Abstract
The incidence of breast cancer increases annually, and it has become common within families of breast cancer patients. Interleukin-2 activates cytotoxic T lymphocytes, which are important for cancer immunity. To identify markers of increased familial breast cancer risk, soluble interleukin-2 receptor levels and immunologic factors were investigated in familial breast cancer and non-familial breast cancer patients. Of 106 untreated breast cancer patients in this study, 24 had familial breast cancer and 82 had non-familial breast cancer. The patients’ soluble interleukin-2 receptor, interleukin-10, vascular endothelial growth factor, interleukin-17, regulatory T cell, myeloid-derived suppressor cell, white blood cell, and C-reactive protein levels, and their neutrophil-to-lymphocyte ratios were measured, and their prognoses were compared according to the soluble interleukin-2 receptor levels. Additionally, postoperative tissues from the patients with high soluble interleukin-2 receptor levels were stained with programmed cell death ligand 1 and cluster of differentiation 8. The soluble interleukin-2 receptor level in the familial breast cancer patients was significantly higher, and it showed significantly stronger correlations with the neutrophil-to-lymphocyte ratio and the interleukin-10, vascular endothelial growth factor, interleukin-17, regulatory T cell, myeloid-derived suppressor cell, white blood cell, and C-reactive protein levels, than in the non-familial breast cancer patients. The regulatory T cell and myeloid-derived suppressor cell levels were significantly higher in the patients with high soluble interleukin-2 receptor levels, and the overall survival and disease-free-survival rates were significantly worse for the familial breast cancer patients than for the non-familial breast cancer patients. Triple-negative breast cancer tissues from the familial breast cancer patients with high soluble interleukin-2 receptor levels stained well for programmed cell death ligand 1 and cluster of differentiation 8. Soluble interleukin-2 receptor levels can be used to predict the prognosis of familial breast cancer patients. Prospectively identifying patients who are less likely to have non-familial breast cancer is vital for improving their overall survival.
Keywords: Familial breast cancer, non-familial breast cancer, soluble interleukin-2 receptor, prognosis, immunity, triple-negative breast cancer
Introduction
Although most cases of breast cancer (BC) occur sporadically in individuals, cases of familial BC (FBC), which refers to a cluster of BC occurrences within a family, are becoming more common. In some of these families, the underlying genetic cause is unknown; however, many FBC cases are caused by mutations in the BRCA1, BRCA2, PTEN, TP53, CDH1, or STK11 genes. Each of these genes is associated with a unique hereditary cancer syndrome. Additional genes, such as CHEK2, BRIP1, RAD51, and ATM can also be associated with some cases of breast and/or gynecologic cancers. 1 Interleukin (IL)-2, which is a cytokine that is important for lymphocyte development, proliferation, and function, is produced by helper cells that differentiate from naïve cells upon stimulation by interferon-γ (IFN-γ) or IL-12. Among other actions, IL-2 is involved in T cell proliferation and activation, promotion of B cell proliferation and antibody production, activation of monocytes/macrophages, and the proliferation/activation of natural killer cells. 2 Additionally, IL-2 is believed to be required for the maintenance of regulatory T cells (Tregs), which release the inhibitory cytokine IL-10 and exhibit immunosuppressive effects. 3 Some BC patients have been shown to have increased IL-2 and soluble IL-2 receptor (sIL-2R) levels.4–7
In this study, the role of sIL-2R was examined in patients with FBC. A deficient DNA mismatch repair (MMR) function due to an MMR gene mutation increases the number of somatic gene mutations and the tumor mutational burden (TMB), leading to the release of neoantigens from cancer cells with many gene mutations. Dendritic cells incorporate and degrade these neoantigens, which are long peptides that bind to human leukocyte antigen (HLA) class II molecules present on the cell surface. The neoantigens are recognized by naïve cluster of differentiation (CD) 4 + T cells, which then produce IL-2. The simultaneously incorporated and degraded short peptides bind to HLA class I molecules on the cell surface and are recognized by naïve CD8 + T cells, which express IL-2R. IL-2 binds to IL-2R to induce the differentiation and proliferation of cytotoxic T lymphocytes (CTLs), which are cancer cell-specific immune cells. Upon activation, CTLs express immune checkpoint molecules, such as programmed cell death 1 (PD-1), on the surface. They also produce IFN-γ, which attacks cancer cells; this induces the cancer cells to express programmed cell death ligand 1 (PD-L1), which suppresses CTL activity.8–10 PD-L1 is a cell surface molecule that is expressed by various cell types, including antigen-presenting cells and vascular endothelial cells; it is also expressed by human tumor cells. 11 Interestingly, BRCA1-deficient (but not BRCA2-deficient) BCs are associated with increased PD-L1 and PD-1 expression, a higher abundance of tumor-infiltrating immune cells, and enrichment of the T cell-inflamed signature. 12
In the present study, the levels of sIL-2R, IL-10, vascular endothelial growth factor (VEGF), IL-17, immunosuppressive Tregs, myeloid-derived suppressor cells (MDSCs), white blood cells (WBCs), and C-reactive protein (CRP), as well as the neutrophil-to-lymphocyte ratios (NLRs), in FBC and non-familial BC (NFBC) patients were measured and compared. Furthermore, PD-L1 and CD8 + staining were performed on tissues from FBC patients with high sIL-2R levels and triple-negative BC (TNBC).
