of a tTP can be assumed to stay below the TTC (stage 4A), there is a low probability of adverse health effects, and further investigation is not required.
General toxicity assessment based on experimental data, the testing strategy for general toxicity (Stage 4B of Figure 7)
When the total consumer exposure to a tTP, or the sum of the group that the tTP belongs to, exceeds the corresponding TTC, a HBGV needs to be established, and in vivo testing is needed following current guidelines. This in vivo testing includes a repeated‐dose toxicity study (e.g. a 90‐day study) according to current standards (including all parameters to be investigated according to the OECD TGs 19 ) (EFSA PPR Panel, 2016). Where applicable, the possibility of investigating additional parameters (e.g. genotoxicity) in the same animal should be considered for animal welfare reasons. As the development of alternative methods is still ongoing, applicants are encouraged to consider existing guidance documents and check for updates on the use of specific in vitro tests before initiating an in vivo test. This could also be an iterative process between the applicant and the authority.
Whenever tTPs show similarities in toxicity based on (qualitative) experimental data (i.e. toxicological profile, same target organ/critical effects), tTPs are considered to belong to a similar group of toxicity. In this case, tTPs should be grouped (adjusted by relative potency factor if there are differences in potency) and the sum of the exposure values compared to the respective reference point (EFSA PPR Panel, 2016).
If the in vivo repeated dose toxicity data available are not suitable to set a HBGV (e.g. at least a 90‐day toxicity), a read‐across approach can be performed, where a HBGV of a structurally similar compound may be used for the tTP using the ECHA Read‐Across Assessment Framework (ECHA, 2017a). In vitro test methods can be useful to support the read‐across hypothesis if the read‐across hypothesis is inconclusive before conducting an (another or a new) in vivo repeated dose toxicity study (e.g. 90‐day toxicity study according to the most recent OECD TGs 11 ).
Evaluation criteria for general toxicity of tTPs
The exposure to a tTP, or the sum of the group that the tTP belongs to, must not exceed the HBGV and/or the level of a tTP must not exceed the corresponding health‐based guideline value for drinking water (HBGLV), assuming an average consumption of 2 L of water per day (stage 4B).
The following scenarios are possible:
-
–
The exposure to a tTP exceeds the HBGV for general toxicity, indicating that the risk has not been characterised as acceptable and mitigation needs to be performed. If it cannot be demonstrated that the exposure levels are below the HBGV, the product‐use‐pattern of the active substance cannot be authorised.
-
–
The exposure to a tTP does not exceed the HBGV, and there is reason to expect that a tTP may have toxicological hazards of concern other than genotoxicity or general toxicity based on the active substance or structurally similar TPs, the available scientific evidence from health authorities such as WHO and ECHA, and/or published peer‐reviewed scientific literature. In this case, the tTP should be further evaluated in subsequent steps, e.g. (developmental) neurotoxicity, immunotoxicity, reproductive and developmental toxicities and carcinogenicity (Tier 3), as this targeted testing may result in a lower HBGV.
-
–
The exposure to a tTP does not exceed the HBGV, there is no reason to expect that a tTP has specific toxicological hazards of concern other than genotoxicity and repeated dose toxicity, and there is no health concern at the estimated levels of exposure. In this case, the exposure is considered acceptable, and no further assessment is needed. A routine monitoring program should be considered and the Occupational Exposure Limit (OEL) should be determined by authorities.
When the above considerations do not lead to clear results (e.g. if the in vivo toxicity data available are not suitable to set a HBGV), a targeted testing may be necessary (Tier 3).
The EFSA Scientific Committee notes that, during the period from birth up to 16 weeks, infants are expected to be exclusively fed on breast milk and/or infant formula. The EFSA Scientific Committee views this period as the time where HBGV for the general population do not apply without further considerations (EFSA Scientific Committee, 2017c).
5.2.3. Tier 3 risk assessment
Tier 3 is a more refined risk assessment and is undertaken when a health concern cannot be excluded from the measured or the estimated levels of exposure or where the Tier 2 assessment does not lead to clear results. Tier 3 provides a more refined risk assessment of tTPs and includes in vivo toxicity testing (other than genotoxicity and repeated dose toxicity) of more specific endpoints according to the most recent OECD TGs. 19 Tier 3 is recommended if there is reason to expect that a tTP may have toxicological hazards of concern based on the active substance or structurally similar TPs, e.g. developmental neurotoxic or immunotoxic or reproductive and developmental or carcinogenic effects (EC, 2021). Appropriate test methods should be used, where in vitro methods should always be considered first. However, it should be noted that a number of toxicological endpoints cannot be (fully) assessed in vitro due to the complexity of the endpoint and the lack of validated and accepted in vitro tests. As the development of alternative methods is still ongoing, applicants are encouraged to consider existing guidance documents and check for updates on the use of specific in vitro tests before initiating an in vivo test. This could also be an iterative process between the applicant and the authority. The appropriate strategy for evaluating these tTPs must be developed on a case‐by‐case basis. A flow chart for Tier 3 is shown in Figure 7.
A risk characterisation (see Section 5.4) should be carried out after conducting the hazard assessment for all relevant human health endpoints and the exposure estimation of tTP. The risk characterisation approach taken in reaching a conclusion needs to be as transparent as possible and needs careful explanation/justification as to uncertainties. Applicants are encouraged to consider existing guidance documents on uncertainty analysis of scientific assessments (ECHA, 2012; EFSA PPR Panel, 2018; EFSA Scientific Committee, 2018a).
The exposure to a tTP must not exceed the HBGV for targeted testing, assuming an average consumption of 2 L of water per day.
The following scenarios are possible:
-
–
The exposure to a tTP exceeds the HBGV for targeted testing, indicating that the risk has not been characterised as acceptable and mitigation needs to be performed. If it cannot be demonstrated that the exposure levels are below the HBGV, the product‐use‐pattern of the active substance cannot be authorised.
-
–
The exposure to a tTP does not exceed the HBGV for targeted testing, and there is no health concern at the estimated levels of exposure. In this case, the exposure is considered acceptable and no further assessment is needed. A routine monitoring program should be considered and the OEL should be determined by authorities.
The EFSA Scientific Committee notes that, during the period from birth up to 16 weeks, infants are expected to be exclusively fed on breast milk and/or infant formula. The EFSA Scientific Committee views this period as the time where HBGV for the general population do not apply without further considerations (EFSA Scientific Committee, 2017c).
5.3. Health‐based guidance values
A HBGV is a science‐based recommendation for the maximum (oral) exposure to a substance that is not expected to result in an appreciable health risk. When a HBGV is needed, the first step should be to consider whether EFSA has already established a HBGV for the tTP. Two main sources of information on the health effects of exposure to chemicals can be used in deriving HBGVs. The first and preferred source is the studies on human populations. However, the availability of such studies is very limited for most chemicals because of the ethical impediments to conducting human toxicological studies and the lack of quantitative information on the concentration to which people have been exposed or on simultaneous exposure to other agents. However, for a few substances, such studies are the primary basis for the development of guidance values. The second and most commonly used source of information is toxicological studies in laboratory animals. Nonetheless, the evidence available from studies in humans and animals to facilitate risk assessment is often limited both in quality and quantity. In order to derive a guidance value to protect human and domesticated animal health, selecting the most appropriate study/studies is necessary. Data from well‐conducted studies in which a clear dose–response relationship has been demonstrated are preferred. Considering all available evidence, if the overall evaluation of tTP leaves no concern for genotoxicity, a HBGV may be established. If concern for genotoxicity remains based on the overall assessment, establishing an HBGV is not considered appropriate. In cases where an HBGV had been previously established for a substance and cannot be confirmed due to newly identified concerns about genotoxicity, it is up to authorities to decide on the regulatory status of the substance during the transitional time that new data are generated (EFSA Scientific Committee, 2017b).
Although NOAELs/LOAELs are commonly used as the point of departure for deriving HBGVs, the preferred approach for deriving a reference value is using a benchmark dose level (BMDL). Calculation of the HBGV for humans and domesticated animals is presented in Table F.1 (see Appendix F). When establishing HBGV values, an appropriate safety margin of at least 100 must be ensured, taking into account the type and severity of effects and the vulnerability of specific groups of the population (EC 1107/2009, EFSA Scientific Committee, 2021c). If specific reference value(s) need to be set, the application of additional UFs considering the duration of treatment (e.g. extrapolation from subacute to subchronic or from subchronic to chronic exposure) and other relevant factors should follow the recommendations by the EFSA Scientific Committee (EFSA Scientific Committee, 2012b). In addition, when the critical effect is judged of particular significance, such as developmental neurotoxic or immunotoxic effects, an increased margin of safety shall be considered and applied if necessary (EC 1107/2009, EFSA PPR Panel, 2016). When an HBGV is established, the risk characterisation is based on the comparison with the estimated intake of the tTP.
Table F.1.
An approach for deriving HBGLVs for humans and food‐producing domesticated animals (adapted from EFSA Scientific Committee, 2012b)
TDI (mg/kg bw per day) = NOAEL or LOAEL or BMDL/UF1 × UF2 × UF3n × UF3n+1 × UF3n+2 etc. UF1 = 10 (to account for interspecies variation) UF2 = 10 (to account for intraspecies variation) UF3n = 1–10 (to account for first data variation issue) UF3n+1 = 2–10 (to account for further data variation issues when needed) UF3n+2 = 2–10 (to account for further data variation issues when needed) UF3n+ values are not applicable for good quality datasets 5) Derive the HBGLV, which considers chronic exposure, as follows: HBGLV = TDI (mg/kg bw per day) × bw × AF/C For humans: bw = body weight [adults = 70 kg (aged above 18 years); toddlers = 12 kg (aged 1–3 years); infants = 5 kg (0–12 months)] AF = allocation factor (see Section F.1.4) C = daily drinking water consumption [default intake = 2 L adult (70 kg bw), 1 L children (12 kg bw), bottle‐fed infants 0.75 L (5 kg bw)] For domesticated animals: Bw = body weight (kg) depends on the category of domesticated animal (see Table F.2) C = daily drinking water consumption (L) depend on the category of domesticated animal (see Table F.2) |
5.4. Human health
The characterisation of the risk for consumers should include the total estimated intake of the tTPs, aggregating all sources of oral intake (dietary and water) (ECHA, 2016a, 2017c). If the total estimated intake exceeds the HBGV, depending on the extent of this exceedance and the nature/severity of the potential adverse effects for the consumers, the scientific output should discuss its implications (EFSA Scientific Committee, 2021c). If the toxicity data are insufficient to decide on a reference value, then a margin of exposure (MoE) can be calculated as the ratio between the reference point (NOAEL, LOAEL, BMDL) to the predicted or estimated exposure (from all sources). If HBGVs are not available for all tTPs originating from one active substance, the lowest available HBGV for the most potent tTP can be used, assuming that all of the tTPs with missing HBGVs are equally potent. This assumption is likely to be conservative since less toxic tTPs may dilute the toxicity of any tTPs that might be more potent (ECHA, 2019b).
For the characterisation of health risks as a result of exposure to tTPs in humans, potential exposure from both drinking water and dietary sources should be taken into account in order to ensure that the total exposure of consumers to a given TP is appropriately assessed. In practice, this means that applicants should screen assessment reports for biocides and PPPs and EFSA conclusions for common eTP, and be aware of the exposure estimates in these reports and water monitoring data covering other substance classes such as pharmaceuticals. The allowable exposure to tTP via water should be calculated based on their respective body weight and daily water intake. The current default water intake for adults is 2 L for adults (70 kg body weight), 1 L for children (12 kg body weight) and 0.75 for infants (5 kg body weight), whereas the water intake for domesticated animals should be based on the animal type (EFSA Scientific Committee, 2012b, Appendix F). Note that once finalised and published, the EFSA Comprehensive Database relevant for pesticide residues made available via PRIMO 4 or later versions should be used for water consumption volumes, food intakes and associated consumers' body weights (EFSA Scientific Committee, 2018b).
Appendix F (see Table F.1) shows the method for deriving HBGLVs, which can be used as an acceptable risk level for water quality monitoring and developing risk management strategies to ensure that concentrations of tTPs are well below health concerns.
5.5. Domesticated animal health
For the characterisation of health risk as a result of exposure to tTPs in domesticated animals, the allowable exposure to tTPs via water should be calculated based on the respective body weight and daily water intake of different animal categories (Appendix F: see Table F.2), and where appropriate, taking into account exposure via all other routes. Criteria for watering livestock should generally take into account the type of animal, the daily water requirements of each species, and the type of feed consumed (Appendix F: see Table F.2). In the absence of appropriate information derived specifically for livestock; it is recommended that drinking water guidelines for human health also be adopted for domesticated animals.
Table F.2.
Average water intake (L/day) for different categories of food‐producing domesticated animals (adapted from ECHA, 2017a,b and updated considering EFSA AHAW Panel, 2020)
| Animal species | Body weight (kg) | Drinking water intake (L/day) based on default body weight |
|---|---|---|
| Beef cattle | 500 | 50 |
| Dairy cattle | 650 | 115 |
| Calf | 200 | 20 |
| Fattening pig | 100 | 10 |
| Breeding pig | 260 | 15 |
| Sheep | 75 | 10 |
| Lamb | 40 | 5 |
| Slaughter goat (= goat kids) | 13 | 1.3 |
| Lactating goat | 70 | 7 |
| Broiler chicken | 1.7 | 0.25 |
| Laying hen | 1.9 | 0.25 |
| Turkey | 7 | 1.0 |
| Horse | 400 | 40 |
| Rabbit | 2.5 | 0.2 |
Appendix F (see Table F.1) shows the method for deriving HBGLVs which can be used as a safe level for water quality monitoring and developing risk management strategies to ensure that concentrations of tTPs are well below health concerns.
6. Recommendations and future perspectives
6.1. National approaches from European Union member states and European non‐member states to minimise entry of pesticides
Different European member states have different approaches to minimise the entry of PPP and biocides into water bodies.
Germany has a regulation for not using pesticides on sealed surfaces, roads or on highly permeable surfaces and plans to reduce overall pesticide use by 30% by 2030. Buffer zones are implemented as well, where either no crops are planted or no pesticide spraying may take place (Mohaupt et al., 2020). Furthermore, cooperation between farmers and drinking water providers has been established to reduce the use of pesticides in water protection zones. Here, farmers will be compensated for potentially lower yields.
The Netherlands use a two tier approach to monitor the use of pesticides. First, environmental (water) concentrations at abstraction points are calculated and corrected by factors representing the specific area, such as degradation. Second, the monitoring data are evaluated and analysed. The Netherlands have been the first to implement emission reduction plans (ERPs), to address exceedance of the WFD thresholds. Another approach is public information, for which a set of 17 information sheets about measures to reduce the emission of pesticides to surface water is freely available online (Adriaanse et al., 2008; Mohaupt et al., 2020).
Denmark set the target to reduce the pesticide load by 40% until 2015 compared to 2011. This goal was met according to the pesticide loads indicator (PLI). In Denmark, also buffer zones as in Germany and Sweden are defined (Mohaupt et al., 2020).
Sweden uses a system to prohibit pesticide use alongside roads, sealed or highly permeable surfaces, similar to Germany. Sweden also uses buffer zones like Denmark and Germany, e.g. 12 m around wells. 30 m around watercourses and wells is not allowed to fill or clean sprayers (Mohaupt et al., 2020).
Belgium sets restrictions in buffer zones, which are 2–30 m large, depending on the land use and the size of the waterbody in this area (Mohaupt et al., 2020).
Other European countries (non‐EU member states) have developed useful approaches for the safe usage of pesticides as well.
Norway uses the ‘Environmental Risk Indicator’ and the ‘Human Health Risk Indicator’. Norway established a ‘Cumulative Environmental Index’ for substances in PPP per year to show the risk for human health and environment over time, considering terrestrial and aquatic effects as well as leaching potential, persistence, bioaccumulation and a potential hazard for all species. Norway also wants to reduce the use of pesticides to protect human health and the environment, which is why pesticides with the highest risk feature the highest tax rates (Spikkerud, 2000).
England implemented a ‘Catchment sensitive farming programme’ to examine the impact of agricultural practice on pesticide pollution and to monitor the success of the used measures. They also have grants for useful measures to support and encourage good agricultural practice (Mohaupt et al., 2020).
6.2. Effect‐based monitoring of water samples using in vitro bioassays
Effect‐based monitoring of water samples using in vitro bioassays can be applied as an additional screening step in specific cases, e.g. if a TP cannot be appropriately identified and/or to investigate combined effects of TP mixtures (in case multiple TPs are formed simultaneously). In principle, this testing strategy should follow more or less the same decision scheme as proposed for the identified TPs, and toxicological endpoints such as genotoxicity, endocrine disruption and neurotoxicity should be assessed. Assessing individual substances takes precedence over testing a mixture whenever this is possible.
6.2.1. Sample pre‐treatment
For hazard assessment of a mixture of TPs in the treated water, prior to the conduct of the bioassays, water samples should be collected after relevant treatment processes and prepared according to the ISO standard on preservation and handling of water samples (ISO 5667‐3:2018) and the ISO standard on biotesting of samples (ISO 5667‐16:2017). It should be taken into account that pre‐treatment, such as concentration of the water sample by solid‐phase extraction, is required to apply sufficiently high concentrations to demonstrate exposure of the cells and bacteria. It is essential to use an optimal extraction method to prevent a reduction in concentration or loss of chemicals and a consequent underestimate of the biological effect as much as possible. Enrichment methods should be non‐discriminatory, compatible with bioassays and (preferably) standardised. It must be taken into account that high enrichments may lead to positive effects of SPE blanks, and therefore appropriate controls should be run in parallel. Knowledge of the limitations of the extraction method used should be recognised and taken into account in the conclusion of the bioassay response.
6.2.2. Genotoxicity testing
For genotoxicity testing, the Ames fluctuation test (ISO 11350:2012), the umu‐test (ISO 13829:2000) and/or the in vitro micronucleus test (ISO 21427‐2:2006) are available as standardised methods for water quality assessment. A mammalian gene mutation test, according to OECD 476 (2016) or OECD 490 (2016), can be considered as well (see Section 5.2), although there might be practical implications when performing these assays with water samples. As a minimum requirement, the genotoxicity testing strategy should cover both gene mutation and structural and numerical chromosomal alterations (clastogenicity and aneugenicity). Water samples (containing a mixture of active substance and one or more TPs) that show a positive response or are considered inconclusive in one or more bioassays are considered genotoxic and warrant further investigation (e.g. chemical characterisation, mechanistic studies) to demonstrate the biological relevance of the response. For water samples that show a negative response in the bioassays, evidence should be provided that the cells and/or bacteria have been exposed at sufficiently high concentrations (e.g. by the occurrence of cytotoxicity).
6.2.3. Neurotoxicity and endocrine disruption
Since to date, no formal guidance documents related to water quality exist for screening for toxicity endpoints other than genotoxicity, it is recommended to follow the recent developments in this field. An example of a testing strategy for neurotoxicity and endocrine disruption in the context of water quality can be found in the guideline that resulted from the joint project Tox‐Box within the funding program Risk Management of new pollutants and pathogens in the water cycle (RiSKWa) (Grummt et al., 2020). The neurotoxicity concept in Tox‐Box is the first innovative step toward evaluating neurotoxicity and has been validated in the follow‐on project Neuro‐Box (Grummt and Kuckelkorn, 2020). Regarding neurotoxicity and endocrine endpoint assessment, when tools such as Tox‐Box or Neuro‐Box are being considered, it must be noted that regulatory guidance for these systems is not yet available, but this might be developed in the future.
To interpret the bioassay results, all the evidence collected should be assessed with a WoE approach, where data from the various in vitro bioassays are weighed according to the strength of the evidence they provide.
Abbreviations
- ADI
acceptable daily intake
- AOP
advanced oxidation process
- AS
active substances
- BCF
bioconcentration factor
- BMDL
benchmark dose level
- CLC
Corine Land Cover
- CLP
classification, labelling and packaging
- CMR
carcinogenic, mutagenic and reprotoxic
- DOC
dissolved organic carbon
- DWD
drinking water directive
- EQS
environmental quality standard
- ERC
ecotoxicological relevant concentration
- ERP
emission reduction plan
- ESAC
EURL ECVAM Scientific Advisory Committee
- ESDAC
European Soil Data Centre
- EST
embryonic stem cell test
- eTP
environmental transformation product
- FOCUS
Forum for the co‐ordination of pesticide fate models and their use
- GAC
granular activated carbon
- GC–MS
gas chromatography–mass spectrometry
- HAA
halo acetic acids
- HBGLV
health‐based guideline value
- HBGV
health‐based guidance value
- HQ
hazard quotient
- ICM
iodinated contrast media
- ITHM
iodo‐trihalomethanes
- LOAEL
lowest‐observed‐adverse‐effect level
- LC–MS
liquid chromatography‐mass spectrometry
- MM
MicroMass test
- MoA
mode of action
- MoE
margin of exposure
- NOAEL
no observed adverse effect level
- NOM
natural organic matter
- OMP
organic micropollutants
- PAT
pesticide application timer
- PEARL
Pesticide Emission Assessment at Regional and Local scales
- PEC
predicted environmental concentration
- PLI
pesticide load indicator
- PPP
plant protection product
- PRZM
pesticide root zone model
- QSAR
quantitative structure–activity relationships
- RV
reference value
- SOM
soil organic matter
- SPE
solid phase extraction
- STU
soil typological unit
- SWAN
surface water assessment enabler
- SWASH
surface water scenario help
- TDI
tolerable daily intake
- TER
toxic exposure ratio
- THM
trihalomethanes
- TK
thymidine Kinase
- TOXSWA
toxic substances in surface waters
- TP
transformation product
- TSAR
tracking System for Alternative methods towards Regulatory acceptance
- TTC
threshold of toxicological concern
- tTP
(water) treatment transformation product
- UV
ultra violet 315–400 nm
- UV‐A
ultra violet 280–315 nm
- UV‐B
ultra violet 100–280 nm
- UV‐C
ultra violet 80 nm
- WoE
weight of evidence
Glossary
- Acceptable daily intake
An estimate of the amount of a substance in food or drinking water that can be consumed daily over a lifetime without presenting an appreciable risk to health. It is usually expressed as milligrams of the substance per kilogram of body weight.
- Assessment group
Chemicals that are considered as a group because they are likely to act on the body in the same way.
- Benchmark dose level
The dose that corresponds to a specific change in an adverse response compared to the response in unexposed subjects, and the lower 95% confidence limit is termed the benchmark dose level (BMDL).
- Dilution factor
The dilution factor describes the ratio of the diluted volume to the initial concentrated volume and the ratio of the initial concentration to the final diluted concentration.
- Environment
means waters (including ground, surface, transitional, coastal and marine), sediment, soil, air, land, wild species of fauna and flora and any interrelationship between them and any relationship with other living organisms.
- Environmental Transformation Product
Metabolites and abiotic transformation products other than transformation products formed by chemical reactions during drinking water treatment.
- General toxicity
includes effects on, e.g. body weight and/or body weight gain, absolute and/or relative organ and tissue weights, alterations in clinical chemistry, urinalysis and/or haematological parameters, functional disturbances in the nervous system as well as in organs and tissues in general and pathological alterations in organs and tissues as examined macroscopicallynand microscopically (ECHA, 2017b).
- Genotoxicity
includes effects on DNA, including gene mutations and chromosome aberrations.
- Hazard quotient
The ratio of the potential exposure to a substance and the level at which no adverse effects are expected.
- Health‐based guidance value (HBGV)
a science‐based recommendation for the maximum (oral) exposure to a substance that is not expected to result in an appreciable health risk, taking into account current safety data, uncertainties in these data and the likely duration of consumption.
- Health‐based guideline value (HBGLV)
for drinking water, similar to HBGV, assuming an average consumption of 2 L of water per day.
- Lowest‐observed‐adverse‐effect level
The lowest level of a substance that has been observed to cause harm in an exposed population.
- Margin of exposure
The ratio between the reference point (NOAEL, LOAEL, BMDL) to the predicted or estimated exposure (from all sources).
- Metabolite
Any intermediate or a degradation product of an active substance, safener or synergist, formed either in organisms or in the environment.
- No‐observed‐adverse‐effect‐level
The highest concentration or amount of a substance at which no detectable adverse effects occur in an exposed population.
- Occupational Exposure Limit (OEL)
Highest allowable concentration of a chemical to which a worker may be exposed over a period. In the context of water treatment transformation products this will be those working in water treatment facilities.
- Plant Protection Products
Products used to protect, preserve or influence the growth of desirable plants or to destroy or control the growth of unwanted plants or parts of plants.
- Precursor
A substance from which another substance is formed.
- Residues
Means one or more substances present in or on plants or plant products, edible animal products, drinking water or elsewhere in the environment and resulting from the use of a plant protection product, including their metabolites, breakdown or reaction products.
- Tolerable daily intake
An estimate of the amount of a substance in food or drinking water which is not added deliberately (e.g. contaminants) and which can be consumed over a lifetime without presenting an appreciable risk to health.
- Transformation product
A product formed from a particular compound (e.g. a pesticide) as a result of metabolism, chemical reactions or environmental processes.
- Threshold of Toxicological Concern
A screening tool that provides conservative exposure limits in the absence of sufficient chemical‐specific toxicological data. It is a science‐based approach for prioritising chemicals with low‐level exposures that require more data over those that can be presumed to present no appreciable human health risk.
- (water) Treatment transformation product
Products formed by chemical reactions during drinking water treatment.
Appendix A – Report on the procedure for creating map material for Exposure assessment (Dilution Factors)
1.
The following steps provide further guidance – in addition to the programmes and models described above – to assess the exposure potential of pesticides. The example provided below describes how to intersect the factors that influence fate and exposure on the European scale in high spatial resolution.
A.1. Material, programme and overall method
In order to evaluate the area of concern in Europe, regarding the input of pesticides, the programme QGIS Desktop 3.2.1 was used. In the project, the coordinate reference system EPSG:3035 was used for visual presentation, since it is well suited for data from the European area.
For Europe, the rivers that have a Strahler Order greater than or equal to 3 were displayed. The Strahler method is a commonly used method for watercourse ordering (Hughes et al., 2011). It also includes lakes connected to the river network. In the Strahler method, all sections without tributaries are assigned the first order. The Strahler Order increases when watercourses of the same order converge. Therefore, the intersection of two first‐order river sections creates a second‐order section. The intersection of 2 second‐order sections creates a third‐order section and so on. On the other hand, the intersection of two sections of different order does not increase the order, but the highest order value is used.
Strahler Order is widely available for many river systems and often already included in freely available data, e.g. from the EEA (used in this project), this does not apply to other network topologies, or if so, then to a lesser extent. The advantages of Strahler's order are mainly the solid mathematical background of the tree structure, so that Strahler's order is commonly used as a calculation method for topology. The disadvantage of this method is the lack of distinction of a main channel, which can lead to problems in large analytical procedures. There are also other network topologies such as the Horton topology. It uses a more natural arrangement of the streams, but still requires a prior radiator arrangement. The method evaluated by Hack is suitable for comparison and analysis of topological flows and can be easily filtered. The disadvantage is that comparing sub‐basins with the same order can be difficult. Weighing up all the advantages and disadvantages; especially considering the free availability of data, since unrestricted data availability was a key criterion, the Strahler order was chosen.
Only rivers with a Strahler Order greater than or equal to 3 were selected, since in Europe many drinking water withdrawals in relation to surface water are obtained through bank filtration or by necessity come from larger rivers with reliable flow. Infrastructure induced bank filtration or direct withdrawals are rather unlikely for small streams and rivers (Strahler Order 1–2), as typically they are subject to strong flow fluctuations.
A buffer of 50 m was placed around all of these water bodies to identify critical areas where pesticide input can occur. The joint style and the end style ‘round’ were chosen in order to obtain a constant distance perpendicular to the initial geometry. The differences between the connection and end styles are only marginal when considering 50 m buffers.
The choice of a 50 m buffer has several reasons. In order to comply with the provisions of the Water Framework Directive, buffer zones of 10 m are applied in Germany, for example. It can be assumed that pesticides applied at a distance of 50 m can also be transported into the respective water body but to a far lesser extent; especially in combination with other factors such as the topographic gradient. Therefore a buffer of 50 m provides more safety in comparison to 10 m. Furthermore, the distance of 50 m was chosen in a rather pragmatic approach, following FOCUS (2007). It states that spray drift is one of the most commonly considered exposure pathways and the FOCUS Drift Calculator is often used to determine the width of buffer zones. PECs can be determined for distances of up to 50 m. These drift values are also confirmed by measurement data in field tests as well. Within these buffer zones, agricultural land use was examined.
Since Europe is divided into three regulatory zones (North, Central and South), each aspect was investigated for each regulatory zone.
In Table A.1 the used data and their sources are listed.
Table A.1.
Used data and sources
| Data | URL | Date/year of publication | Date of download |
|---|---|---|---|
| European counties | https://ec.europa.eu/eurostat/de/web/gisco/geodata/reference-data/administrative-units-statistical-units/countries | 2020 | 14.12.2021 |
| Regulatory Zones | https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/other/EUR25546EN.pdf | 2012 | 4.4.2022 |
| River catchment areas | https://www.eea.europa.eu/data-and-maps/data/european-river-catchments-1 | 28.6.2016 | 26.10.2021 |
| Rivers | https://www.eea.europa.eu/data-and-maps/data/european-catchments-and-rivers-network/rivers/spatialite-file | 2012 | 9.11.2021 |
| Agricultural land use | https://land.copernicus.eu/pan-european/corine-land-cover/clc2018?tab=download | 2018 | 8.11.2021 |
| Population | https://ec.europa.eu/eurostat/de/web/gisco/geodata/reference-data/population-distribution-demography/geostat | 2018 (version from 13.4.2021) | 7.12.2021 |
A.2. European countries
For the data layer with European countries, the data was downloaded from the given URL in Table A.1. The data contained all countries of the world. The attribute table was combined with a self‐made table which assigns the corresponding continent to each European country. This way, all countries in Europe could be selected and saved as a separate layer.
A.3. Regulatory zones
The shape files regarding the regulatory zones were built from the shape files of the European countries, using the given publication as a guide as to which country belongs to which zone. The European Regulatory zones are shown in Figure A.1.
Figure A.1.
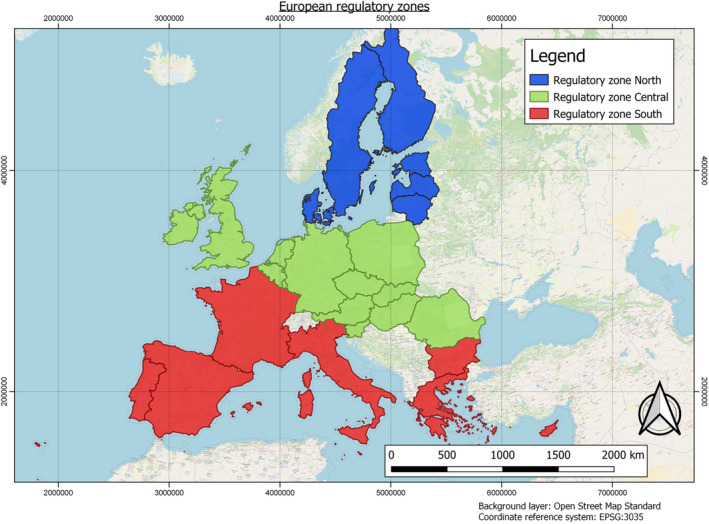
European regulatory zones. The Northern zone is shown in blue, the southern in red and Central Europe is visualised in green
A.4. River catchment areas
The River catchment areas were downloaded from the given URL (see Table A.1). To fix broken geometries, an inverted buffer of 0.01 m was used for further data processing.
A.5. River Strahler order
The data layer contained all European rivers from Strahler Order 1 to 10. To reduce the data load, only rivers with Strahler order greater than or equal to 3 were used. Around these rivers, a buffer of 50 m was placed to identify the area of interest concerning pesticide input. Figures A.2, A.3, A.4–A.4 show the rivers and the catchment areas for the different regulatory zones.
Figure A.2.
Rivers with Strahler Order greater than or equal to 3 in regulatory zone North. In black the river catchment areas are shown
Figure A.3.
Rivers with Strahler Order greater than or equal to 3 in regulatory zone Central. In black the river catchment areas are shown
Figure A.4.
Rivers with Strahler Order greater than or equal to 3 in regulatory zone South. In black the river catchment areas are shown
The GIS analyses showed that the longest river in the regulatory zone North is the River Daugava or Western Dvina with a length of about 370 km through the regulatory zone (total length of the Daugava/Western Dvina: about 1,020 km). This river section has the Strahler Order 7. There is no higher Strahler Order in the northern regulatory zone. The Danube is the longest river in the regulatory zones Central and South with a total length of about 2,850 km and a Strahler Order of 9. Approximately 60% of the Danube flows through the regulatory zone Central; whereas about 20% flows through the regulatory zone South and 20% through Serbia and Croatia, which do not belong to any of the areas investigated. Thus, the zones do differ in the distribution of rivers (only rivers up to SO 7 in North and rivers up to SO 9 in Central and South). The shortest river sections with the lowest considered Strahler Order (SO 3), amount to ~ 100 m in the regulatory zones, therefore this value is used for the consideration of a worst case scenario. In every regulatory zone, the proportion of rivers with Strahler Order 3 is about 50%.
A.6. Land use
To estimate the agricultural land use, Corine Land Cover (CLC) was downloaded from the given URL. The data was then clipped to Europe. The data from Corine Land Cover 2018 were used because they are also used in comparable approaches to determine dilution factors, such as Michel et al. (2022).
In the next step, only the areas with agricultural use were extracted. Table A.2 contains the used Corine Land Cover codes and their descriptions. The total amount of agricultural area in Europe is 24,514,000,000 km2.
Table A.2.
Corine Land Cover agricultural land use
| Corine Land Cover Code | Label 1 | Label 2 | Label 3 |
|---|---|---|---|
| 211 | Agricultural areas | Arable land | Non‐irrigated arable land |
| 212 | Agricultural areas | Arable land | Permanently irrigated land |
| 213 | Agricultural areas | Arable land | Rice fields |
| 221 | Agricultural areas | Permanent crops | Vineyards |
| 222 | Agricultural areas | Permanent crops | Fruit trees and berry plantations |
| 223 | Agricultural areas | Permanent crops | Olive groves |
| 231 | Agricultural areas | Pastures | Pastures |
| 241 | Agricultural areas | Heterogeneous agricultural areas | Annual crops associated with permanent crops |
| 242 | Agricultural areas | Heterogeneous agricultural areas | Complex cultivation patterns |
| 243 | Agricultural areas | Heterogeneous agricultural areas | Land principally occupied by agriculture, with significant areas of natural vegetation |
| 244 | Agricultural areas | Heterogeneous agricultural areas | Agro‐forestry areas |
This layer intersected with the 50 m buffer zones around the rivers. The agricultural area within these buffer zones was calculated (See Table A.3).
Table A.3.
Areas next to rivers including agricultural areas
| Regulatory zone | Area within 50 m adjacent to rivers (km2) | Agricultural area within the buffers (km2) | Percentual area (agriculture/buffer area) (%) | Population 2018 |
|---|---|---|---|---|
| North | 7,346.5 | 2,474.9 | 33.7 | 26,949,500 |
| Central Europe | 13,988.8 | 9,167.3 | 65.5 | 277,063,000 |
| South | 18,149.4 | 10,151.7 | 55.9 | 199,836,000 |
Finally, the sizes of the agricultural areas within the buffer zones and within certain catchment areas were summed by the QGIS Plugin Group Stats based on the catchment area. The area of concern (agricultural use and ≤ 50 m to water bodies) was divided by the size of the catchment area and is presented as a percentage (Figures A.5, A.6, A.7–A.7). The following Figures show the different land use types within each Regulatory Zone (Figures A.5, A.6, A.7, A.8, A.9, A.10, A.11, A.12, A.13, A.14, A.15, A.16, A.17, A.18, A.19, A.20, A.21, A.22, A.23, A.24, A.25, A.26, A.27, A.28–A.28).
Figure A.5.
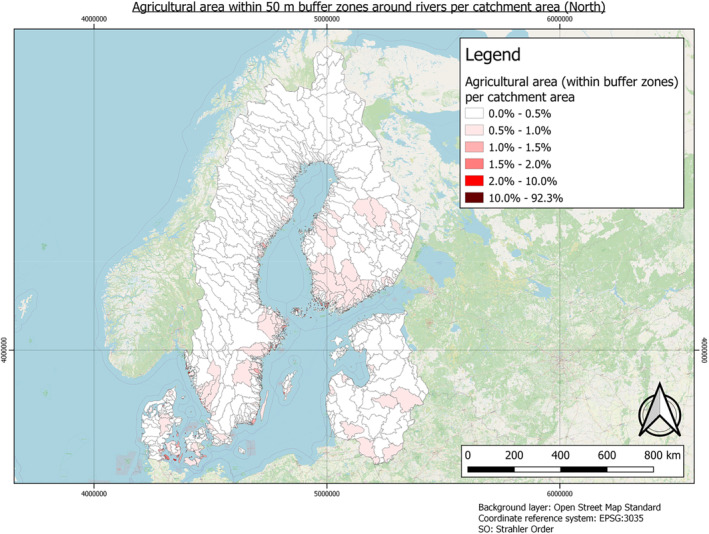
Catchment areas in regulatory zone North with percentage of agricultural area per buffer area adjacent to rivers
Figure A.6.
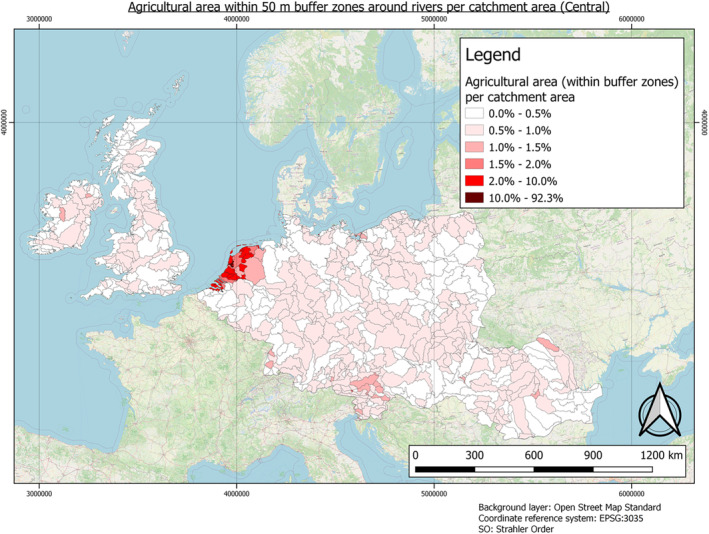
Catchment areas in regulatory zone Central with percentage of agricultural area per buffer area adjacent to rivers
Figure A.7.
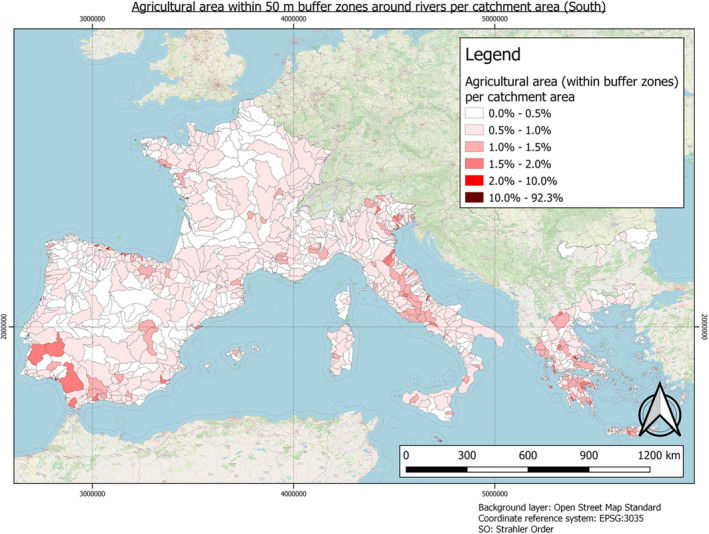
Catchment areas in regulatory zone South with percentage of agricultural area per buffer area adjacent to rivers
Figure A.8.
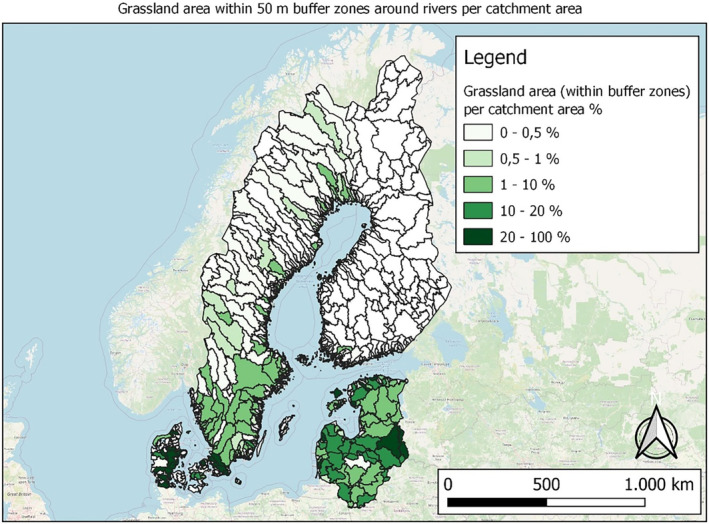
Catchment areas in regulatory zone North with percentage of grassland area per buffer area adjacent to rivers
Figure A.9.
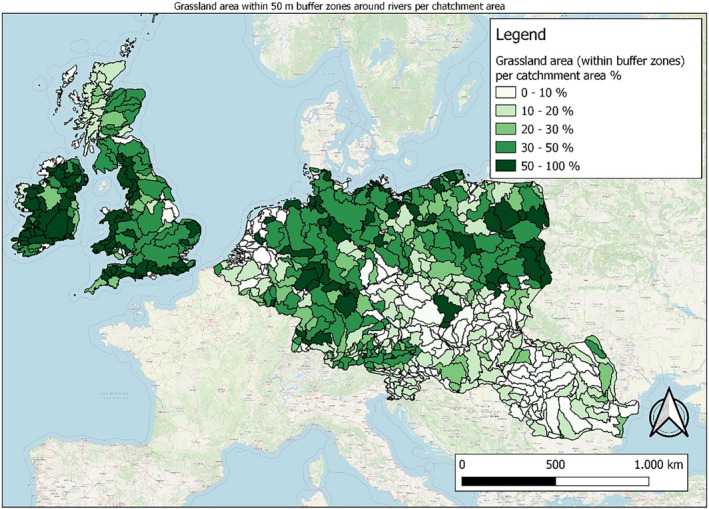
Catchment areas in regulatory zone Central with percentage of grassland area per buffer area adjacent to rivers
Figure A.10.
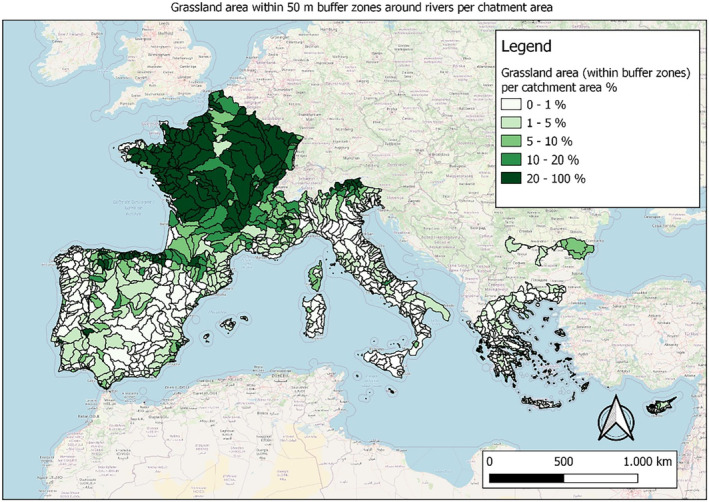
Catchment areas in regulatory zone South with percentage of grassland area per buffer area adjacent to rivers
Figure A.11.
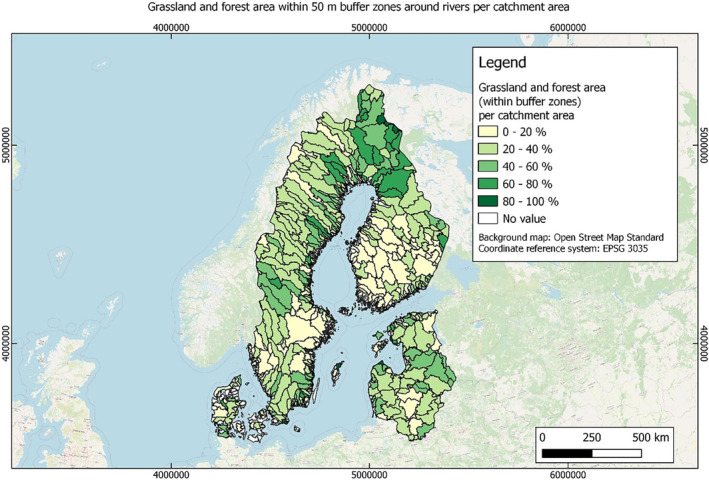
Catchment areas in regulatory zone North with percentage of grassland and forest area per buffer area adjacent to rivers
Figure A.12.
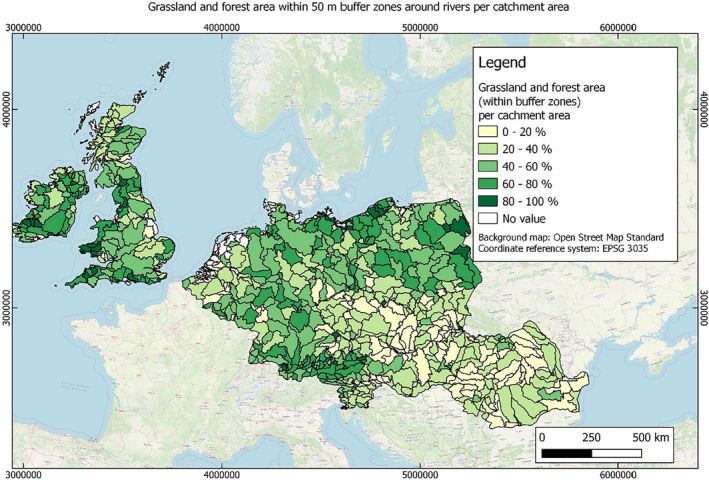
Catchment areas in regulatory zone Central with percentage of grassland and forest area per buffer area adjacent to rivers
Figure A.13.
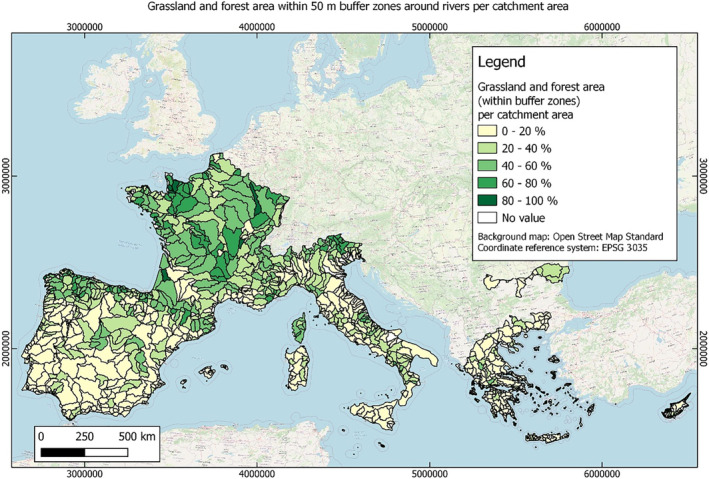
Catchment areas in regulatory zone South with percentage of grassland and forest area per buffer area adjacent to rivers
Figure A.14.
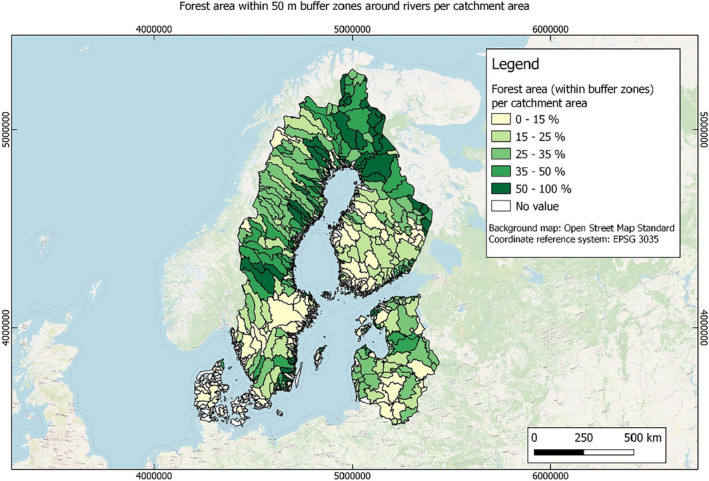
Catchment areas in regulatory zone North with percentage of forest area per buffer area adjacent to rivers
Figure A.15.
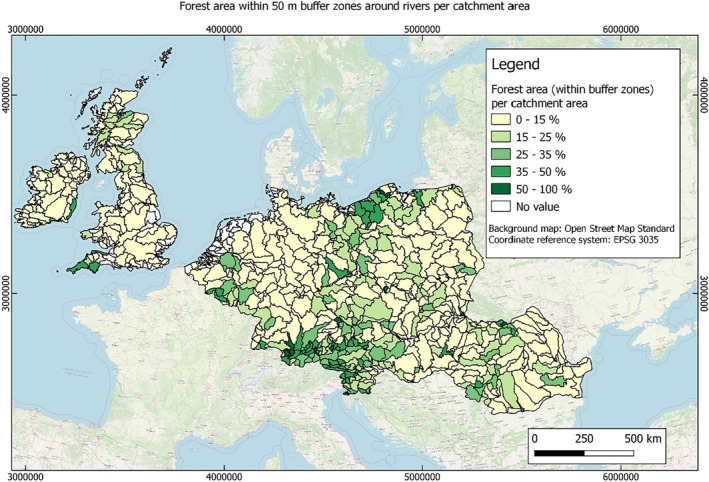
Catchment areas in regulatory zone Central with percentage of forest area per buffer area adjacent to rivers
Figure A.16.