Methods
Patient information
A total of 11 healthy volunteers and 106 patients with histologically confirmed BC who were treated in the Department of Breast Cancer Surgery at Fukushima Medical University (Fukushima, Japan) between January 2011 and June 2016 were enrolled in this study. Staging was performed in accordance with the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis.13,14 Written informed consent was obtained from all the enrolled patients and healthy volunteers. All the procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation at Fukushima Medical University and the Helsinki Declaration of 1975, as revised in 2000. This study was approved by the institutional review board of Fukushima Medical University (approval #-072110) and the local ethics committee (Ethical Review Board of Fukushima Medical University, 2011–2016).
Lynch first defined FBC in 1985. Approximately 80% of BC cases are suggested to be sporadic, with no indications of inheritance. The term “familial” has been applied to the rest of the BC patient population (∼15–20%) and defined as an aggregation of BC within a family (two or more first-degree relatives). Only about 5% of these FBC cases have been proven to be hereditary BC, however. 15
In the current study, FBC was defined as a proband with BC and histologically proven BC in at least two relatives, one of whom is a first-degree relative. The 2020 update of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Genetic/Familial High-Risk Assessment: Breast and Ovarian (now Breast, Ovarian, and Pancreatic) has undergone some major revisions. 16 These revisions include the reorganization of the guidelines according to disease and syndrome type, inclusion of criteria for high-penetrance genes associated with breast and ovarian cancer beyond BRCA1/2, and the addition of pancreatic cancer. The updated guidelines provide recommendations for genetic testing for hereditary cancer syndromes and risk management recommendations for patients diagnosed with syndromes associated with an increased risk of these cancers. In our study, hereditary cancer syndromes were included in the FBC group, and NFBC was defined as only the proband having BC.
Blood samples were collected from the study population, and 1 × 106 peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll density gradient centrifugation method. Aliquots of the PBMCs were then cryopreserved in a freezing medium. Plasma was also separated by centrifugation and stored at −80°C until it could be analyzed by flow cytometry.
Cytokine production
The serum concentrations of sIL-2R, IL-10, VEGF, and IL-17 in the supernatant were measured using an enzyme-linked immunosorbent assay kit (Quantikine; R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer's protocol.
Flow cytometry analysis of the Tregs
To analyze the Treg levels in the samples, a three-color flow cytometry analysis was performed with a mixture of antibodies, including fluorescent isothiocyanate (FITC)-conjugated anti-human CD4, phycoerythrin-conjugated anti-human FoxP3, and allophycocyanin-conjugated anti-human CD25. Data acquisition and analysis were performed on a FACS Aria II flow cytometer (BD Biosciences, San Jose, CA, USA) using FlowJo v10.2 software (Becton, Dickinson and Co., Becton Drive, Franklin Lakes, NJ, USA). The percentage of the Tregs in the samples was calculated as the fraction of the total number of PBMCs.
Flow cytometry analysis of the MDSCs
To analyze the MDSC levels in the samples, a three-color flow cytometry analysis was performed with a mixture of antibodies, including FITC-conjugated antiCD14, phycoerythrin-conjugated antiCD11b, and phycoerythrin cyanin 5.1-conjugated antiCD33. Data acquisition and analysis were performed on a FACS Aria II flow cytometer using FlowJo v10.2 software. The percentage of the MDSCs in the samples was calculated as the fraction of the total number of PBMCs.
Statistical analysis
All the data are presented as the mean ± standard deviation. Differences between the groups were determined using the Student's t-test The relationships between two variables were quantified using Spearman's rank correlation coefficient. The significance of the correlations between the parameters was analyzed using the χ2 test and a t-test To assess the overall survival (OS) and disease-free-survival (DFS) rate, the data that were available until the last follow-up date or at 2500 days were censored. The prognoses of the patients were analyzed using the Kaplan-Meier method, and the log-rank test was used to determine the significance of the differences. A multivariate Cox regression analysis of the survival of the patients with preoperative BC was performed according to the tumor subtype and Ki67 status, as defined in the NCCN Clinical Practice Guidelines in Oncology.17,18 A Cox proportional hazards model was used to examine the simultaneous effects of multiple covariates on survival. The effect of each variable was described with the hazard ratio (HR) and a 95% confidence interval. Statistical significance was set at p <0.05. SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses.
Staining for HE, PD-L1, and CD8
Formalin-fixed and paraffin-embedded breast tumor blocks were selected from primary FBC and NFBC tumors and diagnostic core biopsies with their corresponding primary tumor surgical specimens. The tissue sections were histochemically stained with hematoxylin-eosin (HE). Estrogen receptor, progesterone receptor, Ki67, and HER2 immunohistochemical staining were performed in a pathology lab using routine accredited procedures. Tissues from FBC patients with high sIL-2R levels and TNBC were stained for PD-L1 and CD8. The slides were deparaffinized in toluene and rehydrated in graded alcohols, after which heat-induced epitope retrieval was performed, followed by a PD-L1 IHC protocol using clone E1L3N (rabbit; Cell Signaling Technology, Danvers, MA, USA) and a CD8 IHC protocol using clone (C8/144B) (monoclonal mouse; Dako, Agilent Technologies, Santa Clara, CA, USA).
Results
Clinical features of patients according to subtype
The present study included a total of 106 patients; of these, 24 had FBC and 82 had NFBC. The median ages of the participants were 42.0 years (range, 30 – 63 years) for the FBC patients, 61.0 years (range, 46 – 88 years) for the NFBC patients, and 53.6 years (range, 30 – 74 years) for the healthy volunteers. The FBC group included three patients with stage I BC, 13 with stage II, two with stage III, and six with stage IV, whereas the NFBC group included 16 patients with stage I BC, 35 with stage II, 10 with stage III, and 21 with stage IV (Table 1). None of the patients had received anticancer treatments prior to the study.
Table 1.
IL-2R level on age in FBC versus NFBC by stage and healthy volunteers.
| Stage | FBC | NFBC | Healthy volunteers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | n | % | IL-2R (U/mL) | Age | n | % | IL-2R (U/mL) | Age | n | IL-2R (U/mL) | |
| I | 37.6 | 3 | 13 | 919.6 | 48.3 | 16 | 20 | 698.3 | – | – | – |
| II | 36.6 | 13 | 54 | 1067.7 | 59.1 | 35 | 43 | 794.3 | – | – | – |
| III | 45.5 | 2 | 8 | 1344.4 | 66.6 | 10 | 12 | 981.1 | – | – | – |
| IV | 48.3** | 6 | 25 | 1691.5* | 70.3 | 21 | 25 | 987.9 | – | – | – |
| Total | 42.0* | 24 | 100 | 1255.8* | 61.0 | 82 | 100 | 865.4 | 53.6 | 11 | 667 |
FBC: familial breast cancer; NFBC: non-familial breast cancer; Age: years; n: number; IL-2R: interleukin-2 receptor.
*<0.05, **<0.01.
The p values were determined using the Student's t-test.
Serum sIL-2R levels
As shown in Figure 1, the sIL-2R levels were higher in the patients (FBC and NFBC, 1014.2 ± 79.2 U/mL) than in the healthy volunteers (667.5 ± 42.5 U/mL). The sIL-2R level of the FBC patients (1255.8 ± 176.2) was significantly higher than those of the healthy volunteers (p = 0.04) and NFBC patients (865.4 ± 58.8 U/mL, p = 0.01).
Figure 1.
Results of the sIL-2R evaluation for the healthy volunteers and the patients with FBC + NFBC, FBC alone, and NFBC. The sIL-2R levels were higher in the patients with (FBC+NFBC) than in the healthy volunteers. The sIL-2R levels in the FBC patients were significantly higher than those of the healthy volunteers (*p = 0.0414) and the NFBC patients (**p = 0.0155). The data are presented as the mean ± SD. The P values were determined using the Student's t-test. sIL-2R: soluble interleukin-2 receptor; FBC: familial breast cancer; NFBC: non-familial breast cancer.
sIL-2R correlations
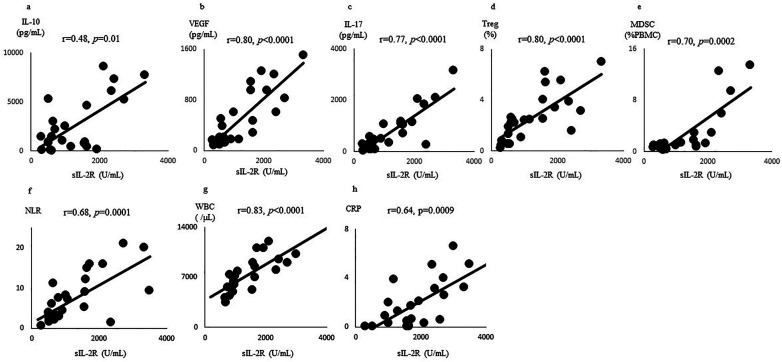
As shown in Figure 2, the sIL-2R level in the FBC patients was significantly positively correlated with IL-10 production (r = 0.48, p = 0.01), the VEGF level (r = 0.80, p < 0.0001), IL-17 production (r = 0.77, p < 0.0001), the Treg level (r = 0.80, p < 0.0001), the MDSC level (r = 0.70, p = 0.0002), the NLR (r = 0.68, p = 0.0001), the WBC count (r = 0.83, p < 0.0001), and the CRP level (r = 0.64, p = 0.0009).