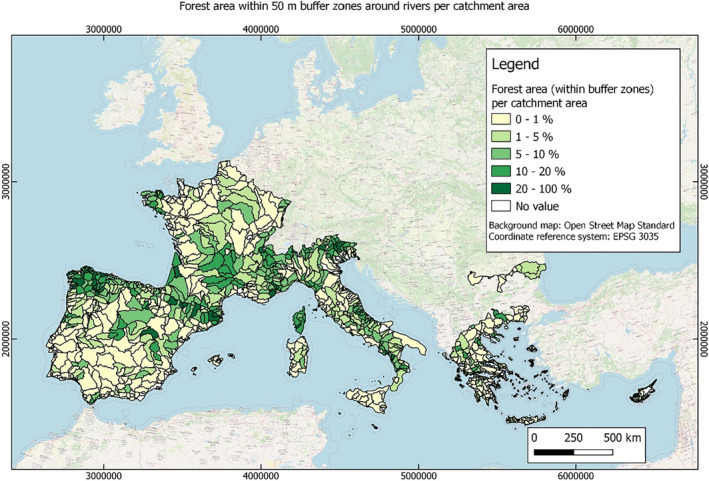
Catchment areas in regulatory zone South with percentage of forest area per buffer area adjacent to rivers
Figure A.17.
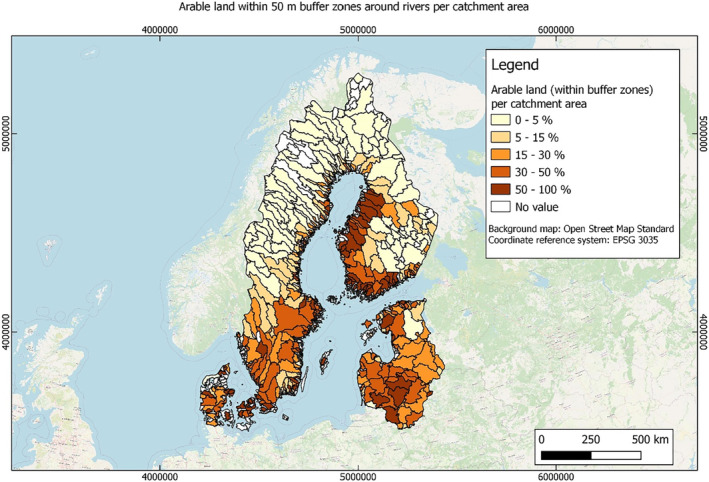
Catchment areas in regulatory zone North with percentage of arable area per buffer area adjacent to rivers
Figure A.18.
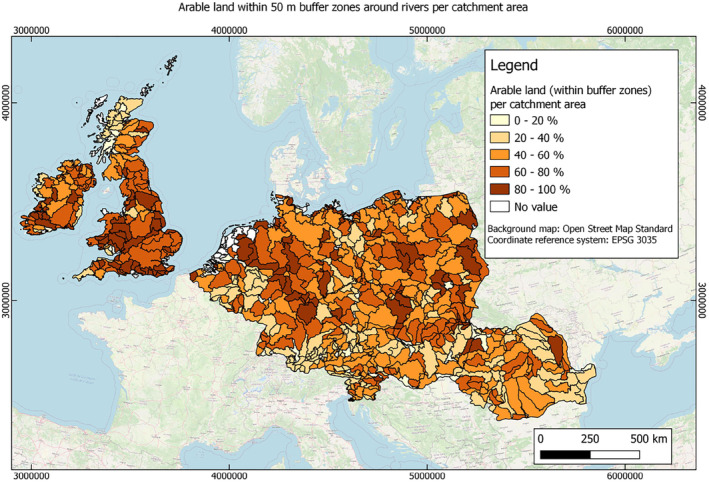
Catchment areas in regulatory zone Central with percentage of arable area per buffer area adjacent to rivers
Figure A.19.
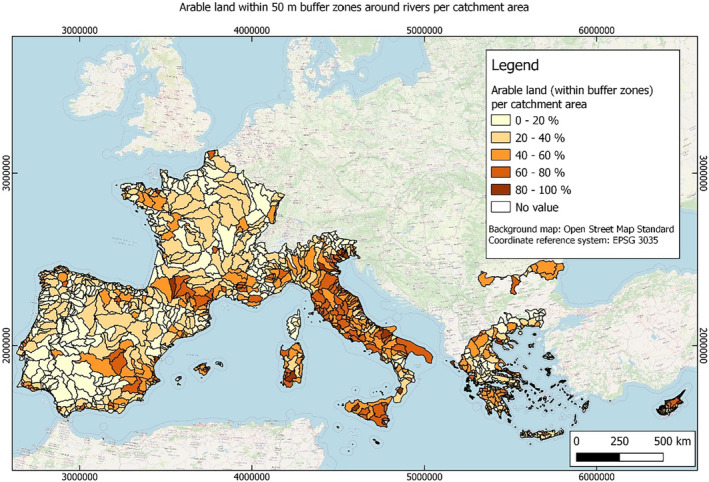
Catchment areas in regulatory zone South with percentage of arable area per buffer area adjacent to rivers
Figure A.20.
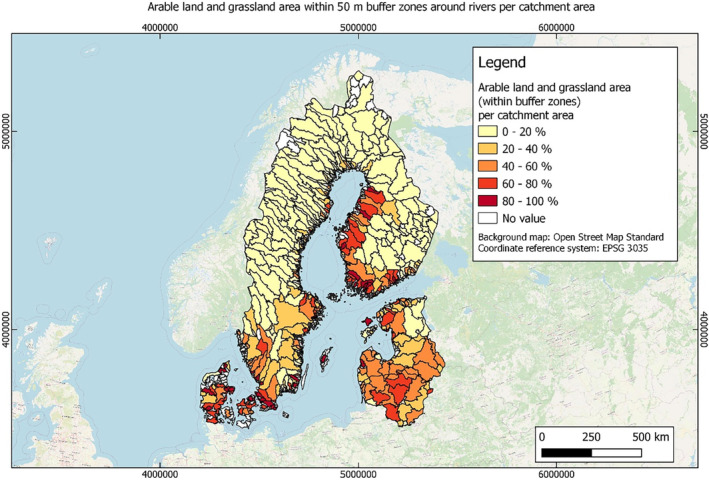
Catchment areas in regulatory zone North with percentage of arable and grassland area per buffer area adjacent to rivers
Figure A.21.
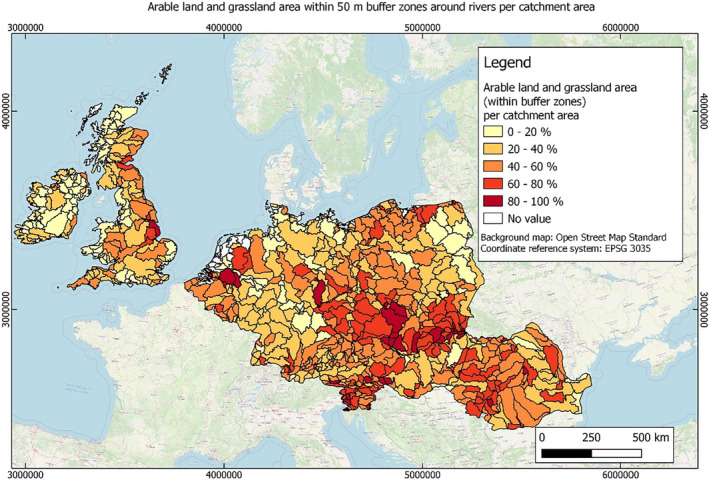
Catchment areas in regulatory zone Central with percentage of arable and grassland area per buffer area adjacent to rivers
Figure A.22.
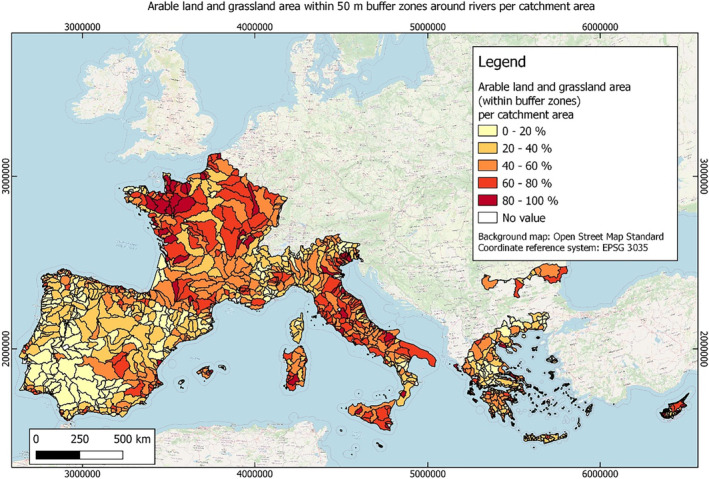
Catchment areas in regulatory zone South with percentage of arable and grassland area per buffer area adjacent to rivers
Figure A.23.
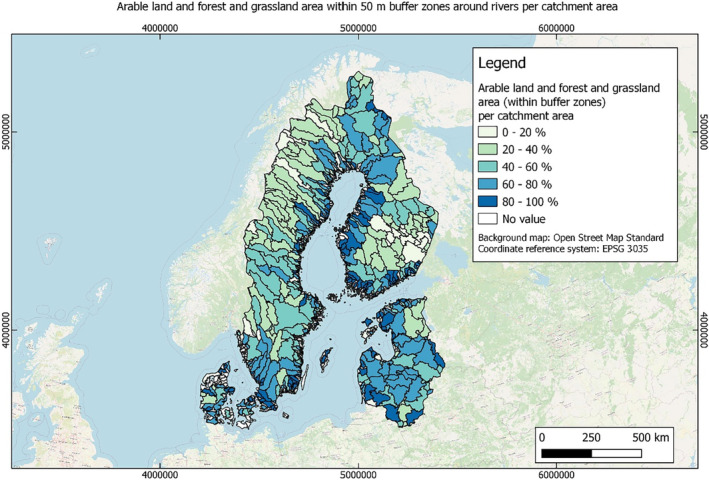
Catchment areas in regulatory zone North with percentage of arable, forest and grassland area per buffer area adjacent to rivers
Figure A.24.
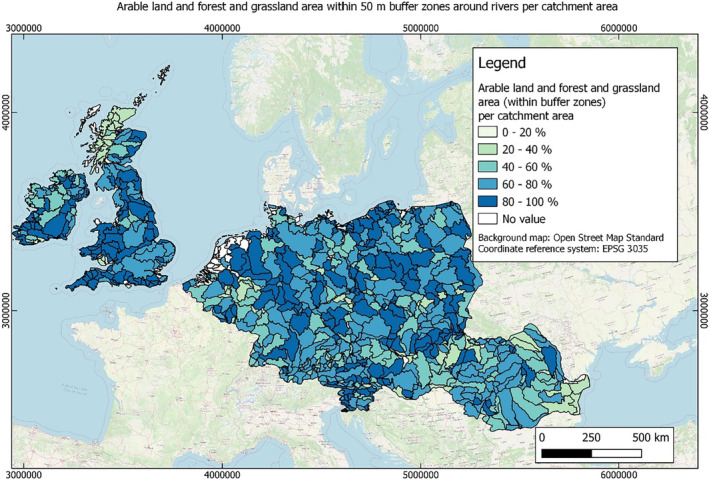
Catchment areas in regulatory zone Central with percentage of arable, forest and grassland area per buffer area adjacent to rivers
Figure A.25.
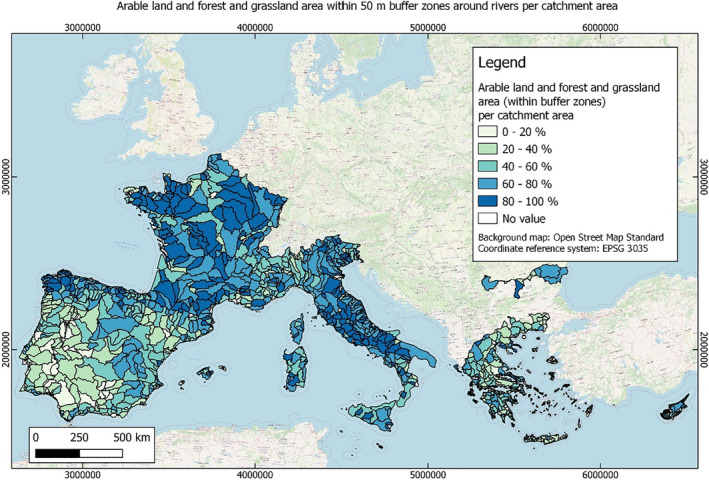
Catchment areas in regulatory zone South with percentage of arable, forest and grassland area per buffer area adjacent to rivers
Figure A.26.
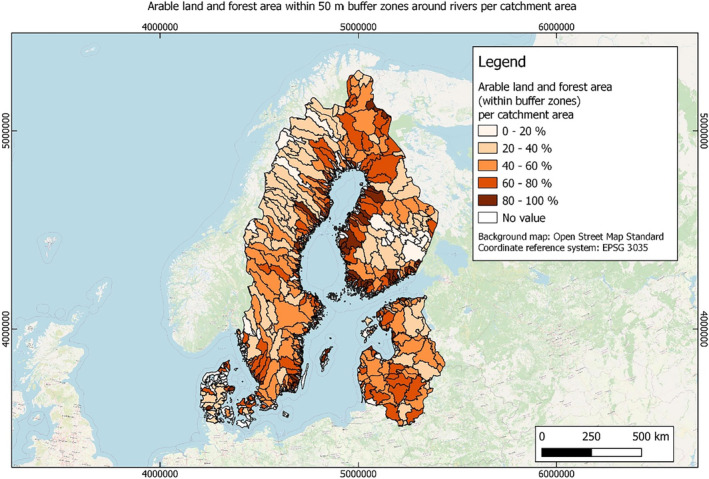
Catchment areas in regulatory zone North with percentage of arable and forest area per buffer area adjacent to rivers
Figure A.27.
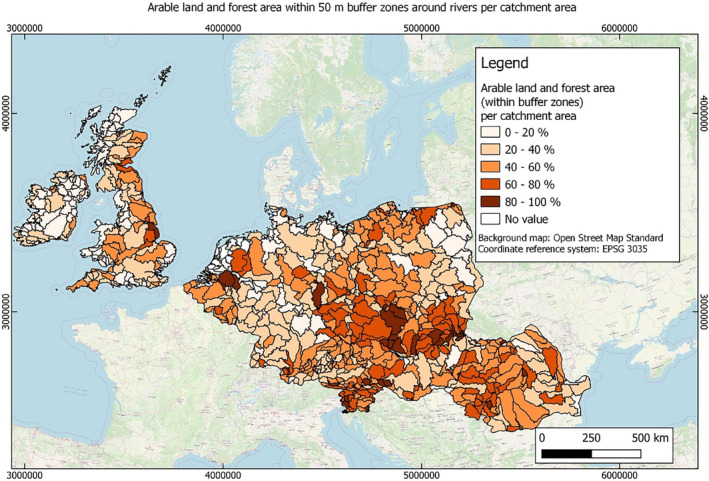
Catchment areas in regulatory zone Central with percentage of arable and forest area per buffer area adjacent to rivers
Figure A.28.
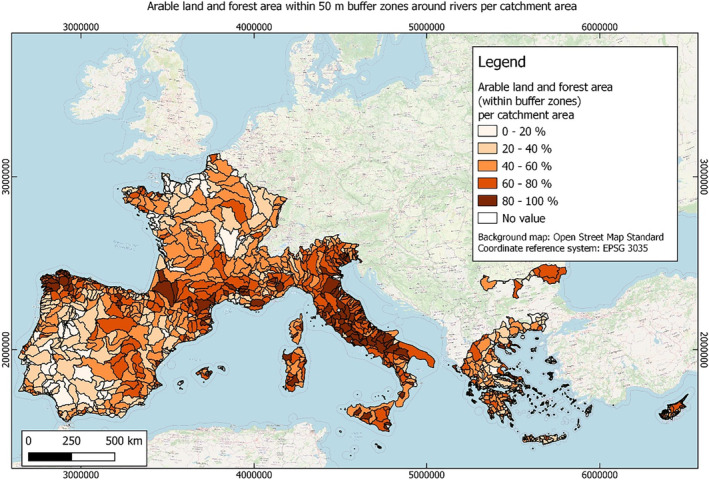
Catchment areas in regulatory zone South with percentage of arable and forest area per buffer area adjacent to rivers
Areas have further been disaggregated to account for the different land use types (Table A.3).
The sizes of the areas with different aspects of interest were calculated for each regulatory zone using the statistics in QGIS and are shown in Table A.4.
Table A.4.
Areas next to rivers including different land use types
| Land use type | Regulatory zone | Area within 50 m around rivers (km2) | Arable land, grassland and forest area within the buffers (km2) | Percentual area (forest/buffer area) |
|---|---|---|---|---|
| Arable land and tree crops | North | 7,346.49 | 1,165.53 | 15.87 |
| Central | 13,998.80 | 7,714.33 | 55.11 | |
| South | 18,149.40 | 5,971.97 | 32.90 | |
| Grassland | North | 7,346.49 | 200.87 | 2.73 |
| Central | 13,998.80 | 3,827.72 | 27.34 | |
| South | 18,149.40 | 1,630.39 | 8.98 | |
| Forest | North | 7,346.49 | 2,204.43 | 30.01 |
| Central | 13,998.80 | 1,937,65 | 13.84 | |
| South | 18,149.40 | 3,540.98 | 19.51 | |
| Grassland and forest | North | 7,346.49 | 2,405.30 | 32.74 |
| Central | 13,998.80 | 5,765.37 | 41.18 | |
| South | 18,149.40 | 5,171.36 | 28.49 | |
| Arable land, tree crops, grassland and forest | North | 7,346.49 | 3,570.83 | 48.61 |
| Central | 13,998.80 | 13,479.70 | 96.29 | |
| South | 18,149.40 | 11,143.33 | 61.40 | |
| Arable land, tree crops and grassland | North | 7,346.49 | 1,366.40 | 18.60 |
| Central | 13,998.80 | 11,542.05 | 82.45 | |
| South | 18,149.40 | 7,602.36 | 41.89 | |
| Arable land and forest | North | 7,346.49 | 3,369.96 | 45.87 |
| Central | 13,998.80 | 9,651.98 | 68.95 | |
| South | 18,149.40 | 9,512.94 | 52.41 |
A.7. Dilution factors in literature
Widely applicable information on dilution factors is scarce, however, an attempt was made by Keller et al. (2014) to calculate dilution factors for domestic wastewater in rivers. The predicted national annual median dilution factor varies across the globe by up to 4 orders of magnitude and within a country during a year by a difference up to 3 orders of magnitude. The maximum monthly difference can vary up to 9 orders of magnitude.
Ort and Siegrist (2009) reported a dilution factor of 1.1 for Switzerland and Baker & Kaprzyk‐Hordem (2013) reported a dilution factor of 15.2 for the United Kingdom. However, since both of latter countries are not part of the Central European regulatory zone, these factors are not considered.
Link et al. (2017) evaluated the fixed dilution factor of 10 used in chemical risk assessment in Germany for wastewater treatment plant effluents and stated, that it sometimes overestimates the dilution potential and underestimates PECs, since about 8% of the calculated dilution factors were below 10 under mean flow conditions (with a median dilution factor of 106). 70% of calculated regional dilution factors were below 10 during low flow conditions.
With respect to standing waters, the role of lakes and ponds in fluvial transfer of pesticides at an interlocking scale from field edge waters to river basins and drinking water supplies is largely unexplored. In their study, Imfeld et al. (2021) outlined that in order to both understand and predict the role of ponds in pesticide transfer at the watershed scale, knowledge gaps still exist. Particularly for smaller standing waters, it is reasonable to assume that processes different from dilution are more decisive.
A.8. Derivation of dilution factors
The worst case dilution factors are based on:
-
–
literature based dilution factors within surface waters
-
–
land use type factors
-
–
regulatory zone factors
The literature based dilution factors within surface waters are then modified with an expert knowledge factor which considers (a) the relevant area of the land use type within a 50 m surface water buffer zone of each European Regulatory Zone (from Table A.2), (b) agricultural area (from Tables A.2 and A.3) and (c) the relevance of the land use type regarding PPP use (arable land and tree crops > grassland > forests) (Tables A.4 and A.6).
Table A.6.
Land use type factors
| Land use type | European Regulatory Zone | ||
|---|---|---|---|
| Central | South | North | |
| Arable and tree crops | 0.84 | 0.86 | 0.90 |
| Grassland | 0.90 | 0.95 | 0.97 |
| Forests | 0.98 | 0.99 | 1.0 |
A.5: Literature based dilution factors within surface waters
| European Regulatory Zone | ||
|---|---|---|
| Central | South | North |
| 2 | 5 | 10 |
A.7: Regulatory Zone factor
| European Regulatory Zone | ||
|---|---|---|
| Central | South | North |
| 0.90 | 0.80 | 1.0 |
A.8: Dilution factors (worst case) for different European Regulatory Zones and land use types
| Land use type | European Regulatory Zone | ||
|---|---|---|---|
| Central | South | North | |
| Arable | 2 | 3 | 9 |
| Tree crops | 2 | 3 | 9 |
| Grassland | 2 | 4 | 10 |
| Forests | 2 | 4 | 10 |
The Corine Land Cover classes used for the category arable land and tree crops are 211 (arable land), 221 (vineyards) and 222 (fruit trees and berry plantations). This includes ornamental flowers as well. For Grassland 321 (pastures) was used and for Forests 331 (broad‐leaved forest), 312 (coniferous forest) and 313 (mixed forest).
The land use type factors are based on the assumption that PPP use differs among land use types and considers the cumulated area of the specific land use type within the buffer zone adjacent to surface waters (see Table A.4). Where PPP use is likely, e.g. arable land, the land use type factor is lower. Also, where more of the specific land use type occurs, this Regulatory Zone will feature a lower land use type factor.
The factor is based on setting the category with the lowest impact to 1 (here: forest areas in the Regulatory Zone North) and relating the other categories based on their relative deviation. Hence, the dilution factor remains unchanged in the Regulatory Zone North with forest land use and is reduced where e.g. large arable areas occur, such as Regulatory Zone Central (compare Table A.4).
The Regulatory Zone factors are based on the density of the surface water area in each zone, which were then translated into factors (see Figures A.2, A.3, A.4–A.4). The factor is based on setting the category with the lowest impact on the dilution factor to 1 (here: Regulatory Zone North due to the highest density of surface water) and relating the other categories based on its relative deviation. Hence, the dilution factor remains unchanged in a Regulatory Zone with high surface water density and is reduced where less water for dilution is available.
The initial surface water dilution factors (DFini) are then multiplied with the land use (FLU) and Regulatory Zone (FRZ) factors and rounded to the nearest whole number to calculate the dilution factor (DF):
Appendix B – Literature overview of the formation of TP during drinking water treatment processes
B.1. Water treatment processes
A large variety of treatment processes can be applied to improve water quality. Which processes are applied largely depends on the characteristics of the source water (surface water, ground water, wastewater, etc.), the use of the treated water (drinking water, household water, water for industrial purposes, etc.) and local circumstances. Generally, the goal of these processes is to remove particles, colour, taste, chemical contaminants and pathogens (also see guidelines for drinking‐water quality).
Disinfection is often considered the most important goal of water treatment, for which several processes can be applied. Often a series of processes (‘barriers’) is applied for safety reasons and to reach sufficient removal of contaminants. Disinfection processes most often applied in the EU are chlorination, ozonation, rapid sand filtration and UV‐irradiation. Advanced oxidation processes (AOPs) are also increasingly applied. Their primary goal is the removal of colour, taste and chemical contaminants like organic micropollutants (OMPs, including active substances from PPP and biocides considered in this guidance; present in ng or μg/L), but they also contribute to the disinfection of the water. Examples of AOPs are UV‐chlorine (UV/Cl), UV‐ozone (UV/O3), UV‐hydrogen peroxide (UV/H2O2) and peroxone (O3/H2O2) processes.
In principle, degradation processes can result in full mineralisation of OMPs, resulting in the formation of CO2 and H2O. However, in practice, full mineralisation is generally not aimed for, as it would require disproportionate amounts of chemicals and/or energy. In most cases, partial degradation is considered sufficient, as it presumably leads to smaller and better degradable transformation products (TP), that can be removed in subsequent filtration or biodegradation processes. It may prove difficult to analyse all potential TP applying target analyses, as for that purpose the identity of these TP will have to be known. In recent years non‐target analyses have been developed and applied for the identification of the formation of TP (Brunner et al., 2020). It has been shown that many different TP with broad ranges of characteristics may be formed, depending on the processes applied, the parent OMPs and the water matrix composition involved.
Apart from the incomplete conversion of OMPs, also other organic carbons present in the water matrix, like natural organic matter (NOM), may be involved in conversion processes. This may result in the formation of byproducts, which are compounds originating from reactions of active compounds and matrix compounds. Furthermore, this NOM may react with parent compounds and TP, resulting in the formation of new TP. It is not always possible to distinguish TP and byproducts (Hebert et al., 2018). In many cases, tens of different TP originating from one parent compound have been identified (Detenchuk et al., 2020, Lebedev et al., 2020), but in many cases identification is difficult or limited. Under different conditions (UV radiation, sunlight, chlorination, etc.), different TP may be found (León et al., 2019). Not only TP originating from the parent compounds, but also those that originate from previously formed TP should be taken into account (Kosma et al., 2016). In this Appendix, a general overview of typical reactions and types of TP that can be expected to be formed during the most important and generally applied disinfection processes will be described. The formation of TP does not necessarily have to be problematic. Whether that is the case depends on their characteristics and concentrations (Brunner et al., 2020) and the impact of further treatment of the water, if applicable.
To obtain information on the possible formation of TP during oxidation and other processes, not only literature on drinking water treatment was included, but also on other water treatment contexts such as wastewater treatment when applicable. The degradation processes taking place in wastewater treatment are comparable to the processes that are applied in drinking water treatment, and also involve (advanced) oxidation processes and biodegradation. Only during the past decade it become possible to identify TP formed. Most literature focuses on a specific parent compound and its possible, identified, TPs. In order to obtain more information about general patterns, also information on wastewater processes was included, when considering degradation pathways and the formation of TP. For example: if a compound can be degraded by activated sludge, it is likely that it can also be degraded, by similar micro‐organisms, during sand filtration. This information is included as background information for this document. It should be noted that most plant protection products and biocidal product dossiers already contain information on the biodegradation that occurs in environmental matrices consequent to the microorganisms present in the soil, natural water and water body sediments. However, this information does not relate to the typical circumstances that occur in a water treatment filter bed.
Sand filtration processes are known to contribute to disinfection, as they remove small particles, including microbial contaminants such as bacteria. For filtration over granular activated carbon (GAC), this is less clear. GAC filtration is generally applied downstream the treatment process, and not as a means of disinfection but to remove organic material. However, it is well known that biodegradation plays an important role in GAC‐filtration. There were two reasons to also include GAC filtration in this study:
During GAC filtration biodegradation processes, comparable to the ones taking place in active sludge or sand filtration, may take place. These can result in the formation of TP.
GAC‐filtration is often applied after oxidation processes to remove TP. These TP may also be degraded in their turn, resulting in new types of TP.
Specific disinfection processes are described below.
B.2. Chlorination
Free chlorine species
Chlorination is often applied for disinfection purposes. Chlorination can be realised by adding chlorine to the water, which results in the formation of hypochlorite according to equation ((1)). After injection of (Cl2)g, NaOCl or Ca(ClO)2 in ammonium free water, hypochlorous acid (HOCl) and hypochlorite ion (ClO−) are the two main free chlorine species under typical drinking water treatment conditions (6 < pH < 9). Hypochlorous acid (HOCl) is the predominant free chlorine species until pH 7.5–7.7 (25–10°C) and ClO− at pH > 7.5–7.7.
| (1) |
| (2) |
Instead of Cl2 also calcium hypochlorite (Ca(OCl)2) or sodium hypochlorite (NaOCl) can be added. These compounds are referred to as ‘free chlorine’.
HOCl is the predominant chlorine species at pH ≤ 7.5 and ClO‐ at pH ≥ 7.5. Cl2 can be neglected under typical drinking water treatment conditions.
If the water contains ammonia, the chlorination must be done at a dose higher than the chlorine demand of the water in order to have a destruction of the chloramines and the presence of free chlorine in the water.
In this paragraph the most important types of reactions and TPs are described, that can be encountered when hypochlorite or chloramine are used as disinfectants. An overview of reactions of chlorine is given by Deborde and von Gunten (2008).
Reactions by free chlorine
The main free chlorine species in reactions with organic compounds under drinking water conditions are HOCl, HOCl/H+, Cl2 and Cl2O (Deborde and von Gunten, 2008; Sivey et al., 2010). HOCl and ClO‐ react with organic compounds by addition, substitution or oxidation. When hypochlorite is applied, both chlorinated and non‐chlorinated TPs will be formed (Kennedy Neth et al., 2017, Li et al., 2017, Yin et al., 2017, Han et al., 2018, Yang et al., 2018, Detenchuk et al., 2020, Pan et al., 2021). An important reason for the formation of chlorinated TP is that chlorine will substitute hydrogen atoms (Du et al., 2018). Examples are the reaction of metformin with sodium hypochlorite, resulting in a cyclic dehyro‐1,2,4‐triazole derivative of metformine and a chloro organic nitrile (R‐C ≡ N) (Armbruster et al., 2015), chlorine substitution, oxidation and joint oxidation with chlorine substitution (Ma et al., 2017) and the formation of chlorinated derivatives of aromatic compounds (Chen et al., 2018, 2020). During the chlorination process apart from Cl and OH radicals (Cl· and HO·) also methyl radicals (·CH3), formaldehyde (H2C=O or its di‐radical precursor ·CH2O·) and chloromethane (ClCH3),open‐ring and ring‐scission products may be involved (Ridgway et al., 2018). Ring opening, decarboxylation, hydrolysis and chlorine substitution reactions, dehydration, deamination and demethylation often are observed in reactions with free chlorine (Negreira et al., 2015, Nika et al., 2017, Gan et al., 2019, Zhang et al., 2020a,b). Sieira et al. (2020) and Tawk et al. (2015) also describe cyclisation reactions and chloroform formation. Furthermore, trichloro acetic acid, dichloro acetic acid and chloral hydrate may be formed (Zhang et al., 2016). Especially with high contents of inorganic compounds, reactive species like chlorine or sulfate radicals may be produced, which may form TP. Li et al. (2015, 2016) describe electrophilic substitution, methoxyl substitution, oxidation of ketone groups, hydrolysis, decarboxylation and ring cleavage reactions and Sun et al. (2019a,b) report C‐C coupling reactions. The TP formed may be toxic (Garcia‐Costa et al., 2021), endocrine disruptive (Jakopin, 2021), genotoxic (Badea et al., 2020) and persistent (Chen et al., 2018). Chlorination TP of antibiotics may still show antibacterial activity (Kennedy Neth et al., 2019).
The interaction of chlorine with nitrogenous constituents in water may involve multiple chlorine addition on the heterocyclic ring and e.g. on aliphatic amine groups (Xiang et al., 2019, Zhang et al., 2020a,b). Chlorination of amine containing compounds results in the substitution of chlorine to the amino group, resulting in the formation of N‐chloramines and hydrolysis of C‐N bonds to form carbonyl derivatives (Cao et al., 2016, Fu et al., 2018, Molé et al., 2019). The main TP of cytosine chlorination appear to be aromatic chloro‐compounds rather than aliphatic N‐chloramines. After dechlorination, these compounds may revert to their initial form. Besides, ring cleavage may occur (Xiang et al., 2019). Xu et al. (2018) have described the formation of chlorinated polycyclid aromatic hydrocarbons as TP. Ul'yanovskii et al. (2022) describe the substitution of an alkylamine group in the aromatic ring by chlorine. Furthermore, they describe the oxidation of a thioether group to sulfoxide. Wang and Helbling (2016) and Yang et al. (2019) report the chlorination of sulfonamides, which involves chlorine substitution, S‐C cleavage, S‐N hydrolysis, desulfonation, oxidation/hydroxylation and conjugation reactions. Selective prediction of the types of TP formed from a certain compound appears to be very difficult.
According to Matsushita et al. (2020) organophosphorous insecticides are partly transformed to their respective oxons during chlorination, replacing the P=S bond with a P=O bond.
Chlorination of sulfonamides, results in SO2 elimination, cyclisation and electrophilic substitution reactions (Nassar et al., 2018).
pH is an important parameter in the formation of TP during chlorination processes, with a higher pH resulting in a decrease in the reaction rate (Carpinteiro et al., 2017, Chen et al., 2021), or different reaction pathways (Liu et al., 2016). This may also be related to the reactivity of the OMPs involved, which may differ depending on pH (Dong et al., 2019).
In the presence of bromide ions (Br‐), which exist in natural water, the reaction mechanism may be fundamentally affected, resulting in the formation of hypobromite (pH dependent HOBr and BrO‐, e.g. used for disinfection of sea water pools) and brominated organic compounds (Liu et al., 2020). These tend to be more toxic than chlorinated compounds (Liu et al., 2020). Besides, these authors found that the presence of Br‐ accelerated the reactions, which was confirmed by Yoom et al. (2018) and by Zhang et al. (2020a,b), who observed a similar effect in the presence of I‐. Iodinated compounds can act as iodine sources and react with chlorine in the presence of organic compounds to yield iodinated by products, which are more cytotoxic and genotoxic than chlorinated products (Sengar and Vijayanandan, 2021).
B.3. Chlorine dioxide
Often chlorine dioxide (ClO2) is applied as pre‐oxidation step. According to Miao et al. (2017), ClO2 preferably reacts with compounds containing phenolic and tertiary amino groups, and is significantly affected by the pH. During these reactions N‐nitrosodimethylamine may be formed. Oxidation of oxcarbazepine by ClO2 resulted in the formation of nitrate, although in lower concentrations than oxidation by ozone. On the other hand, mineralisation capability of ClO2 appeared to be higher than for chlorination. Miao et al. (2017) give an overview of the proposed reaction pathways for ClO2. Hydrolyation in an N‐heterocyclic ring was the first step in the oxidation of oxcarbazepine, followed by N‐heterocyclic ring opening and hexahydropyrimidine moiety re‐arrangement. Finally, hydroxylation took place on the benzaldehyde group. Hörsing et al. (2012) showed the transformation products that were formed applying ozonation, Fenton oxidation, UV and ClO2 to citalopram. Five TPs were formed by loss of a methyl group, a ‐NH2CH3‐ and (CH3)2NH‐group, cleavage of H2O molecules and of a NH(CH3)2O group. According to Han et al. (2017), application of ClO2 causes a low‐level formation of trihalomethanes and haloacetic acids. In case of ClO2, often attention is paid to inorganic DBPs, like chlorite and chlorate, but halogenated and non‐halogenated DBPs can be formed, like polar iodinated DBPs and trihalomethanols, like trichloromethanol and bromodichloromethanol.
Phenolic compounds are mainly converted to unsaturated carbonyl structures by ClO2 treatment (Gan et al., 2019). Chlorine substituted benzoquinones and cyclopent‐4‐ene‐1,3‐diones were shown to be important transformation products after a series of ring opening, decarboxylation, hydrolysis and chlorine substitution reactions. It was shown that by application of ClO2 pre‐oxidation the chloroform formation could be reduced, although more chloral hydrate formation occurred. Recently, Ma et al. (2022) published a review on degradation pathways when ClO2 was applied to micropollutants.
B.4. Chloramination
Apart from OCl− and Cl2, also chloramines can be applied, which is becoming more common (Han et al., 2019; Sieira et al., 2021). They are formed by the reaction of chlorine (Cl2) and ammonia (NH3). Typical chloramines are monochloramine (NH2Cl), dichloramine (NHCl2), trichloramine (NHCl3) (of which only NH2Cl is used in drinking water treatment) and organic chloramines (RHNCl). For disinfection purposes, often NH3 is added to the water after the addition of chlorine. The reaction mechanism for chloramines in general is slower than for free chlorine. Monochloramine is the most effective chloramine for disinfection. At pH values above 7, monochloramine is the most abundant chloramine present.
The main advantage of the use of chloramines instead of chlorine is that with chloramine less disinfection byproducts (DBPs) are formed. With chlorine DBPs like trihalomethanes (THMs) and halogenic acetic acids (HAAs) may be formed during reactions with NOM, which may be carcinogenic (Sinha et al., 2021; Mukhopadhyay et al., 2022). Besides, chloramines will stay in the water for a longer period than chlorine. In general the pH of drinking water will be > 7, to prevent corrosion. In this pH range chlorine will be present in the form of hypochlorite (OCl−), which is less effective as disinfectant than hypochlorous acid. Besides, chloramines provide a better taste and smell than chlorine (Bruchet and Duguet, 2004).
Monochloramination is mainly used as a secondary disinfectant after a ‘primary’ disinfection by chlorine or ozone in order to maintain the bacteriological quality in the distribution network. It has a much lower disinfecting power than chlorine and its low reactivity with organic matter allows to maintain a residual concentration of NH2Cl in the whole network and to limit the formation of disinfection by‐products (THM, HAAs, ...). Monochloramination is not allowed in all EU countries.
Monochloramination is practiced by injecting chlorine and ammonia (or NH4Cl) in a molar ratio ammonia/Chlorine slightly higher than 1 (around 1.2) in order to form only monochloramine. (on‐line injection or preformed monochloramine on site). The main disadvantage of monochloramination is the formation of N‐nitrosodimethylamine (NDMA) during reaction with pesticides (Le Roux et al., 2011).
(Han et al., 2018) and (Han et al., 2019) showed that both reactions with free chlorine and with chloramine result in similar TP, although their reaction kinetics may strongly differ (Sengar and Vijayanandan, 2021). Although reactions with chloramine are slower, they may result in the formation of more cytotoxic or genotoxic TP (Sengar and Vijayanandan, 2021). The main transformation pathways consist of halogenations, hydroxylations and dealkylations and ring opening reactions (Sieira et al., 2021). pH appears to play an important role, as the reaction with HO· can be suppressed under acid or basic conditions (Ye et al., 2021). These authors identified dealkylation and dechlorination‐hydroxylation processes. According to Zhao et al. (2021) chloramination may also result in the formation of N‐nitrosodimethylamine (NDMA). In practice sometimes chloramination is preceded by ClO2 treatment, which may affect the types of TP formed, although this strongly depends on the parent compounds involved, in which the presence of nitrogen appeared to be a determining factor (Shao et al., 2020).
TP of ICM (iodinated contrast media) will react to iodo‐trihalomethanes (ITHM) at high monochloramine doses, but a high‐chlorine dose may suppress their formation. This was attributed to the slow oxidation reaction of HOI to form iodate during chloramination with permits HOI to stay longer in the aqueous solution and react with NOM to form iodinated TP. At a high‐chloramine dose, more HOI will be formed which will react with NOM to form byproducts. However, a higher chlorine dose may lead to oxidation of HIO to iodate thus decreasing their formation (Sengar and Vijayanandan, 2021). The presence of bromine during chloramination will also result in the formation of brominated compounds.
B.5. Ozonation
General process description
Ozonation processes are widely applied in drinking water treatment and more and more in wastewater treatment. Ozonation can be practiced in low dose chemical preoxidation, in interozonation in combination with a GAC filter (MON oxidation and micropollutants) or in final disinfection. These processes are applied not only for disinfection purposes but also for the removal of colour, taste or organic micropollutants (OMPs). Ozone doses in general are based on the concentration of dissolved organic carbon (DOC) in the water, a common dose being about 0.2–0.5 g O3/g DOC (Bourgin et al., 2018).
Ozone reactions
Ozone can react either directly on micropollutants through O3 and indirectly through hydroxyl radicals (OH·) released during the decomposition of ozone in water. The direct action of ozone is considered more selective than the radical action. In ozonation processes, tens or even hundreds of TP may be formed (Deeb et al., 2017, Diehle et al., 2019) (Zoumpouli et al., 2021), often by subsequent reactions taking place. Only a limited number of these TP can be identified (Brunner et al., 2019, Park and Snyder, 2020).
During such processes often phenol and naphtol like compounds can be formed (Bilińska et al., 2019). Ozone can only react with electron rich compounds, which contain e.g. double bonds, aromatic ring structures, deprotonated structures, sulfur, phosphorus, nitrogen and oxygen atoms (Garcia‐Costa et al., 2021). However, during the ozonation process in the water phase also hydroxyl radicals may be formed (Shad et al., 2018), which are much more reactive and react by hydrogen abstraction, radical‐radical interaction, electrophilic addition or electron transfer. Some TP will react further during the process (Mpatani et al., 2021) sometimes by reaction with HO· (Ur Rehman et al., 2019). TP consist of e.g. aldehydes, ketones and carboxylic acids (Hu et al., 2017). Often also isomers of e.g. hydroxylated compounds are identified (Kråkström et al., 2020, 2021). Li et al. (2019) report de‐alkylation, oxidation and ring cleavage reactions.
Ozonation can lead to the formation of N‐nitrosodimethylamine (NDMA), which is a compound with negative effects for human health (Andrzejewski et al., 2008).
Identification of possible TP is important for the toxicological characterisation of the water (Buchner et al., 2019). Gulde et al. (2021) even report a list of 1,749 potential TP from ozonation of 70 parent compounds. Some of these compounds may be persistent or have an adverse effect on the environment (El‐taliawy et al., 2018).
Ozone will attack an olefin bond resulting in the formation of a ketone compound (Ferrando‐Climent et al., 2017). Furthermore, aromatic rings and double bonds may be oxidised (Funke et al., 2021), and so may primary amine groups (Hermes et al., 2020). HO∙ may be involved in hydroxylation reactions. Ozone will also react with aliphatic amines (Lim et al., 2019). All amines are transformed to products containing a nitrogen‐oxygen bond. Tertiary amine groups (R3N) will be oxidised to N‐oxides (R3N‐O) via adduct formation at the lone electron pair of the nitrogen (Kharel et al., 2020). N‐oxides are stable compounds, which may be toxic (Kharel et al., 2020).
It is known that ozone attacks an unsubstituted phenol, resulting in the formation of several TP. For substituted phenol rings a different result may be obtained, depending on the place of substitution. Para substitution of the phenol ring only in some cases prevented the formation of potentially harmful products (Tentscher et al., 2018).
If the parent compounds contain Cl, the structural positioning of this chlorine atom strongly influences its breakdown during ozonation, and thus TP formation (Bourgin et al., 2018).
Andrzejewski et al. (2008) noticed that ozonation of dimethylamine will result in the formation of N‐nitrosodimethylamine (NDMA), the reaction yield increasing with increasing pH values.
Hydroxyl radicals play a very important role during ozonation processes, but their exact contribution strongly depends on pH (Lege et al., 2019). Thus the effectiveness of ozonation, the type and number of TP that can be formed are pH dependent (Hu et al., 2017). Furthermore, it depends on the reactivity of the compound toward reaction with ozone and hydroxyl radicals. If reaction rate constants are known, it can be estimated whether reaction with ozone may easily occur. Cruz‐Alcalde et al. (2017) describe amine α carbon oxidation in combination with hydrolysis taking place. Often direct reaction with O3 (e.g. by addition to a double bond) appears to contribute less to the TP formation than reactions with hydroxyl radicals (Cruz‐Alcalde et al., 2018). The pH of the solution will influence not only the effectiveness and kinetics of ozonation but also the type and number of TP that can be formed (Hu et al., 2017). These authors report that the ozonation reaction rate constant may be 500 times higher at pH 7 than at pH 4. However, according to Daoud et al. (2020) ozonation reactions can be very effective under acidic conditions, whereas neutral or basic environments seemed to be less suitable. This will depend on the OMPs involved.
During ozonation oxidation of amide groups may occur (Funke et al., 2021). Ozonation of phosphorous containing compounds results in the formation of other organic phosphorous compounds and inorganic phosphorous (Xu et al., 2019).
Wirzberger et al. (2021) and Popov et al. (2021) point out that compounds from the water phase may have a substantial influence and lead to unpredictable yields of TP, e.g. by reactions with NOM.
In most cases ozonation is followed by a filtration process, like filtration over granular activated carbon (GAC) or sand filtration. It was found that in general a large part of the TP formed during ozonation can be removed during these filtration processes, either by adsorption and/or by biodegradation (Schollée et al., 2018, 2021, Seiwert et al., 2021). Often, ozonation TP are better biodegradable than their parent compounds. However, hydroxylamines and N‐oxides may be persistent during biological post‐treatment (Seiwert et al., 2021).
B.6. UV disinfection
General process information
UV irradiation often is applied for disinfection purposes. UV‐C radiation has a wavelength of 100–280 nm and is known for the fact that it damages DNA, by initiating a reaction between two molecules of thymine (dimer formation). It is very efficient to inactivate both bacteria and viruses, depending on the UV‐transmittance of the water. UV‐disinfection in general is realised by applying a medium pressure (MP) UV lamp, emitting wavelengths between 200 and 300 nm, or by a low pressure (LP) UV lamp, emitting radiation with a wavelength of 253.7 nm. To prevent byproduct formation at lower wavelengths, MP lamps are equipped with quartz sleeves, cutting off wavelengths lower than 240 nm. In general, a UV dose of about 20–70 mJ/cm2 will be applied for disinfection purposes. These doses are relatively low, and as a result photolysis reactions of OMPs under these circumstances in general are not very effective. UV radiation, however, also is applied in combination with e.g. O3, H2O2 or Cl, resulting in the formation of hydroxyl radicals HO·. Such processes are very effective for the removal of taste, odour and several OMPs. For AOPs UV doses of about 500–600 mJ/cm2 are applied, and under these circumstances, photolysis may significantly contribute to the degradation of compounds. These processes, however, in general are not applied to obtain disinfection.
Formation of transformation products
Some compounds will undergo fast photolysis by UV radiation (Godayol et al., 2015), and sometimes by higher wavelength radiation (Ge et al., 2015). A lot of research has been done into the role of UV radiation in UV‐based advanced oxidation processes, which will be discussed later on. However, in these processes UV doses which are about 10–15 times higher than what is commonly applied in disinfection processes are applied. As a result, during UV disinfection less photolysis will be observed, although it cannot be ruled out. Especially aromatic rings may react under the influence of UV irradiation (Ferrando‐Climent et al., 2017).
Voigt et al. (2021) showed that a high UV doses, possibly in combination with H2O2 or O3, all kinds of TP can be formed from aromatic compounds, e.g. by photolysis.
B.7. Sand and GAC filtration
General process information
Sand filtration (over quartz sand) and filtration over granular activated carbon (GAC) often are applied during drinking water treatment, e.g. for the removal of TP from oxidation processes (Völker et al., 2019). Sand filtration in surface water treatment in general is combined with coagulation/flocculation/sedimentation for the removal of particles. The formed flocculates are then removed by sand filtration. Sand filtration also contributes to lowering the concentration of pathogens in water, as small particles, including many micro‐organisms, may be removed during filtration (Andreoli and Sabogal‐Paz, 2020). Sand filtration also is applied in wastewater treatment, e.g. for the removal of phosphate, but also after ozonation for the removal of OMPs in the effluent. In this case often not pure quartz sand is used.
A typical sand bed in drinking water treatment has a height of 1.5–2.5 m, and is operated with a downward filtration velocity of 3–8 m/h (De Moel et al., 2006).
For the removal of colour, taste and OMPs filtration over GAC is more and more applied in drinking water treatment. Here too, biodegradation processes play an important role.
Formation of TP
In recent years it has become clear that during sand and GAC filtration biodegradation may occur, but this has not yet been investigated to a large extent (Magdeburg et al., 2014, Wang et al., 2019, Nika et al., 2021, Schollée et al., 2021). Also in wastewater treatment biodegradation plays an important role (Nürenberg et al., 2019) by the action of micro‐organisms present in active sludge. Biodegradation by activated sludge is an important treatment step, but not always efficient for the removal of OMPs. Parent compounds often are relatively large molecules that are quite persistent against biodegradation. However, after e.g. oxidation the TP in general are smaller and more hydrophilic and often contain e.g. carboxylate groups, as a result of which these TP are more sensitive to biodegradation (Seiwert et al., 2021). It has been noticed that some TP from e.g. ozonation processes may be removed during sand filtration, probably because of biodegradation, as was shown by Wenk et al. (2020). Often also dual media filtration (sand being one of the media and e.g. anthracite being the other) is applied. However, biodegradation itself may also result in the formation of TP (Gulde et al., 2021). Some of the oxidation products found after ozonation also may be found after biodegradation in a sand filter, e.g. in case of carbamazepine degradation (Kharel et al., 2020).
Di Marcantonio et al. (2020) point out the fact that OMP concentrations in sources for drinking water or wastewater are often in the ng‐L to μg/L range, which is too low for most bacteria to use them as a sole carbon source for growth. Therefore, it is likely that (part of) these OMPs are co‐metabolically degraded, which may also result in the formation of TP. Co‐metabolic degradation implies that the micro‐organisms use not only the OMPs but also require other compounds as a source of carbon. As a result, they can also survive if certain OMPs are temporarily not present in the water. In contrast to co‐metabolic conversion by autotrophic bacteria, heterotrophic bacteria can use organic compounds, including OMPs as primary substrates for cell growth (not requiring additional organic compounds. Heterotrophic bacteria can metabolically transform OMPs and their TP (Wang et al., 2021a,b). However, also in this case, because of the low OMPs concentrations, other carbon sources often will be required. This means that the conversion can be improved by the addition of assimilable organic carbon (AOC). It has been observed that heterotrophic organisms metabolising certain AOC substrates can produce a broad range of enzymes that may be involved in the degradation of similar OMPs. Nitrifying and methanotrophic bacteria may oxidise ammonium and methane, respectively and heterotrophic microorganisms may degrade dissolved and particular organic carbon. The removal process in a sand filter is a combination of adsorption onto sand material, oxidation by iron/manganese oxides, biodegradation by autotrophic bacteria and biodegradation by heterotrophic bacteria.