Figure 2.
Correlations between sIL-2R and IL-10, VEGF, IL-17, Tregs, MDSCs, NLR, WBC, and CRP. The sIL-2R levels of the FBC patients were significantly positively correlated with the (a) IL-10 production, (b) VEGF levels, (c) IL-17 production, (d) Treg levels, (e) MDSC levels, (f) NLR, (g) WBC count, and (h) CRP level. The relationship between two variables was quantified by Spearman's rank correlation coefficient.
sIL-2R: soluble interleukin-2 receptor; IL-10: interleukin-10; VEGF: vascular endothelial growth factor; IL-17: interleukin-17; Treg: regulatory T cell; MDSC: myeloid-derived suppressor cell; NLR: neutrophil to lymphocyte ratio; WBC: white blood cell; CRP: C-reactive protein.
The participants’ sIL-2R levels were classified as high or low based on a cutoff value of 700 U/mL. This cutoff value was chosen based on the median sIL-2R value of 667.50 ± 42.54 U/mL in the healthy subjects. The levels of the Tregs (p = 0.0008) and MDSCs (p = 0.01) were significantly increased in the FBC patients with high serum sIL-2R levels compared to those with low serum sIL-2R levels (Figure 3).
Figure 3.
The Treg and MDSC levels in the FBC patients according to the sIL-2R level based on a cutoff value of 700 U/mL, the levels of (a) Treg (*p = 0.0008) and (b) MDSC (**p = 0.01) were significantly higher in the patients with high sIL-2R levels than in those with low sIL-2R levels. The data are presented as the mean ± SD. The p values were determined using the Student's t-test.
Treg: regulatory T cell; MDSC: myeloid-derived suppressor cell; FBC: familial breast cancer; sIL-2R: soluble interleukin-2 receptor.
Overall and DFS rates of the FBC patients
The FBC patients with high sIL-2R levels (>700 U/mL) showed significantly worse OS (p = 0.0091) and DFS (p = 0.0038) rates than those with low sIL-2R levels (Figure 4). The univariate and multivariate data were analyzed to confer prognostic values to various factors by comparing them to the primary prognostic factors. In the univariate analysis, sIL-2R≥700 U/mL was significantly associated with OS (p = 0.0091) and DFS (p = 0.0038). The multivariate regression analysis showed that the independent, favorable prognostic factors were age (FBS: Stage IV) (p < 0.001; HR, 0.338; 95% CI 0.205 – 0.552), age (FBS: total) (p < 0.05; HR, 0.584; 95% CI 0.380 – 0.901), IL-2R (FBS: Stage IV) (p < 00.5; HR, 0.619; 95% CI 0.402 – 0.955), and IL-2R (FBS: total) (p < 0.05; HR, 0.643; 95% CI 0.432 – 0.959) (Table 1).
Figure 4.
Kaplan-Meier estimates of the overall and disease-free survival rates, according to the surveillance status of the FBC cases. The (a) OS and (b) DFS rates were significantly worse for the FBC patients with high sIL-2R (≥700 U/mL) levels than for those with low sIL-2R (<700 U/mL) levels.
FBC: familial breast cancer; OS: overall survival; DFS: disease-free-survival; sIL-2R: soluble interleukin-2 receptor.
The Kaplan-Meier plot for the DFS rate was dichotomized based on sIL-2R expression above and below the median value of 700 U/mL.