Nika et al. (2021) e.g. showed that N‐desmethyl derivatives and amides were formed during biodegradation. Lege et al. (2019) show that biodegradation processes may result in the formation of N‐dealkylated products. Aerobic microbial degradation may proceed via cleavage of an alkyl chain. Redox conditions also strongly affect the processes taking place: e.g. nitrate reducing conditions may induce a nucleophilic nitrite attack on a molecule. Nitrifying and denitrifying conditions, in both activated sludge and sand filters, may affect the biodegradation of some compounds. Nitrification includes two biological processes, in which ammonia oxidising bacteria and nitrite oxidising bacteria are involved. The ammonia mono‐oxygenase enzyme has a broad substrate specificity, and thus not only catalyses the oxidation of ammonium but also of compounds like alkanes, alkenes, aromatic and alicyclic hydrocarbons, halogenated carbons and sulfonated hydrocarbons. It also has been shown that in this way nitrifying bacteria may be capable of co‐metabolising various OMPs (Wang et al., 2021a,b). However, for some compounds, it has been shown that at least in some cases the nitrification process itself is not involved in biodegradation processes (Castronovo et al., 2017), and that an important byproduct of the biodegradation is sulfamic acid. Biodegradation reactions occurring are e.g. N‐dealkylation (dealkylation of a quaternary ammonium moiety), hydroxylation, amide hydrolysis and N‐dearylation (cleavage of the bond between nitrogen in the amide moiety and the aryl group). According to Mairinger et al. (2021) typical TP of synthetic water‐soluble polymers in wastewater after ozonation followed by sand filtration are sugar derivatives with differing side chains and sulfonic acids. They identified the effect of biodegradation on homologous series of TP, which showed a shift in distribution toward lower mass ranges. Sulfur containing polymeric compounds were potentially transformed via (de)hydration, hydroxylation, decarboxylation and de(hydrogenation) processes.
Biodegradation by methanotrophic bacteria can also be involved in the oxidation of OMPs (Wang et al., 2021a,b). These authors state that the contribution of diverse microbial metabolisms on different OMPs should be further investigated.
It was shown that methanotrophic bacteria are involved in hydroxylation reactions, resulting in the formation of hydroxylated TP. The biodegradation rate and efficiency, however, strongly depend on the OMP composition, and even within the same class of compounds (e.g. sulfonamides) large differences may occur (Wang et al., 2021a,b).
Iodinated contrast media (ICM) may act as an iodine source. Their biotransformation products seem to be highly stable, and it cannot be excluded that they are more toxic than their parent compounds (Sengar and Vijayanandan, 2021). Mechanisms of biodegradation have also been described by Ortiz‐Hernández et al. (2013).
B.8. Advanced oxidation processes
General process information
Advanced oxidation processes are characterised by the formation of very reactive hydroxyl radicals (Gmurek et al., 2019). These dominate the hydroxylation of aromatic rings and cleavage of C‐C bonds in OMPs (Gao et al., 2022). There are AOPs based on UV radiation and non‐photochemical AOPs (Sharma et al., 2018), resulting in different types of TP. Degradation pathways may involve C‐N and C‐H bond cleavage, hydroxylation and the reduction of nitro groups (Elias et al., 2019). Some AOPs, often applied in drinking water treatment, will be discussed here.
O3/H2O2 process
The O3/H2O2 process (or peroxone process) can degrade OMPs by either direct reaction with ozone (see previous paragraph) or by reaction with formed HO∙. The latter process will result in the formation of hydrox‐, keto‐, dechloroethyl‐ and imino‐derivatives of e.g. cyclophosphamide and ifosfamide (Blaney et al., 2019). One of the main reaction pathways is the addition of HO to a C‐C bond. The hydroxyl group then can be oxidised to a carbonyl and carbonoyl oxide group, and ring opening or intramolecular reactions c.q. rearrangement may take place. Furthermore, hydrolysis and hydration may occur (Chow and Leung, 2019).
O3/sunlight and UV/O3 process
The O3/sunlight process was studied by Rivas et al. (2019), Solís et al. (2019). They identified amine oxidation, alkyl chain attack, hydroxylation processes, H abstraction from alcoholic moieties. If present, hydroxyl radicals will attack a sulfonyl‐urea bond in a molecule in such a process (Solís et al., 2019).
Wang et al. (2021a,b) studied the UV/O3 process, in which two types of radicals are formed: HO· and HO2·. Organic acids, aldehydes and ketones were formed. Intermediate groups involved nitro and carbonyl groups.
UV/Cl process
The UV/Cl process, or rather UV/NaOCl process, is a rather new type of AOP, that has gained much attention recently (Trebše et al., 2016, Yang et al., 2021, Zhang et al., 2021). Often a broad range of TP is formed (Pan et al., 2017, Dao et al., 2018). During this process chlorine radicals (Cl·) are formed, that will react with organic compounds by e.g. chlorine substitution, resulting in the formation of chlorinated organic compounds, that often are very harmful and sometimes also persistent (Badea et al., 2020, Chaves et al., 2020). The combined action of active chlorine and UV irradiation results in the formation of e.g. chloro anhydrides and chlorophenols (Trebše et al., 2016). The reactions proceed primarily via chlorine addition and hydrogen abstraction, with the possibility of electron transfer. Mono‐, di and trichloro substituted compounds can be formed. Furthermore, HO·, ClO· and Cl2 −· reactive species are formed, that promote the formation of hydroxy, hydroperoxyl and dealkylated derivatives (Antonopoulou et al., 2020). Double bonds and aromatic rings are most susceptible to attack by HO· and Cl·. According to Osawa et al. (2019a,b) and (Osawa et al., 2019a,b) different TP from UV photolysis and chlorine reactions can be observed. Lee et al. (2020) showed that the contribution of HO· depends on pH values, and may increase at acidic pH values, whereas the contribution of reactive chlorine species may increase at neutral and basic pH values. Hydroxylation, chlorine addition, oxidation, dealkylation and isomerisation can occur. Intermediate products are hydroxyl and hydroperoxyl compounds. ClO· oxidises compounds mainly through electron transfer. With aromatic compounds bearing side alkyl groups hydroxyl products will be formed, and OH insertion both on the aromatic ring and on alkyl groups may occur. Mono‐hydroxylated and mono‐chlorinated products may undergo hydrogen abstraction, resulting in the formation of unstable structures which eventually are turned into compounds with carbonyl functional groups. Furthermore, alcohols or carbonyl compounds may be formed (Cai et al., 2019, 2020, Antonopoulou et al., 2020). Huang et al. (2017) showed that also the benzyl‐nitrogen bond can be cleaved by the UV/Cl process. According to Ra et al. (2019) the UV/Cl treatment of amine moieties results in the formation of mainly TP with primary amines, but also secondary and tertiary amines can be observed. These compounds may be further transformed via the formation and photolysis of new N‐Cl bonds. According to Studziński et al. (2021) chlorinated compounds, in general, are lipophilic, can be bioconcentrated in organic matter, are characterised by significant environmental persistence, and can be spread over considerable distances and are toxic, whereas oxidation products have a significantly smaller impact on the environment. As mentioned above for chlorination processes, also in UV/Cl processes degradation of iodine containing compounds may result in the formation of iodinated TP, although Cl2· and ClO· favoured the generation of chlorine containing products (Zhang et al., 2021). Yan et al. (2020) and Yin et al. (2018) successfully tried to model the structure of TP formed by hydroxylation or chlorine substitution by applying the Frontier Orbital Theory.
Whereas until now mostly UV‐C radiation (100–280 nm) was applied in UV/Cl processes, the rapid development of UV‐LEDs may make these diodes also applicable in AOPs (Kim et al., 2020). This will result in more energy efficient processes, with possibly slightly different photolysis reactions.
Apart from UV‐C radiation, also UV‐B radiation (280–315 nm) may be applied in UV/Cl processes (Lee et al., 2021), which may result in different photolysis products. Sun et al. (2019a,b) describe the application of a solar/chlorine process for the degradation of cyanotoxins. These authors showed that under neutral conditions also ozone was generated, degradation is caused by reactive chlorine, hydroxyl radicals, ozone and solar radiation itself (which has a higher wavelength than UV radiation). Similar conclusions were drawn by Yang et al. (2020), who identified hydroxylation, chlorination, hydrolyzation, N‐demethylation, loss of phenyl group, ring rearrangement and contraction as pathways.
Appendix C – Comparison of OECD transformation and biodegradation tests 307, 308 and 309
1.
C.1: Comparison of OECD transformation and biodegradation tests
| OECD test 307 | OECD test 308 | OECD test 309 | |
|---|---|---|---|
| Matrix | Soil (ay least three types of different soil) | Aquatic sediments and associated waters (at least 2 sediments; different organic carbon content and texture). Fresh and marine if necessary. Not suitable to simulate flowing water | Surface water without particles or sediment and optional additional test with surface sediment (aerobic layers) |
| Concentration | Maximum dosage on the label of compound that is directly applied to the field should be taken | Maximum dosage on the label of compound that is directly applied to the field should be taken | < 1–100 ug/L |
| Test method | Biometer‐type flasks or in flow‐through systems | Incubation apparatus (gas flow‐through and biometer‐type systems) in glass containers or Teflon (batch bottles or centrifuge tubes), depending on Kow). | Batch (with stopper or with cotton) |
| Test time | Max 120 days (if needed, 6–12 months for transformation product monitoring) | Max 100 days, until the degradation pathway and water/sediment distribution pattern are established or when 90% of the test substance has been removed by transformation and/or volatilisation | 2 weeks up to 60 days (batch mode) or several months (depending on lag time) in semi‐continuous mode |
| Compounds to measure | Properties and conditions of soil at start of experiment. Parent compound and TP | Nutrients, properties and conditions of sediment and associated water at start of experiment. Parent compound and TP during experiment (6 points, one concentration). | Nutrients and conditions of surface water at start of experiment. Parent compound and TP if T1/2 > 60 days during experiment (5 points and at least 2 concentrations) |
| Metabolism/Redox | Aerobic/Anaerobic | Aerobic/Anaerobic | Redox should be measured and should be aerobic |
| Test conditions | Dark | Dark | Dark, shaking |
| Test temperature | Controlled temperature (field temp or standard temp of 20°C). | Controlled temperature (10–30°C or standard temp of 20°C) | Controlled temperature (field temp or standard temp of 20–25°C). |
Appendix D – Additional experimental procedures of water treatment processes
1.
Experimental procedures for other treatment processes, that were not prioritised for this guidance but may however be encountered in drinking water treatment, are described here. All experiments are carried out at room temperature.
D.1. Photolysis
Materials
Collimated beam device.
Experiments are carried out in a continuously stirred volume of 150 mL, with a water height of at least 1.6 cm (volume 100–150 mL, diameter of petri dish 8.9 cm). The exact parameters depend on the circumstances, and can be calculated from the spreadsheet of J. Bolton (Bolton and Cater, 1994; Bolton and Stefan, 2002; Bolton and Linden, 2003; Bolton, 2010).
Experimental procedure
Determine the UV spectrum of the test compound between 200 and 350 nm using the initial test concentration of the compound, so its molar extinction coefficient is known.
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the limit of quantification.
Measure the UV‐transmission of the solution at 254 nm.
Apply a UV dose of 600 mJ/cm2, using a medium pressure (MP) UV lamp. The irradiation time required can be calculated using the spreadsheet of J. Bolton. For disinfection also low pressure (LP) lamps can be applied, but MP lamps have a higher risk for TP formation.
Analyse the mother compound and if possible TPs
D.2. UV/H2O2
Materials
Collimated beam device.
Experiments are carried out in a continuously stirred volume of 150 mL, with a water height of at least 1.6 cm (volume 100–150 mL).
Experimental procedure
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the limit of quantification.
Add H2O2 to a concentration of 10 mg/L.
Measure the UV‐transmission of the solution.
Apply an MP UV lamp and UV dose of 600 mJ/cm2. This can be calculated using the spreadsheet of J. Bolton. In principle both LP and MP UV lamps may be used, but the risk of TP formation is higher with MP lamps.
Analyse the parent compound and if possible TPs.
D.3. O3/H2O2
Materials
Adequate safety measures should be taken to prevent people from getting into contact with ozone. Experiments should be carried out in a fume cupboard.
One beaker (1 L) that can be closed
Magnetic stirrer
O3 and H2O2
Experimental procedure
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the limit of quantification.
Add H2O2 to a concentration of about 1 mg/L.
Add O3 to a concentration of about 3.5 mg/L. The mass ratio H2O2: O3 should be about 0.35. Further see the description of ozonation experiments.
D.4. UV/O3
Materials
Collimated beam device.
Experiments are carried out in a continuously stirred volume of 100 mL, with a water height of 1.6 cm (volume 100 mL).
O3 dosing.
Experimental procedure
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the limit of quantificatio. O3 can be dissolved by means of a bubble column (see the description of ozonation processes), either directly from O3 or from O3 that is prepared from air or oxygen. It is also possible to add an aqueous solution of ozone, or ozone gas can be dosed via special devices. The ozone concentration is 1 mg/L.
Measure the UV‐transmission of the solution.
Apply a UV dose of 600 mJ/cm2. This can be calculated using the spreadsheet of J. Bolton. In principle both LP and MP UV lamps may be used, but the formation of TP is higher with MP lamps.
Quench the solution with H2SO3 if necessary. Keep in mind that the presence of H2SO3 may interfere with the analyses, so it is preferred not to quench the solution.
Analyse the parent compound and if possible TPs. Besides, bromate concentrations in the solution should be measured after ozonation.
D.5. UV/Cl
Materials
Collimated beam device.
Experiments are carried out in a continuously stirred volume of 100 mL, with a water height of 1.6 cm (volume 100 mL).
10–15% Sodium or potassium hypochlorite solution. Reactions are carried out at concentrations of about 50 mg free chlorine/L. Check the pH of the solution after addition of hypochlorite solution to achieve the free chlorine level.
Experimental procedure
Prepare a solution of the compound under investigation in standard water, preferably at a concentration of 1,000 × the limit of quantification.
Measure the UV‐transmission of the solution.
Add the required amount of hypochlorite.
Apply a UV dose of 600 mJ/cm2. This can be calculated using the spreadsheet of J. Bolton. In principle both LP and MP UV lamps may be used, but the risk of TP formation is higher with MP lamps.
Quench the solution with H2SO3 if necessary. Keep in mind that the presence of H2SO3 may interfere with the analyses, so it is preferred not to quench the solution.
Analyse the parent compound and if possible transformation products. Besides, bromate and chlorate concentrations in the solution should be measured after ozonation.
Appendix E – Overview of in silico approaches that may be applied
E.1. Hazard prediction
In silico methods refer to any approach performed on a computer or via computer simulation, and computational toxicology has been increasingly used to predict the toxicity of substances (Roncaglioni et al., 2013). Computerised methods are largely used by the pharmaceutical industry to design new molecules, and their use in prioritising risks related to chemicals is increasing. Predictive toxicology allows the replacement, reduction and refinement (3Rs) of the use of animal testing in line with Directive 2010/63/EU, (European Union, 2010), reducing the cost and time effort related to animal testing, promoting the ethical use of the resources invested in research, and speeding up the evaluation of contaminants in the environment. Many regulations makes it therefore a firm legal requirement.
In silico methods can be used in an Integrated Approaches to Testing and Assessment (IATA), which are frameworks for integrating information and include a combination of methods [(Q)SAR, read‐across, in chemico (chemical substance‐based), in vitro (e.g. omics, cell‐based, tissue‐based, etc.), ex vivo (e.g. tissue‐based), in vivo (animal‐based)] or omic technologies (e.g. toxicogenomics), the results of which are integrated. Together with in vitro tests and other biotechnological and computational approaches, in silico methods are considered a New Approach Methodology (NAM) (OECD, 2020).
The REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) legislation enacted in 2006 advocates the use of sufficiently validated computational prediction models based on QSAR (quantitative structure–activity relationship) to fill in physicochemical properties and for some (environmental) toxicity data gaps for the risk assessment of products put on the European market.
The European Union Reference Laboratory on Alternative Methods (EURL ECVAM) established in 2011 and located in Ispra, Italy, supports the development, validation and acceptance of alternative methods. EURL ECVAM is the Commission's Joint Research Centre and its tasks and duties are defined by the Annex VII of the European Directive no 63/2010.
In silico methods have been increasingly used under regulations EC No 1107/2009 and EU No 528/2012 concerning the plant protection products and biocides, for the environmental metabolite prediction and assessment, and the endocrine disruptors assessment.
Regulation EC no 1223/2009 concerning cosmetic products banned the testing of existing cosmetic ingredients and products, as well as the testing of new ingredients, on animals. Consequently, this regulation underlies the necessity to use new approach methods including in silico models to derive safety levels for cosmetics.
Marzo et al. (2016) suggested integrating multiple models to reduce the uncertainty related to in silico predictions. The combination of different tools has been demonstrated to increase the reliability of the predictions in different studies (Rallo et al., 2005; Basant et al., 2016). For that reason, it is recommended to operate all the freely available in silico models (if needed replaced with or complemented with commercials ones) to allow validation of the results and make comparisons between different schemes.
The reliability of in silico approaches depends on the reliability of the experimental data used to develop the model and the concordance between the characteristics of the molecules in the experimental dataset and the target molecule for which properties are being predicted. Therefore, the applicability domain of models, i.e. the range of the unknown target for which the model can provide reliable predictions, should always be considered. In addition, the reliability of the QSAR and read‐across model for a specific endpoint should be considered when using in silico tools for hazard prediction, as not all endpoints can be predicted with the same reliability as bacterial mutagenicity. It is therefore recommended to follow the continuous developments and improvements of in silico methods for toxicity prediction. QSAR models used should follow the agreed OECD principles according to the OECD Guidance on the validation of (Q)SAR models (OECD, 2007). QSAR approaches used should be reported as described in the ECHA (2016b) practical guide and any subsequent updates. Furthermore, it is recommended that at least a rule‐based and statistical‐based model (QSAR) and a read‐across approach are applied in parallel to enhance the reliability of the prediction.
Expert judgement plays a pivotal role in adopting in silico approaches because, even if most of the software is user‐friendly and relatively easy to use, the output should always be critically evaluated to identify outliers, inconsistencies or errors that the model may propose. The prediction, if possible, has to be justified by reasonings and mechanistic interpretations, to increase the reliability of the results.
In silico data can be generated by three main approaches (ECHA, 2008):
-
–
Grouping approaches, which include read‐across and chemical category formation
-
–
QSARs
-
–
Expert systems
Grouping of chemicals
Chemicals can be collected into groups according to comparable physicochemical characteristics, human health or ecotoxicological concerns and/or overlapping environmental fates. The chance to compare the molecules of the group is usually due to similarities in the chemical structure of the compounds, which assure similar reactions of the compounds in the environment or biological systems. OECD and ECHA have published guidance documents to define the grouping of chemical approaches (OECD, 2017; ECHA, 2008). The similarities can be common functional groups, a pattern of change in the group (for example, chain‐length category), or the presence of common precursors. A chemical category consents to extrapolate, interpolate or use the read‐across to obtain missing data. The gathering into groups based on these similarities can allow filling gaps in required data, using different procedures: read‐across, trend analysis and QSARs (OECD, 2022). The read‐across and the Quantitative Structure–Activity Relationship (QSAR) are the main computer‐based methods used in predictive toxicology, and both are considered in this guidance to evaluate the hazard related to TP.
Read‐across
The read‐across technique is based on a correlation of a target molecule with a group of chemical structures, examining similarities or overlapping mechanisms of action. The read‐across is always done for a specific endpoint, allowing the use of existing data for the hazard assessment of unknown chemicals. In other words, the read‐across allows adopting existing experimental data of a group of molecules to predict the activity of an unknown chemical, thus filling data gaps for the hazard assessment. Thanks to endpoint‐specific databases correlated to high‐quality experimental data, the read‐across approach is possible. If the database is not well‐developed, the reliability of the read‐across approach is lower. The results of a read‐across approach must be justified and documented to prove their validity. ECHA has developed a Read‐Across Assessment Framework (RAAF) for the evaluation of read‐across approaches robustly and transparently (ECHA, 2017a) and provides guidelines on the application of alternatives to animal testing (ECHA, 2016). The REACH legislation includes a chapter on grouping and read‐across (REACH, Annex XI, section 1.5). The data gathering has to follow the provided guidelines to use the read‐across results in the REACH regulation.
QSARs
The QSARs methods allow linking the chemical structure of a molecule with the biological activity expected to be produced. The QSAR approach lies in the concept that the toxicity of a compound is a function of its chemical structure, so it uses the recognition of molecular descriptors to predict the toxic characteristics of a target molecule. Quantitative structure–activity relationship (QSAR), a computer‐based method linking chemical structures with biological activities, is used in predictive toxicology.
The method proposed by EFSA Scientific Committee (2011) implies using QSAR tools as a preliminary step to identify hazard compounds, followed by applying read‐across methods to discriminate false negatives and increase the reliability of the methodology. Furthermore, the grouping approach can support the results of QSAR analysis or generate estimated data when suitable QSARs are not available (Worth et al., 2011).
The Organisation for Economic Co‐operation and Development (OECD) indicates the five principles that QSAR models should follow to consider a QSAR model applicable for regulatory purposes (OECD, 2004).
The endpoint has to be clearly defined, as the variety of the experimental systems can differ a lot in terms of protocols and conditions and decrease the reliability of the QSAR prediction. Reliable models are restricted to a specific endpoint, considering a specific mechanism of action (MOA) or a determined effect on a particular tissue or organ under defined conditions.
The algorithm has to be unambiguous to guarantee transparency. This information sometimes is not freely available for commercial models, and this limits the acceptance of the model for regulatory purposes.
The applicability domain has to be defined as every model can generate reliable predictions just for a restricted group of chemicals based on their chemical structures, physicochemical properties and modes‐of‐action (MOAs).
Appropriate measures of goodness‐of‐fit, robustness and predictivity need to be included. The first two parameters represent the internal performance of the model, while the predictivity of the model regards the external validation obtained through the comparison of predictions with experimental data of chemicals that were not included in the development of the model.
A mechanistic interpretation enhances the reliability of QSARs models used for regulatory purposes. This is not always possible, but the interpretation by experts will give a higher value to the prediction.
Expert systems
Expert systems are a diverse group of models consisting of combinations of SARs, QSARs and databases (ECHA, 2008). More information on expert systems can be found in Section 6.1.8 of the guidance published by ECHA (2008).
E.2. Prediction of transformation products
The following scheme can be applied for predicting TP that may be formed during disinfection treatment processes. This can result in a collection of the possible TP created from active substances of plant protection products and biocides found in water. Indeed, even if some reactions have already been identified, the list of responses that can occur can still be undefined. This approach will determine which kind of functional groups could be formed, using a combination of existing literature (such as monitoring data) that gathers the previously identified TP in raw and drinking water, and in silico approaches that predict the chemical structure of the resulting compounds.
The most relevant information on TP formed from specific plant protection products or biocides can be collected by adopting the standard process to perform a systematic literature review as defined by Egger et al. (2008). It consists of a priori definition of inclusion and exclusion criteria, the location of studies, the extraction of the data and the assessment of their quality fixed on pre‐defined schemes.
Formulate the review question: which kind of TPs can be found in drinking water?
Define inclusion and exclusion criteria: include just monitoring data on TPs.
Locate studies.
Select studies based on the defined inclusion criteria; a reason is given.
Assess study quality using a pre‐defined method.
Extract data using a pre‐defined form: molecules, concentrations, endpoints.
Analyse and present results using a pre‐defined method to synthesise the information.
Interpret results considering information gaps for further research.
The simulation provided by computerised (in silico) methods should be considered in parallel to the existing literature data to understand any correspondence between predicted TP and compounds found in the water. The applicability of these methods will depend on different parameters. A prioritised list for the predicted TP is created based on the likelihood of production and the documented detection in the water. It will be evaluated which kind of functional groups are frequently included in a parent compound after water disinfection treatments by investigating the relation of such reactions with the chemical structure of the compounds.
Software
Different in silico tools can be used to gather information on the possible TP formed during disinfection processes of water that contains residues of plant protection products and biocides.
US EPA Chemical Transformation Simulator (CTS) 1.2 is a web‐based tool that predicts transformation pathways of organic chemicals using reaction libraries. Examples are hydrolysis, which could occur during (advanced) oxidation processes and photolysis, possibly involved during UV treatments. It is possible to select the number of subsequent generations of TP to visualise, and the likelihood of being produced is given. Sometimes the main product is made in successive generations as the products of the first generation are unstable and quickly react. It is possible to obtain also information on the physicochemical properties of the predicted compounds. The software includes three modules: Chemical Editor, Physicochemical Properties, Reaction Pathway Simulator (EPA, 2019). The last one is more relevant for the scope of this research and allows to determine potential TP based on specific reaction conditions that are pre‐defined by the user. The tool works for organic chemicals, while organometallics, non‐dissociating salts of organic chemicals, and polymers cannot be processed by the programme, which could be a limitation of the model. The software allows inserting a single chemical or a batch of chemicals (up to 10).
The Chemical Editor (CE) is the first interface before processing one of the three modules. It is the input where the chemical of interest should be inserted using the name of the chemical, the CAS number, the SMILES or the drawing of the chemical structure. As a result, the CE lists for the selected chemical the standardised SMILES string, the preferred common name, the IUPAC name, the chemical formula, the relevant CAS numbers, the average and monoisotopic masses, and the DTXSID (that is the unique substance identifier assigned by EPA's National Center for Computational Toxicology [NCCT]). This process allows for avoiding errors in inserting the molecule, as sometimes a SMILES string is not exclusive of a chemical structure.
The chemical speciation (CS) module uses ChemAxon's Plugin Calculators to generate new and distinct species from a chemical (speciation) in the function of the pH. Furthermore, information about the ionisation constant(s), the dominant tautomer distribution, and the structures for all isomers possibly formed is available, as shown in the scheme in Figure E.1.
Figure E.1.