Immunohistochemistry of HE, PD-L1, and CD8
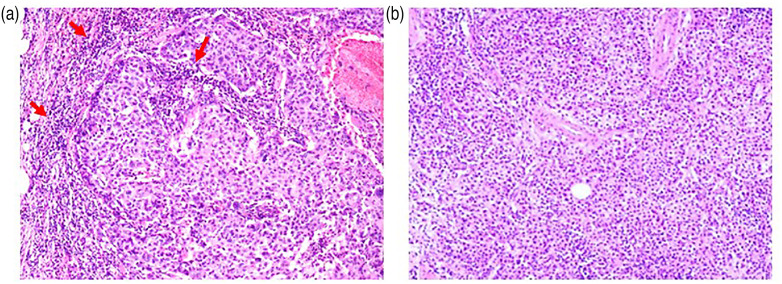
Histopathological analyses were performed on a tissue specimen from an FBC patient diagnosed with invasive ductal carcinoma that was staged as T2N2M1 Stage IV (tumor (T)2, node (N)2, metastasis (M)1, lymphatic invasion (Ly)1, venous invasion (V)2, estrogen receptor (ER) (−) (Allred Score [AS]: 0/8), progesterone receptor (PgR) (−) (AS: 0/8), Ki67 (70%), human epidermal growth factor receptor 2 (HER2) (−), and grade 3 [poorly differentiated]) (Figure 5(a)) and a specimen from an NFBC patient diagnosed with invasive ductal carcinoma that was staged as T2N1M1 Stage IV (Ly1, V1, ER (+) (AS: 5/8), PgR (+) (AS: 5/8), Ki67 (27%), HER2 (+), grade 1 [well differentiated]) (Figure 5(b)).
Figure 5.
Representative hematoxylin-eosin (HE) staining images breast tumor tissue staining. Tumor-infiltrating lymphocytes in breast tumors are shown in tissue sections stained with HE. More immune cells (red arrows) are shown in (a) the familial breast cancer specimens than in (b) the non-familial breast cancer HE specimens. The images are shown at 100× magnification.
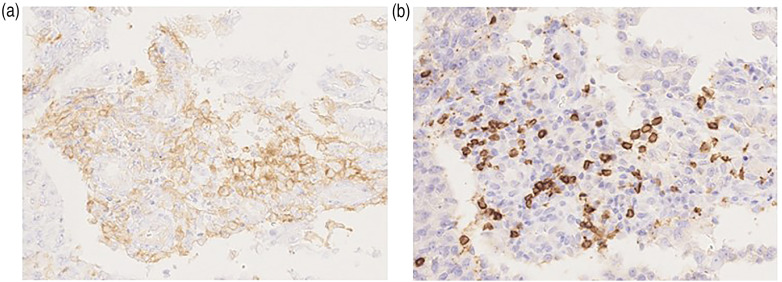
The HE analysis of the FBS patient specimens shows more stromal and intratumoral tumor-infiltrating lymphocytes (TILs) around the cancer cells than that of the NFBC patients. The immunohistochemical analyses of the biopsy specimens from the FBC patients with high sIL-2R levels and TNBC show more PD-L1 (Figure 6(a)) and CD8 (Figure 6(b)) staining than that of the specimens from the NFBC patients with low sIL-2R levels and non-TNBC. A comparative analysis of the TIL scoring from the histochemically and immunohistochemically stained tumor sections was not performed in this study.
Figure 6.
Representative immunohistochemistry images for the PD-L1 and CD8 cells. The epithelial compartment is positive for (a) PD-L1, whereas the infiltrating immune cells are positive for (b) CD8. The images are shown at 200× magnification.
PD-L1: programmed cell death ligand 1; CD8: cluster of differentiation 8.
Discussion
BC is a heterogeneous disease with multiple molecular subtypes. In this study, there was not a significant difference in the tumor subtypes between the FBC and NFBC patients (Table 2). Since Perou et al.17,18 used cDNA microarrays and performed gene expression profiling (GEP) of BC specimens in 2000, intrinsic subtype classification based on GEP has attracted attention. Based on this classification method, BC is divided into different biological subtypes, such as luminal A, luminal B, HER2-enriched, basal-like, and normal breast-like. In the Sankt Gallen consensus meetings of 2011 and 2013, a substitute definition of the intrinsic subtypes based on the ER/PgR/HER2/Ki67 status, which is mainly composed of immunohistochemical examinations performed as part of the common pathological examinations, was adopted.19–21 The subtype definitions in this study include ER-/PgR-/HER2 (TNBC), ER−/PgR−/HER2+, ER+/PgR+/HER2+, ER+ and/or PgR+/HER2−, luminal A-like (high ER/PR and clearly low Ki-67 or grade), and luminal B-like (lower ER/PR with a clearly high Ki-67 and histological grade 3 in the clinical grouping) (Table 2). 22 The HER2-enriched and basal-like subtypes should be defined by genetic analysis, but we did not do this. Our analysis showed that 13 of the 24 FBC cases were of the luminal A subtype, which makes it very unlikely that they were BRCA1/2, PALB2, or ATM cases. The sIL-2R levels were not linked to luminal A as much as the other types, however. We tried to evaluate the subtype prognostic factors according to stage, but we were unable to identify any prognostic factors that were significantly different from each other because of the small number of FBC patients. The results of this study should be confirmed by further studies with a larger number of subjects.
Table 2.