The Physicochemical Properties (PCP) Module allows the characterisation of the transformation of the parental chemical profile, based on a consensus approach that unifies multiple physicochemical calculators, as shown in Figure E.2. It is possible to generate specific physicochemical properties of interest by selecting calculators and properties. The PCP includes a collection of information from:
-
–
EPI Suite: it uses a QSAR approach that recognises fragment descriptors;
-
–
Toxicity Estimation Software Tool (TEST): it implements fragment‐based QSARs, hierarchical clustering with structural similarity based on a variety of two‐dimensional physicochemical descriptors, and nearest neighbour;
-
–
ChemAxon plug‐in calculators: based on a QSAR approach including atom or fragment descriptors;
-
–
OPEn structure–activity/property Relationship App (OPERA) uses a weighted nearest neighbour approach to construct QSAR models based on two‐dimensional physicochemical descriptors.
Figure E.2.
Given a parental compound and determined reaction conditions, the Reaction Pathway Simulator (RPS) Module predicts TP. It works using a series of libraries built on experimental data, and it recognises reactive functional groups that are susceptible to be processed through, for example, reduction and hydrolysis. Different pathways are included or excluded for the specific chemical based on the available experimental data. For the included reaction schemes, a relative reaction rate (rank) is assigned, leading to a prediction of the percentage production of each TP. Thus, the tool already gives an evaluation of the likelihood of being produced. Once the TP are predicted, it is possible to see the calculated physicochemical properties of the parental compound and its TP. The workflow to generate TP is shown in Figure E.3. In this sense, this module is the more interesting, as it allows to predict new products and characterise them.
Figure E.3.
BioTransformer https://biotransformer.ca/ is an open‐access software tool for in silico metabolism prediction and metabolite identification. It combines a knowledge‐based and a machine learning‐based approach to predict the metabolism of small molecules.
The current version of the BioTransformer is 3.1.0. It includes:
The MetXBioDB biotransformation database, which provides information about metabolic reactions;
a reaction knowledge base of generic biotransformation rules;
a reasoning engine that implements both generic and transformer‐specific algorithms to predict metabolites.
It consists of the Metabolism Prediction Tool (BMPT) and the Metabolite Identification Tool (BMIT). The BMPT machine learning system uses a set of random forest and ensemble prediction models to predict the metabolism in humans, which is not relevant for this research. Still, it also includes an evaluation of the environmental microbial transformation, including aerobic and anaerobic degradation of small molecules in soil and water. The IUPAC name of the chemical of interest has to be inserted. The computation can take some minutes to hours, depending on the compound. The BMPT calculates specific metabolites using mass spectrometry (MS) data (EPA, 2019).
Biocatalysis/Biodegradation Database (BBD), developed by the University of Minnesota and now maintained by the Swiss Federal Institute of Aquatic Science and Technology (EAWAG). It offers rules‐based pathway predictions for the microbial degradation of chemical compounds in the environment, allowing us to understand the enzyme‐catalysed biodegradation processes. The information collected in this database has been used to develop the UM‐Pathway Prediction System (UM‐PPS). It is a computational metabolic pathway predictor based on metabolic rules related to organic functional groups, which allow the prediction of the microbial metabolism for chemicals that have not been studied yet. This tool thus enables filling data for chemicals.
EnviPath https://envipath.org/ is a database and prediction system which predicts the microbial transformation of organic chemicals, showing the experimental biotransformation pathways that are involved and the relative rule‐based reasonings. Information about the enzyme‐catalysed reactions of environmental xenobiotics allows for predicting TP formation in the environment and may give insight into reaction products derived from the disinfection of water. Different responses in the background can be overlapped by reactions, such as hydrolysis or photolysis, that occur during disinfection processes. The database includes 1,500 microbial catabolic reactions and ~ 220 biotransformation pathways organised in packages. The software reads SMILES format, or it allows to draw the molecule in the editor. The experimental data are reported if the paths are included in the database. Otherwise, a prediction is given. It is possible to consult all the transformation rules and specific pathways in the prediction output. EnviPath offers users the possibility of building their databases with biotransformation data and integrating it with Eawag‐BBD data (Wicker et al., 2016).
US EPA Estimation Programmes Interface (EPI) SuiteTM is a Windows® based tool that collects physical and chemical properties and estimations on the environmental fate of chemicals. It has been developed based on EPA and Syracuse Research Corp (SRC). It is used as a screening tool, and if experimental data are available, it is recommended to adopt them. Version 4.11 is available (EPA, 2022a,b). It uses a single input to run the following estimation programmes:
-
–
BIOWIN™: it estimates aerobic and anaerobic biodegradability of organic chemicals using seven different models. Two of these are the original Biodegradation Probability Programme (BPP™). The seventh and newest model estimates anaerobic biodegradation potential.
-
–
BioHCwin: it estimates biodegradation half‐life for compounds containing only carbon and hydrogen (i.e. hydrocarbons).
-
–
WATERNT™: it estimates water solubility directly using a ‘fragment constant’ method similar to that used in the KOWWIN™ programme. This model could be relevant to understanding if the TP persists in the drinking water after treatment processes.
-
–
HYDROWIN™: estimates aqueous hydrolysis rate constants and half‐lives for the following chemical classes: esters, carbamates, epoxides, halomethanes, selected alkyl halides, and phosphorus esters. It estimates rate constants for acid‐ and base‐catalysed hydrolysis, except for phosphorus esters and not neutral hydrolysis. In addition, HYDROWIN™ identifies various chemical structure classes for which hydrolysis may be significant (i.e. carbamates) and gives relevant experimental data.
-
–
STPWIN™: using several outputs from EPI Suite™, this programme predicts the removal of a chemical in a typical activated sludge‐based sewage treatment plant. Values are given for total removal and three processes that may reduce the contaminants: biodegradation, sorption to sludge and air stripping. The programme assumes a standard system design and set of default operating conditions.
Except for the BioHCWIN model, EPI Suite™ and the individual programmes included within the software are owned by the U.S. Environmental Protection Agency and are protected by copyright throughout the world®.
Appendix F – Derivation of HBGLV for human health and food‐producing domesticated animals health
1.
F.1. Guidance on selected default values
F.1.1. Body weight
Applicants are encouraged to consider the existing guidance documents of EFSA (EFSA Scientific Committee, 2012b) on the selected default values and check for updates on default body weight. Note that once finalised and published, the extraction of the EFSA Comprehensive Database relevant for pesticide residues made available via PRIMO 4 or later versions should be used for human consumers' body weights.
F.1.2. Water intake
According to WHO (2011), there is variation in both the volume of water consumed daily and the body weight of consumers. It is therefore necessary to apply some assumptions to determine a guideline value. The default assumption for consumption by an adult is 2 l of water per day, that WHO (2011) associated with a default assumption for body weight of 60 kg. In some cases, the guideline value is based on children, where they are considered to be particularly vulnerable to a particular substance. In this event, a default intake of 1 L is assumed that WHO, 2011 associated with a body weight of 10 kg; where the most vulnerable group is considered to be bottle‐fed infants, where an intake of 0.75 L was assumed for a body weight of 5 kg.
The EFSA Scientific Committee (EFSA Scientific Committee, 2012b), recommends a 2 L default value for chronic daily total liquid intake (i.e. milk, tap water, other beverages) for European adults, including the elderly. For children's total liquid intake, the reader is referred to the opinion on dietary reference values for water of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), where adequate intake levels are provided according to their age (EFSA NDA Panel, 2010). Once finalised and published, the extraction of the EFSA Comprehensive Database relevant for pesticide residues made available via PRIMO 4 or later versions should be used for human consumers.
F.1.3. Uncertainty factors (UFs) used in establishing health‐based guideline values (HBGVs)
Uncertainty factors (UFs) (also called assessment factors, safety factors, adjustment factors or extrapolation factors) are used to derive HBGV by extrapolating from experimental animal data to humans (IPCS, 2009). UFs are intended to cover the uncertainty and variability arising from the inter‐species differences, intra‐species differences and data variation, for example use of LOAEL and extrapolation from subchronic or subacute to chronic exposure. Applicants are encouraged to consider the existing guidance documents of EFSA (e.g. EFSA Scientific Committee, 2012b) and ECHA (e.g. ECHA, 2012) on the selected default values and check for updates on UFs.
F.1.4. Allocation factor (AF)
According to WHO (2011), drinking water is usually not the only source of human exposure to the chemicals for which guideline values have been derived. In many cases, exposure to or intake of chemical contaminants via drinking water is much less than that via other sources, such as food, air and consumer products (WHO, 2011). Therefore, WHO recommends considering the proportion of the ADI or TDI that may be attributed to different sources in developing guideline values and risk management strategies. This approach ensures that total daily intake from all sources does not exceed the ADI or TDI. As the primary sources of exposure to chemicals are generally food (e.g. pesticide residues) and water, it is important to quantify the exposures from both sources. In the absence of adequate exposure data, the default allocation of the total daily intake of drinking water is 20%, which reflects a reasonable level of exposure, while still being protective (WHO, 2011). In some circumstances, there is clear evidence that exposure from food is very low, such as for some of the DBPs, in such cases, the allocation may be as high as 80%, which still allows for some exposure from other sources (WHO, 2011). The higher allocation factor (80%) may also apply to different TP that are formed from a same (structurally similar) active substance during various water treatment processes (relative exposure route needs to be established). Alternatively, a lower allocation factor (20%) may apply to same TP formed from different active substances. In the case of some pesticides, that are likely to be present as residues in food, from where there is significant exposure, the allocation for water may be as low as 1% (WHO, 2011). The values chosen are, in most cases sufficient to account for additional routes of intake (i.e. inhalation and dermal absorption) of contaminants in water, under certain circumstances (e.g. limited ventilation), inhalation and dermal exposure may be taken into account in adapting the guideline values to local conditions. Allocation factors may not be needed when consumer intakes via food can be calculated using relevant food residues data and intake calculations such as those that can be calculated using the PRIMO tool.
F.2. Health‐based guidance values for domesticated food‐producing animals
No background material available on HBGVs for domesticated animals could be found.
Appendix G – Public consultation on the draft ECHA/EFSA Guidance on the impact of water treatment processes on residues of active substances or their metabolites in water abstracted for the production of drinking water
Comments received
These are available on OpenEFSA under the section Public consultations
Replies to comments received
These can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.8194
Supporting information
Public consultation on the draft ECHA/EFSA Guidance on the impact of water treatment processes on residues of active substances or their metabolites in water abstracted for the production of drinking water
Suggested citation: ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) , Hofman‐Caris, R ., Dingemans, M ., Reus, A ., Shaikh, S. M ., Muñoz Sierra, J ., Karges, U ., aus der Beek, T ., Nogueiro, E ., Lythgo, C ., Parra Morte, J. M ., Bastaki, M ., Serafimova, R ., Friel, A ., Court Marques, D ., Uphoff, A ., Bielska, L ., Putzu, C ., … Papadaki, P . (2023). Guidance document on the impact of water treatment processes on residues of active substances or their metabolites in water abstracted for the production of drinking water. EFSA Journal, 21(8), 1–108. 10.2903/j.efsa.2023.8194
For information on the applicability of this document under the Biocidal Products Regulation and the Plant Protection Products Regulation, please visit the websites of ECHA and the European Commission (Food Safety, Plants).
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00127
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: ECHA and EFSA wish to thank the following for the support provided to this scientific output: Stefan Kools, Emile Cornelissen, Kees van Leeuwen, Thomas ter Laak, Adele Ferrario, Christine Kübeck, Andreas Nahrstedt, Elke Dopp, Ulrich Borchers, Mark Egsmose, Roberto Lava, Gabriella Fait, Laura Padovani, Chiara Pecorini. ECHA and EFSA wish to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 14 July 2023
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.OJ L 309/1, 24.11.2009, pp. 1–50.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products.
E.g. botanical active substances.
Please refer to the below list as well as to Section 2.3 of this guidance document and more extensively to European Comission (2021) and EFSA PPR Panel (2013a,b) for definition of ‘of no concern’.
The term ‘metabolites or transformation products’ is used here as indicated in the BPR Guidance ‘Introduction to guidance on the BPR, Part A, volumes I‐IV’, Chapter 4: https://echa.europa.eu/documents/10162/2324906/introduction_part_a_en.pdf/2c188c12-a366-5e45-f843-10abf1e37b6a?t=1648535130674
According to Article 3(f) of the Biocidal Products Regulation (528/2012/EC, BPR) ‘substance of concern’ means any substance, other than the active substance, which has an inherent capacity to cause an adverse effect, immediately or in the more distant future, on humans, in particular vulnerable groups, animals or the environment and is present or is produced in a biocidal product in sufficient concentration to present risks of such an effect.
Guidance on biocides legislation: https://echa.europa.eu/guidance-documents/guidance-on-biocides-legislation
The Human Health WG‐I‐2022 meeting supported the application of the SANCO guidance for biocides. Public minutes: https://webgate.ec.europa.eu/s-circabc/w/browse/e2ca2f52-18d9-487f-916c-803acfca6ae9 Guidance document on the assessment of the relevance of metabolites in groundwater of substances regulated under Regulation (EC) No 1107/2009 https://ec.europa.eu/food/system/files/2021-10/pesticides_ppp_app-proc_guide_fate_metabolites-groundwtr-rev11.pdf
It should be noted that there were discussions among Competent Authorities on biocides on this approach (CA‐June21‐Doc.4.3), and the approach may be adapted in the future.
Often referred to as metabolites. As some TPs are formed in the environment without the involvement of any organism for their formation, this term (metabolite) is not necessarily accurate and we use ‘environmental TP’ to cover both.
The ‘limit of quantification’ (LOQ) of an analytical procedure can be described as the lowest concentration of the analyte in a sample that can be quantified with sufficient recovery and precision as an exact value (Guidance Document on Pesticide Analytical methods for Risk Assessment and post‐approval Control and Monitoring Purposes; SANTE/2020/12830, Rev. 1, 24 February 2021). It is the lowest concentration of a substance that can be measured with certainty using standard tests.
The 100 mJ/cm2 specified is for the irradiation dose recalculated at 254 nm following the common practice when using MP UV lamps.
D5 and D6 refer to the number of isotope labelled deuterium atoms (instead of hydrogen) and C8 to the number of 13 C atoms in the molecule. This is used to distinguish the labelled model compounds from possibly present unlabeled compounds.
The EFSA PPR Panel Guidance on the establishment of the residue definition for dietary risk assessment (2016) focuses on parent active substances and their metabolites. However, metabolites may differ from transformation products in terms of overlap of physico‐chemical properties with the parent compound, the number of compounds that is formed and exposure scenario. Therefore, not all steps from this EFSA Guidance are included in the proposed approach for transformation products.
References
- Adriaanse PI, Linders JBHJ, van den Berg GA, Boesten JJTI, van der Bruggen MWP, Jilderda K, Luttik R, Merkens WSW, Stienstra YJ and Teunissen RJM, 2008. Development of an assessment methodology to evaluate agricultural use of plant protection products for drinking water production from surface waters: a proposal for the registration procedure in The Netherlands. Alterra. [Google Scholar]
- Andreoli FC and Sabogal‐Paz LP, 2020. Household slow sand filter to treat groundwater with microbiological risks in rural communities. Water Research, 186, 116352. [DOI] [PubMed] [Google Scholar]
- Andrzejewski P, Kasprzyk‐Hordern B and Nawrocki J, 2008. N‐nitrosodimethylamine (NDMA) formation during ozonation of dimethylamine‐containing waters. Water Research, 42, 863–870. [DOI] [PubMed] [Google Scholar]
- Antonopoulou M, Ioannidis N, Kaloudis T, Triantis TM and Hiskia A, 2020. Kinetic and mechanistic investigation of water taste and odor compound 2‐isopropyl‐3‐methoxy pyrazine degradation using UV‐A/Chlorine process. Science of the Total Environment, 732, 138404. [DOI] [PubMed] [Google Scholar]
- APHA (American Public Health Association) , 1998. Standard methods for the examination of water and waste water. American Public Health Association. [Google Scholar]
- Armbruster D, Happel O, Scheurer M, Harms K, Schmidt TC and Brauch HJ, 2015. Emerging nitrogenous disinfection byproducts: transformation of the antidiabetic drug metformin during chlorine disinfection of water. Water Research, 79, 104–118. [DOI] [PubMed] [Google Scholar]
- ATSDR , 2022. Toxicological Profile for N‐Nitrosodimethylamine (NDMA). Draft for Public Comment. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp141.pdf [Google Scholar]
- Badea SL, Geana EI, Niculescu VC and Ionete RE, 2020. Recent progresses in analytical GC and LC mass spectrometric based‐methods for the detection of emerging chlorinated and brominated contaminants and their transformation products in aquatic environment. Science of the Total Environment, 722, 137914. [DOI] [PubMed] [Google Scholar]
- Baker D and Kaprzyk‐Hordem R, 2013. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: Ne developments. Science Total Environmental, 454–455, 442–456. 10.1016/j.scitotenv.2013.03.043 [DOI] [PubMed] [Google Scholar]
- Basant N, Gupta S and Singh KP, 2016. QSAR modeling for predicting reproductive toxicity of chemicals in rats for regulatory purposes. Toxicology Research, 5, 1029–1038. 10.1039/c6tx00083e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been F, Kruve A, Vughs D, Meekel N, Reus A, Zwartsen A, Wessel A, Fischer A, ter Laak T and Brunner A, 2021. Risk‐based prioritization of suspects detected in riverine water using complementary chromatographic techniques. Water Research, 204, 117612. 10.1016/j.watres.2021.117612 [DOI] [PubMed] [Google Scholar]
- Bilińska L, Blus K, Gmurek M and Ledakowicz S, 2019. Brine recycling from industrial textile waste water treated by ozone. By‐products accumulation. Part 1: multi recycling loop. Water (Switzerland), 11. [Google Scholar]
- Blaney L, Lawler DF and Katz LE, 2019. Transformation kinetics of cyclophosphamide and ifosfamide by ozone and hydroxyl radicals using continuous oxidant addition reactors. Journal of Hazardous Materials, 364, 752–761. [DOI] [PubMed] [Google Scholar]
- Bletsou AA, Jeon J, Hollender J, Archontaki E and Thomaidis NS, 2015. Targeted and non‐targeted liquid chromatography‐mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC ‐ Trends in Analytical Chemistry, 66, 32–44. [Google Scholar]
- Bolton JR, 2010. Ultraviolet applications handbook. ICC Lifelong Learn Inc. [Google Scholar]
- Bolton JR and Cater SR, 1994. In: Heiz GR, Zepp RG and Crosby DG (eds). Aquatic and surface photochemistry. Lewis Publishers, Boca Raton, Florida. pp. 467–490. [Google Scholar]
- Bolton JR and Linden KG, 2003. Standardization of methods for fluence (UV Dose) determination in bench‐scale UV experiments. Journal of Environmental Engineering, 129, 209–215. [Google Scholar]
- Bolton JR and Stefan MI, 2002. Fundamental photochemical approach to the concepts of fluence (UV dose) and electrical energy efficiency in photochemical degradation reactions. Research on Chemical Intermediates, 28, 857–870. [Google Scholar]
- Bolton JR, Linden KG, Kuo J, Chen CL and Nellor M, 2005. Discussion of "Standardized collimated beam testing protocol for water/wastewater ultraviolet disinfection" by Jeff Kuo, Ching‐lin Chen, and Margaret Nellor. Journal of Environmental Engineering, 131, 827–829. [Google Scholar]
- Bourgin M, Beck B, Boehler M, Borowska E, Fleiner J, Salhi E, Teichler R, von Gunten U, Siegrist H and McArdell CS, 2018. Evaluation of a full‐scale wastewater treatment plant upgraded with ozonation and biological post‐treatments: abatement of micropollutants, formation of transformation products and oxidation by‐products. Water Research, 129, 486–498. [DOI] [PubMed] [Google Scholar]
- Bruchet A and Duguet JP, 2004. Role of oxidants and disinfectants on the removal, masking and generation of tastes and odours. Water Science and Technology, 49, 297–306. [PubMed] [Google Scholar]
- Brunner AM, Vughs D, Siegers W, Bertelkamp C, Hofman‐Caris R, Kolkman A and ter Laak T, 2019. Monitoring transformation product formation in the drinking water treatments rapid sand filtration and ozonation. Chemosphere, 214, 801–811. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Bertelkamp C, Dingemans MML, Kolkman A, Wols B, Harmsen D, Siegers W, Martijn BJ, Oorthuizen WA and ter Laak TL, 2020. Integration of target analyses, non‐target screening and effect‐based monitoring to assess OMP related water quality changes in drinking water treatment. Science of the Total Environment, 705, 135779. [DOI] [PubMed] [Google Scholar]
- Buchner EM, Happel O, Schmidt CK, Scheurer M, Schmutz B, Kramer M, Knauer M, Gartiser S and Hollert H, 2019. Approach for analytical characterization and toxicological assessment of ozonation products in drinking water on the example of acesulfame. Water Research, 153, 357–368. [DOI] [PubMed] [Google Scholar]
- Cai WW, Peng T, Zhang JN, Hu LX, Yang B, Yang YY, Chen J and Ying GG, 2019. Degradation of climbazole by UV/chlorine process: kinetics, transformation pathway and toxicity evaluation. Chemosphere, 219, 243–249. [DOI] [PubMed] [Google Scholar]
- Cai WW, Peng T, Yang B, Xu C, Liu YS, Zhao JL, Gu FL and Ying GG, 2020. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: experimental and theoretical studies. Chemical Engineering Journal, 402, 126224. [Google Scholar]
- Cao F, Zhang M, Yuan S, Feng J, Wang Q, Wang W and Hu Z, 2016. Transformation of acetaminophen during water chlorination treatment: kinetics and transformation products identification. Environmental Science and Pollution Research, 23, 12303–12311. [DOI] [PubMed] [Google Scholar]
- Carpinteiro I, Rodil R, Quintana JB and Cela R, 2017. Reaction of diazepam and related benzodiazepines with chlorine. Kinetics, transformation products and in‐silico toxicological assessment. Water Research, 120, 280–289. [DOI] [PubMed] [Google Scholar]
- Castronovo S, Wick A, Scheurer M, Nödler K, Schulz M and Ternes TA, 2017. Biodegradation of the artificial sweetener acesulfame in biological wastewater treatment and sandfilters. Water Research, 110, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves FP, Gomes G, Della‐Flora A, Dallegrave A, Sirtori C, Saggioro EM and Bila DM, 2020. Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: effect of pH, estrogenic activity, transformation products and toxicity. Science of the Total Environment, 746, 141041. [DOI] [PubMed] [Google Scholar]
- Chen WL, Cheng JY and Lin XQ, 2018. Systematic screening and identification of the chlorinated transformation products of aromatic pharmaceuticals and personal care products using high‐resolution mass spectrometry. Science of the Total Environment, 637–638, 253–263. [DOI] [PubMed] [Google Scholar]
- Chen WL, Ling YS, Lee DJH, Lin XQ, Chen ZY and Liao HT, 2020. Targeted profiling of chlorinated transformation products and the parent micropollutants in the aquatic environment: a comparison between two coastal cities. Chemosphere, 242, 125268. [DOI] [PubMed] [Google Scholar]
- Chen M, Wei D, Wang F, Yin J, Li M and Du Y, 2021. Bioassay‐ and QSAR‐based screening of toxic transformation products and their formation under chlorination treatment on levofloxacin. Journal of Hazardous Materials, 414, 125495. [DOI] [PubMed] [Google Scholar]
- Chow CH and Leung KSY, 2019. Removing acesulfame with the peroxone process: transformation products, pathways and toxicity. Chemosphere, 221, 647–655. [DOI] [PubMed] [Google Scholar]
- Cruz‐Alcalde A, Sans C and Esplugas S, 2017. Priority pesticides abatement by advanced water technologies: the case of acetamiprid removal by ozonation. Science of the Total Environment, 599–600, 1454–1461. [DOI] [PubMed] [Google Scholar]
- Cruz‐Alcalde A, Sans C and Esplugas S, 2018. Priority pesticide dichlorvos removal from water by ozonation process: reactivity, transformation products and associated toxicity. Separation and Purification Technology, 192, 123–129. [Google Scholar]
- Dao YH, Tran HN, Tran‐Lam TT, Pham TQ and Le GT, 2018. Degradation of paracetamol by an UV/chlorine advanced oxidation process: influencing factors, factorial design, and intermediates identification. International Journal of Environmental Research and Public Health, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud F, Zuehlke S, Spiteller M and Kayser O, 2020. Ozonation of rivaroxaban production waste water and comparison of generated transformation products with known in vivo and in vitro metabolites. Science of the Total Environment, 714, 136825. [DOI] [PubMed] [Google Scholar]
- De Greef, J. and De Nijs, A. , 1990. Risk assessment of new chemical substances. Dilution of effluents in The Netherlands (report 670208001).
- De Moel PJ, Verberk JQJC and Van Dijk JC, 2006. Principles and Practices. Sdu Editors Delft University of Technology. [Google Scholar]
- Deborde M and von Gunten U, 2008. Reactions of chlorine with inorganic and organic compounds during water treatment‐Kinetics and mechanisms: a critical review. Water Research, 42, 13–51. [DOI] [PubMed] [Google Scholar]
- Deeb AA, Stephan S, Schmitz OJ and Schmidt TC, 2017. Suspect screening of micropollutants and their transformation products in advanced wastewater treatment. Science of the Total Environment, 601–602, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Detenchuk EA, Trebše P, Marjanović A, Kosyakov DS, Ul'yanovskii NV, Kralj MB and Lebedev AT, 2020. Transformation of resveratrol under disinfection conditions. Chemosphere, 260, 127557. [DOI] [PubMed] [Google Scholar]
- Di Marcantonio C, Bertelkamp C, van Bel N, Pronk TE, Timmers PHA, van der Wielen P and Brunner AM, 2020. Organic micropollutant removal in full‐scale rapid sand filters used for drinking water treatment in The Netherlands and Belgium. Chemosphere, 260, 127630. [DOI] [PubMed] [Google Scholar]
- Diehle M, Gebhardt W, Pinnekamp J, Schäffer A and Linnemann V, 2019. Ozonation of valsartan: structural elucidation and environmental properties of transformation products. Chemosphere, 216, 437–448. [DOI] [PubMed] [Google Scholar]
- Directive (EU) , 2020. 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance).
- Dong F, Li C, Crittenden J, Zhang T, Lin Q, He G, Zhang W and Luo J, 2019. Sulfadiazine destruction by chlorination in a pilot‐scale water distribution system: kinetics, pathway, and bacterial community structure. Journal of Hazardous Materials, 366, 88–97. [DOI] [PubMed] [Google Scholar]
- Du E, Li J, Zhou S, Zheng L and Fan X, 2018. Transformation of naproxen during the chlorination process: products identification and quantum chemistry validation. Chemosphere, 211, 1007–1017. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) , 2008. Guidance on information requirements and chemical safety assessment Chapter R.6: QSARs and grouping of chemicals. ECHA, Helsinki, Finland. 134 pp. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r6_en.pdf/77f49f81-b76d-40ab-8513-4f3a533b6ac9?t=1322594777272 [Google Scholar]
- ECHA (European Chemicals Agency) , 2012. Guidance on information requirements and chemical safety assessment. Chapter R.19: Uncertainty analysis. ECHA, Helsinki, Finland. 36 pp. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r19_en.pdf/d5bd6c3f-3383-49df-894e-dea410ba4335?t=1353935215756 [Google Scholar]
- ECHA (European Chemicals Agency) , 2016a. Guidance on Information Requirements and Chemical Safety Assessment Part E: Risk Characterisation. ECHA, Helsinki, Finland. 49 pp. Available online: 1da6cadd‐895a‐46f0‐884b‐00307c0438fd (europa.eu) [Google Scholar]
- ECHA (European Chemicals Agency) , 2016b. Practical Guide How to use and report (Q)SARS. ECHA, Helsinki, Finland. 37 pp. version 3.1 July 2016. ISBN: 978–92–9247‐809‐4. 10.2823/81818 Available online: https://echa.europa.eu/documents/10162/13655/pg_report_qsars_en.pdf/407dff11-aa4a-4eef-a1ce-9300f8460099 [DOI]
- ECHA (European Chemicals Agency) , 2017a. Read‐Across Assessment Framework (RAAF). ECHA, Helsinki, Finland. 60 pp. Available online: 614e5d61‐891d‐4154‐8a47‐87efebd1851a (europa.eu) [Google Scholar]
- ECHA (European Chemicals Agency) , 2017b. Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7a: Endpoint specific guidance, Version 6.0 July 2017. ECHA, Helsinki, Finland. 610 pp. Available online: Guidance on IR&CSA ‐ Chapter R.7a (europa.eu).
- ECHA (European Chemicals Agency) , 2017c. Guidance on the biocidal products regulation—volume III human health‐assessment & evaluation (Parts B+ C). ECHA, Helsinki, Finland. 427 pp. Available online: https://echa.europa.eu/documents/10162/23036412/biocides_guidance_human_health_ra_iii_part_bc_en.pdf/30d53d7d-9723-7db4-357a-ca68739f5094 [Google Scholar]
- ECHA (European Chemicals Agency) , 2022. Guidance on the Biocidal Products Regulation, Volume III: Human health Part A: Information requirements, Version 2 March 2022. ECHA, Helsinki, Finland. 121 pp. Available online: https://echa.europa.eu/documents/10162/2324906/bpr_guidance_vol_iii_part_a_en.pdf/05e4944d-106e-9305-21ba-f9a3a9845f93?t=1648536087369
- EEA (European Environment Agency) , Kristensen P., et al., 2018. European waters: Assessment of status and pressures 2018. Luxembourgh Report No.: EEA Report 07/2018. 90 pp. EEA, Copenhagen, Denmark. Available online: European waters – Assessment of status and pressures 2018 — European Environment Agency (europa.eu) [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2020. Scientific Opinion on the health and welfare of rabbits farmed in different production systems. EFSA Journal 2020;18(1):5944, 96 pp. 10.2903/j.efsa.2020.5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA External Scientific Report , 2019. Evaluation of the applicability of existing (Q)SAR models for predicting the genotoxicity of pesticides and similarity analysis related with genotoxicity of pesticides for facilitating of grouping and read across. EFSA Supporting Publication 2019;EN‐1598, 221 pp. 10.2903/j.efsa.2019.1598 [DOI] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies) , 2010. Scientific Opinion on Dietary reference values for water. EFSA Journal 2010;8(3):1459, 48 pp. 10.2903/j.efsa.2010.1459 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013a. Scientific Opinion on the report of the FOCUS groundwater working group (FOCUS, 2009): assessment of lower tiers. EFSA Journal 2013;11(2):3114, 29 pp. 10.2903/j.efsa.2013.3114 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013b. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 268 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2016. Guidance on the establishment of the residue definition for dietary risk assessment. EFSA Journal 2016;14(12):4549, 129 pp. 10.2903/j.efsa.2016.4549 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2018. EFSA Scientific opinion on pesticides in foods for infants and young children. EFSA Journal 2018;16(6):5286, 75 pp. 10.2903/j.efsa.2018.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2021. Statement of the PPR Panel on a framework for conducting the environmental exposure and risk assessment for transition metals when used as active substances in plant protection products (PPP). EFSA Journal 2021;19(3):6498, 88 pp. 10.2903/j.efsa.2021.6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Scientific Opinion on exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750, 103 pp. 10.2903/j.efsa.2012.2750 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017a. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. https://doi.org10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017b. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017c. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2019a. Genotoxicity assessment of chemical mixtures. EFSA Journal 2019;17(1):5519, 11 pp. 10.2903/j.efsa.2019.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2019b. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2019c. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal 2019;17(6):5708, 17 pp. 10.2903/j.efsa.2019.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2021a. Scientific Opinion on the guidance on aneugenicity assessment. EFSA Journal 2021;19(8):6770, 27 pp. 10.2903/j.efsa.2021.6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2021b. Statement on the derivation of Health‐Based Guidance Values (HBGVs) for regulated products that are also nutrients. EFSA Journal 2021;19(3):6479, 39 pp. 10.2903/j.efsa.2021.6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD and Altman DG, 2008. Systematic Reviews in Health Care: Meta‐Analysis in Context. Systematic Reviews in Health Care: Meta‐Analysis in Context: Second Edition. 2nd edn. Wiley Blackwell. pp. 1–487. 10.1002/9780470693926 [DOI] [Google Scholar]
- Elias MT, Chandran J, Aravind UK and Aravindakumar CT, 2019. Oxidative degradation of ranitidine by UV and ultrasound: identification of transformation products using LC‐Q‐ToF‐MS. Environmental Chemistry, 16, 41–54. [Google Scholar]
- El‐taliawy H, Casas ME and Bester K, 2018. Removal of ozonation products of pharmaceuticals in laboratory Moving Bed Biofilm Reactors (MBBRs). Journal of Hazardous Materials, 347, 288–298. [DOI] [PubMed] [Google Scholar]
- EPA U.S. Environmental Protection Agency , 2019. User's Guide for the Chemical Transformation Simulator (CTS), Version 1.0 Chemical Transformation Simulator: A Cheminformatics Tool for Predicting Transformation Pathways and Physicochemical Properties. 2019 U.S. Environmental Protection Agency. DOI: User's Guide for the Chemical Transformation Simulator (CTS) Version 1.0 (epa.gov).
- EPA U.S. Environmental Protection Agency , 2022a. EPI Suite™‐Estimation Programme Interface | US EPA. [Google Scholar]
- EPA U.S. Environmental Protection Agency , 2022b. ToxRefDB ‐ Release user‐friendly web‐based tool for mining ToxRefDB|Science Inventory|US EPA.
- European Commission , 2014. Assessing Potential for Movement of Active Substances and their Metabolites to Ground Water in the EU. Report of the FOCUS Ground Water Work Group, EC Document Reference Sanco/13144/2010 version 3, 613 pp.
- European Commission , 2016. Guidance Document On Semiochemical Active Substances And Plant Protection Products. SANTE/12815/2014 rev. 5.2 May 2016.
- European Commission , 2021. Guidance Document On The Assessment Of The Relevance Of Metabolites In Groundwater Of Substances Regulated Under Regulation (EC) No 1107/2009, Sanco/221/2000, rev.11, 21 October 2021. Available online: pesticides_ppp_app‐proc_guide_fate_metabolites‐groundwtr‐rev11.pdf (europa.eu)
- European Union , 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (Text with EEA relevance).
- European Union Commission Regulation , 2013a. No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market (Text with EEA relevance).
- European Union Commission Regulation , 2013b. No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market (Text with EEA relevance).
- Ferrando‐Climent L, Gonzalez‐Olmos R, Anfruns A, Aymerich I, Corominas L, Barceló D and Rodriguez‐Mozaz S, 2017. Elimination study of the chemotherapy drug tamoxifen by different advanced oxidation processes: transformation products and toxicity assessment. Chemosphere, 168, 284–292. [DOI] [PubMed] [Google Scholar]
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2000. FOCUS groundwater scenarios in the EU plant protection product review process. Report of the FOCUS Groundwater Scenarios Workgroup, EC Document Reference Sanco/321/2000, 197.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2003. FOCUS Surface Water Scenarios in the EU Evaluation Process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios, EC Document Reference SANCO/4802/2001‐rev.2. 245 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2007. Landscape And Mitigation Factors In Aquatic Ecological Risk Assessment. Volume 2. Detailed Technical Reviews. Final Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v2.0., ed. E. Commission, 169 pp.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2014. Generic Guidance for Tier 1 FOCUS Ground Water Assessments. Version 2.2. EC Document Reference SANCO/321/2000 rev.2.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2015. Generic guidance for FOCUS surface water Scenarios. V1.4.
- Fu W, Li B, Yang J, Yi H, Chai L and Li X, 2018. New insights into the chlorination of sulfonamide: smiles‐type rearrangement, desulfation, and product toxicity. Chemical Engineering Journal, 331, 785–793. [Google Scholar]
- Funke J, Prasse C, Dietrich C and Ternes TA, 2021. Ozonation products of zidovudine and thymidine in oxidative water treatment. Water Research, X 11, 100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W, Ge Y, Zhu H, Huang H and Yang X, 2019. ClO2 pre‐oxidation changes the yields and formation pathways of chloroform and chloral hydrate from phenolic precursors during chlorination. Water Research, 148, 250–260. [DOI] [PubMed] [Google Scholar]
- Gao YQ, Zhang J, Zhou JQ, Chu WH and Gao NY, 2022. An expected formation of TCNM from chlorination of bisphenol A with ultrasonic pre treatment: a new nitrogen source for N‐DBP from N2 in air. Chemical Engineering Journal, 429, 132326. [Google Scholar]
- Garcia‐Costa AL, Alves A, Madeira LM and Santos MSF, 2021. Oxidation processes for cytostatic drugs elimination in aqueous phase: a critical review. Journal of Environmental Chemical Engineering, 9, 104709. [Google Scholar]
- Ge L, Na G, Zhang S, Li K, Zhang P, Ren H and Yao Z, 2015. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: direct photodegradation, hydroxyl‐radical oxidation, and antibacterial activity changes. Science of the Total Environment, 527–528, 12–17. [DOI] [PubMed] [Google Scholar]
- Gmurek M, Gomes JF, Martins RC and Quinta‐Ferreira RM, 2019. Comparison of radical‐driven technologies applied for paraben mixture degradation: mechanism, biodegradability, toxicity and cost assessment. Environmental Science and Pollution Research, 26, 37174–37192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godayol A, Gonzalez‐Olmos R, Sanchez JM and Antico E, 2015. Assessment of the effect of UV and chlorination in the transformation of fragrances in aqueous samples. Chemosphere, 125, 25–32. [DOI] [PubMed] [Google Scholar]
- Gros M, Petrović M and Barceló D, 2007. Wastewater treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro river basin (Northeast Spain). Environmental Toxicology and Chemistry, 26, 1553–1562. 10.1897/06-495R.1 [DOI] [PubMed] [Google Scholar]
- Grummt and Kuckelkorn , 2020. RiSKWa – Verbundprojekt NeuroBox Methodische Weiterentwicklung zur Bewertung von neurotoxischen Effekten im Wasserkreislauf. Methodische Weiterentwicklung der dreistufigen In‐vitro‐Testbatterie zur Erfassung neurotoxischer Wirkungen und Koordination des Verbundes. https://www.umweltbundesamt.de/sites/default/files/medien/5620/dokumente/bmbf_fkz_02wrs1419a-f_neurobox_schlussbericht_0.pdf [Google Scholar]
- Grummt T, Braunbeck T, Hollert H, Kramer M, 2020. Tox Box Guideline: Hazard‐based risk management of anthropogenic trace substances in drinking water to secure a long‐term drinking water supply. Available online: Leitfaden_TOXBOX_engl.indd (umweltbundesamt.de)
- Gulde R, Rutsch M, Clerc B, Schollée JE, von Gunten U and McArdell CS, 2021. Formation of transformation products during ozonation of secondary wastewater effluent and their fate in post‐treatment: from laboratory‐ to full‐scale. Water Research, 200, 117200. [DOI] [PubMed] [Google Scholar]
- Han J, Zhang X, Liu J, Zhu X and Gong T, 2017. Characterization of halogenated DBPs and identification of new DBPs trihalomethanols in chlorine dioxide treated drinking water with multiple extractions. Journal of Environmental Sciences, 58, 83–92. [DOI] [PubMed] [Google Scholar]
- Han Y, Ma M, Li N, Hou R, Huang C, Oda Y and Wang Z, 2018. Chlorination, chloramination and ozonation of carbamazepine enhance cytotoxicity and genotoxicity: multi‐endpoint evaluation and identification of its genotoxic transformation products. Journal of Hazardous Materials, 342, 679–688. [DOI] [PubMed] [Google Scholar]
- Han Y, Ma M, Oda Y, Rao K, Wang Z, Yang R and Liu Y, 2019. Insight into the generation of toxic products during chloramination of carbamazepine: kinetics, transformation pathway and toxicity. Science of the Total Environment, 679, 221–228. [DOI] [PubMed] [Google Scholar]
- Hebert A, Feliers C, Lecarpentier C, Neale PA, Schlichting R, Thibert S and Escher BI, 2018. Bioanalytical assessment of adaptive stress responses in drinking water: a predictive tool to differentiate between micropollutants and disinfection by‐products. Water Research, 132, 340–349. [DOI] [PubMed] [Google Scholar]
- Hermes N, Jewell KS, Falås P, Lutze HV, Wick A and Ternes TA, 2020. Ozonation of sitagliptin: removal kinetics and elucidation of oxidative transformation products. Environmental Science and Technology, 54, 10588–10598. [DOI] [PubMed] [Google Scholar]
- Hollender J, Schymanski E, Singer H and Ferguson PL, 2017. Nontarget screening with high resolution mass spectrometry in the environment: ready to go? Environ Sci Technol., 51, 20–11512. 10.1021/acs.est.7b02184 [DOI] [PubMed] [Google Scholar]
- Hollender J, van Bavel B, Dulio V, Farmen E, Furtmann K, Koschorreck J, Kunkel U, Krauss M, Munthe J, Schlabach M, Slobodnik J, Stroomberg G, Ternes T, Thomaidis NS, Togola A and Tornero V, 2019. High resolution mass spectrometry‐based non‐target screening can support regulatory environmental monitoring and chemicals management. Environmental Science Europe, 31, 42. 10.1186/s12302-019-0225-x [DOI] [Google Scholar]
- Hörsing M, Kosjek T, Andersen HR, Heath E and Ledin A, 2012. Fate of citalopram during water treatment with O3, ClO2, UV and fenton oxidation. Chemosphere, 89, 2–135. [DOI] [PubMed] [Google Scholar]
- Hu R, Zhang L and Hu J, 2017. Investigation of ozonation kinetics and transformation products of sucralose. Science of The Total Environment, 603–604, 8–17. [DOI] [PubMed] [Google Scholar]
- Huang N, Wang T, Wang WL, Wu QY, Li A and Hu HY, 2017. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: synergistic effect, transformation products and toxicity evaluation. Water Research, 114, 246–253. [DOI] [PubMed] [Google Scholar]
- Hughes RM, Kaufmann PR and Weber MH, 2011. National and regional comparisons between Strahler order and stream size. Journal of the North American Benthological Society, 30, 103–121. [Google Scholar]
- Imfeld G, Payraudeau S, Tournebize J, Simeoni‐Sauvage S, Macary F, Chaumont C, Probst A, Sanchez Perez JM, Bahi A, Chaumet B, Gilevska T, Alexandre H and Probst JL, 2021. The role of ponds in pesticide dissipation at the agricultural catchment scale: a critical review. Water, 13, 1202. 10.3390/w13091202.hal-03209526 [DOI] [Google Scholar]
- IPCS , 2009. Principles and methods for the risk assessment of chemicals in food. (Environmental Health Criteria; 240). ISBN 978 92 4 157240 8. ISSN 0250‐863X.
- Jakopin Z, 2021. Assessment of the endocrine‐disrupting potential of halogenated parabens: an in silico approach. Chemosphere, 264, 128447. [DOI] [PubMed] [Google Scholar]
- Kadmi Y, Favier L and Wolbert D, 2015. N‐nitrosamines, emerging disinfection by‐products of health concern: an overview of occurrence, mechanisms of formation, control and analysis in water. Water, Science and Technology, 15, 1–25. [Google Scholar]
- Keller VDJ, Williams RJ, Lofthouse C and Johnson AC, 2014. Worldwide estimation of river concentrations of any chemical originating from sewage‐treatment plants using dilution factors. Environmental Toxicology Chemistry, 33, 447–452. 10.1002/etc.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy Neth NL, Carlin CM and Keen OS, 2017. Doxycycline transformation and emergence of antibacterially active products during water disinfection with chlorine. Environmental Science: Water Research and Technology, 3, 1086–1094. [Google Scholar]
- Kennedy Neth NL, Carlin CM and Keen OS, 2019. Emerging investigator series: transformation of common antibiotics during water disinfection with chlorine and formation of antibacterially active products. Environmental Science: Water Research and Technology, 5, 1222–1233. [Google Scholar]
- Kharel S, Stapf M, Miehe U, Ekblad M, Cimbritz M, Falås P, Nilsson J, Sehlén R and Bester K, 2020. Ozone dose dependent formation and removal of ozonation products of pharmaceuticals in pilot and full‐scale municipal wastewater treatment plants. Science of the Total Environment, 731, 139064. [DOI] [PubMed] [Google Scholar]
- Kim TK, Kim T, Cha Y and Zoh KD, 2020. Energy‐efficient erythromycin degradation using UV‐LED (275 nm)/chlorine process: radical contribution, transformation products, and toxicity evaluation. Water Research, 185, 116159. [DOI] [PubMed] [Google Scholar]
- Kosma CI, Lambropoulou DA and Albanis TA, 2016. Analysis, occurrence, fate and risks of proton pump inhibitors, their metabolites and transformation products in aquatic environment: a review. Science of the Total Environment, 569–570, 732–750. [DOI] [PubMed] [Google Scholar]
- Kråkström M, Saeid S, Tolvanen P, Kumar N, Salmi T, Kronberg L and Eklund P, 2020. Ozonation of carbamazepine and its main transformation products: product determination and reaction mechanisms. Environmental Science and Pollution Research, 27, 23258–23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kråkström M, Saeid S, Tolvanen P, Kumar N, Salmi T, Kronberg L and Eklund P, 2021. Identification and Quantification of Transformation Products Formed during the Ozonation of the Non‐steroidal Anti‐inflammatory Pharmaceuticals Ibuprofen and Diclofenac. Science and Engineering, Ozone. [Google Scholar]
- Le Roux J, Gallard H and Croué JP, 2011. Chloramination of nitrogenous contaminants (pharmaceuticals and pesticides): NDMA and halogenated DBPs formation. Water Research, 45, 3164–3174. [DOI] [PubMed] [Google Scholar]
- Le Roux JL, Gallard H, Croué JP, Papot S and Deborde M, 2012. NDMA formation by chloramination of ranitidine: kinetics and mechanism. Environmental Science and Technology, 46, 11095–11103. [DOI] [PubMed] [Google Scholar]
- Lebedev AT, Bavcon Kralj M, Polyakova OV, Detenchuk EA, Pokryshkin SA and Trebše P, 2020. Identification of avobenzone by‐products formed by various disinfectants in different types of swimming pool waters. Environment International, 137, 105495. [DOI] [PubMed] [Google Scholar]
- Lee M, Blum LC, Schmid E, Fenner K and von Gunten U, 2017. A computer‐based prediction platform for the reaction of ozone with organic compounds in aqueous solution: kinetics and mechanisms. Environmental Science. Processes and Impacts, 19, 465–476. [DOI] [PubMed] [Google Scholar]
- Lee YM, Lee G, Kim MK and Zoh KD, 2020. Kinetics and degradation mechanism of Benzophenone‐3 in chlorination and UV/chlorination reactions. Chemical Engineering Journal, 393, 124780. [Google Scholar]
- Lee JY, Lee YM, Kim TK, Choi K and Zoh KD, 2021. Degradation of cyclophosphamide during UV/chlorine reaction: kinetics, by products, and their toxicity. Chemosphere, 268, 128817. [DOI] [PubMed] [Google Scholar]
- Lege S, Eisenhofer A, Heras JEY and Zwiener C, 2019. Identification of transformation products of denatonium – occurrence in wastewater treatment plants and surface waters. Science of the Total Environment, 686, 140–150. [DOI] [PubMed] [Google Scholar]
- León C, Boix C, Beltrán E, Peñuela G, López F, Sancho JV and Hernández F, 2019. Study of cyanotoxin degradation and evaluation of their transformation products in surface waters by LC‐QTOF MS. Chemosphere, 229, 538–548. [DOI] [PubMed] [Google Scholar]
- Li J, Ma LY, Xu L and Shi ZG, 2015. A novel two‐dimensional liquid‐chromatography method for online prediction of the toxicity of transformation products of benzophenones after water chlorination. Analytical and Bioanalytical Chemistry, 407, 6137–6148. [DOI] [PubMed] [Google Scholar]
- Li J, Ma LY and Xu L, 2016. Transformation of benzophenone‐type UV filters by chlorine: kinetics, products identification and toxicity assessments. Journal of Hazardous Materials, 311, 263–272. [DOI] [PubMed] [Google Scholar]
- Li AJ, Wu P, Law JCF, Chow CH, Postigo C, Guo Y and Leung KSY, 2017. Transformation of acesulfame in chlorination: Kinetics study, identification of by products, and toxicity assessment. Water Research, 117, 157–166. [DOI] [PubMed] [Google Scholar]
- Li LP, Kwan JKC and Yeung KL, 2019. An investigation of the transformation, kinetics and bioactivity of ozone treatment of DEET in water. Chemical Engineering Journal, 368, 10–17. [Google Scholar]
- Lim S, McArdell CS and von Gunten U, 2019. Reactions of aliphatic amines with ozone: kinetics and mechanisms. Water Research, 157, 514–528. [DOI] [PubMed] [Google Scholar]
- Link L, von der Ohe PC, Voß K and Schäfer RB, 2017. Comparison of dilution factors for German wastewater treatment plant effluents in receiving streams to the fixed dilution factor from chemical risk assessment. Science of the Total Environment, 598, 805–813. [DOI] [PubMed] [Google Scholar]
- Liu W, Wei D, Liu Q and Du Y, 2016. Transformation pathways and acute toxicity variation of 4‐hydroxyl benzophenone in chlorination disinfection process. Chemosphere, 154, 491–498. [DOI] [PubMed] [Google Scholar]
- Liu Q, Xu X, Wang L and Wang D, 2020. Transformation reactivity of parent polycyclic aromatic hydrocarbons and the formation trend of halogenated polycyclic aromatic hydrocarbons in the presence of bromide ion during chlorination. Chemical Engineering Journal, 400, 125901. [Google Scholar]
- Ma L, Li J and Xu L, 2017. Aqueous chlorination of fenamic acids: kinetic study, transformation products identification and toxicity prediction. Chemosphere, 175, 114–122. [DOI] [PubMed] [Google Scholar]
- Ma X, Chen H, Chen R and Hu X, 2022. Direct and activated chlorine dioxide oxidation for micropollutant abatement: a review on kinetics, reactive sites, and degradation pathway. Water (Switzerland), 14. [Google Scholar]
- Magdeburg A, Stalter D, Schlüsener M, Ternes T and Oehlmann J, 2014. Evaluating the efficiency of advanced wastewater treatment: target analysis of organic contaminants and (geno‐)toxicity assessment tell a different story. Water Research, 50, 35–47. [DOI] [PubMed] [Google Scholar]
- Mairinger T, Loos M and Hollender J, 2021. Characterization of water‐soluble synthetic polymeric substances in wastewater using LC‐HRMS/MS. Water Research, 190, 116745. [DOI] [PubMed] [Google Scholar]
- Marzo M, Kulkarni S, Manganaro A, Roncaglioni A, Wu S, Barton‐Maclaren TS and Benfenati E, 2016. Integrating in silico models to enhance predictivity for developmental toxicity. Toxicology, 370, 127–137. 10.1016/j.tox.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Matsushita T, Honda S, Kuriyama T, Fujita Y, Kondo T, Matsui Y, Shirasaki N, Takanashi H and Kameya T, 2018. Identification of mutagenic transformation products generated during oxidation of 3‐methyl‐4‐nitrophenol solutions by orbitrap tandem mass spectrometry and quantitative structure–activity relationship analyses. Water Research, 129, 347–356. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Fujita Y, Omori K, Huang Y, Matsui Y and Shirasaki N, 2020. Effect of chlorination on anti‐acetylcholinesterase activity of organophosphorus insecticide solutions and contributions of the parent insecticides and their oxons to the activity. Chemosphere, 261, 127743. [DOI] [PubMed] [Google Scholar]
- Miao HF, Han HH, Ji XP, Lu MF, Huang ZX and Ruan WQ, 2017. Oxidative transformation of oxcarbazepine by Cl2, ClO2 and O3: characteristics and pathways. Water Science and Technology: Water Supply, 17, 84–94. [Google Scholar]
- Michel A, Armbruster D, Benz‐Birck A, Deppermann N, Doetzer R, Flörs M, Frericks M, Li S, Gebler S, Schröder T and Seitz W, 2022. Proposal for a tiered approach to evaluate the risk of transformation products formed from pesticide residues during drinking water treatment. Environmental Sciences Europe, 34, 110. [Google Scholar]
- Mohaupt V, Völker J, Altenburger R, Birk S, Kirst I, Kühnel D and Whalley C, 2020. Pesticides in European rivers, lakes and groundwaters–Data assessment. ETC/ICM Technical Report 1/2020: European Topic Centre on Inland, Coastal and Marine waters, 86 pp.