The clinical features of the patients according to subtype.
| FBC (n = 24) | NFBC (n = 82) | |
|---|---|---|
| ER − /PgR − /HER2− (TNBC) | 2 | 12 |
| ER − /PgR − /HER2+ | 2 | 5 |
| ER + /PgR + /HER2+ | 1 | 4 |
| ER + and/or PgR + /HER2− | 2 | 11 |
| Luminal A-like | 13 | 28 |
| Luminal B-like | 4 | 22 |
ER: estrogen receptor; PgR: progesterone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer; luminal A: high receptor, low proliferation, low grade, luminal B: low receptor, high proliferation, high grade, n: number, +: positive, −: negative.
Serum sIL-2R levels are higher in several malignancies than in healthy individuals. Breast cancer patients have been shown to have increased serum levels of sIL-2R, 23 as well as IL-10, VEGF, IL-17, Tregs, and MDSCs.24–28 Various studies have indicated that sIL-2R in cancer patients shows correlations with IL-10, VEGF, and IL-17 and relationships with Tregs and MDSCs.29–31 In the current study, the FBC patients with high sIL-2R levels had significantly worse OS and DFS rates compared to those with low sIL-2R levels. Furthermore, our results show that stage was related to sIL-2R levels. Several studies linking sIL-2R levels with breast cancer and stage also showed a link with age.6,32,33 We found that the sIL-2R level was correlated with stage IV in the FBC group. Additionally, age was correlated with the sIL-2R level in the FBC group, but not in the NFBC.
Compared with NFBC, FBC is comprised of cancer cells that; (1) contain more mutations; (2) release neoantigens and appear to have CD8 + T cells in and around the microenvironment that express IL-2 receptors; and (3) release more sIL-2Rs into the blood. Moreover, FBC patients show simultaneous increases in IL-10 levels and the numbers of immunosuppressive cells, such as Tregs and MDSCs. In the present study, the sIL-2R level in the FBC patients was significantly and positively correlated with IL-10, VEGF, IL-17, Tregs, and MDSCs. The significantly positive correlation between sIL-2R and IL-10 indicates a disruption of the Th2>Th1 balance, which leads to Th2 predominance and suppression of cellular immunity. The significantly positive correlations that sIL-2R shows with VEGF and IL-17 suggest further tumor development, as high levels of both VEGF and IL-17 increase the number of immunosuppressive cells.
Conversely, our results do not show a significant positive correlation between sIL-2R and IL-10, VEGF, IL-17, Tregs, or MDSCs for the NFBC patients. This suggests that the inflammatory and immune responses are more dramatic in the cancer microenvironment of FBC than of NFBC. High sIL-2R levels significantly increased the number of Tregs and MDSCs in the FBC patients, but not in the NFBC patients. This implies that preventing the growth of immunosuppressive cells, such as Tregs and MDSCs, in FBC patients is important. Moreover, high sIL-2R levels were indicative of a significantly worse prognosis for FBC patients than for NFBC patients. Elevated sIL-2R levels may predispose FBC patients to cancer growth, rather than cancer suppression. IL-2 was administered to mice with spontaneous mammary cancer, which is an appropriate mouse model of FBC, and it resulted in a significant prolongation of the average survival time. 34
PD-L1 is expressed in 20% of TNBCs, suggesting that it is a therapeutic target in TNBCs. In this study, an immunohistochemical analysis confirmed that there was PD-L1 and CD8 expression in the FBC tissues with high sIL-2R levels, but less CD8 staining in the NFBC tissues. This indicates that the expression of PD-LI is similar in both FBC and NFBC; however, in the cancer microenvironment of FBC, a Th2>Th1 imbalance occurred because the NLR, WBC, CRP, and IL-10 levels, which are indices of inflammation, showed positive correlations. Furthermore, Tregs and MDSCs were present. Therefore, in and around the microenvironment, more CD8 + T cells appear to be induced and developed to attack the cancer. Although the direct relationship between IL-2 and PD-L1 is unclear, a combination of IL-2 and PD-L1 inhibitors has been used as a treatment regimen for chronic infections and cancer. 35 Regardless of the Treg or MDSC levels, immune checkpoint inhibitors should be effective when CD8 + T cells proliferate.
When treating FBC patients, we should consider the relationship between cancer and immunity, as well as that between cancer and genetics. In the future, shifting the Th1/Th2 balance toward Th1-dominance to inactivate immunosuppressive cells may enhance the treatment efficacy of immune checkpoint inhibitors in patients with FBC. It has been reported that BRCA1- and BRCA2-deficient breast cancers are associated with features of genomic instability, including increased mutation burden. In such cases, identification of the genes associated with an increased TMB would enable the prediction of sensitivity to immune checkpoint inhibitors and, therefore, the development of more efficacious therapies for FBC patients.