- Molé RA, Good CJ, Stebel EK, Higgins JF, Pitell SA, Welch AR, Minarik TA, Schoenfuss HL and Edmiston PL, 2019. Correlating effluent concentrations and bench‐scale experiments to assess the transformation of endocrine active compounds in wastewater by UV or chlorination disinfection. Chemosphere, 226, 565–575. [DOI] [PubMed] [Google Scholar]
- Mpatani FM, Aryee AA, Kani AN, Han R, Li Z, Dovi E and Lingbo Q, 2021. A review of treatment techniques applied for selective removal of emerging pollutant‐trimethoprim from aqueous systems. Journal of Cleaner Production, 308, 127359, ISSN 0959‐6526. 10.1016/j.jclepro.2021.127359. Available online: https://www.sciencedirect.com/science/article/pii/S095965262101578X [DOI] [Google Scholar]
- Mukhopadhyay A, Duttagupta S and Mukherjee A, 2022. Emerging organic contaminants in global community drinking water sources and supply: a review of occurrence, processes and remediation. Journal of Environmental Chemical Engineering, 10, 107560. [Google Scholar]
- Nassar R, Rifai A, Trivella A, Mazellier P, Mokh S and Al‐Iskandarani M, 2018. Aqueous chlorination of sulfamethazine and sulfamethoxypyridazine: kinetics and transformation products identification. Journal of Mass Spectrometry, 53, 614–623. [DOI] [PubMed] [Google Scholar]
- Negreira N, Regueiro J, López de Alda M and Barceló D, 2015. Degradation of the anticancer drug erlotinib during water chlorination: non‐targeted approach for the identification of transformation products. Water Research, 85, 103–113. [DOI] [PubMed] [Google Scholar]
- Nika MC, Bletsou AA, Koumaki E, Noutsopoulos C, Mamais D, Stasinakis AS and Thomaidis NS, 2017. Chlorination of benzothiazoles and benzotriazoles and transformation products identification by LC‐HR‐MS/MS. Journal of Hazardous Materials, 323, 400–413. [DOI] [PubMed] [Google Scholar]
- Nika MC, Aalizadeh R and Thomaidis NS, 2021. Non‐target trend analysis for the identification of transformation products during ozonation experiments of citalopram and four of its biodegradation products. Journal of Hazardous Materials, 419, 126401. [DOI] [PubMed] [Google Scholar]
- Nürenberg G, Kunkel U, Wick A, Falås P, Joss A and Ternes TA, 2019. Nontarget analysis: a new tool for the evaluation of wastewater processes. Water Research, 163, 114842. [DOI] [PubMed] [Google Scholar]
- OECD , 2004. OECD principles for the validation, for regulatory purposes, of (quantitative) structure‐activity relationships models. Biotechnology, (November), 1–2. Available online: www.oecd.org/dataoecd/33/37/37849783.pdf
- OECD , 2007. Guidance document on the validation of (Quantitative) Structure‐Activity Relationship [(Q)SAR] models 30 March 2007. Series on Testing and Assessment Number 69. ENV/JM/MONO(2007)2. Organisation for Economic Cooperation and Development, Paris, France. Available online: https://www.oecd.org
- OECD , 2017. Guidance on Grouping of Chemicals, Second Edition, OECD Series on Testing and Assessment, No. 194, OECD Publishing, Paris, 141 pp. Available online: 9789264274679-en.pdf(oecd-ilibrary.org).
- OECD , 2020. Overview of Concepts and Available Guidance related to Integrated Approaches to Testing and Assessment (IATA), OECD Series on Testing and Assessment, No. 329, Environment, Health and Safety, Environment Directorate, OECD. Available online: Overview of Concepts and Available Guidance related to Integrated Approaches to Testing and Assessment (IATA) (oecd.org).
- OECD , 2022. [Internet] Grouping of Chemicals: Chemical Categories and Read‐Across. Available online: Grouping of Chemicals: Chemical Categories and Read‐Across – OECD.
- OECD 471 , 2020. OECD Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 26 June 2020. Available online: Test No. 471: Bacterial Reverse Mutation Test (oecd-ilibrary.org).
- OECD 474 , 2016. OECD Test No. 474: Mammalian Eryhrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 29 July 2016. Available online: 9789264264762-en.pdf(oecd-ilibrary.org).
- OECD 475 , 2016. OECD Test No. 475: Mammalian Bone Marrow Chromosomal Aberration test. OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 29 July 2016. Available online: 9789264264786-en.pdf(oecd-ilibrary.org).
- OECD 476 , 2016. OECD Test No. 476: In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris 29 July 2016. Available online: 9789264264809-en.pdf(oecd-ilibrary.org).
- OECD 487 , 2016. OECD Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 29 July 2016. Available online: 9789264264861-en.pdf(oecd-ilibrary.org) [Google Scholar]
- OECD 488 , 2022. OECD Test No. 488: Transgenic Rodent Somatic Germ Cell Mutation Assays, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris 29 July 2016. Available online: Test No. 488 on Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays (oecd‐ilibrary.org).
- OECD 489 , 2016. OECD Test No. 489: In vivo Mammalian Alkaline Comet Assay. OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 29 July 2016. Available online: 9789264264885-en.pdf(oecd-ilibrary.org) [Google Scholar]
- OECD 490 , 2016. OECD Test No. 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris: 29 July 2016. Available online: 9789264264908-en.pdf(oecd-ilibrary.org) [Google Scholar]
- Ort C and Siegrist H, 2009. Assessing wastewater dilution in small rivers with high resolution conductivity probes. Water Science and Technology, 59, 1593–1601. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Hernández ML, Enrique SS, Dantan E and Castrejon‐Godinez ML, 2013. Pesticide biodegradation: mechanisms, genetics and strategies to enhance the process, Biodegradation. Life of Science, 251–287. [Google Scholar]
- Osawa RA, Carvalho AP, Monteiro OC, Oliveira MC and Florêncio MH, 2019a. Degradation of duloxetine: identification of transformation products by UHPLC‐ESI(+)‐HRMS/MS, in silico toxicity and wastewater analysis. Journal of Environmental Sciences (China), 82, 113–123. [DOI] [PubMed] [Google Scholar]
- Osawa RA, Carvalho AP, Monteiro OC, Oliveira MC and Florêncio MH, 2019b. Transformation products of citalopram: identification, wastewater analysis and in silico toxicological assessment. Chemosphere, 217, 858–868. [DOI] [PubMed] [Google Scholar]
- Pan Y, Cheng S, Yang X, Ren J, Fang J, Shang C, Song W, Lian L and Zhang X, 2017. UV/chlorine treatment of carbamazepine: transformation products and their formation kinetics. Water Research, 116, 254–265. [DOI] [PubMed] [Google Scholar]
- Pan X, Wei J, Zou M, Chen J, Qu R and Wang Z, 2021. Products distribution and contribution of (de)chlorination, hydroxylation and coupling reactions to 2,4‐dichlorophenol removal in seven oxidation systems. Water Research, 194, 116916. [DOI] [PubMed] [Google Scholar]
- Park M and Snyder SA, 2020. Statistical profiling for identifying transformation products in an engineered treatment process. Chemosphere, 251, 126401. [DOI] [PubMed] [Google Scholar]
- Popov M, Kragulj Isakovski M, Molnar Jazić J, Tubić A, Watson M, Šćiban M and Agbaba J, 2021. Fate of natural organic matter and oxidation/disinfection by‐products formation at a full‐scale drinking water treatment plant. Environmental Technology (United Kingdom), 42, 3475–3486. [DOI] [PubMed] [Google Scholar]
- Ra J, Yoom H, Son H, Hwang TM and Lee Y, 2019. Transformation of an amine moiety of atenolol during water treatment with Chlorine/UV: reaction kinetics, products, and mechanisms. Environmental Science and Technology, 53, 7653–7662. [DOI] [PubMed] [Google Scholar]
- Rallo R, Espinosa G and Giralt F, 2005. Using an ensemble of neural based QSARs for the prediction of toxicological properties of chemical contaminants. Process Safety and Environmental Protection, 83, 387–392. 10.1205/psep.04389 [DOI] [Google Scholar]
- Ridgway H, Orbell J and Gray S, 2018. Chlorination of oxybenzone and prediction of transformation products using non‐equilibrium “forced” molecular dynamics. Desalination and Water Treatment, 114, 31–50. [Google Scholar]
- Rivas FJ, Solís RR, Beltrán FJ and Gimeno O, 2019. Sunlight driven photolytic ozonation as an advanced oxidation process in the oxidation of bezafibrate, cotinine and iopamidol. Water Research, 151, 226–242. [DOI] [PubMed] [Google Scholar]
- Roncaglioni A, Toropov AA, Toropova AP and Benfenati E, 2013. In silico methods to predict drug toxicity. Current Opinion in Pharmacology. Elsevier Ltd. 10.1016/j.coph.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Schollée JE, Bourgin M, von Gunten U, McArdell CS and Hollender J, 2018. Non‐target screening to trace ozonation transformation products in a wastewater treatment train including different post‐treatments. Water Research, 142, 267–278. [DOI] [PubMed] [Google Scholar]
- Schollée JE, Hollender J and McArdell CS, 2021. Characterization of advanced wastewater treatment with ozone and activated carbon using LC‐HRMS based non‐target screening with automated trend assignment. Water Research, 200, 117209. [DOI] [PubMed] [Google Scholar]
- Seiwert B, Nihemaiti M, Bauer C, Muschket M, Sauter D, Gnirss R and Reemtsma T, 2021. Ozonation products from trace organic chemicals in municipal wastewater and from metformin: peering through the keyhole with supercritical fluid chromatography‐mass spectrometry. Water Research, 196, 117024. [DOI] [PubMed] [Google Scholar]
- Sengar A and Vijayanandan A, 2021. Comprehensive review on iodinated X‐ray contrast media: complete fate, occurrence, and formation of disinfection by products. Science of the Total Environment, 769, 144846. [DOI] [PubMed] [Google Scholar]
- Shad A, Li C, Zuo J, Liu J, Dar AA and Wang Z, 2018. Understanding the ozonated degradation of sulfadimethoxine, exploration of reaction site, and classification of degradation products. Chemosphere, 212, 228–236. [DOI] [PubMed] [Google Scholar]
- Shao KL, Ye ZX, Huang H and Yang X, 2020. ClO2 pre‐oxidation impacts the formation and nitrogen origins of dichloroacetonitrile and dichloroacetamide during subsequent chloramination. Water Research, 186, 116313. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ahmad J and Flora SJS, 2018. Application of advanced oxidation processes and toxicity assessment of transformation products. Environmental Research, 167, 223–233. [DOI] [PubMed] [Google Scholar]
- Sieira BJ, Montes R, Touffet A, Rodil R, Cela R, Gallard H and Quintana JB, 2020. Chlorination and bromination of 1,3‐diphenylguanidine and 1,3‐di‐o‐tolylguanidine: kinetics, transformation products and toxicity assessment. Journal of Hazardous Materials, 385, 121590. [DOI] [PubMed] [Google Scholar]
- Sieira BJ, Quintana JB, Cela R and Rodil R, 2021. Reaction of phenazone‐type drugs and metabolites with chlorine and monochloramine. Science of the Total Environment, 757, 143770. [DOI] [PubMed] [Google Scholar]
- Sinha R, Gupta AK and Ghosal PS, 2021. A review on Trihalomethanes and Haloacetic acids in drinking water: global status, health impact, insights of control and removal technologies. Journal of Environmental Chemical Engineering, 9, 106511. [Google Scholar]
- Sivey JD, McCullough CE and Roberts AL, 2010. Chlorine monoxide (Cl2O) and molecular chlorine (Cl2) as active chlorinating agents in reaction of dimethenamid with aqueous free chlorine. Environmental Science and Technology, 44, 3357–3362. [DOI] [PubMed] [Google Scholar]
- Solís RR, Gimeno O, Rivas FJ and Beltrán FJ, 2019. Simulated solar driven photolytic ozonation for the oxidation of aqueous recalcitrant‐to‐ozone tritosulfuron. Transformation products and toxicity. Journal of Environmental Management, 233, 513–522. [DOI] [PubMed] [Google Scholar]
- Studziński W, Gackowska A and Kudlek E, 2021. Determination of environmental properties and toxicity of octyl‐dimethyl‐para‐aminobenzoic acid and its degradation products. Journal of Hazardous Materials, 403, 123856. [DOI] [PubMed] [Google Scholar]
- Sun J, Bu L, Chen S, Lu X, Wu Y, Shi Z and Zhou S, 2019a. Oxidation of Microcystic‐LR via the solar/chlorine process: radical mechanism, pathways and toxicity assessment. Ecotoxicology and Environmental Safety, 183, 109509. [DOI] [PubMed] [Google Scholar]
- Sun X, Wei D, Liu W, Geng J, Liu J and Du Y, 2019b. Formation of novel disinfection by‐products chlorinated benzoquinone, phenyl benzoquinones and polycyclic aromatic hydrocarbons during chlorination treatment on UV filter 2,4‐dihydroxybenzophenone in swimming pool water. Journal of Hazardous Materials, 367, 725–733. [DOI] [PubMed] [Google Scholar]
- Tawk A, Deborde M, Labanowski J and Gallard H, 2015. Chlorination of the β‐triketone herbicides tembotrione and sulcotrione: kinetic and mechanistic study, transformation products identification and toxicity. Water Research, 76, 132–142. [DOI] [PubMed] [Google Scholar]
- Tentscher PR, Bourgin M and Von Gunten U, 2018. Ozonation of para‐substituted phenolic compounds yield sp‐benzoquinones, other cyclic α,β‐unsaturated ketones, and substituted catechols. Environmental Science and Technology, 52, 4763–4773. [DOI] [PubMed] [Google Scholar]
- Tentscher PR, Lee M and Von Gunten U, 2019. Micropollutant oxidation studied by quantum chemical computations: methodology and applications to thermodynamics, kinetics, and reaction mechanisms. Accounts of Chemical Research, 52, 605–614. [DOI] [PubMed] [Google Scholar]
- Trebše P, Polyakova OV, Baranova M, Kralj MB, Dolenc D, Sarakha M, Kutin A and Lebedev AT, 2016. Transformation of avobenzone in conditions of aquatic chlorination and UV‐irradiation. Water Research, 101, 95–102. [DOI] [PubMed] [Google Scholar]
- Ul'yanovskii NV, Kosyakov DS, Sypalov SA, Varsegov IS, Shavrina IS and Lebedev AT, 2022. Antiviral drug Umifenovir (Arbidol) in municipal wastewater during the COVID‐19 pandemic: estimated levels and transformation. Science of the Total Environment, 805, 150380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ur Rehman SW, Wang H, Yao W, Deantes‐Espinosa VM, Wang B, Huang J, Deng S, Yu G and Wang Y, 2019. Ozonation of the algaecide irgarol: kinetics, transformation products, and toxicity. Chemosphere, 236, 124374. [DOI] [PubMed] [Google Scholar]
- Verlicchi P, Al Aukidy M, Jelic A, Petrović M and Barcelóce D, 2014. Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: a case study of a catchment area in the Po Valley (Italy). Science of The Total Environment, 470–471, 844–854. 10.1016/j.scitotenv.2013.10.026 [DOI] [PubMed] [Google Scholar]
- Voigt M, Bartels I, Schmiemann D, Votel L, Hoffmann‐Jacobsen K and Jaeger M, 2021. Metoprolol and its degradation and transformation products using AOPS‐assessment of aquatic ecotoxicity using QSAR. Molecules, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völker J, Stapf M, Miehe U and Wagner M, 2019. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon. Environmental Science and Technology, 53, 7215–7233. [DOI] [PubMed] [Google Scholar]
- Wang M and Helbling DE, 2016. A non‐target approach to identify disinfection byproducts of structurally similar sulfonamide antibiotics. Water Research, 102, 241–251. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Wang Y and Jiang G, 2019. Suspect screening analysis of the occurrence and removal of micropollutants by GC‐QTOF MS during wastewater treatment processes. Journal of Hazardous Materials, 376, 153–159. [DOI] [PubMed] [Google Scholar]
- Wang X, Yu N, Yang J, Jin L, Guo H, Shi W, Zhang X, Yang L, Yu H and Wei S, 2020. Suspect and non‐target screening of pesticides and pharmaceuticals transformation products in wastewater using QTOF‐MS. Environmental International, 137, 105599. 10.1016/j.envint.2020.105599 [DOI] [PubMed] [Google Scholar]
- Wang J, de Ridder D, van der Wal A and Sutton NB, 2021a. Harnessing biodegradation potential of rapid sand filtration for organic micropollutant removal from drinking water: a review. Critical Reviews in Environmental Science and Technology, 51, 2086‐2118.lege. [Google Scholar]
- Wang N, Lv G, He L and Sun X, 2021b. New insight into photodegradation mechanisms, kinetics and health effects of p‐nitrophenol by ozonation in polluted water. Journal of Hazardous Materials, 403, 123805. [DOI] [PubMed] [Google Scholar]
- Wenk, J. , Zoumpouli G. A., Chew J. Y. M., 2020. Ozonation in the framework of sustainable future water management. Advances in Science, Technology and Innovation. 99–102. [Google Scholar]
- WHO , 2011. Guidelines for drinking‐water quality. 4th Edition. Available online: https://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf [Google Scholar]
- WHO/IPCS , 2009. Assessment of combined exposures to multiple chemicals: report of a WHO/IPCS international workshop on aggregate/cumulative risk assessment. (IPCS harmonization project document; no. 7) ISBN 978 92 4 156383 3.
- Wicker J, Lorsbach T, Gütlein M, Schmid E, Latino D, Kramer S and Fenner K, 2016. enviPath ‐ the environmental contaminant biotransformation pathway resource. Nucleic Acids Research, 44, D502–D508. 10.1093/nar/gkv1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirzberger V, Klein M, Woermann M, Lutze HV, Sures B and Schmidt TC, 2021. Matrix composition during ozonation of N‐containing substances may influence the acute toxicity towards Daphnia magna. Science of the Total Environment, 765, 142727. [DOI] [PubMed] [Google Scholar]
- Worth A, Fuart‐Gatnik M, Lapenna S and Serafimova R, 2011. Applicability of QSAR analysis in the evaluation of developmental and neurotoxicity effects for the assessment of the toxicological relevance of metabolites and degradates of pesticide active substances for dietary risk assessment. EFSA Supporting Publications, 8. 10.2903/sp.efsa.2011.en-169 [DOI] [Google Scholar]
- Xiang Y, Deng Z, Yang X, Shang C and Zhang X, 2019. Transformation of adenine and cytosine in chlorination — an ESI‐tqMS investigation. Chemosphere, 234, 505–512. [DOI] [PubMed] [Google Scholar]
- Xu X, Xiao R, Dionysiou DD, Spinney R, Fu T, Li Q, Wang Z, Wang D and Wie Z, 2018. Kinetics and mechanisms of the formation of chlorinated and oxygenated polycyclic aromatic hydrocarbons during chlorination. Chemical Engineering Journal, 351, 248–257. [Google Scholar]
- Xu ZB, Wang WL, Huang N, Wu QY, Lee MY and Hu HY, 2019. 2‐Phosphonobutane‐1,2,4‐tricarboxylic acid (PBTCA) degradation by ozonation: kinetics, phosphorus transformation, anti‐precipitation property changes and phosphorus removal. Water Research, 148, 334–343. [DOI] [PubMed] [Google Scholar]
- Yan X, Chen H, Lin T, Chen W, Xu H and Tao H, 2020. UV/chlorination of sulfamethazine (SMZ) and other prescription drugs: kinetics, transformation products and insights into the combined toxicological assessment. Environmental Technology (United Kingdom): 1–54. [DOI] [PubMed]
- Yang B, Xu C, Kookana RS, Williams M, Du J, Ying G and Gu F, 2018. Aqueous chlorination of benzodiazepines diazepam and oxazepam: kinetics, transformation products and reaction pathways. Chemical Engineering Journal, 354, 1100–1109. [Google Scholar]
- Yang Y, Shi J, Yang Y, Yin J, Zhang J and Shao B, 2019. Transformation of sulfamethazine during the chlorination disinfection process: transformation, kinetics, and toxicology assessment. Journal of Environmental Sciences (China), 76, 48–56. [DOI] [PubMed] [Google Scholar]
- Yang B, Peng T, Cai WW and Ying GG, 2020. Transformation of diazepam in water during UV/chlorine and simulated sunlight/chlorine advanced oxidation processes. Science of the Total Environment, 746, 141332. [DOI] [PubMed] [Google Scholar]
- Yang T, Mai J, Wu S, Liu C, Tang L, Mo Z, Zhang M, Guo L, Liu M and Ma J, 2021. UV/chlorine process for degradation of benzothiazole and benzotriazole in water: efficiency, mechanism and toxicity evaluation. Science of the Total Environment, 760, 144304. [DOI] [PubMed] [Google Scholar]
- Ye B, Liu Z, Zhu X, Wu H, Liang Z, Wang W, Wu Q, Hu H and Zhang X, 2021. Degradation of atrazine (ATZ) by ammonia/chlorine synergistic oxidation process. Chemical Engineering Journal, 415, 128841. [Google Scholar]
- Yin J, Niu Y and Shao B, 2017. Products of methotrexate during chlorination. Journal of Environmental Sciences (China), 55, 100–108. [DOI] [PubMed] [Google Scholar]
- Yin K, He Q, Liu C, Deng Y, Wei Y, Chen S, Liu T and Luo S, 2018. Prednisolone degradation by UV/chlorine process: influence factors, transformation products and mechanism. Chemosphere, 212, 56–66. [DOI] [PubMed] [Google Scholar]
- Yoom H, Shin J, Ra J, Son H, Ryu D, Kim C and Lee Y, 2018. Transformation of methylparaben during water chlorination: effects of bromide and dissolved organic matter on reaction kinetics and transformation pathways. Science of the Total Environment, 634, 677–686. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang X, Yang H and Xie YF, 2016. Chlorination of oxybenzone: kinetics, transformation, disinfection by products formation, and genotoxicity changes. Chemosphere, 154, 521–527. [DOI] [PubMed] [Google Scholar]
- Zhang H, Guo C, Lv J, Hou S, Zhang Y, Gao J and Xu J, 2020a. Aqueous chlorination of ephedrine: kinetic, reaction mechanism and toxicity assessment. Science of the Total Environment, 740, 140146. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin T, Chen H, Chen W, Xu H and Tao H, 2020b. DNA pyrimidine bases in water: insights into relative reactivity, byproducts formation and combined toxicity during chlorination. Science of the Total Environment, 717, 137205. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wei D, Yu Q and Du Y, 2021. Characterization of UV and chlorine contributions to transformation of 2,3,4‐trihydroxybenzophenone under combined UV‐chlorine treatment. Chemosphere, 263, 128310. [DOI] [PubMed] [Google Scholar]
- Zhao B, Zhou J and Nakada N, 2021. N‐nitrosodimethylamine formation potential (NDMA‐FP) of ranitidine remains after chlorination and/or photo‐irradiation: identification of transformation products in combination with NDMA‐FP test. Chemosphere, 267, 129200. [DOI] [PubMed] [Google Scholar]
- Zoumpouli GA, Zhang Z, Wenk J and Prasse C, 2021. Aqueous ozonation of furans: kinetics and transformation mechanisms leading to the formation of α,β‐unsaturated dicarbonyl compounds. Water Research, 203, 117487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Public consultation on the draft ECHA/EFSA Guidance on the impact of water treatment processes on residues of active substances or their metabolites in water abstracted for the production of drinking water