This study was preliminary and should be validated by studies with a larger number of subjects. The best way to test the effect of sIL-2R as a predictive and prognostic marker for patients with FBC would be a controlled randomized clinical trial with measurable and clinically relevant outcomes.
In the present study, sIL-2R was shown to be a reliable marker for FBC. Suppression of cellular immunity and an increased number of immunosuppressive cells brought about by high sIL-2R levels may be related to immunological mechanisms. The possibility of targeting IL-2R expression and immune checkpoints needs to be investigated in the future.
Furthermore, various immunological parameters, including immune cells and cytokines associated with the illness duration in patients with severe coronavirus disease-2019 (COVID-19), have been investigated recently. The significant association between illness duration and sIL-2Rα levels in patients with severe COVID-19 implies that T cells are involved in the evolution of COVID-19. 36 sIL-2R may be a biomarker for identification and predicting the clinical progression of the disease in FBC patients with severe COVID-19.
Acknowledgements
The authors thank the patients and their family members for their participation in this study and the study coordinator for recruiting the study participants. This article was dedicated to the families lost in the 2011 Great East Japan Earthquake and Tsunami. This study was part of the Okinawa—Fukushima Obesity Project.
Author biographies
Kenji Gonda is a specially appointed professor at Fukushima Medical University, working at Daido Central Hospital and acting as director. He is a medical doctor and has a PhD. His specialties are oncology surgery, clinical genetics, and tumor immunology. He is a certified breast cancer specialist and is studying familial breast cancer. He investigates the increasing pharmacological effects of hereditary breast cancer in Okinawa. He is a member of ASCO (American Society of Clinical Oncology), AACR (American Association for Cancer Research), and ASHG (American Society of Human Genetics).
Shoichiro Horita works as a lecturer at the Department of Bioregulation and Pharmacological Medicine, Fukushima Medical University. He holds a PhD from the University of Tokyo and studied at Oxford University, specializing in the interrelationships between in vivo molecules and cell channel mutations, and investigates interleukin and cell channel mutations.
Yuko Maejima is a specially appointed professor in the Division of Obesity and Inflammation Analysis, in the Department of Bioregulation and Pharmacological Medicine, Fukushima Medical University, holds her PhD, and specializes in the development and construction of the oxytocin neural network. Oxytocin might become a biomarker for tumors, including breast cancer, and thus she investigates the relationship between oxytocin and interleukin.
Seiichi Takenoshita is the president of Fukushima Medical University and also the president of the Board. He was previously a professor of oncology surgery at Fukushima Medical University. He is a medical doctor and has a PhD. He is currently in charge of all clinical research at the university. Of these, genetic studies of tumors including breast cancer, immunological studies, and radiological studies are also included. Immediately after the nuclear power plant accident following the Great East Japan Earthquake on March 11, 2011, he spearheaded the construction of a medical system.
Kenju Shimomura is a professor at the Department of Bioregulation and Pharmacological Medicine, Fukushima Medical University. He is a medical doctor and has a PhD. Endocrine and metabolic diseases such as diabetes and obesity that he is investigating are involved in the development of breast cancer, and assorted drugs are used to regulate various biological functions and physiological functions in breast cancer and other tumors. He is involved in research to unravel and control the pathology.
Footnotes
Authors’ contributions: KG recruited the patients and performed the clinical investigations. KG, SH, YM, KS, and ST conceived and designed the study and drafted the manuscript. Each author certifies that all the investigations were conducted in conformity with the ethical principles. All the authors have read and approved the final manuscript.
Consent for publication: Written informed consent for the publication of this case report and any accompanying images was obtained from all patients.
Availability of data and materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The name of the study for the Fukushima Medical University IRB was ‘The study of immune suppression, inflammation, and nutritional damage in cancer patients’.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Kenji Gonda https://orcid.org/0000-0002-7671-1808
References
- 1.The Genetic and Rare Diseases Information Center (GARD), a program of the National Center for Advancing Translational Sciences (NCATS), the National Institutes of Health (NIH). Familial breast cancer, https://rarediseases.info.nih.gov/diseases/10415/familial-breast-cancer (Last updated: 8/30/2017, accessed 20 August 2021).
- 2.Cerretti DP, McKereghan K, Larsen A, et al. Cloning, sequence, and expression of bovine interleukin 2. Proc Natl Acad Sci USA 1986; 83: 3223–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3- expressing regulatory T cells. Nat Immunol 2005; 6: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 4.Coventry BJ, Weeks SC, Heckford SE, et al. Lack of IL-2 cytokine expression despite Il-2 messenger RNA transcription in tumor-infiltrating lymphocytes in primary human breast carcinoma: selective expression of early activation markers. J Immunol 1996; 156: 3486–3492. [PubMed] [Google Scholar]
- 5.García-Tuñón I, Ricote M, Ruiz A, et al. Interleukin-2 and its receptor complex (alpha, beta and gamma chains) in in situ and infiltrative human breast cancer: an immunohistochemical comparative study. Breast Cancer Res 2004; 6: R1–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesarová P, Kvasnicka J, Umlaufová A, et al. Soluble TNF and IL-2 receptors in patients with breast cancer. Med Sci Monit 2000; 6: 661–667. [PubMed] [Google Scholar]
- 7.Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm 2005; 3: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen DL, Mahmud SA, Vang KB, et al. Identification of cellular sources of IL-2 needed for regulatory T cell development and homeostasis. J Immunol 2018; 200: 3926–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol 2018; 36: 411–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kintsler S, Cassataro MA, Drosch M, et al. Expression of programmed death ligand (PD-L1) in different tumors. Comparison of several current available antibody clones and antibody profiling. Ann Diagn Pathol 2019; 41: 24–37. [DOI] [PubMed] [Google Scholar]
- 12.Wen WX, Leong CO. Association of BRCA1- and BRCA2-deficiency with mutation burden, expression of PD-L1/PD-1, immune infiltrates, and T cell-inflamed signature in breast cancer. PLoS One 2019; 14: e0215381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018; 16: 1362–1389. [DOI] [PubMed] [Google Scholar]
- 14.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN Clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw 2009; 7: 1060–1096. [DOI] [PubMed] [Google Scholar]
- 15.Lynch HT, Lynch JF. Breast cancer genetics. In: 1st International Research Conference on Familial Cancer September 16–21, 1985 (eds Müller HR, Weber W.), 1985, pp. 20–24, Basel: Karger AG. [Google Scholar]
- 16.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1. 2020. J Natl Compr Canc Netw 2020; 18: 380–391. [DOI] [PubMed] [Google Scholar]
- 17.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752. [DOI] [PubMed] [Google Scholar]
- 18.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol 2011; 22: 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol 2015; 26: 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer 2017. Ann Oncol 2017; 28: 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami S, Hirayama R, Satomi A, et al. Serum soluble interleukin-2 receptor levels in patients with breast cancer. Breast Cancer 1997; 4: 25–28. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharjee HK, Bansal VK, Nepal B, et al. Is interleukin 10 (IL10) expression in breast cancer a marker of poor prognosis? Indian J Surg Oncol 2016; 7: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llanes-Fernández L, Alvarez-Goyanes RI, Arango-Prado Mdel C, et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast 2006; 15: 482–489. [DOI] [PubMed] [Google Scholar]
- 26.Maniati E, Hagemann T. IL-17 mediates resistance to anti-VEGF therapy. Nat Med 2013; 19: 1092–1094. [DOI] [PubMed] [Google Scholar]
- 27.Plitas G, Konopacki C, Wu K, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity 2016; 45: 1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonda K, Shibata M, Ohtake T, et al. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett 2017; 14: 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mindiola R, Caulejas D, Núñez-Troconis J, et al. Increased number of IL-2, IL-2 receptor and IL-10 positive cells in premalignant lesions of the cervix. Invest Clin 2008; 49: 533–545. [PubMed] [Google Scholar]
- 30.Nukui A, Masuda A, Abe H, et al. Increased serum level of soluble interleukin-2 receptor is associated with a worse response of metastatic clear cell renal cell carcinoma to interferon alpha and sequential VEGF-targeting therapy. BMC Cancer 2017; 17: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Jang SW, Lee W, et al. PTEN Drives Th17 cell differentiation by preventing IL-2 production. J Exp Med 2017; 214: 3381–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwa HL, Kuo WH, Chang LY, et al. Prediction of breast cancer and lymph node metastatic status with tumour markers using logistic regression models. J Eval Clin Pract 2008; 14: 275–280. [DOI] [PubMed] [Google Scholar]
- 33.Lv M, Xiaoping X, Cai H, et al. Cytokines as prognostic tool in breast carcinoma. Front Biosci (Landmark Ed) 2011; 16: 2515–2526. [DOI] [PubMed] [Google Scholar]
- 34.Semushina SG, Aronov DA, Moiseeva EV. Local interleukin-2 immunotherapy of breast cancer: benefit and risk in a spontaneous mouse model. Pathol Oncol Res 2019; 25: 945–951. [DOI] [PubMed] [Google Scholar]
- 35.West EE, Jin HT, Rasheed AU, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest 2013; 123: 2604–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma A, Zhang L, Ye X, et al. High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front Immunol 2021; 12: 626235. [DOI] [PMC free article] [PubMed] [Google Scholar]